Published online Mar 27, 2023. doi: 10.4240/wjgs.v15.i3.387
Peer-review started: October 31, 2022
First decision: January 3, 2023
Revised: January 11, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 27, 2023
Processing time: 147 Days and 6.3 Hours
Surgical site infections (SSIs) are the commonest healthcare-associated infection. In addition to increasing mortality, it also lengthens the hospital stay and raises healthcare expenses. SSIs are challenging to predict, with most models having poor predictability. Therefore, we developed a prediction model for SSI after elective abdominal surgery by identifying risk factors.
To analyse the data on inpatients undergoing elective abdominal surgery to identify risk factors and develop predictive models that will help clinicians assess patients preoperatively.
We retrospectively analysed the inpatient records of Shaanxi Provincial People’s Hospital from January 1, 2018 to January 1, 2021. We included the demographic data of the patients and their haematological test results in our analysis. The attending physicians provided the Nutritional Risk Screening 2002 (NRS 2002) scores. The surgeons and anaesthesiologists manually calculated the National Nosocomial Infections Surveillance (NNIS) scores. Inpatient SSI risk factors were evaluated using univariate analysis and multivariate logistic regression. Nomograms were used in the predictive models. The receiver operating characteristic and area under the curve values were used to measure the specificity and accuracy of the model.
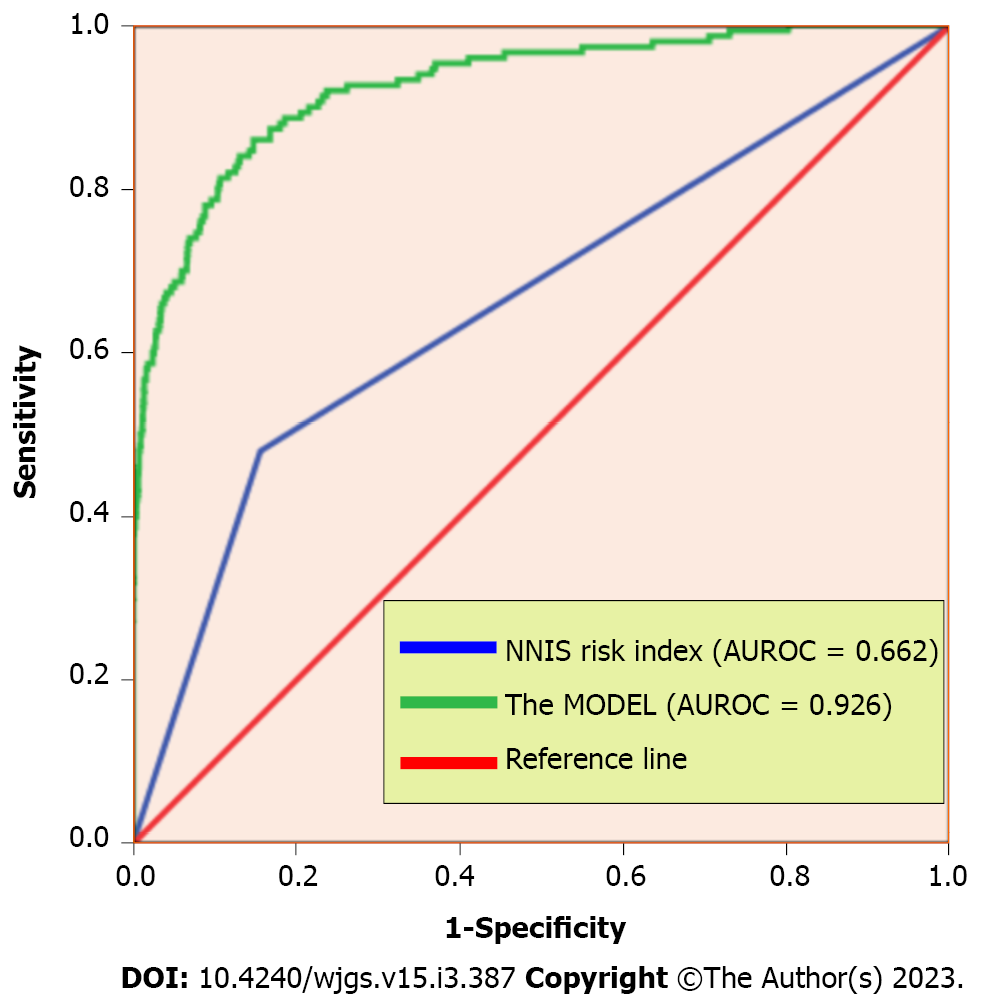
A total of 3018 patients met the inclusion criteria. The surgical sites included the uterus (42.2%), the liver (27.6%), the gastrointestinal tract (19.1%), the appendix (5.9%), the kidney (3.7%), and the groin area (1.4%). SSI occurred in 5% of the patients (n = 150). The risk factors associated with SSI were as follows: Age; gender; marital status; place of residence; history of diabetes; surgical season; surgical site; NRS 2002 score; preoperative white blood cell, procalcitonin (PCT), albumin, and low-density lipoprotein cholesterol (LDL) levels; preoperative antibiotic use; anaesthesia method; incision grade; NNIS score; intraoperative blood loss; intraoperative drainage tube placement; surgical operation items. Multivariate logistic regression revealed the following independent risk factors: A history of diabetes [odds ratio (OR) = 5.698, 95% confidence interval (CI): 3.305-9.825, P = 0.001], antibiotic use (OR = 14.977, 95%CI: 2.865-78.299, P = 0.001), an NRS 2002 score of ≥ 3 (OR = 2.426, 95%CI: 1.199-4.909, P = 0.014), general anaesthesia (OR = 3.334, 95%CI: 1.134-9.806, P = 0.029), an NNIS score of ≥ 2 (OR = 2.362, 95%CI: 1.019-5.476, P = 0.045), PCT ≥ 0.05 μg/L (OR = 1.687, 95%CI: 1.056-2.695, P = 0.029), LDL < 3.37 mmol/L (OR = 1.719, 95%CI: 1.039-2.842, P = 0.035), intraoperative blood loss ≥ 200 mL (OR = 29.026, 95%CI: 13.751-61.266, P < 0.001), surgical season (P < 0.05), surgical site (P < 0.05), and incision grade I or III (P < 0.05). The overall area under the receiver operating characteristic curve of the predictive model was 0.926, which is significantly higher than the NNIS score (0.662).
The patient’s condition and haematological test indicators form the bases of our prediction model. It is a novel, efficient, and highly accurate predictive model for preventing postoperative SSI, thereby improving the prognosis in patients undergoing abdominal surgery.
Core Tip: Herein, we retrospectively analysed the data, including patient personal information, test indicators, and surgical information, of patients undergoing elective abdominal surgery and used univariate and multivariate logistic regression analyses to assess risk factors for surgical site infection (SSI) in hospitalised patients. Nomograms were used in the prediction models. Subject working characteristics and area under the curve were used to measure the accuracy of the model up to 97%. R language was used to create a web page for dynamic predictive analysis of abdominal SSIs. A new predictive approach for preventing abdominal SSIs is made easier and more precise.
- Citation: Zhang J, Xue F, Liu SD, Liu D, Wu YH, Zhao D, Liu ZM, Ma WX, Han RL, Shan L, Duan XL. Risk factors and prediction model for inpatient surgical site infection after elective abdominal surgery. World J Gastrointest Surg 2023; 15(3): 387-397
- URL: https://www.wjgnet.com/1948-9366/full/v15/i3/387.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i3.387
Surgical site infection (SSI) is the commonest healthcare-associated infection[1] that helps determine patient prognosis. SSIs occur in 2%-5% of inpatients undergoing surgery in the United States[2]. The incidence of SSI ranges from 2% to 10% in Europe[3-5], while in China, it ranges from 4% to 6%[6,7]. Patients undergoing complex surgeries associated with high-risk factors are more likely to develop SSI[8]. SSI results in a prolonged hospital length of stay (LOS). It burdens patients physically, psychologically, and economically[9].
Patients with abdominal symptoms requiring abdominal surgeries, such as gastric surgery, colorectal surgery, appendix surgery, etc., have a higher incidence of postoperative infection because the human gastrointestinal tract is a cavity that communicates with the outside world, comprising a wide variety of intestinal flora, which can cause infections[10,11]. The National Quality Partnership, as part of the Surgical Care Improvement Project (SCIP), aims to prevent postoperative SSI. Several preoperative quality indicators, namely preoperative oxygen inhalation, normal body temperature maintenance, adequate circulating glucose, sterile drapes, surgical gowns, wound-protection devices, antimicrobial-coated sutures, incisional wound irrigation, and prophylactic negative-pressure wound therapy, lower the risk of SSI[12]. Despite these efforts, the LOS remained high, and the SSI remained unaffected. The National Nosocomial Infections Surveillance (NNIS) risk index is a traditional tool used to predict SSI[13]. The model comprises the American Society of Anaesthesiologists’ preoperative assessment score, incision grade, and surgery time, with the score ranging from 0 to 3. These three elements, however, are insufficient to construct a prediction model. Grant et al[14] later developed a prediction model with an area under the receiver operating characteristic curve (AUROC) of 0.65, higher than that of the NNIS. Despite its ease of use, this model could only be applied to colorectal surgery. Therefore, our goal was to establish a novel, efficient, and highly accurate predictive model to prevent postoperative SSI in patients undergoing abdominal surgery.
The clinical data of 3018 patients who underwent abdominal surgeries from January 2018 to January 2021 at Shaanxi Provincial People's Hospital were retrospectively analysed. We included patients aged > 18 years and < 100 years in the study. This study was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from the patients and their families before surgery. SSI was diagnosed if one of the following occurred: Incision infection, deep incision infection, and organ-space infection[15]. The infection prevention and control staff manually diagnosed SSI. This study was approved by the Ethics Committee of Shaanxi Provincial People's Hospital.
The hospital information system (HIS) was used to obtain the following patient-related data: Basic information: Age, gender, marital status, place of residence, and a history of diabetes and hypertension.
Scores: Nutritional Risk Screening 2002 (NRS 2002) and NNIS.
Preoperative biochemical index: Red blood cell, white blood cell (WBC), haemoglobin, procalcitonin (PCT), albumin (ALB), triglyceride, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol, and total cholesterol levels.
Hospitalisation information: Preoperative duration (days from admission to surgery), preoperative antibiotic use, surgical season, anaesthesia method (general anaesthesia or non-general anaesthesia), incision grade (I, II, or III), intraoperative blood loss, intraoperative irrigation, tension reduction suture, incision drainage, multiple tissue excision, and the surgical site.
The 22.0 and R 4.2.1 were used to perform statistical analyses. The chi-square test or Fisher's exact test was used to compare enumeration data, and the t-test was used to compare measurement data. SSI was the dependent variable, and the other variables were the independent variables. Significant indicators of SSI after abdominal surgery (P < 0.05) were identified using the univariate analysis, and multivariate logistic regression was used to identify independent risk factors for SSI after abdominal SSI (P < 0.05). The "rms" package in R 4.2.1 was used to display the prediction model as a nomogram based on independent risk factors. A nomogram was used to calculate the probability of SSI after abdominal surgery. Scores are assigned to each index. Higher probabilities were associated with a higher score. Receiver operating characteristic curves were constructed, and the area under the curve (AUC) values were calculated. The higher the value, the higher the model’s accuracy. The datasets analysed in the current study are not publicly available due to the hospital’s restrictions on public resources and confidentiality requirements; however, they are available from the corresponding author upon reasonable request.
A total of 3018 patients were included in this study. Of these, 150 patients were diagnosed with SSI, and 2868 were diagnosed with nonsurgical site infection. The median age of the patients was 45 years. Of the 3018 patients, 900 (29.8%) were males, 2118 (70.2%) were females, 1622 (53.7%) patients lived in urban areas, and 1396 (46.3%) patients lived in rural areas. A total of 539 (17.8%) patients had hypertension, and 402 (13.3%) patients had diabetes. The surgical site distribution was as follows: The uterus (42.2%), the liver (27.6%), the gastrointestinal tract (19.1%), the appendix (5.9%), the kidney (3.7%), and the groin area (1.4%).
Univariate and multivariate logistic regression analyses were performed on SSI development after abdominal surgery. Univariate analyses revealed that gender; age; marital status; place of residence; history of diabetes; the NRS 2002 score; the NNIS score; preoperative WBC, PCT, ALB, and LDL; preoperative antibiotic use; anaesthesia method, incision grade; intraoperative blood loss; intraoperative drainage; multiple tissue excision; surgical season; and surgical site were significantly associated with postoperative abdominal incision infection (P < 0.05) (Table 1).
| Factors | SSI (n = 150) | NSSI (n = 2868) | X2 | P value | |
| Gender | Male | 85 | 815 | 54.356 | < 0.001 |
| Female | 65 | 2053 | |||
| Age | < 70 yr | 83 | 2145 | 27.927 | < 0.001 |
| ≥ 70 yr | 67 | 723 | |||
| Marriage | Married | 132 | 2678 | 10.006 | 0.007 |
| Single | 7 | 108 | |||
| Others | 11 | 82 | |||
| Residence | Rural | 83 | 1313 | 5.232 | 0.022 |
| Urban | 67 | 1555 | |||
| Antibiotic use | Yes | 14 | 66 | 27.316 | < 0.001 |
| No | 136 | 2802 | |||
| Hypertension | Yes | 20 | 519 | 2.204 | 0.138 |
| No | 130 | 2349 | |||
| Diabetes | Yes | 46 | 356 | 41.137 | < 0.001 |
| No | 104 | 2512 | |||
| Preoperative duration | < 7 d | 108 | 2248 | 3.391 | 0.066 |
| ≥ 7 d | 42 | 620 | |||
| NRS 2002 | < 3 | 104 | 2652 | 44.853 | < 0.001 |
| ≥ 3 | 46 | 216 | |||
| NNIS | < 2 | 80 | 2420 | 96.634 | < 0.001 |
| ≥ 2 | 70 | 448 | |||
| RBC (1012/L) | < 4 | 86 | 1565 | 0.44 | 0.507 |
| ≥ 4 | 64 | 1303 | |||
| WBC (109/L) | < 10 | 86 | 1943 | 7.017 | 0.008 |
| ≥ 10 | 64 | 925 | |||
| HB (g/L) | < 120 | 67 | 1473 | 2.555 | 0.11 |
| ≥ 120 | 83 | 1395 | |||
| PCT (μg/L) | < 0.05 | 58 | 1824 | 37.748 | < 0.001 |
| ≥ 0.05 | 92 | 1044 | |||
| ALB (g/L) | < 35 | 105 | 1344 | 30.574 | < 0.001 |
| ≥ 35 | 45 | 1524 | |||
| TG (mmol/L) | < 1.7 | 124 | 1503 | 0.433 | 0.511 |
| ≥ 1.7 | 26 | 365 | |||
| LDL (mmol/L) | < 3.37 | 95 | 1456 | 9.011 | 0.003 |
| ≥ 3.37 | 55 | 1412 | |||
| HDL (mmol/L) | < 1.55 | 79 | 1722 | 3.222 | 0.073 |
| ≥ 1.55 | 71 | 1146 | |||
| TC (mmol/L) | < 6.45 | 117 | 2149 | 0.718 | 0.397 |
| ≥ 6.45 | 33 | 719 | |||
| Blood loss (mL) | < 200 | 87 | 2401 | 65.118 | < 0.001 |
| ≥ 200 | 63 | 467 | |||
| Drainage | Yes | 103 | 1129 | 50.661 | < 0.001 |
| No | 47 | 1739 | |||
| Tension suture | Yes | 7 | 72 | Fisher | 0.112 |
| No | 143 | 2796 | |||
| Flushing | Yes | 86 | 1596 | 0.164 | 0.685 |
| No | 64 | 1272 | |||
| Item | Single | 41 | 1443 | 30.12 | < 0.001 |
| Multiple | 109 | 1425 | |||
| Anesthesia | General | 133 | 1969 | 26.525 | < 0.001 |
| N-general | 17 | 899 | |||
| Incision | I | 8 | 95 | 228.143 | < 0.001 |
| II | 103 | 2702 | |||
| III | 39 | 71 | |||
| Season | Spring | 36 | 825 | 301.157 | < 0.001 |
| Summer | 41 | 68 | |||
| Autumn | 42 | 382 | |||
| Winter | 31 | 1593 | |||
| Surgical site | Uterus | 22 | 1252 | 188.267 | < 0.001 |
| Liver | 12 | 821 | |||
| Gastrointestinal | 76 | 501 | |||
| Appendix | 27 | 152 | |||
| Kidney | 5 | 107 | |||
| Groin | 8 | 35 |
Multivariate analysis revealed that diabetes [odds ratio (OR) = 5.698, 95% confidence interval (CI): 3.305-9.825, P = 0.001]; antibiotic use (OR = 14.977, 95%CI: 2.865-78.299, P = 0.001); an NRS 2002 score of ≥ 3 (OR = 2.426, 95%CI: 1.199-4.909, P = 0.014); an NNIS score of ≥ 2 (OR = 2.362, 95%CI: 1.019-5.476, P = 0.045); PCT ≥ 0.05 μg/L (OR = 1.687, 95%CI: 1.056-2.695, P = 0.029); LDL < 3.37 mmol/L (OR = 1.719, 95%CI: 1.039-2.842, P = 0.035); surgical sites, such as the gastrointestinal tract (OR = 3.646, 95%CI: 1.097-12.121, P = 0.035), appendix (OR = 23.056, 95%CI: 6.944-76.548, P < 0.001), kidney (OR = 6.256, 95%CI: 1.377-29.361, P < 0.020), and the groin area (OR = 53.589, 95%CI: 10.354-277.357, P < 0.001); surgical seasons, including summer (OR = 18.948, 95%CI: 9.537-37.648, P < 0.001), autumn (OR = 2.648, 95%CI: 1.454-4.823, P = 0.001), and winter (OR = 0.481, 95%CI: 0.266-0.872, P = 0.016); incision grade III (OR = 11.226, 95%CI: 1.689-74.630, P = 0.012); general anaesthesia (OR = 3.334, 95%CI: 1.134-9.806, P = 0.029); intraoperative blood loss > 200 mL (OR = 29.026, 95%CI: 13.751-61.266, P < 0.001) were independent risk factors for SSI (Table 2).
| Factors | OR | 95%CI | P value | |
| The history of diabetes | 5.698 | 3.305 | 9.825 | 0.001 |
| The use of antibiotic | 14.977 | 2.865 | 78.299 | 0.001 |
| NRS 2002 ≥ 3 | 2.426 | 1.199 | 4.909 | 0.014 |
| NNIS ≥ 2 | 2.362 | 1.019 | 5.476 | 0.045 |
| PCT ≥ 0.05 μg/L | 1.687 | 1.056 | 2.695 | 0.029 |
| LDL < 3.37 mmol/L | 1.719 | 1.039 | 2.842 | 0.035 |
| General anesthesia | 3.334 | 1.134 | 9.806 | 0.029 |
| Blood loss ≥ 200 mL | 29.026 | 13.751 | 61.266 | < 0.001 |
| Surgical site | ||||
| Uterus | Ref. | |||
| Gastrointestinal | 3.646 | 1.097 | 12.121 | 0.035 |
| Appendix | 23.056 | 6.944 | 76.548 | < 0.001 |
| Kidney | 6.256 | 1.377 | 29.361 | 0.020 |
| Groin | 53.589 | 10.354 | 277.357 | < 0.001 |
| Incision | ||||
| I | Ref. | |||
| III | 11.226 | 1.689 | 74.630 | 0.012 |
| Season | ||||
| Spring | Ref. | |||
| Summer | 18.948 | 9.537 | 37.648 | < 0.001 |
| Autumn | 2.648 | 1.454 | 4.823 | 0.001 |
| Winter | 0.481 | 0.266 | 0.872 | 0.016 |
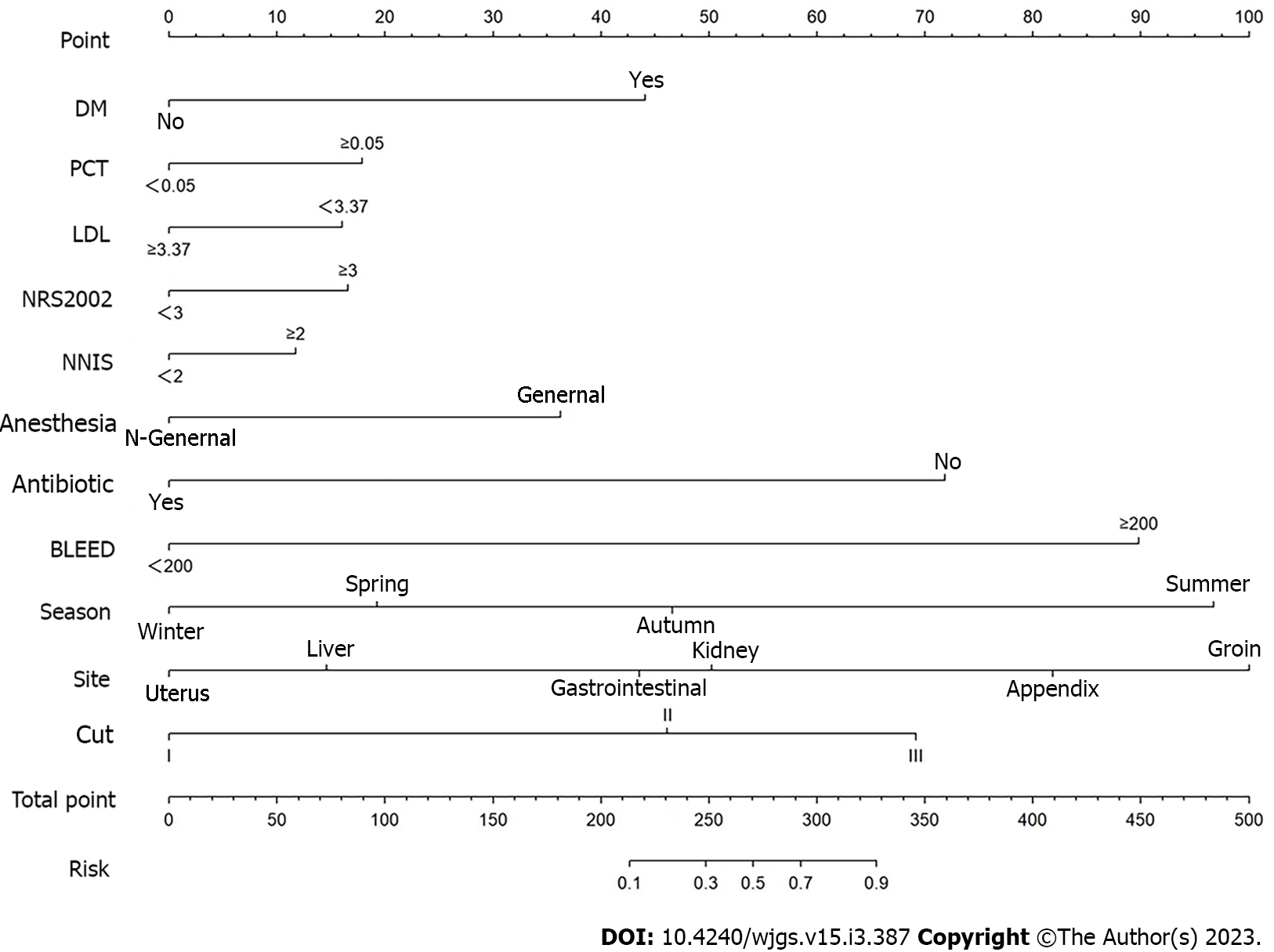
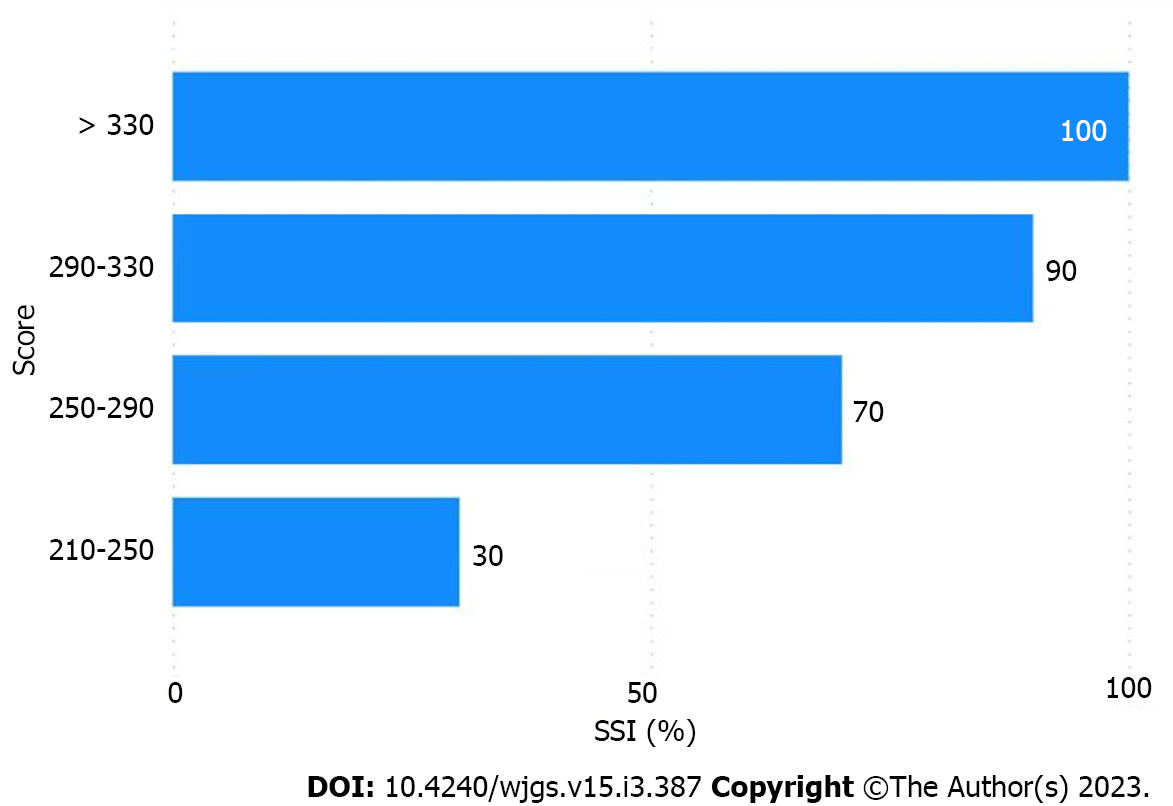
The multivariate analysis results were incorporated into the nomogram to construct a predictive model of SSI after abdominal surgery using R 4.2.1 (Figure 1). The following points were assigned to the patients based on the nomogram: 0 points for patients without a history of diabetes and 43 points for patients with a history of diabetes; 0 points for patients with a PCT level within the normal range and 19 points for patients with an abnormal PCT level; 0 points for patients with an LDL of ≥ 3.37 mmol/L and 16 points for patients with an LDL of < 3.37 mmol/L; 0 points for patients with an NRS 2002 score of < 2 and 17 points for patients with an NRS 2002 score of ≥ 3; 0 points for patients with an NNIS score of < 2 and 12 points for patients with an NNIS score of ≥ 2; 0 points for patients who received non-general anaesthesia and 38 points for patients who received general anaesthesia; 0 points for preoperative antibiotic use and 71 points for no preoperative antibiotic use; 0 points for patients with an intraoperative blood loss of < 200 mL and 91 points for patients with an intraoperative blood loss of ≥ 200 mL; 0 points if the surgical season was winter, 20 points if the surgical season was spring, 45 points if the surgical season was autumn, and 96 points if the surgical season was summer; in terms of the surgical site, the points were assigned as follows: 0 points for the uterus, 15 points for the liver, 45 points for the stomach, 51 points for the kidney, 82 points for the appendix, and 98 points for the groin area; in terms of the incision grade the points were assigned as follows: 0 points for grade I incision, 48 points for grade II incision, and 68 points for grade III incision. The total score was 500. The predictive value of SSI after abdominal surgery was 90% when the score was > 328. Overall, the predictive model had a significantly higher AUC value (0.926) than that of the NNIS (0.662) (Figure 2). SSI occurrence was significantly associated with the SSI risk score obtained on logistic regression. Particularly, the model was associated with an increased incidence of SSI (30%, 70%, 90%, and 100% for score cut-offs of 210-250, 250-290, 290-330, and > 330, respectively) as the SSI score increased in the validation cohort (Figure 3). Based on these results, we set up an online tool to better predict SSI risk after abdominal surgery established on the nomogram in this study (https://drzhangjinssi.shinyapps.io/DynNo/mapp/).
SSI after abdominal surgery results in prolonged hospital LOS and significant hospitalisation costs[16]. A survey reported that the additional expenditure per SSI patient could support the hospitalisation costs of 13 normal surgical patients[8]. Therefore, the significance of SSI for hospitals, countries, and patients is obvious[17]. Over the past few years, several SSI prediction models have been developed to help clinicians identify high-risk patients who might benefit from early intervention. Due to its simplicity and convenience, the NNIS risk index is currently the method that is most frequently used. Its three variables, however, are insufficient for a precise evaluation[18,19]. Mu et al[20] established an SSI prediction model based on patient data from 39 countries between 2006 and 2008 [area under the receiver operating characteristic curve (AUROC) = 0.67]. An accurate prediction model might be created using data from 39 additional; however, using such a model in clinical settings could be inconvenient. Although Van Walraven et al[21] established a prediction model with an AUROC of 0.80; this model required substantial patient information. Medical personnel are overworked in settings where electronic medical records are not being used. Therefore, it is necessary to construct a prediction model which is accurate and easy to use. In this study, the SSI prediction model is relatively novel and efficient. It can be used to predict SSI after abdominal surgery, and the necessary information involved is within the scope of implementation, making it applicable. In this study, the SSI-related factors were retrospectively examined from the perspectives of fundamental preoperative patient data, preoperative blood test indicators, surgery-related data, and the overall patient condition score, including age, gender, marital status, WBC count, and intraoperative blood loss. Additionally, we included various comprehensive and representative factors, including the NRS 2002 and NNIS scores. Our model is innovative compared with other models[22,23]. Besides objective test indicators and the patient’s personal information, the doctor can establish overall control and evaluate the patient’s condition. This model is more practical and credible, as shown by the entire procedure and the AUROC result.
The predictability of the SSI prediction model was comprehensively evaluated using univariate regression, multivariate logistic regression, and R 4.2.1 “rms”. Identifying patients at high risk for SSI is important; however, intervention should be the primary action following identification. The SCIP items must first be completed, albeit not all of them need to be covered[24,25]. Furthermore, when patients undergo elective surgeries, the model should be used comprehensively to determine the probability of infection. SSI is more likely to occur when the prediction score is high, and precautions must be taken accordingly. Improving the patient’s nutrition, appropriate anaesthesia methods, and reducing intraoperative blood loss will help prevent SSIs. Patients with an SSI monitor for post-discharge wound surveillance could help identify and manage the condition at the earliest using intelligent identification programs available in some developed regions of the world. This would improve the effectiveness of hospital visits and foster better communication between doctors and patients[26,27]. Additionally, a preoperative plan devised by a multidisciplinary team could lower the occurrence of SSI, particularly in critically ill patients, as well as help in a comprehensive assessment and symptomatic treatment[28]. There are four aspects to predicting SSI preoperatively: Assessment, intervention, diagnosis, and treatment, which are equally essential for managing SSI[29]. Multidisciplinary discussions and comprehensive step-by-step assessments can help lower the incidence of SSI, thereby improving patient satisfaction and recovery indexes.
The efficacy of our model has been verified; however, it has a few limitations. First, professionals diagnosed and selected the patients for this study; however, there may still be artificial errors that affect our model. Second, as the study was a retrospective analysis, potential selection bias could exist. The prediction model was created based on a broad cohort of patients undergoing abdominal surgery. The model needs constant improvement to be clinically used because the data were only from one institution, and the sample size was insufficient. This challenge could be categorised under clinical big data analysis, as reported by Ejaz et al[16]. Lastly, in terms of data analysis, several missing variables were excluded, and the model establishment expression form needs improvement.
The following will be considered in our future studies: (1) As a result of the promotion of diagnosis-related groups payment system for hospitalised patients[30], the International Classification of Diseases code[31] will become increasingly standardised as it can be used to screen cases; (2) More validation cohorts need to be included, and patient information can be collected from different regions of the country and globally, making the model more convincing and resilient; (3) The patients’ missing data needs to be handled appropriately. Chen et al[1] suggested that other variables can be used to replace the factors with too many missing values. As a fundamental step, clinicians need to strengthen their ability to write medical records; and (4) The text content in the model will be embedded later and then applied to the entire HIS, making the process more efficient and accurate.
SSI prediction models are useful for hospitalised patients and have recently undergone continuous development. However, they lack reliability due to their complex and dynamic nature. Herein, we established a novel model for predicting SSI after abdominal surgery and verified its efficiency and accuracy in preventing postoperative SSI. We anticipate that our study will help improve patient prognosis after abdominal surgery.
Surgical site infections (SSIs) can increase mortality and prolong the length of hospital stay, thereby increasing healthcare costs. Therefore, it is much necessary to develop a prediction model after elective abdominal surgeries in order to identify risk factors of SSI.
To establish a predictive model for SSI which is more easily assess the risk of it. And provide timely interventions for high-risk patients to improve the quality of care so as to reduce medical costs and ease the burden on patients.
The present study aimed to develop a realistic, feasible, valid and unique model for predicting the risk of elective abdominal SSI.
This observational study was conducted from January 1, 2018 to January 1, 2021 using patient demographic data and haematological test results. Inpatient SSI risk factors were evaluated using univariate analysis and multivariate logistic regression. Nomograms were used in the predictive models. The receiver operating characteristic and area under the curve values were used to measure the specificity and accuracy of the model.
The key findings indicated that the surgical sites included the uterus (42.2%), the liver (27.6%), the gastrointestinal tract (19.1%), the appendix (5.9%), the kidney (3.7%), and the groin area (1.4%). SSI occurred in 5% of the patients (n = 150). Multivariate logistic regression revealed the following independent risk factors: A history of diabetes, antibiotic use, a Nutritional Risk Screening 2002 score of ≥ 3, general anaesthesia, a National Nosocomial Infections Surveillance (NNIS) score of ≥ 2, procalcitonin ≥ 0.05 μg/L, low-density lipoprotein cholesterol < 3.37 mmol/L, intraoperative blood loss ≥ 200 mL, surgical season, surgical site, and incision grade (all P < 0.05. The overall area under the receiver operating characteristic curve of the predictive model was 0.926, which was significantly higher than that of the NNIS (0.662).
The patient’s condition and haematological test indicators formed the bases of our prediction model. It is a novel, efficient, and highly accurate predictive model for preventing postoperative SSI, thereby improving the prognosis in patients undergoing abdominal surgery.
This study developed the accurate model for predicting the risk of elective abdominal SSI. We plan to make larger multi-centre and large sample studies in order to obtain more realistic and valid data results.
The authors would like to thank Professor Xiang-Long Duan for contribution of this writing support and selfless help. In particular, the authors would like to thank the editors and reviewers of World Journal of Gastrointestinal Surgery for the recognition of our research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Musa Y, Nigeria; Sultan AAEA, Egypt S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Chen W, Lu Z, You L, Zhou L, Xu J, Chen K. Artificial Intelligence-Based Multimodal Risk Assessment Model for Surgical Site Infection (AMRAMS): Development and Validation Study. JMIR Med Inform. 2020;8:e18186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Waltz PK, Zuckerbraun BS. Surgical Site Infections and Associated Operative Characteristics. Surg Infect (Larchmt). 2017;18:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Strobel R, Kreis M, Lauscher JC. [Surgical site infections-Prevention and treatment strategies]. Chirurg. 2021;92:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Lo Giudice D, Trimarchi G, La Fauci V, Squeri R, Calimeri S. Hospital infection control and behaviour of operating room staff. Cent Eur J Public Health. 2019;27:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Danwang C, Bigna JJ, Tochie JN, Mbonda A, Mbanga CM, Nzalie RNT, Guifo ML, Essomba A. Global incidence of surgical site infection after appendectomy: a systematic review and meta-analysis. BMJ Open. 2020;10:e034266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Shao J, Zhang H, Yin B, Li J, Zhu Y, Zhang Y. Risk factors for surgical site infection following operative treatment of ankle fractures: A systematic review and meta-analysis. Int J Surg. 2018;56:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Shen YM. [Strategies on the prevention and treatment of surgical site infection and the resulting wound]. Zhonghua Shao Shang Za Zhi. 2021;37:207-212. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Zhou J, Ma X. Cost-benefit analysis of craniocerebral surgical site infection control in tertiary hospitals in China. J Infect Dev Ctries. 2015;9:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Li PY, Yang D, Liu D, Sun SJ, Zhang LY. Reducing Surgical Site Infection with Negative-Pressure Wound Therapy After Open Abdominal Surgery: A Prospective Randomized Controlled Study. Scand J Surg. 2017;106:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt). 2010;11:79-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Bressan AK, Aubin JM, Martel G, Dixon E, Bathe OF, Sutherland FR, Balaa F, Mimeault R, Edwards JP, Grondin SC, Isherwood S, Lillemoe KD, Saeed S, Ball CG. Efficacy of a Dual-ring Wound Protector for Prevention of Surgical Site Infections After Pancreaticoduodenectomy in Patients With Intrabiliary Stents: A Randomized Clinical Trial. Ann Surg. 2018;268:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 398] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 13. | Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, Banerjee SN, Edwards JR, Tolson JS, Henderson TS. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:152S-157S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 1021] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Grant R, Aupee M, Buchs NC, Cooper K, Eisenring MC, Lamagni T, Ris F, Tanguy J, Troillet N, Harbarth S, Abbas M. Performance of surgical site infection risk prediction models in colorectal surgery: external validity assessment from three European national surveillance networks. Infect Control Hosp Epidemiol. 2019;40:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Endo S, Tsujinaka T, Fujitani K, Fujita J, Tamura S, Yamasaki M, Kobayashi S, Akamaru Y, Mizushima T, Shimizu J, Umeshita K, Ito T, Mori M, Doki Y. Risk factors for superficial incisional surgical site infection after gastrectomy: analysis of patients enrolled in a prospective randomized trial comparing skin closure methods. Gastric Cancer. 2016;19:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ejaz A, Schmidt C, Johnston FM, Frank SM, Pawlik TM. Risk factors and prediction model for inpatient surgical site infection after major abdominal surgery. J Surg Res. 2017;217:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Isbell KD, Hatton GE, Wei S, Green C, Truong VTT, Woloski J, Pedroza C, Wade CE, Harvin JA, Kao LS. Risk Stratification for Superficial Surgical Site Infection after Emergency Trauma Laparotomy. Surg Infect (Larchmt). 2021;22:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 626] [Reference Citation Analysis (0)] |
| 19. | Ercole FF, Starling CE, Chianca TC, Carneiro M. Applicability of the national nosocomial infections surveillance system risk index for the prediction of surgical site infections: a review. Braz J Infect Dis. 2007;11:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 20. | Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol. 2011;32:970-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 21. | Van Walraven C, Musselman R. The Surgical Site Infection Risk Score (SSIRS): A Model to Predict the Risk of Surgical Site Infections. PLoS One. 2013;8:e67167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Bucher BT, Ferraro JP, Finlayson SRG, Chapman WW, Gundlapalli AV. Use of Computerized Provider Order Entry Events for Postoperative Complication Surveillance. JAMA Surg. 2019;154:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Haya N, Feiner B, Baessler K, Christmann-Schmid C, Maher C. Perioperative interventions in pelvic organ prolapse surgery. Cochrane Database Syst Rev. 2018;8:CD013105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Awad SS. Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect (Larchmt). 2012;13:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 25. | Humphreys H. Preventing surgical site infection. Where now? J Hosp Infect. 2009;73:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Koek MB, Wille JC, Isken MR, Voss A, van Benthem BH. Post-discharge surveillance (PDS) for surgical site infections: a good method is more important than a long duration. Euro Surveill. 2015;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Kummerow Broman K, Gaskill CE, Faqih A, Feng M, Phillips SE, Lober WB, Pierce RA, Holzman MD, Evans HL, Poulose BK. Evaluation of Wound Photography for Remote Postoperative Assessment of Surgical Site Infections. JAMA Surg. 2019;154:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Lin DM, Carson KA, Lubomski LH, Wick EC, Pham JC. Statewide Collaborative to Reduce Surgical Site Infections: Results of the Hawaii Surgical Unit-Based Safety Program. J Am Coll Surg. 2018;227:189-197.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Noorit P, Siribumrungwong B, Thakkinstian A. Clinical prediction score for superficial surgical site infection after appendectomy in adults with complicated appendicitis. World J Emerg Surg. 2018;13:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Malik AT, Li M, Khan SN, Alexander JH, Li D, Scharschmidt TJ. Are current DRG-based bundled payment models for revision total joint arthroplasty risk-adjusting adequately? Bone Joint J. 2020;102-B:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Bao W, Lin H, Zhang Y, Wang J, Zhang S. Medical code prediction via capsule networks and ICD knowledge. BMC Med Inform Decis Mak. 2021;21:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |