Published online Feb 27, 2023. doi: 10.4240/wjgs.v15.i2.163
Peer-review started: September 23, 2022
First decision: October 5, 2022
Revised: October 21, 2022
Accepted: January 3, 2023
Article in press: January 3, 2023
Published online: February 27, 2023
Processing time: 156 Days and 19.4 Hours
Pancreatic malignancy is still the most lethal gastrointestinal malignancy. It has a very poor prognosis with low survival rate. Surgery is still the main treatment option for pancreatic malignancy. Most patients already have locally advanced and even late stage disease due to non-specific abdominal symptoms. Even though some cases are still suitable for surgical treatment, due to its aggressiveness adjuvant chemotherapy is becoming the standard treatment for controlling the disease. Radiofrequency ablation (RFA) is a thermal therapy that has been used as one of the standard treatments for liver malignancy. It can also be performed intraoperatively. There are several reports on percutaneous RFA treatment for pancreatic malignancy using transabdominal ultrasound and guided by computed tomography scan. However, due to its anatomical location and the risk of high radiation exposure, these methods seem to be very limited. Endoscopic ultrasound (EUS) has been widely used for pancreatic abnormality evaluation due to its ability to detect more accurately, especially small pancreatic lesions, compared to other imaging modalities. By the EUS approach, it is easier to achieve good visualization of tumor ablation and necrosis as the echoendoscope position is closer to the tumor area. Based on studies and a recent meta-analysis, EUS-guided RFA is a promising treatment approach for most pancreatic malignancy cases, but most studies only collected data from a small sample size. Larger studies are needed before clinical recommendations can be made.
Core Tip: Pancreatic cancer is still the most lethal gastrointestinal malignancy. Most patients are diagnosed at the late stage of the disease. Surgery is still the definitive treatment for managing pancreatic cancer. However, skill, experience, and expertise are required of the surgeon due to its high risk of complications. Recently, endoscopic ultrasound has been used for managing pancreatic cancer. Studies have shown its practicability, efficacy, and benefit in combination with standard chemotherapy. It would need larger studies before it can be recommended as standard management in clinical practice.
- Citation: Lesmana CRA. Impact of endoscopic ultrasound-guided radiofrequency ablation in managing pancreatic malignancy. World J Gastrointest Surg 2023; 15(2): 163-168
- URL: https://www.wjgnet.com/1948-9366/full/v15/i2/163.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i2.163
Pancreatic malignancy is still the most lethal gastrointestinal malignancy, and it is ranked seventh for mortality. It has a very poor prognosis with a low survival rate[1]. There are many risk factors that might contribute to pancreatic cancer development, such as genetics, obesity, diabetes mellitus, smoking, chronic pancreatitis, fatty pancreas, and heavy alcohol consumption[2]. It has been classified into two categories, i.e., exocrine cancer, which is dominated by pancreatic adenocarcinoma, and endocrine cancer, also known as pancreatic neuroendocrine tumor (pNET). In the clinical stage, it is classified into type IA and IB (considered as resectable disease with a maximum 4 cm diameter), type II (locally advanced with diameter > 4 cm with possible lymph nodes involvement), type III (unresectable with vascular involvement), and type IV (metastatic cancer)[3].
Pancreatoduodenectomy (Whipple) operation is still the best option to prolong survival. However, most cases already come in at the late stage, and overall mortality remains low. Endoscopic ultrasound (EUS) is an innovation dedicated to managing pancreatobiliary disorders[4]. In the history of developing the EUS procedure, a diagnostic comparison study by Palazzo et al[5] showed that EUS had higher accuracy to detect small pancreatic lesions when compared to computed tomography (CT) scan or ultrasonography. Based on the pioneering study by Vilmann et al[6] in which the EUS-guided pancreas biopsy technique used a catheter with aspiration needle, therapeutic EUS has been recently developed not only for biliary drainage procedure but also for managing pancreatic malignancy through direct tumor ablation therapy or radiofrequency ablation (RFA) in a multidisciplinary context and evaluation[4,7].
The RFA procedure is an electrocautery-based technique that results in tissue necrosis. It has been used widely for managing unresectable primary as well as secondary liver cancer, where it has been previously reported by Nießen et al[8]. The local recurrence was primarily dependent on tumor size. Another RFA innovation study has been reported by Gervais et al[9] for managing renal cell carcinoma up to 5 cm in size with overall median survival of 9.9 mo. No recurrent disease in patients with technically successful treatment, no metastasis during treatment course, and no dialysis was needed in post ablation patients.
Surgery is still the main treatment option for pancreatic malignancy. However, due to its aggressiveness, neo-adjuvant chemotherapy is becoming the standard treatment for controlling the disease. Most patients are diagnosed at an advanced stage of cancer. In surgical treatment, tumor size and margin, vascular involvement, and lymph nodes are important parameters for the patient’s outcome[10]. A questionnaire-based retrospective study was conducted for pancreatic cancer with 6-year follow-up on patient outcomes, and it showed that the 30-d mortality rate was 5.3% with median survival of only 16.3 mo. Three out of twenty patients who had 5-year survival with positive histology results had recurrent disease in the 6-year follow-up. Some of these patients already showed locally advanced disease as there was evidence of positive margin. The median survival was lower in patients with positive margin compared to patients with negative margin (13.9 mo vs 20.6 mo)[11].
Another study looking at patient survival after pancreatic head resection for ductal adenocarcinoma observed an overall mortality rate of 4.10% and 3-year and 5-year survival rates of only 31.50% and 11.86%, respectively. In this study, 81.50% of patients already had obstructive jaundice condition. The pathology results of tumor differentiation revealed that 52.40% of patients were already at G2 intermediate differentiation, 42.00% of patients at G3 poor differentiation (42.00%), and 2.60% of patients at G4 differentiation[12].
A recent systematic review on quality of life in patients who underwent pancreatoduodenectomy showed that there was a decrease in physical functioning 3 mo after operation. Mental health issues were the only parameter shown to be stable 3 mo after operation. Several parameters, such as fatigue, postoperative pain, dyspnea, insomnia, loss of appetite, and bowel movement problems, were reported as negative influences after the operation, even though most parameters were resolved within 3 to 6 mo. This might become an important issue since most patients are offered for chemotherapy after the operation[13].
There are different ablation methods following temperature increase or impedance and probes (surgical or endoscopic using catheters or needles), such as chemical ablation and thermal ablation (cryoablation and hyperthermic ablation). RFA is one of the thermal therapies that has been used as one of the standard treatments for liver malignancy[14]. A prospective study by Curley et al[15], which was looking at the role of RFA treatment for primary as well as metastatic liver malignancies, showed that this procedure was effective and safe for tumor destruction with low tumor recurrence rate and no mortality related to the procedure.
An experimental study by Date et al[16] on pig pancreas with surrounding organ and vessels were ablated using temperature changing evaluation showed that temperature is the most important parameter to achieve complete ablation. With localized ablation therapy, there was no damage at the duodenal site or the other parts of the pancreas. Another innovative study by Hadjicostas et al[17] reported their experience in performing intraoperative RFA concomitantly with surgery for locally advanced and unresectable pancreatic cancer patients. RFA seemed to be a promising treatment as it could control the tumor growth.
A case series by Varshney et al[18] on RFA treatment guided by CT scan during the operation for unresectable pancreatic cancer showed that tumor necrosis could be achieved without any mortality events related to the procedure. The percutaneous RFA approach using abdominal ultrasound has also been reported by D’Onofrio et al[19] in patients with locally advanced pancreatic adenocarcinoma, where a 93% technical success rate was reported without any complications. The survival rate was recorded to be longer than 6 mo. However, there are limitations for the percutaneous RFA treatment approach. RFA treatment using transabdominal ultrasound is sometimes difficult due to overlying abdominal gas, and there is a risk of radiation exposure when using a CT scan-guided approach. On the other hand, Karim et al[20] reported several technical complications after the Whipple procedure, such as wound infection in 23.5% of patients and pancreatic leak in 21.4% of patients. Other complications noted in this study were lung complications (17.3%) and intra-abdominal collection (12.2%).
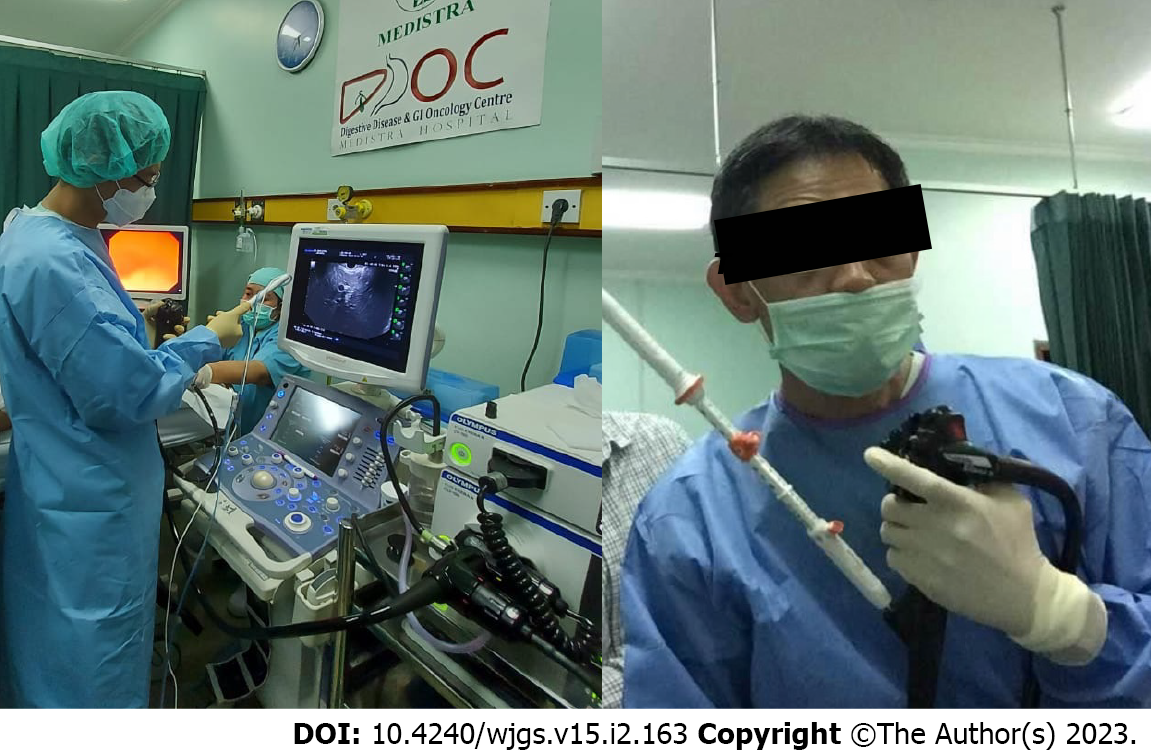
EUS has been widely used for pancreatic abnormality evaluation due to its ability to detect lesions more accurately, especially small pancreatic lesions, compared to other imaging modalities[21]. One pioneer animal experimental study by Goldberg et al[22] showed that EUS-guided RFA (EUSRA) can be successfully performed with a good necrosis coagulation target area. Recently, a needle dedicated for EUSRA was developed (Figure 1), where it showed a 100% technical success rate in animal models. There are four types of EUS-guided radiofrequency dedicated needles or probes, namely the 19 G fine needle aspiration (Radionics, Inc., Burlington, MA, United States), the Habib catheter (EMcision Ltd., London, United Kingdom), the Hybrid cryothermy probe (Hybrid-Therma; ERBE, Tubingen, Germany), and the EUSRA needle (STARmed, Koyang, Korea). The only bipolar probe is Hybrid cryothermy. Both the Hybrid cryothermy probe and EUSRA needle have internal cooling system. The cooling system uses a water-based cooled needle (cool-tip system). This system uses the electrical current from a generator with a monopolar electrode because bipolar pancreatic probes under endoscopic control do not exist. The electrode types are single internally cooled electrodes, cluster internal cooled electrode systems, and variations (StarBurst from RITA and LeVeen from Boston Scientific). All RFA needles or probe are connected to the generators to deliver a thermal effect to the lesion[23].
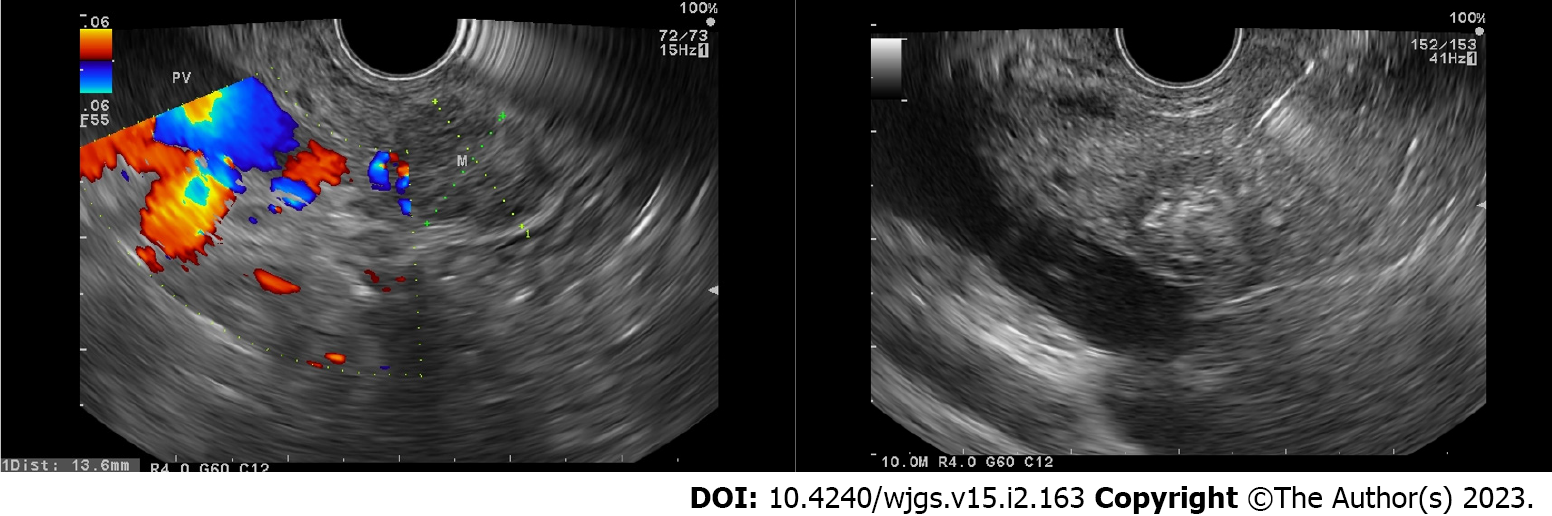
By using the EUS approach, it is easier to achieve good visualization of the tumor ablation and necrosis as the echoendoscope position is closer to the tumor area (Figure 2). Several case series have been reported to have a high technical success rate (73%-100%). However, several adverse events (AE) have also been noted, such as abdominal pain, bleeding, hyperamylasemia, obstructive jaundice, duodenal stricture, pancreatitis, pancreatic duct stenosis, and bacteremia[24,25]. In 2016, Lakhtakia et al[26] reported their experience using the EUSRA procedure in 3 patients with insulinoma. After a 12-mo follow-up, patients were still asymptomatic with a normoglycemic condition. A multi-center pilot study was conducted on the use of EUSRA in pancreatic cystic neoplasms and pNETs, where EUSRA was completed in all cases, and no major complications were observed after the procedure. There was complete resolution in 2 patients as well as cyst reduction in 3 patients after a 3-6-mo follow-up. Patients with pNETs showed a good response as tumor necrosis was recorded[27].
A pilot study by Rossi et al[28] on the feasibility, efficacy, and safety of EUSRA for secreting pNET patients showed that serum hormone levels reverted to normal within 24 h, and the symptoms regressed. After a 34-mo follow-up, no mortality was recorded, and tumor shrinkage and disappearance were noted after 24 mo. A case series by de Nucci et al[29] on patients with pNETs showed that complete ablation can be achieved within one session with a short period of hospitalization. Another prospective study by Song et al[30] using the EUSRA treatment approach for unresectable pancreatic cancer showed that the procedure was performed successfully in 6 cases, and systemic chemotherapy was completed on the same day in 3 patients. In this study, there were no major AEs even though 2 patients experienced mild abdominal pain.
Recently, a meta-analysis on EUSRA efficacy in pancreatic tumor management was performed with 13 studies included in the analysis. Based on this meta-analysis, the technical success rate was 100%, and the overall clinical success rate was 91.8%. Abdominal pain was the most common AE observed (9.82%), whereas perforation and infection were found in 1 patient, and pancreatitis was noted in 2 patients. This analysis concluded that EUSRA is a promising treatment strategy. However, most studies only collected a small sample size[31].
A recent longitudinal cohort study by Thosani et al[32] in 10 patients with pancreatic adenocarcinoma, where one to four RFA sessions per patient were performed, revealed that CA 19-9 levels decreased after 12 treatment sessions. Tumor size reduction of more than 50% was recorded in 3 patients. The median survival was 20.5 mo, whereas median survival of 13.4 mo was recorded after RFA treatment. All patients also underwent systemic chemotherapy. No significant complications were recorded in this study. A recent clinical case series study by Rossi et al[33] in elderly patients with pancreatic insulinoma showed that the EUSRA procedure was a safe procedure for elderly patients at high surgical risk. In this study, no major complications occurred during the procedure.
EUSRA is a promising treatment approach for pancreatic malignancy. However, further larger studies are needed, especially in pancreatic adenocarcinoma. The role of EUSRA in combination with systemic chemotherapy might become a new approach for managing unresectable pancreatic cancer. It may also become a promising combination strategy for tumor downstaging where it can be followed by surgery for possible tumor elimination or cure.
I would like to thank Prof. Ho Khek Yu, National University Hospital Singapore and Prof. Dong Wan Seo, Asan Medical Center, Seoul, South Korea, who have given their support for the development of endoscopic ultrasound procedures in our center, Digestive Disease and Gastrointestinal Oncology Center, Medistra Hospital, Jakarta.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arcidiacono PG, Italy; Sahewalla A, India; Yao J, China S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 2. | Ranganath R, Chu Q. Global trends in pancreas cancer among Asia-Pacific population. J Gastrointest Oncol. 2021;12:S374-S386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 3. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1518] [Article Influence: 253.0] [Reference Citation Analysis (1)] |
| 4. | Nakai Y, Takahara N, Mizuno S, Kogure H, Koike K. Current Status of Endoscopic Ultrasound Techniques for Pancreatic Neoplasms. Clin Endosc. 2019;52:527-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Palazzo L, Roseau G, Gayet B, Vilgrain V, Belghiti J, Fékéte F, Paolaggi JA. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy. 1993;25:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 252] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 410] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 7. | Yoon WJ, Daglilar ES, Kamionek M, Mino-Kenudson M, Brugge WR. Evaluation of radiofrequency ablation using the 1-Fr wire electrode in the porcine pancreas, liver, gallbladder, spleen, kidney, stomach, and lymph nodes: A pilot study. Dig Endosc. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Nießen A, Hackert T. State-of-the-art surgery for pancreatic cancer. Langenbecks Arch Surg. 2022;407:443-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1261] [Article Influence: 180.1] [Reference Citation Analysis (39)] |
| 11. | Speer AG, Thursfield VJ, Torn-Broers Y, Jefford M. Pancreatic cancer: surgical management and outcomes after 6 years of follow-up. Med J Aust. 2012;196:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Distler M, Rückert F, Hunger M, Kersting S, Pilarsky C, Saeger HD, Grützmann R. Evaluation of survival in patients after pancreatic head resection for ductal adenocarcinoma. BMC Surg. 2013;13:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | van Dijk SM, Heerkens HD, Tseng DSJ, Intven M, Molenaar IQ, van Santvoort HC. Systematic review on the impact of pancreatoduodenectomy on quality of life in patients with pancreatic cancer. HPB (Oxford). 2018;20:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 562] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 15. | Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 805] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 16. | Date RS, Biggins J, Paterson I, Denton J, McMahon RF, Siriwardena AK. Development and validation of an experimental model for the assessment of radiofrequency ablation of pancreatic parenchyma. Pancreas. 2005;30:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Hadjicostas P, Malakounides N, Varianos C, Kitiris E, Lerni F, Symeonides P. Radiofrequency ablation in pancreatic cancer. HPB (Oxford). 2006;8:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Varshney S, Sewkani A, Sharma S, Kapoor S, Naik S, Sharma A, Patel K. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7:74-78. [PubMed] |
| 19. | D'Onofrio M, Crosara S, De Robertis R, Butturini G, Salvia R, Paiella S, Bassi C, Mucelli RP. Percutaneous Radiofrequency Ablation of Unresectable Locally Advanced Pancreatic Cancer: Preliminary Results. Technol Cancer Res Treat. 2017;16:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Karim SAM, Abdulla KS, Abdulkarim QH, Rahim FH. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): Cross sectional study. Int J Surg. 2018;52:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | DiMagno EP, DiMagno MJ. Endoscopic Ultrasonography: From the Origins to Routine EUS. Dig Dis Sci. 2016;61:342-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Lakhtakia S, Seo DW. Endoscopic ultrasonography-guided tumor ablation. Dig Endosc. 2017;29:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Choi JH, Seo DW, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy. 2018;50:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 25. | Yousaf MN, Ehsan H, Muneeb A, Wahab A, Sana MK, Neupane K, Chaudhary FS. Role of Radiofrequency Ablation in the Management of Unresectable Pancreatic Cancer. Front Med (Lausanne). 2020;7:624997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Lakhtakia S, Ramchandani M, Galasso D, Gupta R, Venugopal S, Kalpala R, Reddy DN. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc. 2016;83:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | Rossi S, Viera FT, Ghittoni G, Cobianchi L, Rosa LL, Siciliani L, Bortolotto C, Veronese L, Vercelli A, Gallotti A, Ravetta V. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | de Nucci G, Imperatore N, Mandelli ED, di Nuovo F, d'Urbano C, Manes G. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors: a case series. Endosc Int Open. 2020;8:E1754-E1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Song TJ, Seo DW, Lakhtakia S, Reddy N, Oh DW, Park DH, Lee SS, Lee SK, Kim MH. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 31. | Dhaliwal A, Kolli S, Dhindsa BS, Choa J, Mashiana HS, Ramai D, Chandan S, Bhogal N, Sayles H, Bhat I, Singh S, Adler DG. Efficacy of EUS-RFA in pancreatic tumors: Is it ready for prime time? Endosc Int Open. 2020;8:E1243-E1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Thosani N, Cen P, Rowe J, Guha S, Bailey-Lundberg JM, Bhakta D, Patil P, Wray CJ. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) for advanced pancreatic and periampullary adenocarcinoma. Sci Rep. 2022;12:16516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Rossi G, Petrone MC, Capurso G, Partelli S, Falconi M, Arcidiacono PG. Endoscopic ultrasound radiofrequency ablation of pancreatic insulinoma in elderly patients: Three case reports. World J Clin Cases. 2022;10:6514-6519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |