Published online Feb 27, 2023. doi: 10.4240/wjgs.v15.i2.193
Peer-review started: August 23, 2022
First decision: November 5, 2022
Revised: November 19, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: February 27, 2023
Processing time: 187 Days and 23.8 Hours
Superior mesenteric artery syndrome (SMAS) is a rare condition causing fun
To analyze the clinical features, risk factors, and prevention of SMAS after laparoscopic-assisted radical right hemicolectomy.
We retrospectively analyzed clinical data of 256 patients undergoing laparoscopic-assisted radical right hemicolectomy in the Affiliated Hospital of Southwest Medical University from January 2019 to May 2022. The occurrence of SMAS and its countermeasures were evaluated. Among the 256 patients, SMAS was confirmed in six patients (2.3%) by postoperative clinical presentation and imaging features. All six patients were examined by enhanced computed tomo
In the experimental group, the aortomesenteric angle and distance after surgery were significantly decreased than those before surgery (P < 0.05). The aortomesenteric angle, distance and BMI were significantly higher in the control group than in the experimental (P < 0.05). There was no significant difference in the type of lymphadenectomy and surgical approach between the two groups (P > 0.05).
The small preoperative aortomesenteric angle and distance and low BMI may be important factors for the complication. Over-cleaning of lymph fatty tissues may also be associated with this complication.
Core Tip: This study retrospectively analyzed 256 patients undergoing laparoscopic-assisted radical right hemicolectomy, and six patients developed superior mesenteric artery syndrome (SMAS). The preoperative and postoperative aortomesenteric angle and distance were compared in the six patients, and 20 patients without postoperative SMAS were randomly selected for comparative analysis with 6 patients developed SMAS. The results and literature review suggest possible reasons and preventative measures for SMAS after right hemicolectomy.
- Citation: Xie J, Bai J, Zheng T, Shu J, Liu ML. Causes of epigastric pain and vomiting after laparoscopic-assisted radical right hemicolectomy - superior mesenteric artery syndrome. World J Gastrointest Surg 2023; 15(2): 193-200
- URL: https://www.wjgnet.com/1948-9366/full/v15/i2/193.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i2.193
A series of symptoms may occur after right hemicolectomy. They include nausea, bilious vomiting, epigastric pain, and postprandial abdominal fullness and distension. A total of 256 cases of laparoscopic-assisted radical right hemicolectomy was performed between January 2019 and May 2022 at the Affiliated Hospital of Southwestern Medical University, with six cases of postoperative complications of persistent upper gastrointestinal obstruction and a final diagnosis of superior mesenteric artery syndrome (SMAS). Several factors have been identified that have an impact on the occurrence of SMAS. The most common is significant weight loss, which leads to loss of retroperitoneal fat. These predisposing factors include wasting diseases (burns, cancer, and endocrine disorders), severe injuries (head or spinal trauma, and application of a body cast), dietary disorders (anorexia nervosa and malabsorptive diseases), and postoperative states (treatment for scoliosis, and abdominal surgery)[1]. Postoperative SMAS following intra-abdominal procedures is extremely rare. In this paper, we analyze cases and review the relevant literature to discuss the possible causes and preventative measures of SMAS after right hemicolectomy.
Among the 256 patients in this group who underwent laparoscopic-assisted radical right hemi
Patients developed upper gastrointestinal obstruction symptoms after 5–10 d postoperatively. In patients with postoperative gastric tube drainage, the drainage continuously exceeded 500–800 mL/d. The patients experienced epigastric distention, eructation, and vomiting after meals or removal of the gastric tube. The vomiting volume was large, similar to pyloric obstruction. The vomit contained bile, partially excluding pyloric obstruction and gastric emptying disorder. Two cases displayed an associated 10%–18% weight loss and electrolyte disturbances. The prominent feature of this group of cases was that the obstructive symptoms were position-related. The symptoms decreased or dis
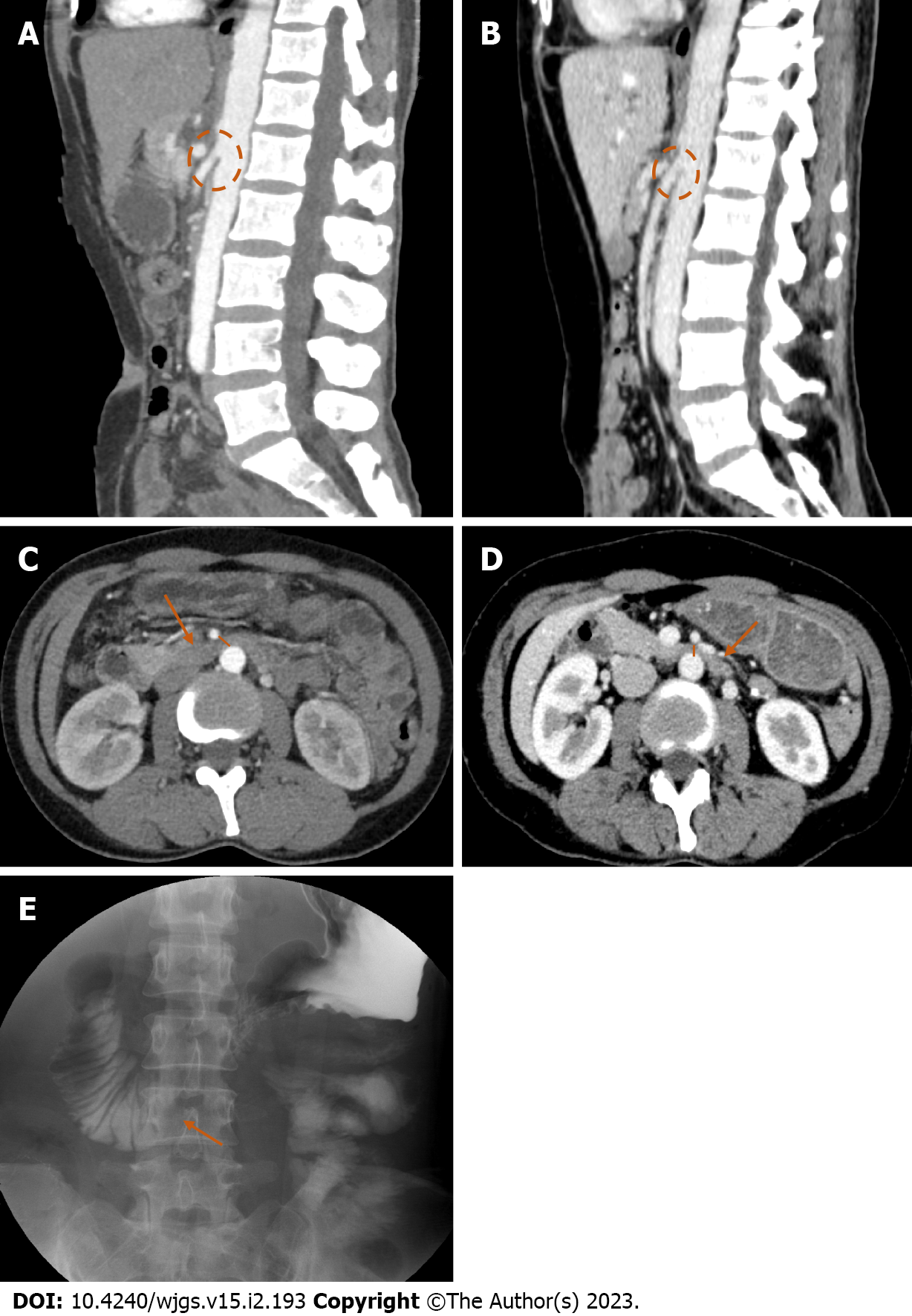
All six patients underwent abdominal contrast-enhanced computed tomography (CT) before surgery. After surgery, three of the patients were simultaneously examined by abdominal contrast-enhanced CT and an upper gastrointestinal series. The other three patients received abdominal contrast-enhanced CT only. Figure 1A–1E shows the image of a patient with postoperative SMAS.
Based on the clinical and radiological findings, a diagnosis of SMAS was suspected. All six cases were initially treated conservatively with gastric tube placement, fasting, and increased rehydration. These patients were gradually introduced to enteral feeding. In one case, endoscopic nasojejunal tube feeding was performed. Two cases did not improve with conservative treatment and were treated with duodenojejunostomy or gastrojejunostomy. Electrolyte abnormalities were carefully treated. All six patients showed weight gain and symptom resolution, corroborating our diagnosis.
Of the six patients, five had ascending colon cancer and one had ascending colon lymphoma. All six patients were examined by CT before and after surgery. The median age of patients with postoperative SMAS was 50.3 ± 13.0 years, including 130 men and 126 women, and the median age of all patients in the same period was 61.8 ± 13.8 years, including four men and two women.
There was little difference in sex and age between patients with postoperative SMAS and all surgical patients in the same period. It seemed that sex and age were not risk factors for SMAS. Patients who developed SMAS after surgery were used as the experimental group. A simple random sampling method was used to select a control group of 10 male and 10 female patients who underwent surgery at the same time but did not develop SMAS and received preoperative abdominal enhanced CT. Arterial phase images of both groups were reconstructed by sagittal multiplanar reformation. The angle and distance between the superior mesenteric artery (SMA) and abdominal aorta (AA) were measured before and after surgery in the experimental group and before surgery in the control group. The preoperative body mass index (BMI) of the experimental and control groups was calculated. The type of lymphadenectomy and surgical approach in the experimental group and the control group were recorded. Types of lymphadenectomy include D3 lymphadenectomy and D2 lymphadenectomy. There were three surgical approaches , the lateral approach , intermediate and caudal approach. SPSS 23.0 software was used for statistical analyses of the above risk factors. The Kolmogorov–Smirnov method was used to test whether the measurement data conformed to the normal distribution. The data conforming to the normal distribution was expressed as mean ± standard deviation. The t test was used to compare the differences in angle and distance preoperatively and postoperatively in the experimental group. The differences in angle, distance and BMI between the experimental and control groups were compared by independent sample t test, and the differences in type of lymphadenectomy and surgical approach between the two groups were compared by χ2 test. Receiver operating characteristic curves were used to analyze the optimal diagnostic threshold and diagnostic efficiency of statistically significant parameters. The difference was statistically significant at P < 0.05.
In the experimental group of six cases, the angle between the SMA and AA was 18°–29° (mean 23.50° ± 4.23°) in the preoperative period and 10°–18° (mean 14.67° ± 3.08°) in the postoperative period. The distance between the SMA and the AA was 7–11 mm (mean 9.33 ± 1.37 mm) in the preoperative period and 3–8 mm (mean 5.17 ± 1.72 mm) in the postoperative period. Preoperative BMI ranged from 16.8 to 25.1 kg/m2 (mean 18.82 ± 3.13 kg/m2). Five patients received D3 lymphadenectomy and one D2 lymphadenectomy. There were three surgical approaches: Lateral in two cases, intermediate in one, and caudal approach in three. In the control group, the angle between the SMA and AA of the 20 patients ranged from 19° to 49° (mean 36.35° ± 8.13°). The distance between SMA and AA ranged from 7 to 25 mm (mean 14.45 ± 4.44 mm). BMI ranged from 18.7 to 27.2 kg/m2 (mean 22.85 ± 2.33 kg/m2). Fifteen patients received D3 lymphadenectomy and five D2 lymphadenectomy. There were three surgical approaches: Lateral in five cases, intermediate in eight, and caudal approach in seven. In the experimental group, the angle and distance after surgery were significantly decreased than those before surgery (P < 0.05) (Table 1). The angle, distance and BMI were significantly higher in the control group than in the experimental (P < 0.05) (Table 2). There was no significant difference in the type of lymphadenectomy and surgical approach between the two groups (P > 0.05). The area under receiver operating characteristic curve for aortomesenteric angle, distance and BMI was 0.913, 0.888, 0.867, and cutoff of the aortomesenteric angle, distance and BMI to identify the control and experimental groups was 29.50°, 11.50 mm, 18.45 kg/m2 respectively (Table 3).
| Preoperative | Postoperative | t | P value | |
| Angle (°) | 23.50 ± 4.23 | 14.67 ± 3.08 | 12.562 | 0.000 |
| Distance (mm) | 9.33 ± 1.37 | 5.17 ± 1.72 | 6.934 | 0.001 |
| Risk factor | Experimental group (n = 6) | Control group (n = 20) | t/χ2 | P value | |
| Angle (°) | 23.50 ± 4.23 | 36.35 ± 8.13 | 3.686b | 0.001 | |
| Distance (mm) | 9.33 ± 1.37 | 14.45 ± 4.44 | 4.491b | 0.000 | |
| BMI (kg/m2) | 18.82 ± 3.13 | 22.85 ± 2.33 | 3.436b | 0.002 | |
| Type of lymphadenectomy | 0.000a | 1.000 | |||
| D3 | 5 | 15 | |||
| D2 | 1 | 5 | |||
| Surgical approach | 1.219a | 0.544 | |||
| Lateral approach group | 2 | 5 | |||
| Intermediategroup | 1 | 8 | |||
| Caudal approach group | 3 | 7 |
| Risk factor | AUC | SE | P value | 95%CI | Cut off | Sensitivity | Specificity |
| Angle | 0.913 | 0.056 | 0.003 | 0.803–1.000 | 29.50° | 0.800 | 1.000 |
| Distance | 0.888 | 0.065 | 0.005 | 0.760–1.000 | 11.50 mm | 0.750 | 1.000 |
| BMI | 0.867 | 0.123 | 0.007 | 0.625–1.000 | 18.45 kg/m2 | 1.000 | 0.833 |
SMAS is a rare medical condition that describes the clinical symptoms resulting from vascular compression of the third part of the duodenum in the angle between the SMA and AA. This syndrome is also known as aortomesenteric artery compression, arteriomesenteric duodenal compression, Wilkie’s syndrome, and cast syndrome. The incidence of SMAS reported in previous studies has ranged from 0.13%–0.78%[2]. The symptoms of SMAS can be vague, chronic, and significantly overlap with more common gastrointestinal disorders, such as gastritis, peptic ulcer disease, irritable bowel syndrome, and gastroparesis[3]. Chief complaints of patients with SMAS include early satiety, postprandial pain or discomfort, nausea and bilious emesis that often develop after a meal, bloating, eructation, and reflux. The latter is classically relieved by lying in the left lateral decubitus position or follows an episode of emesis[4-6]. Death in SMAS is due to aspiration pneumonia, acute gastric rupture, severe electrolyte imbalance, hypokalemia, and cardiovascular collapse[7]. The normal anatomical aortomesenteric angle and aortomesenteric distance is 25°–60° and 10–28 mm, respectively. An aortomesenteric angle of 22°–25° and distance of 8 mm correlates with symptoms of SMAS[3,8]. The diagnosis of SMAS must be based on clinical symptomatology correlated with radiographic information[9]. Once diagnosed, SMAS can be safely treated conservatively, including by nasogastric decompression and correction of electrolytes and intravenous hydration, followed by enteral nutrition through a nasojejunal tube or parenteral nutrition if necessary. Operative management is indicated only when conservative management fails[10]. The multiple surgical approaches include lysis of the ligament of Treitz, gastrostomy tube placement, or proximal bypass of the common channel to the distal stomach or duodenum (i.e., duodenojejunostomy and/or gastrojejunostomy)[11]. Once the third portion of the duodenum is bypassed, the symptoms resolve quickly.
Postoperative SMAS following intra-abdominal procedures is extremely rare, but has previously been reported following colectomy[12], proctoright hemicolectomy[13], retroperitoneal sarcoma resection[14] and Roux-en-Y gastric bypass[15]. Corrective spinal surgery for scoliosis, which requires relative lengthening of the spine and results in the narrowing of the aortomesenteric angle, is the most frequently cited cause of postoperative SMAS with an estimated incidence of 1%–4.7%[16].
Many previous studies have described the common causes for the occurrence of SMAS, but further research will be needed to investigate the etiopathogenesis of SMAS after right hemicolectomy. In this study, we discussed the cases of SMAS occurring after right hemicolectomy and reviewed the relevant literature to suggest five possible reasons and preventative measures.
First, the unifying theme for most cases of postoperative SMAS is a sudden major rearrangement of intra-abdominal anatomy[14]. The postoperative CT in this group showed that the position of intestinal structures in the abdominal cavity was changed. The right hemicolectomy disrupted the suspension of the transverse colon from the hepatic region of the colon, resulting in prolapse of the anastomosed colonic segment and excessive pulling of the colonic mesenteric root, resulting in the compression of the duodenal root. The six patients had no clinical manifestation of SMAS before the surgery. Postoperative visceral prolapse and further depletion of mesenteric fat resulted in reduction of the aortomesenteric angle and distance significantly. Six patients with postoperative SMAS were selected as the study subjects. The control group comprised 20 patients who had undergone surgery at the same time but who did not have postoperative SMAS. All patients underwent abdominal contrast-enhanced CT before surgery. The statistical results showed that the aortomesenteric angle and distance were smaller in the experimental group than in the control group. Thus, the pre-existing small preoperative aortomesenteric angle and distance were anatomical factors leading to SMAS. Further reductions in angle and distance after right hemicolectomy led to the development of SMAS symptoms.
Second, careful analysis of the surgical data of all patients revealed over-cleaning of lymph fatty tissues in the six patients. Five patients who underwent D3 clearance and the other who underwent D2 clearance also had a partially dissected naked surface of the SMA. Over-cleaning of lymph fatty tissues may have contributed to the postoperative SMAS in this group of patients. However, the type of lymphadenectomy in the experimental and control groups did not differ significantly, which may be due to the small sample size, leading to the lack of strict statistical significance of the conclusions. More evidence needs to be accumulated and observed in more cases.
Third, during right hemicolectomy, the electric knife dissociated the second and third part of the duodenum, resulting in injury of the duodenal intestinal plexus. This may affect peristalsis and tone of the duodenum, inducing the development of SMAS[17].
Fourth, intestinal peptides influence gastric function. Reduced sources of intestinal peptides after right hemicolectomy may inhibit the movement of the duodenum and affect its digestion and absorption, inducing the development of SMAS. Finally, local mesenteric traction of tissue near the SMA due to confined abdominal exudate and peritoneal adhesions after right hemicolectomy may be a contributing factor to SMAS.
Patients with an angle between the SMA and AA < 29.50°, distance < 11.50 mm, and especially those with BMI < 18.45 kg/m2 are at greater risk of developing SMAS after right hemicolectomy. To reduce the incidence, early nutrition should be enhanced to reduce visceral fat consumption. Intraoperative preservation of some peritoneal structures to enhance the support of mesenteric vessels as much as possible is prudent. Other important aspects are: To reduce SMAS to prevent postoperative adhesions by standardizing surgery; to ensure that the anastomosis is tension-free and has good blood flow; correctly placing the drainage tube; accelerating healing of the anastomosis; reducing the occurrence of peri-anastomotic infection; and avoiding adhesions that can form a mass that pulls the superior mesenteric vessels. Reduction of the number of intraoperative electrocautery procedures can reduce damage to the intestinal wall plexus. Finally, the use of pro-gastrointestinal drugs postoperatively can increase propulsive gastrointestinal motility.
SMAS is an uncommon phenomenon. Postoperative SMAS following right hemicolectomy is rarer. We reported six cases of SMAS after laparoscopic-assisted radical right hemicolectomy and reviewed the literature to analyze potential risk factors or determining factors for the occurrence of SMAS. Some suggestions were put forward to reduce the occurrence of SMAS. Future studies should explore whether the occurrence of obstruction can be reduced in patients prone to SMAS after right hemicolectomy by improving reconstruction of the anastomotic colonic segment to reduce its pull on the superior mesenteric vessels or by prophylactic release of the ligament of Treitz.
Superior mesenteric artery syndrome after laparoscopic-assisted radical right hemicolectomy is a rare complication and can often be unrecognized by radiologists and clinicians.
Help people understand postoperative superior mesenteric artery syndrome.
Potential risk factors for the development of superior mesenteric artery syndrome were analyzed through case discussions and review of the literature.
The preoperative and postoperative aortomesenteric angle and distance were compared in the experimental group of 6 patients, and 20 patients without postoperative SMAS in the 256 patients were randomly selected for comparative analysis with 6 patients developed SMAS.
In the experimental group, the aortomesenteric angle and distance after surgery were significantly decreased than those before surgery. The aortomesenteric angle, distance and BMI were significantly higher in the control group than in the experimental. There was no significant difference in the type of lymphadenectomy and surgical approach between the two groups.
The small preoperative aortomesenteric angle and distance and low BMI may be important factors for the complication. Over-cleaning of lymph fatty tissues may also be associated with this complication.
Future studies should explore whether the occurrence of obstruction can be reduced in patients prone to SMAS after right hemicolectomy by improving reconstruction of the anastomotic colonic segment to reduce its pull on the superior mesenteric vessels or by prophylactic release of the ligament of Treitz.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Chitul A, Romania; Nakamura K, Japan; Reddy NNR, India; Socea B, Romania S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Wilkinson R, Huang CT. Superior mesenteric artery syndrome in traumatic paraplegia: a case report and literature review. Arch Phys Med Rehabil. 2000;81:991-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Zaraket V, Deeb L. Wilkie's Syndrome or Superior Mesenteric Artery Syndrome: Fact or Fantasy? Case Rep Gastroenterol. 2015;9:194-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Neri S, Signorelli SS, Mondati E, Pulvirenti D, Campanile E, Di Pino L, Scuderi M, Giustolisi N, Di Prima P, Mauceri B, Abate G, Cilio D, Misseri M, Scuderi R. Ultrasound imaging in diagnosis of superior mesenteric artery syndrome. J Intern Med. 2005;257:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Merrett ND, Wilson RB, Cosman P, Biankin AV. Superior mesenteric artery syndrome: diagnosis and treatment strategies. J Gastrointest Surg. 2009;13:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Shah D, Naware S, Thind S, Kuber R. Superior mesenteric artery syndrome: an uncommon cause of abdominal pain mimicking gastric outlet obstruction. Ann Med Health Sci Res. 2013;3:S24-S26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Lamba R, Tanner DT, Sekhon S, McGahan JP, Corwin MT, Lall CG. Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics. 2014;34:93-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11:3307-3310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 8. | Rabie ME, Ogunbiyi O, Al Qahtani AS, Taha SB, El Hadad A, El Hakeem I. Superior Mesenteric Artery Syndrome: Clinical and Radiological Considerations. Surg Res Pract. 2015;2015:628705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Warncke ES, Gursahaney DL, Mascolo M, Dee E. Superior mesenteric artery syndrome: a radiographic review. Abdom Radiol (NY). 2019;44:3188-3194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Welsch T, Büchler MW, Kienle P. Recalling superior mesenteric artery syndrome. Dig Surg. 2007;24:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Baker MT, Lara MD, Kothari SN. Superior mesenteric artery syndrome after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2:667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Fearon NM, Mohan HM, Winter DC. Wilkie's syndrome causing persistent vomiting post-colectomy. Int J Surg Case Rep. 2013;4:1071-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Matheus Cde O, Waisberg J, Zewer MH, Godoy AC. Syndrome of duodenal compression by the superior mesenteric artery following restorative proctocolectomy: a case report and review of literature. Sao Paulo Med J. 2005;123:151-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Wong LH, Sutton TL, Spurrier RG, Zigman AF, Mayo SC. Post-Operative Superior Mesenteric Artery Syndrome Following Retroperitoneal Sarcoma Resection. Clin Pract. 2020;11:2-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Clapp B, Applebaum B. Superior mesenteric artery syndrome after Roux-en-y gastric bypass. JSLS. 2010;14:143-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Qian BP, Ji ML, Jiang J, Zhu ZZ, Wan B, Qiu Y. Anatomic relationship between superior mesenteric artery and aorta before and after surgical correction of thoracolumbar kyphosis. J Spinal Disord Tech. 2013;26:E293-E298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Hemingway DM, Finlay IG. Effect of colectomy on gastric emptying in idiopathic slow-transit constipation. Br J Surg. 2000;87:1193-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |