Published online Feb 27, 2023. doi: 10.4240/wjgs.v15.i2.142
Peer-review started: October 13, 2022
First decision: November 15, 2022
Revised: November 23, 2022
Accepted: January 11, 2023
Article in press: January 11, 2023
Published online: February 27, 2023
Processing time: 136 Days and 20.1 Hours
Borderline resectable pancreatic cancer (BRPC) is a complex clinical entity with specific biological features. Criteria for resectability need to be assessed in combination with tumor anatomy and oncology. Neoadjuvant therapy (NAT) for BRPC patients is associated with additional survival benefits. Research is currently focused on exploring the optimal NAT regimen and more reliable ways of assessing response to NAT. More attention to management standards during NAT, including biliary drainage and nutritional support, is needed. Surgery remains the cornerstone of BRPC treatment and multidisciplinary teams can help to evaluate whether patients are suitable for surgery and provide individualized management during the perioperative period, including NAT responsiveness and the selection of surgical timing.
Core Tip: Borderline resectable pancreatic cancer (BRPC) is a type of pancreatic cancer with specific biological characteristics. To date, there is no unified comprehensive management for this disease. Thus, an evaluation of BRPC resection and neoadjuvant therapy (NAT) is needed. This review summarizes new resection methods and different NAT schemes, including treatment efficacy evaluation and management. This study also discusses the current progress of surgical treatment and the use of multidisciplinary teams to provide comprehensive multimodal management of BRPC treatment.
- Citation: Wu HY, Li JW, Li JZ, Zhai QL, Ye JY, Zheng SY, Fang K. Comprehensive multimodal management of borderline resectable pancreatic cancer: Current status and progress. World J Gastrointest Surg 2023; 15(2): 142-162
- URL: https://www.wjgnet.com/1948-9366/full/v15/i2/142.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i2.142
Pancreatic cancer (PC) has the poorest prognosis of all common cancers with a 5-year relative survival rate of only 11%. In the United States, the PC incidence rate ranked 10th among males and 8th among females in 2022[1]. Even with recent advances in treatment, survival has not improved significantly in the last 10 years[2]. The only potentially curative treatment for this disease is surgical resection, however, 80%–85% of patients are not candidates for resection due to nonspecific symptoms and a lack of early diagnostic methods[3].
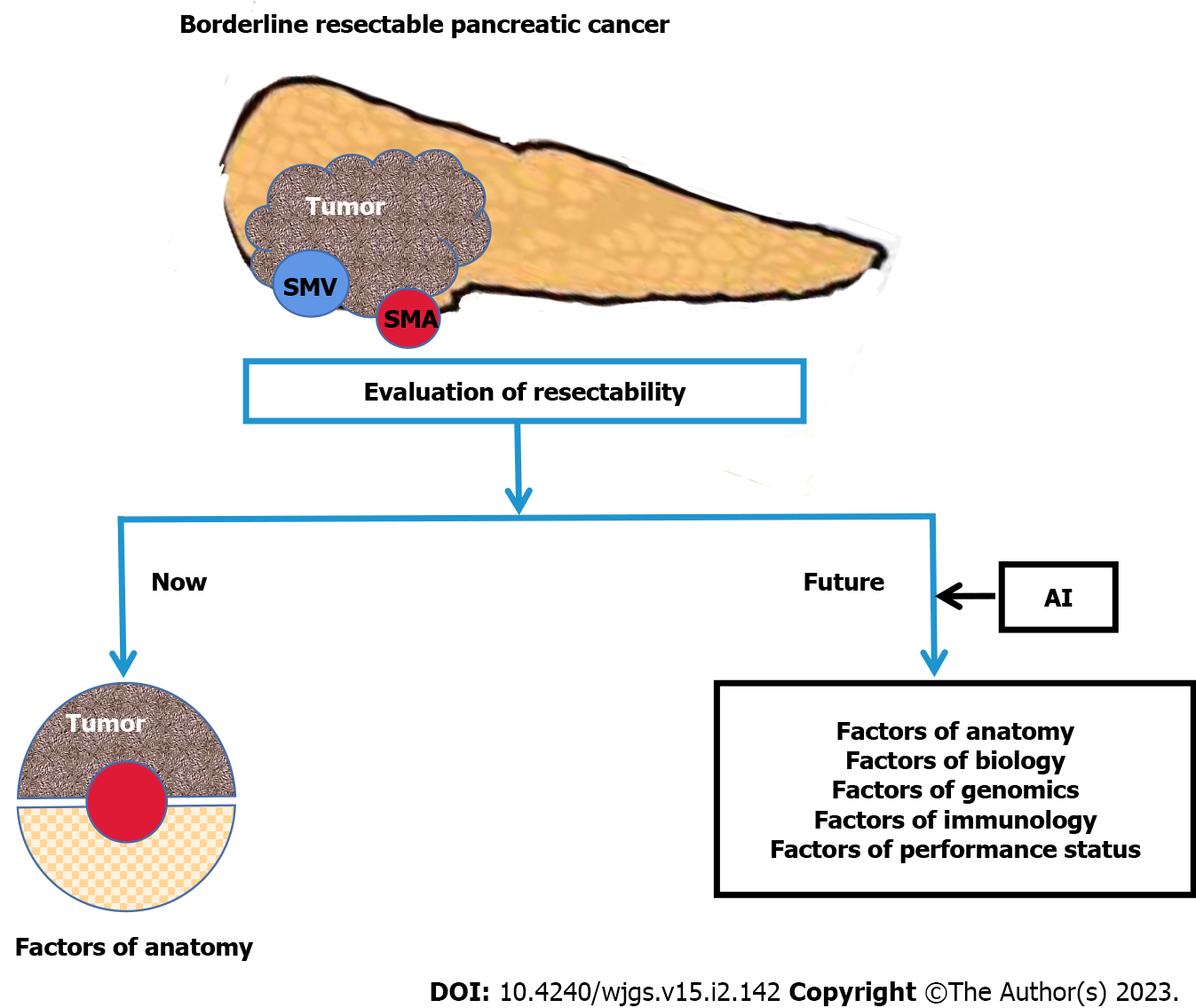
It is critical to identify specific borderline resectable pancreatic cancer (BRPC) patients who could be eligible for radical surgery. This disease is currently classified into four types for clinical management: RPC, BRPC, and unresectable PC, including locally advanced pancreatic cancer (LAPC) and metastatic PC. BRPC is further divided into PC with arterial invasion and PC with superior mesenteric vein (SMV) /portal vein (PV) invasion only[4]. The indications for surgery have expanded over the past few decades with advances in surgical techniques and improved preoperative imaging precision. The term “borderline resectable” was first introduced by the MD Anderson Cancer Center group in 2006[5]. Although surgery is technically feasible because it is localized and does not metastasize, BRPC carries a high risk of positive margins due to vascular infiltration[6,7]. In other words, the microsco
Historically, the resectability of PC has been dependent on the contiguous relationship between the celiac axis (CA), the superior mesenteric artery (SMA)/SMV, the PV, and the common hepatic artery (CHA). Improvements in surgical methods and post-surgical care have meant that tumor infiltration of the SMV/PV, SMA, HA, or CA is no longer a surgical contraindication. With the development of surgical techniques, especially increase in safety of vessel reconstruction, the range of resectability as defined by anatomy has expanded over the past 20 years[9-12]. The National Comprehensive Cancer Network (NCCN) guideline is the most widely used standard for the resectability of anatomy for BRPC patients because it distinguishes between pancreatic body/tail and pancreatic head/uncinate process tumors[13]. To assure uniformity and standardization in reporting and trial enrollment, this standard also eliminates the anatomically ambiguous terminology previously used to describe the interface between the tumor and nearby blood vessels (such as vascular “abutment,” “encasement,” “occlusion,” and “impingement”), in favor of defining ≥ 180° as a detailed degree of the interface between the tumor and each vessel[14].
BRPC is shown to be a particularly aggressive disease that may not benefit from upfront surgery. Biological parameters are used to evaluate resectability[15]. Some patients have no peripheral vascular invasion and imaging results indicate that the lesions can be respected and preoperative carbohydrate antigen 19-9 (CA19-9) levels are significantly increased. Other patients have positron emission tomography/computed tomography (PET/CT) results that indicate that the regional lymph nodes (LN) are suspicious and positive. Thus, a new international consensus on BRPC classification was suggested by the International Association of Pancreatology (IAP) in 2017[4].
IAP-BR-criteria added biological factors including CA19-9 ≥ 500 IU/mL and regional LN metastasis by biopsy or PET/CT into the BRPC definition based on anatomic criteria (BRPC type-a), defined as BRPC type-b. BRPC type-a with the addition of conditional factors such as Eastern Cooperative Oncology Group Performance Status (ECOG PS) 2 or more is defined as BRPC type-c. In a cohort of 369 patients with RPC, Kato et al[16] compared IAP-BR-criteria and NCCN-BR-criteria and found that IAP-BR-criteria were more effective at predicting prognosis.
In 2021, potential modifications to the current resectability classification based on IAP-BR-criteria, including additional candidate factors, were discussed at the Japanese Society of Hepato-Biliary-Pancreatic Surgery[17]. At this meeting, further candidate factors were proposed. PET/CT maximum standardized uptake values (SUV max) of the primary tumor were suggested for use as biological indicators to evaluate resectability and therapy response after NAT, while carcinoembryonic antigen, cancer antigen 125, or pancreatic cancer-associated antigen-2 were proposed for use as surrogate tumor markers in Lewis’s antigen-negative patients. For conditional host-related factors, age, Charlson-Dayo comorbidity, and markers of the systemic inflammatory response such as the modified Glasgow Prognostic Score or the neutrophil/Lymphocyte ratio (NLR) were also suggested for evaluating the resectability. More interestingly, new prognostic “genomic” markers that include germline deoxyribonucleic acid (DNA) damage repair mutations such as S100A2, S100A4, KRAS, and therapeutic target markers, including microsatellite instability, BRCA1, BRCA2, and other homologous recombination deficiency gene mutations, were also considered to have application potential and to be of value for further research.
Several genomic, transcriptomic, morphological, proteomic, metabolomic, and immune subtyping methods for PC were reviewed by Huang et al[18], of which the immune and morphological subtypes are associated with prognosis. Wartenberg et al[19] used immunohistochemical staining of immune cells in the tumor microenvironment to classify PC into three types: Immune-escape, immune-rich, and immune-exhausted. The immune-rich subtype was associated with a better prognosis. N Kalimuthu et al[20] classified PC into gland-forming and non-gland forming subtypes by histopathology and reported that patients with < 40% non-gland forming subtypes had superior overall survival (OS). The other classification subtypes were primarily used to predict the feasibility of chemotherapy and the efficacy of immunotherapy or targeted therapies.
In summary, resectability aims to make resection “meaningful” and promote a more favorable prognosis rather than maintaining a single focus on the resection rate. The various subtyping approaches for PC should be integrated and simplified using multiomics. Current studies still rely solely on the anatomical definition of BRPC (Figure 1). Future research needs to consider BRPC bio
To date, approximately 20% of PC patients are surgical candidates at the moment of diagnosis, 30% are BRPC or LAPC, and 50% have metastatic disease and are ineligible for surgery[3]. The architectural features of BRPC are such that even after extensive dissection of the nerve plexus along arteries, upfront surgery without NAT frequently results in a high R1 resection rate[6,7]. The current standard for BRPC treatment typically starts with preoperative chemotherapy or chemoradiotherapy, because NAT could degrade the stage and improve the R0 resection rate[21-25]. In addition to controlling local disease, the absence of tumor progression following NAT is a selection criterion for identifying tumors that are less biologically invasive or respond well to systemic therapy. Thus, this treatment can help to identify optimal surgical candidates. Moreover, because many postoperative patients cannot accept adjuvant therapy due to complications, NAT can help to reduce the incidence of pancreatic fistula and increase the completion rate of multimodality therapy. In summary, NAT is primarily used to select suitable surgery candidates and improve the tolerance of preoperative treatment[7,26,27].
Long-term results of the PREOPANC trial were published in 2022[27]. The findings revealed a significant difference in the OS of patients who received NAT (3 cycles of neoadjuvant gemcitabine + 2 cycles of radiotherapy with 36 Gy) and those who received upfront surgery. At a median follow-up of 59 mo, the neoadjuvant chemoradiotherapy group had a median survival time (MST) of 15.7 mo, while the upfront surgery group had an MST of 14.3 mo (HR, 0.73; 95%CI, 0.56–0.96; P = 0.025). Moreover, the 5-year OS rate for patients who underwent NAT or upfront surgery was 20.5% and 6.5%, respectively. The NAT group also had a higher R0 resection rate than the upfront surgery group (72% vs 43%, P < 0.001). Although single gemcitabine is no longer a standard treatment for PC patients, the PREOPANC trial showed that gemcitabine combined with NAT, surgery, and adjuvant chemotherapy was effective against BRPC. van Dam et al[28] conducted a meta-analysis and subgroup analysis of 5 randomized controlled trials (RCTs) in which BRPC was included and found that NAT was associated with higher OS of patients with BRPC (HR 0.61, 95%CI 0.44–0.85; P = 0.004; I² = 59%). Both neoadjuvant chemotherapy (NACT) (HR 0.54, 95%CI 0.34–0.87; P = 0.01; I² = 64%) and neoadjuvant chemoradiation (NACRT) resulted in a higher OS than upfront surgery (HR 0.74, 95%CI 0.58–0.95; P = 0.02; I² = 7%). Since NAT improves the R0 resection rate and OS, European Society of Medical Oncology (ESMO)[29] and NCCN guidelines[13] recommend NAT over upfront surgery for BRPC.
Due to the lack of RCT data, there is no consensus on a preferred NAT regimen. However, NCCN guidelines[13] recommend fluorouracil-leucovorin-irinotecan-oxaliplatin/modified fluorouracil-leucovorin-irinotecan-oxaliplatin (FOLFIRINOX/mFOLFIRINOX) ± radiation or gemcitabine/nab-paclitaxel (GNP) ± radiation as the first-line regimens[13]. A SWOG-1505 preliminary analysis was reported more recently. This study evaluated mFOLFIRINOX and GNP in the NAT setting of RPC and found that the GNP arm was associated with a higher median disease-free survival (DFS) (14.2 vs 10.9 mo, P = 0.87) and a complete or moderate pathological response (42% vs 25%) than the mFOLFIRINOX arm. However, the resection rate, R0 resection rate, and 2-year OS did not differ between the groups[30]. Of note, there was no significant difference in the toxicity profile of the GNP and mFOLFIRINOX groups in this research. A phase II study conducted by Kondo et al[31] included 47 patients with BRPC-arterial contact who received six cycles of a neoadjuvant gemcitabine, nab-paclitaxel plus S-1 regimen. The R0 rate was 86%, and the 2-year OS rate and median OS time among 47 eligible patients were 70.1% and 41.0 mo, respectively. More RCTs are needed to compare these two first-line neoadjuvant che
For PC patients with known BRCA1/2 or PALB2 mutations, NCCN guidelines recommend FOLFIRINOX/mFOLFIRINOX or GNP plus platinum complex treatment[13]. Similar to breast cancer, key proteins involved in homologous recombination during PC include BRCA1 and BRCA2, and PALB2 is a critical regulator of BRCA2 function[32-34]. Prior studies have shown that platinum-based treatment increases the OS of patients with advanced PC who have germline mutations in BRCA1, BRCA2, or PALB2[35-37]. A retrospective study found that patients with these mutations had an overall response rate (ORR) of 58% while those in the control group had an ORR of 21% (P = 0.0022). In addition, the real-world progression-free survival was 10.1 mo for patients with these mutations and 6.9 mo for controls (HR 0.43; 95%CI 0.25–0.74; P = 0.0068)[38].
The role of radiation or chemoradiation during NAT for BRPC remains unclear[39]. NACRT uses radiotherapy to sterilize tumor boundaries that are in contact with vasculature and chemotherapy to treat any undiagnosed micro-metastatic disease. Intensity-modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT), three-dimensional conventional radiation therapy (3D-CRT), and intraoperative radiation therapy (IORT), have all been used for patients with BRPC[40]. Retrospective evidence suggests that NACRT may be associated with a higher proportion of margin-negative resections than NACT[41].
Studies have further divided the current NACRT regimen into concurrent chemoradiotherapy with or without induction chemotherapy (Table 1). Until now, RCTs that directly compare NACRT and NACT are lacking in patients with BRPC. A021501 study enrolled 126 patients with BRPC, of whom 70 (55.6%) and 56 (44.4%) were randomized into an NACT arm and an NACRT arm, respectively. The NACT arm received eight doses of mFOLFIRINOX while the NACRT arm received eight doses of mFOLFIRINOX followed by a hypofractionated protocol that uses SBRT 33-40 Gy or image-guided radiotherapy (RT) with 25 Gy. After NAT, patients without disease progression underwent a pancreatectomy along with four additional doses of oxaliplatin-calcium-folinate-fluorouracil (FOLFOX6). Of the first 30 patients in each arm, the R0 rate was achieved in 10 (33%) and 17 (57%) patients in the NACRT and NACT arms, respectively, resulting in the closure of the NACRT arm and continuation of the NACT arm. The 18-mo OS rates were 66.7% and 47.3% in the NACT and NACRT arms, respectively, while the median OS of patients was 29.8 and 17.1 mo in the NACT and NACRT arms, respectively. These findings suggested that NACT alone was associated with a more favorable prognosis than NACRT among patients with BRPC[42].
| Ref. | P | N | Induction chemotherapy | Concurrent chemotherapy | SNT/TNT | RT method | Total RT dose (Gy) | Resection rate (%) | R0 rate (%) | Median OS (mo) |
| Katz et al[147] | II | 22 | FOLFIRINOX | Cape | SNT | 3D-CRT/IMRT | 50.4 | 68 | 93 | 21.7 |
| Nagakawa et al[148] | II | 27 | GEM | GEM+S-1 | SNT | IMRT | 50.4 | 70.3 | 94.7 | 22.4 |
| Masui et al[149] | II | 30 | GEM | GEM | SNT | 3D-CRT | 39 | 50 | 83 | 13.8 |
| IMRT | 42 | 67 | 83 | 32 | ||||||
| Murphy et al[150] | II | 48 | FOLFIRINOX | Cape | TNT | Proton | 25 | 67 | 97 | 37.7 |
| IMRT | 58 | |||||||||
| Tran et al[151] | II | 25 | FOLFIRINOX | GEM | SNT | IMRT | 50 | 52 | 100 | 24.2 |
| Versteijne et al[152] | III | 54 | GEM | GEM | SNT | 3D-CRT | 36 | 61 | 79 | 16 |
| Takahashi et al[153] | II | 41 | NR | S-1 | SNT | 3D-CRT | 50.4 | 85.4 | 74.3 | 30.8 |
| Hayashi et al[154] | II | 45 | NR | S-1/GEM | SNT | 3D-CRT | 50.4 | 62.2 | 96.4 | 17.3 |
| Sharp et al[155] | II | 126 | mFOLFIRINOX | NR | SNT | SBRT/HIGRT | 33-40 / 25 | 35 | 251 | 17.1 |
| Ghaneh P et al[43] | III | 88 | NR | Cape | SNT | 3D-CRT | 50.4 | NR | 191 | NR |
ESPAC-5F is a four-arm, multicenter, phase II trial that evaluated different approaches to NAT among patients with BRPC. Ninety patients were randomly assigned to four arms: Upfront surgery, two cycles of gemcitabine + capecitabine followed by surgery, four cycles of FOLFIRINOX followed by surgery, and NACRT with 50.4 Gy over 28 fractions with concurrent capecitabine followed by surgery. There was no statistical difference in R0 rates between the arms (14%, 17%, 18%, and 37% for the upfront surgery, gemcitabine + capecitabine arm, FOLFIRINOX, and NACRT arms, respectively). There was a trend toward a higher R0 rate in the NACRT arm, but the intention-to-treatment (ITT) R0 rate was 19%, which was like the ITT R0 rates of the other three arms. It is worth noting that patients in the NAT arms had a higher 1-year OS than those in the upfront surgery arm (77% vs 42%, HR = 0.27; P < 0.001), but the NACRT arm did not confer a more prominent survival benefit than other arms[43]. Multiple single-institution retrospective studies have evaluated the efficacy of neoadjuvant SBRT for BRPC and LAPC, showing excellent R0 resection rates and promising OS[44-46]. These results contradict the A021501 study, potentially because the A021501 study had low rates of pancreatectomy (35%) and treatment completion (18%). It is worth mentioning that SBRT has a theoretical synergy with emerging immunotherapies, the effectiveness and safety of which have been confirmed by previous basic research and clinical studies[47,48].
In conclusion, the superiority of NACT and NACRT for BRPC remains unclear and there is still controversy about the most appropriate NAT regimen. Current research findings suggest that the clinical benefit of NAT over upfront surgery is tentatively certain. BRPC patients could actively participate in NACRT/SBRT/IMRT-related clinical trials but all radiotherapy procedures should be performed in a large experienced pancreatic center. A robust NAT study would ideally compare NACT with NACRT including 3D-CRT, IMRT, or SBRT.
Most trials for BRPC have performed surgical resection between short-course NAT (SNT) and adjuvant therapy. As the biological behavior of PC has become better understood, some researchers have proposed a new modality called total NAT (TNT). TNT was designed to provide postoperative adjuvant therapy in a preoperative setting and includes concurrent chemoradiotherapy delivered before or after systemic chemotherapy[49]. The theoretical advantages of TNT over SNT include its ability to reduce the risk of delayed chemotherapy for postoperative patients and improve patient compliance and tolerance and ensure drug dose and intensity. A retrospective study found that TNT increased the surgical resection and R0 rates among BRPC and LAPC patients[50]. However, another study reported that while TNT improved the complete pathological remission rate, there was no statistical difference in the median OS between the two groups compared with the SNT mode[51]. In the SWOG-1505 trial, less than half of the patients completed systematic treatment, causing some doubt about whether TNT can help patients to receive the maximum amount of systemic therapy[30]. The use of the TNT mode for PC patients is still in its initial stages. The problems associated with TNT are the same as those of NAT, and this treatment modality still lacks a highly accurate and effective evaluation mechanism.
While findings from RCTs of adjuvant chemotherapy given to patients receiving upfront surgery have suggested that the ideal time frame for perioperative systemic chemotherapy is 6 mo[52], there is no agreement on the length and cycle of NAT for BRPC patients. To make this decision, NAT endpoints will need to be defined to assess responsiveness and tumor re-staging during treatment. NAT patients are typically evaluated every 2 mo to measure treatment toxicity and assess objective clinical, radiologic, biochemical, or metabolic responses. However, there are few effective biomarkers or imaging techniques available to monitor treatment responses among patients with pancreatic neoplasms[51,53].
CA19-9 and CT are the only techniques widely used to assess objective response rates following NAT due to the absence of relevant high-quality study findings. However, there is a growing consensus that CT has important limitations including its inability to distinguish between the tumor and fibrosis or inflammation or to accurately determine tumor responsiveness to NAT[54,55]. Studies indicate that a significant proportion of unresectable patients, assessed by CT using the Response Evaluation Criteria In Solid Tumors (RECIST) criteria following NAT, finally achieve an R0 resection[56,57].
Several radiologic approaches are being used to evaluate NAT responses including dual-energy CT (DECT), 18Ffluorodeoxyglucose-PET/CT (FDG-PET/CT), endoscopic ultrasound (EUS), and diffusion-weighted magnetic resonance imaging-MRI (DWI-MRI). Since variations in SUVs may represent the metabolic response of cancer to chemotherapy and FDG uptake is highly correlated with tumor load and viability, FDG-PET/CT is used to evaluate NAT efficacy toward a variety of solid malignancies[58-60]. The metabolic response observed by FDG-PET/CT offers a functional assessment of tumor responsiveness compared to RECIST criteria. Akita et al[58] demonstrated that maximal SUVs and tumor size were dramatically reduced following NACRT in BRPC and RPC patients. However, pancreatic tissues incorporate lots of stroma and a high infiltration of inflammatory cells such as macrophages and neutrophils and NAT can further promote both inflammatory cell infiltration and fibrosis. As a result, posttherapy SUV may not accurately reflect the pathological response. In addition, Akita et al[58] found that a favorable period for FDG-PET/CT assessment is 8 wk post-radiation. A histological inspection of the resected specimens at that time did not reveal inflammatory alterations in the peripancreatic tissues.
The differentiation of tumor composition can be discriminated by DECT which uses simultaneous scanning with special stages of electricity. DECT can precisely differentiate between PC and continual mass-forming chronic pancreatitis. Moreover, the iodine concentration for the duration of DECT can differentiate between pancreatic patients who successfully respond to chemotherapy and those who don’t. This finding suggests that DECT may be used to identify fibrosis caused by NAT[61,62].
DWI-MRI can recognize tissue diffusivity characteristics and be used to perform quantitative and qualitative tumor evaluations. Cuneo et al[63] reported that tumor apparent diffusion coefficient (ADC) values of DWI-MRI were specifically linked to the amount of tumor cell destruction. Responders and non-responders had different pretreatment ADC values, suggesting that the ADC values of DWI-MRI prior to NAT can predict the histologic response of BRPC patients.
EUS is rarely used to evaluate NAT response in PCs. The influence of stroma is weak so the value of elastography for chemotherapy and radiation therapy remains unknown[64].
Radiomics is a quantitative image analysis technology that mines the in-depth features of images, allowing for the extraction of data on tumor intensity, shape, size, or volume from digital images. This technique is used as a biomarker for disease diagnosis, grading, prognosis evaluation, and responses to treatment and can both support personalized clinical decisions and improve individualized treatment options[65]. The role of radiomics to evaluate NAT responsiveness has been studied extensively in other solid tumors. Braman et al[66] found that a combined intertumoral and peritumoral radiomic feature identified by contrast-enhanced MRI can predict the complete response of breast cancer patients following NAT. Several studies have assessed the use of radiomics to aid PC prognosis and NAT responsiveness (Table 2). Ciaravino et al reported that 17 LAPC or BRPC patients reached the resectable stage after NAT, and CT texture analysis showed that there was a statistically significant difference between kurtosis before and after NAT (P = 0.0046)[67]. A prospective study by Borhani et al[68] included 39 patients with RPC or BRPC, all of whom had completed surgery after NAT. This study reported that the histologic response could be assessed by pretreatment mean positive pixel (MPP) at a fine- and medium-level filtration, pretreatment kurtosis at a medium-level filtration and changes in kurtosis, and higher MPP was related to favorable histologic response (OR 1.06; 95%CI, 1.002-1.12).
| Ref. | N | Imaging modality | Segmentation method | Feature extraction software | Extracted features | Statistically significant features | Extracted features type | % RQS (points) |
| Yue et al[156] | 25 | PET | Semi-automated | 3D kernel-based approach | 12 | 3 | Second-order texture features | 25% (9) |
| Chen et al[157] | 20 | CT | Manual | In-house developed software | 8 | 4 | First-order texture features | 14% (5) |
| Ciaravino et al[67] | 17 | CECT | Manual | MaZda | 5 | 1 | First-order texture features | 17% (6) |
| Kaissis et al[158] | 55 | MRI | Manual | Pyradiomics | 1606 | 13 | Shape features; First-order texture features; Second-order texture features; Filtered image features | 36% (13) |
| Nasief et al[70] | 90 | CT | Manual | IBEX | 1300 | 13 | Shape features; First-order texture features; Second-order texture features; Customised features1 | 33% (12) |
| Borhani et al[68] | 39 | CECT | Manual | TexRAD | 6 | 4 | First-order texture features; Filtered image features | 6% (2) |
Currently, artificial intelligence (AI) technologies, including deep learning and machine learning, are also being developed to evaluate NAT efficacy in PC patients. One study divided 81 PC patients receiving NAT into a response group (333 images) and a non-response group (443 images). A model using only the deep learning (convolutional neural network) had an area under the curve (AUC) of 0.738, while a combined model incorporating CA19-9 and deep learning had an AUC of 0.785[69]. Not only does the discrimination and accuracy of this model need to be improved, but also small sample size, and heterogeneity caused by different NAT receptors, resectability, and CT slice thicknesses limited the generalizability of the study. New research has combined radiomics with AI. Delta radiomics is a quantitative approach used to assess the treatment-induced net change of radiomic features in a set of longitudinal images. This technique could theoretically be used for the early prediction of NAT treatment responsiveness. Nasief et al[70] analyzed 28 daily CT sets collected during routine CT-guided CRT along with pathological treatment response data from 90 patients with RPC or BRPC to obtain delta-radiomic features related to therapy response. The results showed that 13 delta-radiomic features passed the T-test and linear-mixed-effects models and changed significantly after 2-4 wk of treatment. The best-performing machine learning model for differentiating good vs poor responders was designed using the normalized-entropy-to-standard-deviation-difference, kurtosis, and coarseness. The AUC of this model was 0.94, but due to the limitation of sample size and the lack of biological interpretability of machine learning model, delta radiomics model distance to practical clinical application, additional studies are needed to validate the reproducibility of the model and to address the issues of model interpretability as well as visualization applications.
While CA19-9 is typically used to track the effectiveness of NAT among patients with BRPC, the predictive value of this marker remains limited. The relevance of a NAT-induced decrease in CA19-9 Levels has not been clearly defined, and the cut-off CA19-9 value for diagnosing NAT responders remains controversial. Prior studies have shown that a 20%–50% drop in CA19-9[71-74] or an absolute value of 72-400 (U/mL) CA19-9 after NAT[75-77] was associated with resectability or a favorable prognosis following resection. In addition, among patients who are Lewis’s antigen expression negative or have abnormal bilirubin levels due to cholestasis, serum CA19-9 is inapplicable. Thus, more accurate and focused biomarkers are required to evaluate NAT responsiveness[78-80]. While circulating cell-free DNA, circulating tumor cells, exosomes, and ephrin typeA receptor 2 in tumor-derived extracellular vesicles all show good correlations with NAT responsiveness, larger sample RCTs are required to validate their roles[81-83]. For BRPC patients who received gemcitabine and S1 followed by radiotherapy, the augmentation of partial response rates after NAT was associated with positive expression of human equilibrative nucleoside transporter 1 and negative expression of thymidylate synthase[84]. Moreover, Glazer et al[85] demonstrated that the NLR is associated with the OS of BRPC patients who undergo surgical resection after NAT.
Non-invasive and accurate tumor restaging after NAT may be possible through AI approaches such as machine learning and deep learning, combined with different device-based radiomics and novel biomarkers. This will inform the choice of timing for post-NAT surgery among BRPC patients.
Malnutrition is a common problem among PC patients, two-thirds of whom are diagnosed with anorexia at the first visit[86]. NAT significantly alters the nutritional status of patients with esophageal and gastric cancer, which impacts postoperative recovery and surgery rates[87]. However, there is a lack of high-level evidence-based studies on the changes in nutritional status among BRPC patients during NAT as well as the optimal strategy for nutritional status assessment and support.
A retrospective study published in 2020 showed that the preoperative nutritional risk, an independent prognostic factor for OS (HR 5.24, P = 0.013), was significantly higher among patients receiving NAT (P = 0.026)[88]. Moreover, Kim et al[89] found that the average pre-NACT prognostic nutritional index (PNI) was higher than the post-NACT PNI, with a difference of 2.98. Moreover, if the change value of PNI, obtained by pre-NAT PNI minus post-NAT PNI, is lower than -1.94, it is a risk factor for the OS of PC patients following NAT. Thus, a nutritional evaluation of BRPC patients should be routinely performed, especially those who have received NAT.
In addition to scoring using conventional nutritional screening tools, CT-based body composition analysis is being increasingly used to evaluate the nutritional status of patients with PC. Several studies of body composition have shown that sarcopenia, defined by a reduction in the skeletal muscle index of the third lumbar spine, and an increase in the visceral fat area and subcutaneous fat area, are high-risk factors for postoperative pancreatic leakage and can affect the long-term prognosis of PC patients[90-94]. Sandini et al[95] found that patients with BRPC or LAPC who received NAT had a significant loss of adipose tissue. However, there was little reduction in lean body mass. This study also showed that NAT-induced increases in muscle mass were a reliable indicator of respectability.
At present, there is no unified standard management for the nutritional support of BRPC patients during NAT. An expert consensus from Spain in 2021[96] recommended that BRPC patients receiving NAT should receive a nutritional screening by Malnutrition Universal Screening Tool (MUST) before NAT, receive nutrition support with a MUST score ≥ 1, and be taking oral nutritional supplements. A position paper from the International Study Group on Pancreatic Surgery for nutritional support and therapy in pancreatic surgery[97] recommended that when one of the following criteria is met, nutritional support for patients with PC during NAT should be seriously considered: (1) Weight loss > 15%, (2) A BMI < 18.5 kg/m², (3) A subjective global assessment score C or nutritional risk score > 5, or (4) A serum albumin < 30 g/L (no evidence of liver or renal dysfunction).
Nutritional therapy during NAT for BRPC patients should be individualized to each patient’s performance status. New nutritional screening tools should be designed to incorporate body composition analysis and corresponding cutoffs should be developed for clinical application.
Most pancreatic tumors are located in the head of the pancreas, which is prone to malignant biliary obstruction (MBO) and affects hepatic function, coagulation, and fibrinolysis. While it remains unknown whether early preoperative biliary drainage (PBD) or straightforward surgery is the better option[98-100], it is reasonable for BRPC patients to choose PBD. Since NAT is routinely required for patients with BRPC and the NAT period is generally 2-6 mo[101], PBD should be performed to ensure that chemotherapy can be safely completed without interruption from cholangitis or hepatic insufficiency while waiting for surgery[102].
There are two critical types of PBD: Percutaneous biliary drainage (PTBD) and endoscopic retrograde biliary drainage (ERBD), including endoscopic biliary stenting (EBS) and endoscopic naso-biliary drainage (ENBD)[103]. Before the introduction of ERBD, PTBD was the preferred type of biliary drainage. However, retrospective studies from Japan showed lower survival and higher rates of peritoneal recurrence among patients treated with PTBD than those receiving ERBD[104,105]. In addition, in a retrospective study of patients undergoing PTBD or ERBD, the PTBD group had significantly higher hepatic metastasis, more wound infections, and lower OS[100]. Sasahira et al[106] found that ENBD was associated with much less dysfunction than EBS in MBO. However, ENBD may not be suitable for long-term preoperative cure because of its impact on patient quality of life and disruptions in the enterohepatic circulation of bile salts[107].
Thus, EBS is repeatedly used when PBD is performed, and the used stents can be extensively divided into plastic stents (PS) and self-expandable metal stents (SEMS), including full-covered SEMS (FCSEMS), partially covered SEMS (PCSEMS) and uncovered SEMS (USEMS). Some studies have found that FCSEMS is more effective than PS for MBO among BRPC patients receiving NAT. Several RCTs have verified that the median patency of SEMS is 4–9 mo or more, which is notably longer than that of PS[108-111]. A recent RCT from Japan which included patients with BRPC who required PBD before GNP based NAT, illustrated that the rate of stent dysfunction was drastically lower in the FCSEMS arm than in the PS arm (18.2% vs 72.8%, P = 0.015), and showed that FCSEMS and PS had a similar safety profile and medical costs[112]. A retrospective study of 749 patients with MBO found that covered SEMS (CSEMS) and USEMS had similar rates of clinical success in bile-duct obstruction treatment and patency duration. However, the USEMS arm was associated with less tumor growth than the CSEMS arm (76% vs 9%, P < 0.001)[113]. While no studies have directly compared PCSEMS and FCSEMS, a decision on the choice of biliary stents for BRPC patients receiving NAT would ideally be made following a joint evaluation by a surgeon and a pathologist.
EUS-guided biliary drainage (EUS-BD) has emerged as a positive approach for biliary drainage when ERCP is unsuccessful and can reduce the likelihood of pancreatitis, injury to the pancreatic tissue, and irritation. However, due to the risk of potential bile leakage and the high demand at the endoscopist level, more studies are needed to compare the efficacy and safety of EUS-BD with other methods for preoperative biliary drainage[114].
While study findings remain insufficient, available data suggest that SEMS is more suitable in PBD for patients with BRPC during NAT. However, the choice of PS is most suitable when the window period for preoperative therapy is short. More high-quality studies are required to demonstrate the most appropriate method for PBD during NAT.
Lymph node recurrence is an important part of the postoperative recurrence of PC[115]. There remains some controversy about the scope of surgical lymph node dissection, and most researchers believe that expanding regional lymph node dissection cannot improve patient prognosis. However, a few studies indicate that there is value in expanding dissection[116,117]. Lymph nodes in the arterial and portal regions are the main sites for the local recurrence of PC[118]. A series of meta-analyses showed no significant increase in the median survival time and 1-, 3-, and 5-year survival rates of patients receiving extended lymphadenectomy in pancreaticoduodenectomy (EPD) versus standard lymphadenectomy in pancreaticoduodenectomy (SPD) and an increased risk of complications[119,120]. The standard lymph node dissection ranges are 5, 6, 8a, 12b1, 12b2, 12c, 13a, 13b, 14a, 14b, 17a, and 17b for pancreatic head cancer. For cancers of the pancreatic body and tail, dissection of stations 10, 11, and 18 is standard and dissection of station 9 is only recommended for patients with cancer of the pancreatic body[121]. No definitive studies have illustrated the benefit of expanded lymph node dissection for BRPC patients. The concept of Heidelberg triangle surgery was proposed by the University of Heidelberg in Germany[122]. In addition to conventional lymph node dissection, all lymph nodes, vessels, and nerve tissue in the Heidelberg triangle can be dissected.
One study found that about one in five patients who received pancreatoduodenectomy (PD), distal pancreatectomy (DP), or DP with abdominal axis resection (DP-CAR) for pancreatic tumors had lymph node recurrence. Of these, peri-pancreatic head (peri-Ph), para-aortic, and SMA lymph node recurrences were the most common, accounting for 12%, 11%, and 10%, respectively[123]. The precise type of lymph node dissection should be chosen according to the tumor’s location and other characteristics.
BRPC is often associated with the invasion of important vessels such as the celiac trunk and common hepatic artery, resulting in low clinical resection rates. Studies illustrate that combined external pancreatic atherectomy in patients with tumor invasion of SMA, CA, and HA is often associated with more postoperative complications and higher mortality rates and has no impact on survival. Thus, reconstructive pancreatic resection with arterial invasion is not recommended for this patient population[124,125]. However, some recent studies have questioned these results, suggesting that in highly specialized pancreatic centers, even atherectomy can be performed safely and promote long-term outcomes similar to standard surgery for radical cure[126,127]. Distal pancreatectomy with abdominal axis resection DP-CAR or modified Appleby resection improves the safety of BRPC combined with atherectomy. Since this technique allows en block resection of the celiac axis and the common hepatic artery, arterial reconstruction is not required. However, the feasibility and effectiveness of this procedure remain to be evaluated in future clinical studies.
Many studies have focused on how to increase the rate of radical resection and thus improve survival among patients with BRPC. The depth of arterial tumor invasion has a greater impact on radical resection than the circumferential size of the tumor invasion[128]. Arterial wall invasion in PC is often confined to the arterial epithelium and rarely breaks through the outer elastic layer of dense connective tissue. Some studies have proposed the concept of “arterial sheath debridement,” using the loose tissue between the arterial epithelium and the outer elastic layer as the anatomical plane to debride the peri-arterial nerve fiber connective tissue, to obtain radical resection while avoiding severe complications associated with arterial resection[129]. However, the current understanding of treatment for junctional resectable patients and the common use of preoperative radiotherapy have shown an increase in peri-arterial tissue inflammation and fibrosis. This has made it difficult to free the affected artery and completely debride peri-arterial tissue invaded by the tumor during surgery.
Most PC patients have SMV-portal vein axis involvement. However, radical surgery combined with vein resection and reconstruction is shown to be safe and feasible with a good prognosis in several studies[130,131]. Dua et al[132] propose various vascular anastomoses such as longitudinal vascular suture, transverse vascular suture, end-to-end vascular anastomosis, vessel wall patch repair, and autograft or artificial vascular reconstruction. The most appropriate approach is chosen based on the circumference and length of the invading vessels. There is no consensus about the best mechanism for revascularization, but direct suturing, patch repair, and autograft are preferred because of the increased risk of thrombosis associated with artificial implants.
Patients who undergo pancreatic tumor resection are prone to local recurrence and distant metastasis after surgery. Local control of the tumor is essential to prolonging survival and improving quality of life. Previous studies have shown that neoadjuvant chemotherapy, radiotherapy, and extracorporeal irradiation therapy can improve local and regional control and survival. However, external-beam radiotherapy is limited in its clinical application by the challenge of delivering sufficient doses of radiation. In contrast, IORT has the unique advantage of delivering the maximum dose of ionizing radiation precisely to the tumor, tumor bed, surrounding lymph node area, superior mesenteric margin, portal vein, and areas at high risk for recurrence, while surgically removing radiation-sensitive organs such as the small intestine from the radiation field to minimize damage to surrounding normal tissue. In addition, the surgeon and radiation therapist can coordinate intraoperatively under direct vision to determine the exact location and extent of the irradiated target area to avoid missing risk areas. Simultaneous completion of surgery and radiotherapy can significantly shorten the treatment course of patients. IORT patients have a median survival time of 19.1 mo, a 2-year survival rate of 42.1%, and a 2-year local control rate of 83.7% after resection, which are all higher than patients in the control group. In addition, the pain relief rate after IORT is 94.9%[133]. Harrison et al[134] found that after FOLFIRINOX-based NAT, survival rates at 12, 24, 48 and 60 mo were 99%, 79%, 47%, and 28%, respectively, for all forms of resection plus IORT (10 Gy). For patients who only received IORT (20 Gy), the survival rates at 12, 24, 48, and 60 mo were 98%, 49%, 13%, and 9%, respectively. The overall complication rate of IORT was 26.7%, including gastroparesis, gastrointestinal bleeding, pancreatic leakage, and celiac leakage. Clinical and experimental studies have shown that IORT at 10–20 Gy is still well tolerated by organs, even in patients with combined revascularisation[135]. These findings suggest that patients with postoperative pathology showing residual tumors visible to the naked eye at the margins or positive margins on frozen pathology, or patients with moderate or severe pain and ineffective pain relief, can improve their prognosis and quality of survival following combined IORT.
125I particle implantation directly implants particles into tumor tissues to achieve precise treatment of tumor. Gamma rays released by these particles reach tumor cells with reduced decay and a high effective dose, causing tumor tissues to receive more radiation and undergo higher levels of necrosis. The gamma-ray irradiation distance is short so most of the energy can be absorbed by tumor tissues, minimizing the damage to surrounding normal tissues. In addition, toxic side effects, including radiation-induced inflammation, seed displacement, pancreatic fistula, bleeding, and gastrointestinal obstruction are minimal[136]. A refined and standardized treatment approach with reasonable preoperative planning of the particle number and distribution and accurate prediction of energy distribution will improve treatment efficacy and reduce the incidence of acute adverse effects. By maximizing the radiation dose to the tumor and reducing radiation damage to the surrounding normal tissues, local invasion of the tumor is significantly inhibited and patient OS is increased. However, there is still a lack of corresponding research to support whether BRPC patients will benefit from this treatment.
Intraoperative cryoablation therapy is a tumor treatment technique based on the idea that physical action kills cells. Lesion tissue is repeatedly frozen and thawed, tumor cells appear to be dehydrated and burst, and the microstructure inside the broken tumor cells can activate the immune system to control tumor progression. In addition, platelets in the blood vessels around the tumor accumulate and form thrombi to destroy the blood supply to the tumor, thus indirectly killing the tumor cells. Com
Irreversible electroporation (IRE), also known as NanoKnife, is a new non-thermal physical ablation technique. By applying short and high pulse voltages between two electrodes made of unique materials, the original membrane potential of the cell is altered, creating irreversible nanoscale pores in the lipid bilayer of the membrane and causing disruption of cellular homeostasis that leads to cell death.
This ablation method only causes cell death in a specific area while preserving the integrity of the tissue scaffold and the fibrous structure of the cells. In contrast, the adjacent tissues, including blood vessels and surrounding normal tissues are not damaged, avoiding the "heat sink effect" in the ablation area and facilitating tissue repair. IRE is used to achieve local ablation by disrupting cellular homeostasis and destroying or controlling tumor growth. This technique is more selective to tissues and cells than other modalities and can protect the surrounding blood vessels, bile ducts, and other important tissues, and cause the physiological death of cells in the ablation area to avoid excessive tissue necrosis and increase the body’s immune burden. The addition of IRE to conventional therapy promotes significantly longer patient survival than that of historic controls[139]. Papoulas et al[140] showed that intraoperative IRE and PD can be used successfully in appropriate BRPC patients to achieve clear microdissection margins, minimizing the risk of local recurrence and improving outcomes.
MDTs have become a popular way to guarantee the best care for cancer patients and reliably improve the diagnosis and treatment of PC[141-143]. Unfortunately, there is a lack of data on the role and criteria of MDTs in PC. Syed et al[144] found that the multidisciplinary pancreas conference led to a significantly higher rate of adjuvant chemotherapy initiation than has been previously reported. Hansen et al[145] analyzed 7,015 patients with diagnosed or suspected pancreatic and duodenal tumors who received MDTs and compared the results from similar patients seen at the same hospital before the implementation of MDTs. In this study, patients with advanced stages of disease who received MDTs had a higher rate of surgery, including vascular reconstruction, and there was no increase in morbidity and mortality. Neither study identified long-term survival benefits of MDTs for PC patients.
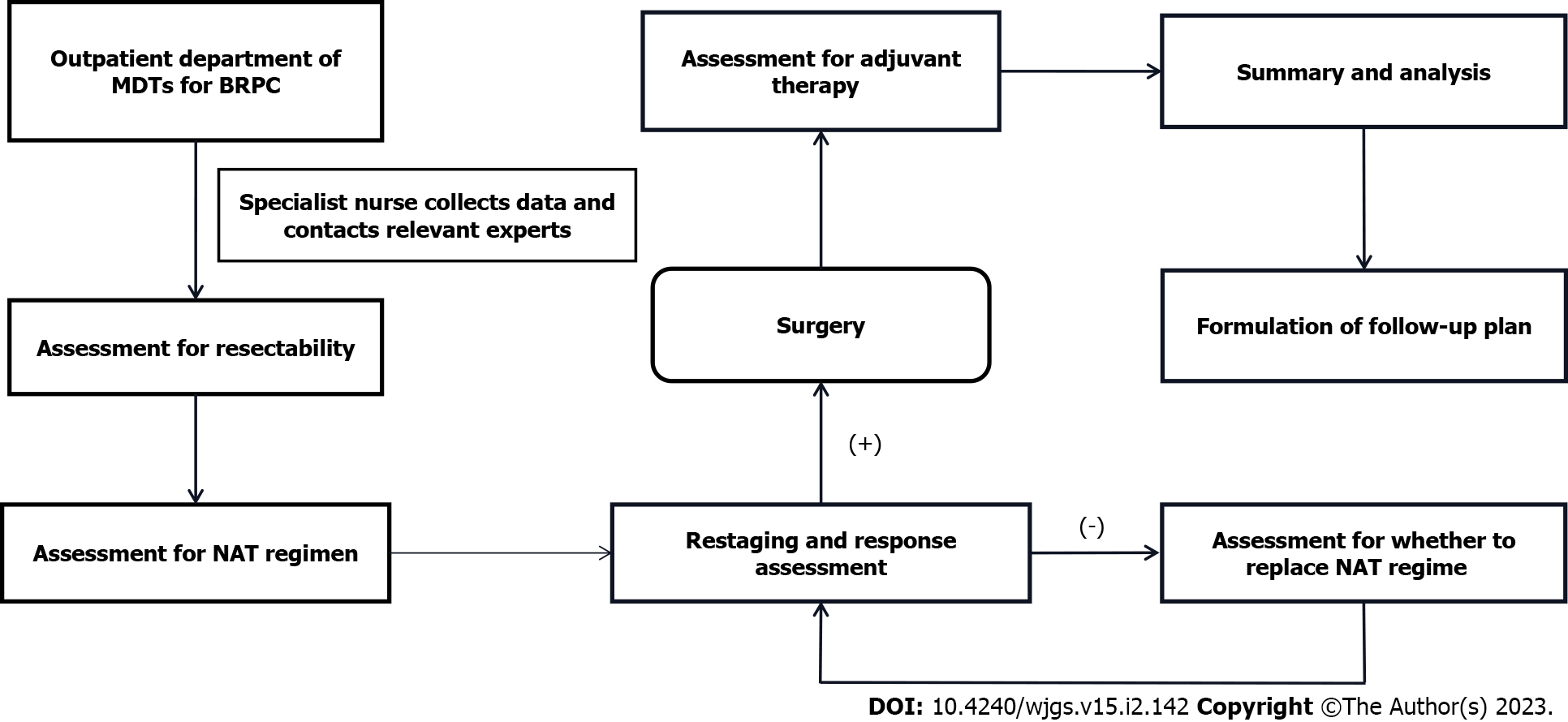
As discussed previously, there is no broad consensus on the standard of care for BRPC, so the core task of MDTs is diagnosis and assessment for resectability and treatment, including the selection of a surgical scheme and NAT regimen and the evaluation of NAT responsiveness[146]. The MDT mode established around BRPC should: (1) Build on the outpatient department of MDTs and involve experts from different clinical specialties and subspecialties, including but not limited to pancreatic surgery, gastroenterology, radiology, medical oncology, pathology, nutriology, therapeutic radiology, and anesthesiology, (2) Ensure that specialist nurses in the clinic serve as the hub for various experts, (3) Ensure stability and communication between team core members, (4) Require that medical records are quantitatively evaluated and a long-term follow-up system is established, and (5) Ensure that MDT time, personnel, location, and equipment are fixed (Figure 2). MDTs can formulate a regular review plan for patients, dynamically evaluate treatment effects and adverse events, adjust the treatment plan as needed, and terminate treatment if necessary. The MDT can also carry out multidisciplinary research, including clinical trials.
A 2019 study found that MDTs from different centers varied substantially in resectability rates for non-metastatic PC[146]. The researchers suggested that for patients with PC, uniform MDT patterns and criteria require further exploration. Large sample-sized and multicenter studies are required. In addition, it is necessary to reduce the heterogeneity that results from differences in the equipment used across centers.
BRPC management has entered the era of multimodality therapy with a single surgical treatment. In the past 20 years, more studies have identified that surgical treatment for PC is insufficient. Even extensive surgery is a local treatment, while cancer, especially PC, is a systemic disease. Thus, appropriate management for BRPC should not only focus on improving surgical rates but also assess how to maximize the survival benefit of radical surgery through the rational selection of patients along with individualized neoadjuvant regimens and surgical modalities.
This review provides a comprehensive discussion of current multimodality treatment regimens for patients with BRPC, including the assessment of resectability, the overall management of NAT, advances in surgical modalities, and preliminary exploration of MDTs. Several clinical trials are exploring optimal NAT regimens, which confer a long-term survival benefit for BRPC patients, the results of these trials can be followed in the future. Using precision medicine, the assessment of resectability at the molecular and genetic levels becomes possible, suggesting that molecular targeted therapy or immunotherapy could be a breakthrough for BRPC treatment. The combination of AI and mul
Radical resection is currently the cornerstone of PC treatment, and intraoperative adjuvant therapy regimens continue to evolve. The first step to maximize the benefits of surgery is to accurately select “suitable surgical candidates”. MDTs need to focus on the full personalized management of patients with BRPC, using radiology combined with tumor biology and general status to assess and evaluate resectability and multimodality treatment options.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bencini L, Italy; Isaji S, Japan S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11406] [Article Influence: 3802.0] [Reference Citation Analysis (4)] |
| 2. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3408] [Article Influence: 486.9] [Reference Citation Analysis (1)] |
| 3. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1660] [Article Influence: 332.0] [Reference Citation Analysis (1)] |
| 4. | Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW, Kishiwada M, Kitagawa H, Michalski CW, Wolfgang CL. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 504] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 5. | Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 648] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 6. | Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, Merchant NB, Minter RM, Tamm EP, Sahani DV, Simeone DM. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014;270:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 7. | Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM, Pisters PW, Posner MC, Berlin JD, Ahmad SA. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 8. | Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 602] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 9. | Allema JH, Reinders ME, van Gulik TM, van Leeuwen DJ, de Wit LT, Verbeek PC, Gouma DJ. Portal vein resection in patients undergoing pancreatoduodenectomy for carcinoma of the pancreatic head. Br J Surg. 1994;81:1642-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Fuhrman GM, Leach SD, Staley CA, Cusack JC, Charnsangavej C, Cleary KR, El-Naggar AK, Fenoglio CJ, Lee JE, Evans DB. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 1996;223:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 253] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA, Evans DB. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935-49; discussion 949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 407] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 12. | Mikulic D, Mrzljak A. Borderline resectable pancreatic cancer and vascular resections in the era of neoadjuvant therapy. World J Clin Cases. 2021;9:5398-5407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 13. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 689] [Article Influence: 172.3] [Reference Citation Analysis (0)] |
| 14. | Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley RM, Conlon K, Cruz LF, Dervenis C, Fingerhutt A, Friess H, Gouma DJ, Hartwig W, Lillemoe KD, Montorsi M, Neoptolemos JP, Shrikhande SV, Takaori K, Traverso W, Vashist YK, Vollmer C, Yeo CJ, Izbicki JR; International Study Group of Pancreatic Surgery. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 651] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 15. | Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, Aitini E, Barni S; Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente (GISCAD). FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015;44:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Kato Y, Yamada S, Tashiro M, Sonohara F, Takami H, Hayashi M, Kanda M, Kobayashi D, Tanaka C, Nakayama G, Koike M, Fujiwara M, Kodera Y. Biological and conditional factors should be included when defining criteria for resectability for patients with pancreatic cancer. HPB (Oxford). 2019;21:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Oba A, Del Chiaro M, Satoi S, Kim SW, Takahashi H, Yu J, Hioki M, Tanaka M, Kato Y, Ariake K, Wu YHA, Inoue Y, Takahashi Y, Hackert T, Wolfgang CL, Besselink MG, Schulick RD, Nagakawa Y, Isaji S, Tsuchida A, Endo I. New criteria of resectability for pancreatic cancer: A position paper by the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS). J Hepatobiliary Pancreat Sci. 2022;29:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Huang X, Zhang G, Liang T. Subtyping for pancreatic cancer precision therapy. Trends Pharmacol Sci. 2022;43:482-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L, Eichmann MD, Worni M, Gloor B, Perren A, Karamitopoulou E. Integrated Genomic and Immunophenotypic Classification of Pancreatic Cancer Reveals Three Distinct Subtypes with Prognostic/Predictive Significance. Clin Cancer Res. 2018;24:4444-4454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 20. | N Kalimuthu S, Wilson GW, Grant RC, Seto M, O'Kane G, Vajpeyi R, Notta F, Gallinger S, Chetty R. Morphological classification of pancreatic ductal adenocarcinoma that predicts molecular subtypes and correlates with clinical outcome. Gut. 2020;69:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Tran Cao HS, Zhang Q, Sada YH, Silberfein EJ, Hsu C, Van Buren G 2nd, Chai C, Katz MHG, Fisher WE, Massarweh NN. Value of lymph node positivity in treatment planning for early stage pancreatic cancer. Surgery. 2017;162:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Lim KH, Chung E, Khan A, Cao D, Linehan D, Ben-Josef E, Wang-Gillam A. Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist. 2012;17:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Pingpank JF, Hoffman JP, Ross EA, Cooper HS, Meropol NJ, Freedman G, Pinover WH, LeVoyer TE, Sasson AR, Eisenberg BL. Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. J Gastrointest Surg. 2001;5:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Takahashi H, Ogawa H, Ohigashi H, Gotoh K, Yamada T, Ohue M, Miyashiro I, Noura S, Kishi K, Motoori M, Shingai T, Nakamura S, Nishiyama K, Yano M, Ishikawa O. Preoperative chemoradiation reduces the risk of pancreatic fistula after distal pancreatectomy for pancreatic adenocarcinoma. Surgery. 2011;150:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Tzeng CW, Tran Cao HS, Lee JE, Pisters PW, Varadhachary GR, Wolff RA, Abbruzzese JL, Crane CH, Evans DB, Wang H, Abbott DE, Vauthey JN, Aloia TA, Fleming JB, Katz MH. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18:16-24; discussion 24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Thanikachalam K, Damarla V, Seixas T, Dobrosotskaya I, Wollner I, Kwon D, Winters K, Raoufi M, Li J, Siddiqui F, Khan G. Neoadjuvant Phase II Trial of Chemoradiotherapy in Patients With Resectable and Borderline Resectable Pancreatic Cancer. Am J Clin Oncol. 2020;43:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Tienhoven G, van Eijck CHJ; Dutch Pancreatic Cancer Group. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol. 2022;40:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 409] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 28. | van Dam JL, Janssen QP, Besselink MG, Homs MYV, van Santvoort HC, van Tienhoven G, de Wilde RF, Wilmink JW, van Eijck CHJ, Groot Koerkamp B; Dutch Pancreatic Cancer Group. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomised controlled trials. Eur J Cancer. 2022;160:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 29. | Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, Arnold D; ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56-v68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 927] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 30. | Ahmad SA, Duong M, Sohal DPS, Gandhi NS, Beg MS, Wang-Gillam A, Wade JL 3rd, Chiorean EG, Guthrie KA, Lowy AM, Philip PA, Hochster HS. Surgical Outcome Results From SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg. 2020;272:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 31. | Kondo N, Uemura K, Sudo T, Hashimoto Y, Sumiyoshi T, Okada K, Seo S, Otsuka H, Murakami Y, Takahashi S. A phase II study of gemcitabine/nab-paclitaxel/S-1 combination neoadjuvant chemotherapy for patients with borderline resectable pancreatic cancer with arterial contact. Eur J Cancer. 2021;159:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 744] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 33. | Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, Livingston DM. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 642] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 34. | Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 928] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 35. | Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 36. | Reiss KA, Yu S, Judy R, Symecko H, Nathanson KL, Domchek SM. Retrospective Survival Analysis of Patients With Advanced Pancreatic Ductal Adenocarcinoma and Germline BRCA or PALB2 Mutations. JCO Precis Oncol. 2018;2:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Pishvaian MJ, Joseph Bender R, Matrisian LM, Rahib L, Hendifar A, Hoos WA, Mikhail S, Chung V, Picozzi V, Heartwell C, Mason K, Varieur K, Aberra M, Madhavan S, Petricoin E 3rd, Brody JR. A pilot study evaluating concordance between blood-based and patient-matched tumor molecular testing within pancreatic cancer patients participating in the Know Your Tumor (KYT) initiative. Oncotarget. 2017;8:83446-83456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Wattenberg MM, Asch D, Yu S, O'Dwyer PJ, Domchek SM, Nathanson KL, Rosen MA, Beatty GL, Siegelman ES, Reiss KA. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020;122:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 39. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 752] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 40. | Turner KM, Delman AM, Kharofa JR, Smith MT, Choe KA, Olowokure O, Wilson GC, Patel SH, Sohal D, Ahmad SA. Radiation therapy in borderline resectable pancreatic cancer: A review. Surgery. 2022;172:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 41. | Janssen QP, van Dam JL, Kivits IG, Besselink MG, van Eijck CHJ, Homs MYV, Nuyttens JJME, Qi H, van Santvoort HJ, Wei AC, de Wilde RF, Wilmink JW, van Tienhoven G, Groot Koerkamp B. Added Value of Radiotherapy Following Neoadjuvant FOLFIRINOX for Resectable and Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis. Ann Surg Oncol. 2021;28:8297-8308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Katz MHG, Shi Q, Meyers J, Herman JM, Chuong M, Wolpin BM, Ahmad S, Marsh R, Schwartz L, Behr S, Frankel WL, Collisson E, Leenstra J, Williams TM, Vaccaro G, Venook A, Meyerhardt JA, O'Reilly EM. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2022;8:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 179] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 43. | Ghaneh P, Palmer D, Cicconi S, Jackson R, Halloran CM, Rawcliffe C, Sripadam R, Mukherjee S, Soonawalla Z, Wadsley J, Al-Mukhtar A, Dickson E, Graham J, Jiao L, Wasan HS, Tait IS, Prachalias A, Ross P, Valle JW, O'Reilly DA, Al-Sarireh B, Gwynne S, Ahmed I, Connolly K, Yim KL, Cunningham D, Armstrong T, Archer C, Roberts K, Ma YT, Springfeld C, Tjaden C, Hackert T, Büchler MW, Neoptolemos JP; European Study Group for Pancreatic Cancer. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 157] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 44. | Shaib WL, Hawk N, Cassidy RJ, Chen Z, Zhang C, Brutcher E, Kooby D, Maithel SK, Sarmiento JM, Landry J, El-Rayes BF. A Phase 1 Study of Stereotactic Body Radiation Therapy Dose Escalation for Borderline Resectable Pancreatic Cancer After Modified FOLFIRINOX (NCT01446458). Int J Radiat Oncol Biol Phys. 2016;96:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Zakem SJ, Mueller AC, Meguid C, Torphy RJ, Holt DE, Schefter T, Messersmith WA, McCarter MD, Del Chiaro M, Schulick RD, Goodman KA. Impact of neoadjuvant chemotherapy and stereotactic body radiation therapy (SBRT) on R0 resection rate for borderline resectable and locally advanced pancreatic cancer. HPB (Oxford). 2021;23:1072-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Mellon EA, Hoffe SE, Springett GM, Frakes JM, Strom TJ, Hodul PJ, Malafa MP, Chuong MD, Shridhar R. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 47. | Mills BN, Qiu H, Drage MG, Chen C, Mathew JS, Garrett-Larsen J, Ye J, Uccello TP, Murphy JD, Belt BA, Lord EM, Katz AW, Linehan DC, Gerber SA. Modulation of the Human Pancreatic Ductal Adenocarcinoma Immune Microenvironment by Stereotactic Body Radiotherapy. Clin Cancer Res. 2022;28:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 48. | Liu L, Huang X, Shi F, Song J, Guo C, Yang J, Liang T, Bai X. Combination therapy for pancreatic cancer: anti-PD-(L)1-based strategy. J Exp Clin Cancer Res. 2022;41:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 49. | De Simoni O, Scarpa M, Soldà C, Bergamo F, Lonardi S, Fantin A, Pilati P, Gruppo M. Could Total Neoadjuvant Therapy Followed by Surgical Resection Be the New Standard of Care in Pancreatic Cancer? J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, Goenka AH, Hallemeier CL, Haddock MG, Harmsen WS, Mahipal A, McWilliams RR, Halfdanarson TR, Grothey AF. Factors Predicting Response, Perioperative Outcomes, and Survival Following Total Neoadjuvant Therapy for Borderline/Locally Advanced Pancreatic Cancer. Ann Surg. 2021;273:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 280] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 51. | Kim RY, Christians KK, Aldakkak M, Clarke CN, George B, Kamgar M, Khan AH, Kulkarni N, Hall WA, Erickson BA, Evans DB, Tsai S. Total Neoadjuvant Therapy for Operable Pancreatic Cancer. Ann Surg Oncol. 2021;28:2246-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Labori KJ. Short-Course or Total Neoadjuvant Chemotherapy in Resectable and Borderline Resectable Pancreatic Cancer - Current Status and Future Perspectives. Front Surg. 2022;9:839339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Alva-Ruiz R, Yohanathan L, Yonkus JA, Abdelrahman AM, Gregory LA, Halfdanarson TR, Mahipal A, McWilliams RR, Ma WW, Hallemeier CL, Graham RP, Grotz TE, Smoot RL, Cleary SP, Nagorney DM, Kendrick ML, Truty MJ. Neoadjuvant Chemotherapy Switch in Borderline Resectable/Locally Advanced Pancreatic Cancer. Ann Surg Oncol. 2022;29:1579-1591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 54. | Dholakia AS, Hacker-Prietz A, Wild AT, Raman SP, Wood LD, Huang P, Laheru DA, Zheng L, De Jesus-Acosta A, Le DT, Schulick R, Edil B, Ellsworth S, Pawlik TM, Iacobuzio-Donahue CA, Hruban RH, Cameron JL, Fishman EK, Wolfgang CL, Herman JM. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol. 2013;2:413-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Zins M, Matos C, Cassinotto C. Pancreatic Adenocarcinoma Staging in the Era of Preoperative Chemotherapy and Radiation Therapy. Radiology. 2018;287:374-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 56. | Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, Wang H, Abbruzzese J, Pisters PW, Vauthey JN, Charnsangavej C, Tamm E, Crane CH, Balachandran A. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749-5756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 57. | Yasuta S, Kobayashi T, Aizawa H, Takahashi S, Ikeda M, Konishi M, Kojima M, Kuno H, Uesaka K, Morinaga S, Miyamoto A, Toyama H, Takakura N, Sugimachi K, Takayama W. Relationship between surgical R0 resectability and findings of peripancreatic vascular invasion on CT imaging after neoadjuvant S-1 and concurrent radiotherapy in patients with borderline resectable pancreatic cancer. BMC Cancer. 2020;20:1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Akita H, Takahashi H, Ohigashi H, Tomokuni A, Kobayashi S, Sugimura K, Miyoshi N, Moon JH, Yasui M, Omori T, Miyata H, Ohue M, Fujiwara Y, Yano M, Ishikawa O, Sakon M. FDG-PET predicts treatment efficacy and surgical outcome of pre-operative chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Eur J Surg Oncol. 2017;43:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Kukar M, Alnaji RM, Jabi F, Platz TA, Attwood K, Nava H, Ben-David K, Mattson D, Salerno K, Malhotra U, Kanehira K, Gannon J, Hochwald SN. Role of Repeat 18F-Fluorodeoxyglucose Positron Emission Tomography Examination in Predicting Pathologic Response Following Neoadjuvant Chemoradiotherapy for Esophageal Adenocarcinoma. JAMA Surg. 2015;150:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Humbert O, Riedinger JM, Charon-Barra C, Berriolo-Riedinger A, Desmoulins I, Lorgis V, Kanoun S, Coutant C, Fumoleau P, Cochet A, Brunotte F. Identification of Biomarkers Including 18FDG-PET/CT for Early Prediction of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Clin Cancer Res. 2015;21:5460-5468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Yin Q, Zou X, Zai X, Wu Z, Wu Q, Jiang X, Chen H, Miao F. Pancreatic ductal adenocarcinoma and chronic mass-forming pancreatitis: Differentiation with dual-energy MDCT in spectral imaging mode. Eur J Radiol. 2015;84:2470-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Noda Y, Goshima S, Miyoshi T, Kawada H, Kawai N, Tanahashi Y, Matsuo M. Assessing Chemotherapeutic Response in Pancreatic Ductal Adenocarcinoma: Histogram Analysis of Iodine Concentration and CT Number in Single-Source Dual-Energy CT. AJR Am J Roentgenol. 2018;211:1221-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 63. | Cuneo KC, Chenevert TL, Ben-Josef E, Feng MU, Greenson JK, Hussain HK, Simeone DM, Schipper MJ, Anderson MA, Zalupski MM, Al-Hawary M, Galban CJ, Rehemtulla A, Feng FY, Lawrence TS, Ross BD. A pilot study of diffusion-weighted MRI in patients undergoing neoadjuvant chemoradiation for pancreatic cancer. Transl Oncol. 2014;7:644-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, Plaza C, de Vicente E, Prados S, Tabernero S, Barbacid M, Lopez-Rios F, Hidalgo M. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 65. | Avanzo M, Stancanello J, El Naqa I. Beyond imaging: The promise of radiomics. Phys Med. 2017;38:122-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 317] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 66. | Braman NM, Etesami M, Prasanna P, Dubchuk C, Gilmore H, Tiwari P, Plecha D, Madabhushi A. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. 2017;19:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 440] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 67. | Ciaravino V, Cardobi N, DE Robertis R, Capelli P, Melisi D, Simionato F, Marchegiani G, Salvia R, D'Onofrio M. CT Texture Analysis of Ductal Adenocarcinoma Downstaged After Chemotherapy. Anticancer Res. 2018;38:4889-4895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Borhani AA, Dewan R, Furlan A, Seiser N, Zureikat AH, Singhi AD, Boone B, Bahary N, Hogg ME, Lotze M, Zeh HJ III, Tublin ME. Assessment of Response to Neoadjuvant Therapy Using CT Texture Analysis in Patients With Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. AJR Am J Roentgenol. 2020;214:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Watson MD, Baimas-George MR, Murphy KJ, Pickens RC, Iannitti DA, Martinie JB, Baker EH, Vrochides D, Ocuin LM. Pure and Hybrid Deep Learning Models can Predict Pathologic Tumor Response to Neoadjuvant Therapy in Pancreatic Adenocarcinoma: A Pilot Study. Am Surg. 2021;87:1901-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Nasief H, Zheng C, Schott D, Hall W, Tsai S, Erickson B, Allen Li X. A machine learning based delta-radiomics process for early prediction of treatment response of pancreatic cancer. NPJ Precis Oncol. 2019;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 71. | Satoi S, Yamamoto T, Yamaki S, Sakaguchi T, Sekimoto M. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2020;4:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 72. | Takahashi H, Yamada D, Asukai K, Wada H, Hasegawa S, Hara H, Shinno N, Ushigome H, Haraguchi N, Sugimura K, Yamamoto K, Nishimura J, Yasui M, Omori T, Miyata H, Ohue M, Yano M, Sakon M, Ishikawa O. Clinical implications of the serum CA19-9 level in "biological borderline resectability" and "biological downstaging" in the setting of preoperative chemoradiation therapy for pancreatic cancer. Pancreatology. 2020;20:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 73. | Tanaka M, Heckler M, Mihaljevic AL, Sun H, Klaiber U, Heger U, Büchler MW, Hackert T. CT response of primary tumor and CA19-9 predict resectability of metastasized pancreatic cancer after FOLFIRINOX. Eur J Surg Oncol. 2019;45:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Heger U, Sun H, Hinz U, Klaiber U, Tanaka M, Liu B, Sachsenmaier M, Springfeld C, Michalski CW, Büchler MW, Hackert T. Induction chemotherapy in pancreatic cancer: CA 19-9 may predict resectability and survival. HPB (Oxford). 2020;22:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 75. | Bednar F, Zenati MS, Steve J, Winters S, Ocuin LM, Bahary N, Hogg ME, Zeh HJ 3rd, Zureikat AH. Analysis of Predictors of Resection and Survival in Locally Advanced Stage III Pancreatic Cancer: Does the Nature of Chemotherapy Regimen Influence Outcomes? Ann Surg Oncol. 2017;24:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | van Veldhuisen E, Vogel JA, Klompmaker S, Busch OR, van Laarhoven HWM, van Lienden KP, Wilmink JW, Marsman HA, Besselink MG. Added value of CA19-9 response in predicting resectability of locally advanced pancreatic cancer following induction chemotherapy. HPB (Oxford). 2018;20:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | Sadot E, Doussot A, O'Reilly EM, Lowery MA, Goodman KA, Do RK, Tang LH, Gönen M, D'Angelica MI, DeMatteo RP, Kingham TP, Jarnagin WR, Allen PJ. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol. 2015;22:3512-3521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 78. | Aoki S, Motoi F, Murakami Y, Sho M, Satoi S, Honda G, Uemura K, Okada KI, Matsumoto I, Nagai M, Yanagimoto H, Kurata M, Fukumoto T, Mizuma M, Yamaue H, Unno M; Multicenter Study Group of Pancreatobiliary Surgery (MSG-PBS). Decreased serum carbohydrate antigen 19-9 Levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: a multicenter case-control study of 240 patients. BMC Cancer. 2019;19:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 79. | Luo G, Fan Z, Cheng H, Jin K, Guo M, Lu Y, Yang C, Fan K, Huang Q, Long J, Liu L, Xu J, Lu R, Ni Q, Warshaw AL, Liu C, Yu X. New observations on the utility of CA19-9 as a biomarker in Lewis negative patients with pancreatic cancer. Pancreatology. 2018;18:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 80. | Luo G, Liu C, Guo M, Cheng H, Lu Y, Jin K, Liu L, Long J, Xu J, Lu R, Ni Q, Yu X. Potential Biomarkers in Lewis Negative Patients With Pancreatic Cancer. Ann Surg. 2017;265:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 81. | Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC, Stephens BM, Huang J, Semaan A, Guerrero PA, Kamyabi N, Zhao J, Hurd MW, Koay EJ, Taniguchi CM, Herman JM, Javle M, Wolff R, Katz M, Varadhachary G, Maitra A, Alvarez HA. Circulating Nucleic Acids Are Associated With Outcomes of Patients With Pancreatic Cancer. Gastroenterology. 2019;156:108-118.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 82. | Gemenetzis G, Groot VP, Yu J, Ding D, Teinor JA, Javed AA, Wood LD, Burkhart RA, Cameron JL, Makary MA, Weiss MJ, He J, Wolfgang CL. Circulating Tumor Cells Dynamics in Pancreatic Adenocarcinoma Correlate With Disease Status: Results of the Prospective CLUSTER Study. Ann Surg. 2018;268:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 83. | Liang K, Liu F, Fan J, Sun D, Liu C, Lyon CJ, Bernard DW, Li Y, Yokoi K, Katz MH, Koay EJ, Zhao Z, Hu Y. Nanoplasmonic Quantification of Tumor-derived Extracellular Vesicles in Plasma Microsamples for Diagnosis and Treatment Monitoring. Nat Biomed Eng. 2017;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 84. | Felix K, Hinz U, Dobiasch S, Hackert T, Bergmann F, Neumüller M, Gronowitz S, Bergqvist M, Strobel O. Preoperative Serum Thymidine Kinase Activity as Novel Monitoring, Prognostic, and Predictive Biomarker in Pancreatic Cancer. Pancreas. 2018;47:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Glazer ES, Rashid OM, Pimiento JM, Hodul PJ, Malafa MP. Increased neutrophil-to-lymphocyte ratio after neoadjuvant therapy is associated with worse survival after resection of borderline resectable pancreatic ductal adenocarcinoma. Surgery. 2016;160:1288-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 529] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 87. | Awad S, Tan BH, Cui H, Bhalla A, Fearon KC, Parsons SL, Catton JA, Lobo DN. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 88. | Trestini I, Paiella S, Sandini M, Sperduti I, Elio G, Pollini T, Melisi D, Auriemma A, Soldà C, Bonaiuto C, Tregnago D, Avancini A, Secchettin E, Bonamini D, Lanza M, Pilotto S, Malleo G, Salvia R, Bovo C, Gianotti L, Bassi C, Milella M. Prognostic Impact of Preoperative Nutritional Risk in Patients Who Undergo Surgery for Pancreatic Adenocarcinoma. Ann Surg Oncol. 2020;27:5325-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Kim KH, Hwang HK, Kang IC, Lee WJ, Kang CM. Oncologic impact of preoperative prognostic nutritional index change in resected pancreatic cancer following neoadjuvant chemotherapy. Pancreatology. 2020;20:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Tanaka K, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, Kobayashi D, Tanaka C, Nakayama G, Koike M, Fujiwara M, Kodera Y. Pancreatic Fat and Body Composition Measurements by Computed Tomography are Associated with Pancreatic Fistula After Pancreatectomy. Ann Surg Oncol. 2021;28:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Shi H, Wei Y, Cheng S, Lu Z, Zhang K, Jiang K, Xu Q. Survival prediction after upfront surgery in patients with pancreatic ductal adenocarcinoma: Radiomic, clinic-pathologic and body composition analysis. Pancreatology. 2021;21:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 92. | Basile D, Parnofiello A, Vitale MG, Cortiula F, Gerratana L, Fanotto V, Lisanti C, Pelizzari G, Ongaro E, Bartoletti M, Garattini SK, Andreotti VJ, Bacco A, Iacono D, Bonotto M, Casagrande M, Ermacora P, Puglisi F, Pella N, Fasola G, Aprile G, Cardellino GG. The IMPACT study: early loss of skeletal muscle mass in advanced pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2019;10:368-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 93. | Pecorelli N, Carrara G, De Cobelli F, Cristel G, Damascelli A, Balzano G, Beretta L, Braga M. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. 2016;103:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 94. | Tumas J, Tumiene B, Jurkeviciene J, Jasiunas E, Sileikis A. Nutritional and immune impairments and their effects on outcomes in early pancreatic cancer patients undergoing pancreatoduodenectomy. Clin Nutr. 2020;39:3385-3394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 95. | Sandini M, Patino M, Ferrone CR, Alvarez-Pérez CA, Honselmann KC, Paiella S, Catania M, Riva L, Tedesco G, Casolino R, Auriemma A, Salandini MC, Carrara G, Cristel G, Damascelli A, Ippolito D, D'Onofrio M, Lillemoe KD, Bassi C, Braga M, Gianotti L, Sahani D, Fernández-Del Castillo C. Association Between Changes in Body Composition and Neoadjuvant Treatment for Pancreatic Cancer. JAMA Surg. 2018;153:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 96. | Carrato A, Cerezo L, Feliu J, Macarulla T, Martín-Pérez E, Vera R, Álvarez J, Botella-Carretero JI. Clinical nutrition as part of the treatment pathway of pancreatic cancer patients: an expert consensus. Clin Transl Oncol. 2022;24:112-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 97. | Gianotti L, Besselink MG, Sandini M, Hackert T, Conlon K, Gerritsen A, Griffin O, Fingerhut A, Probst P, Abu Hilal M, Marchegiani G, Nappo G, Zerbi A, Amodio A, Perinel J, Adham M, Raimondo M, Asbun HJ, Sato A, Takaori K, Shrikhande SV, Del Chiaro M, Bockhorn M, Izbicki JR, Dervenis C, Charnley RM, Martignoni ME, Friess H, de Pretis N, Radenkovic D, Montorsi M, Sarr MG, Vollmer CM, Frulloni L, Büchler MW, Bassi C. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2018;164:1035-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 98. | van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 696] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 99. | Scheufele F, Schorn S, Demir IE, Sargut M, Tieftrunk E, Calavrezos L, Jäger C, Friess H, Ceyhan GO. Preoperative biliary stenting vs operation first in jaundiced patients due to malignant lesions in the pancreatic head: A meta-analysis of current literature. Surgery. 2017;161:939-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 100. | Strom TJ, Klapman JB, Springett GM, Meredith KL, Hoffe SE, Choi J, Hodul P, Malafa MP, Shridhar R. Comparative long-term outcomes of upfront resected pancreatic cancer after preoperative biliary drainage. Surg Endosc. 2015;29:3273-3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Ielpo B, Caruso R, Duran H, Diaz E, Fabra I, Malavé L, Ferri V, Alvarez R, Cubillo A, Plaza C, Lazzaro S, Kalivaci D, Quijano Y, Vicente E. A comparative study of neoadjuvant treatment with gemcitabine plus nab-paclitaxel vs surgery first for pancreatic adenocarcinoma. Surg Oncol. 2017;26:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 102. | Kitahata Y, Kawai M, Tani M, Hirono S, Okada K, Miyazawa M, Shimizu A, Yamaue H. Preoperative cholangitis during biliary drainage increases the incidence of postoperative severe complications after pancreaticoduodenectomy. Am J Surg. 2014;208:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Dhir V, Artifon EL, Gupta K, Vila JJ, Maselli R, Frazao M, Maydeo A. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 104. | Murakami Y, Uemura K, Hashimoto Y, Kondo N, Nakagawa N, Sasaki H, Hatano N, Kohmo T, Sueda T. Does preoperative biliary drainage compromise the long-term survival of patients with pancreatic head carcinoma? J Surg Oncol. 2015;111:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Uemura K, Murakami Y, Satoi S, Sho M, Motoi F, Kawai M, Matsumoto I, Honda G, Kurata M, Yanagimoto H, Nishiwada S, Fukumoto T, Unno M, Yamaue H. Impact of Preoperative Biliary Drainage on Long-Term Survival in Resected Pancreatic Ductal Adenocarcinoma: A Multicenter Observational Study. Ann Surg Oncol. 2015;22 Suppl 3:S1238-S1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Sasahira N, Hamada T, Togawa O, Yamamoto R, Iwai T, Tamada K, Kawaguchi Y, Shimura K, Koike T, Yoshida Y, Sugimori K, Ryozawa S, Kakimoto T, Nishikawa K, Kitamura K, Imamura T, Mizuide M, Toda N, Maetani I, Sakai Y, Itoi T, Nagahama M, Nakai Y, Isayama H. Multicenter study of endoscopic preoperative biliary drainage for malignant distal biliary obstruction. World J Gastroenterol. 2016;22:3793-3802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 107. | Kawakami H, Kondo S, Kuwatani M, Yamato H, Ehira N, Kudo T, Eto K, Haba S, Matsumoto J, Kato K, Tsuchikawa T, Tanaka E, Hirano S, Asaka M. Preoperative biliary drainage for hilar cholangiocarcinoma: which stent should be selected? J Hepatobiliary Pancreat Sci. 2011;18:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 108. | Gardner TB, Spangler CC, Byanova KL, Ripple GH, Rockacy MJ, Levenick JM, Smith KD, Colacchio TA, Barth RJ, Zaki BI, Tsapakos MJ, Gordon SR. Cost-effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest Endosc. 2016;84:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 109. | Kubota K, Sato T, Watanabe S, Hosono K, Kobayashi N, Mori R, Taniguchi K, Matsuyama R, Endo I, Nakajima A. Covered self-expandable metal stent deployment promises safe neoadjuvant chemoradiation therapy in patients with borderline resectable pancreatic head cancer. Dig Endosc. 2014;26:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Tsuboi T, Sasaki T, Serikawa M, Ishii Y, Mouri T, Shimizu A, Kurihara K, Tatsukawa Y, Miyaki E, Kawamura R, Tsushima K, Murakami Y, Uemura K, Chayama K. Preoperative Biliary Drainage in Cases of Borderline Resectable Pancreatic Cancer Treated with Neoadjuvant Chemotherapy and Surgery. Gastroenterol Res Pract. 2016;2016:7968201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Katsinelos P, Paikos D, Kountouras J, Chatzimavroudis G, Paroutoglou G, Moschos I, Gatopoulou A, Beltsis A, Zavos C, Papaziogas B. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: a comparative study of patency and cost effectiveness. Surg Endosc. 2006;20:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Tamura T, Itonaga M, Ashida R, Yamashita Y, Hatamaru K, Kawaji Y, Emori T, Kitahata Y, Miyazawa M, Hirono S, Okada KI, Kawai M, Shimokawa T, Yamaue H, Kitano M. Covered self-expandable metal stents vs plastic stents for preoperative biliary drainage in patient receiving neo-adjuvant chemotherapy for borderline resectable pancreatic cancer: Prospective randomized study. Dig Endosc. 2021;33:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 113. | Lee JH, Krishna SG, Singh A, Ladha HS, Slack RS, Ramireddy S, Raju GS, Davila M, Ross WA. Comparison of the utility of covered metal stents vs uncovered metal stents in the management of malignant biliary strictures in 749 patients. Gastrointest Endosc. 2013;78:312-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 114. | Hasegawa S, Endo I, Kubota K. Plastic or self-expandable metal stent: Which is the most suitable for patients with pancreatic head cancer in the upcoming era of neoadjuvant chemotherapy? Dig Endosc. 2022;34:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Asiyanbola B, Gleisner A, Herman JM, Choti MA, Wolfgang CL, Swartz M, Edil BH, Schulick RD, Cameron JL, Pawlik TM. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. J Gastrointest Surg. 2009;13:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Jang JY, Kang JS, Han Y, Heo JS, Choi SH, Choi DW, Park SJ, Han SS, Yoon DS, Park JS, Yu HC, Kang KJ, Kim SG, Lee H, Kwon W, Yoon YS, Han HS, Kim SW. Long-term outcomes and recurrence patterns of standard vs extended pancreatectomy for pancreatic head cancer: a multicenter prospective randomized controlled study. J Hepatobiliary Pancreat Sci. 2017;24:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 117. | Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, Miyagawa S, Yamaguchi A, Ishiyama S, Takeda Y, Sakoda K, Kinoshita T, Yasui K, Shimada H, Katoh H. Standard vs extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 118. | Choi SH, Kim HY, Hwang HK, Kang CM, Lee WJ. Oncologic Impact of Local Recurrence in Resected Pancreatic Cancer and Topographic Preference in Local Recurrence Patterns According to Tumor Location. Pancreas. 2020;49:1290-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Michalski CW, Kleeff J, Wente MN, Diener MK, Büchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 120. | Dasari BV, Pasquali S, Vohra RS, Smith AM, Taylor MA, Sutcliffe RP, Muiesan P, Roberts KJ, Isaac J, Mirza DF. Extended Versus Standard Lymphadenectomy for Pancreatic Head Cancer: Meta-Analysis of Randomized Controlled Trials. J Gastrointest Surg. 2015;19:1725-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 121. | Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, Andrén-Sandberg A, Asbun HJ, Bockhorn M, Büchler MW, Conlon KC, Fernández-Cruz L, Fingerhut A, Friess H, Hartwig W, Izbicki JR, Lillemoe KD, Milicevic MN, Neoptolemos JP, Shrikhande SV, Vollmer CM, Yeo CJ, Charnley RM; International Study Group on Pancreatic Surgery. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156:591-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 472] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 122. | Hackert T, Strobel O, Michalski CW, Mihaljevic AL, Mehrabi A, Müller-Stich B, Berchtold C, Ulrich A, Büchler MW. The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford). 2017;19:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 123. | Imamura T, Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, Ohgi K, Uesaka K. Reconsidering the Optimal Regional Lymph Node Station According to Tumor Location for Pancreatic Cancer. Ann Surg Oncol. 2021;28:1602-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 124. | Glebova NO, Hicks CW, Tosoian JJ, Piazza KM, Abularrage CJ, Schulick RD, Wolfgang CL, Black JH 3rd. Outcomes of arterial resection during pancreatectomy for tumor. J Vasc Surg. 2016;63:722-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 125. | Jegatheeswaran S, Baltatzis M, Jamdar S, Siriwardena AK. Superior mesenteric artery (SMA) resection during pancreatectomy for malignant disease of the pancreas: a systematic review. HPB (Oxford). 2017;19:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 126. | Rangelova E, Wefer A, Persson S, Valente R, Tanaka K, Orsini N, Segersvärd R, Arnelo U, Del Chiaro M. Surgery Improves Survival After Neoadjuvant Therapy for Borderline and Locally Advanced Pancreatic Cancer: A Single Institution Experience. Ann Surg. 2021;273:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 127. | Takahashi S. How to treat borderline resectable pancreatic cancer: current challenges and future directions. Jpn J Clin Oncol. 2018;48:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 128. | Watanabe S, Kobayashi N, Kubota K, Sato T, Kato S, Hosono K, Shimamura T, Inayama Y, Nakajima A, Endo I. A novel scoring system for arterial invasion of pancreatic body and tail cancer based on multidetector row computed tomography and biomarkers. Pancreatology. 2013;13:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 129. | Diener MK, Mihaljevic AL, Strobel O, Loos M, Schmidt T, Schneider M, Berchtold C, Mehrabi A, Müller-Stich BP, Jiang K, Neoptolemos JP, Hackert T, Miao Y, Büchler MW. Periarterial divestment in pancreatic cancer surgery. Surgery. 2021;169:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 130. | Müller SA, Hartel M, Mehrabi A, Welsch T, Martin DJ, Hinz U, Schmied BM, Büchler MW. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg. 2009;13:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 131. | Yekebas EF, Bogoevski D, Cataldegirmen G, Kunze C, Marx A, Vashist YK, Schurr PG, Liebl L, Thieltges S, Gawad KA, Schneider C, Izbicki JR. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg. 2008;247:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 132. | Dua MM, Tran TB, Klausner J, Hwa KJ, Poultsides GA, Norton JA, Visser BC. Pancreatectomy with vein reconstruction: technique matters. HPB (Oxford). 2015;17:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 133. | Li Y, Feng Q, Jin J, Shi S, Zhang Z, Che X, Zhang J, Chen Y, Wu X, Chen R, Li S, Wang J, Li G, Li F, Dai M, Zheng L, Wang C. Experts' consensus on intraoperative radiotherapy for pancreatic cancer. Cancer Lett. 2019;449:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 134. | Harrison JM, Wo JY, Ferrone CR, Horick NK, Keane FK, Qadan M, Lillemoe KD, Hong TS, Clark JW, Blaszkowsky LS, Allen JN, Castillo CF. Intraoperative Radiation Therapy (IORT) for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma (BR/LA PDAC) in the Era of Modern Neoadjuvant Treatment: Short-Term and Long-Term Outcomes. Ann Surg Oncol. 2020;27:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 135. | Calvo FA, Asencio JM, Roeder F, Krempien R, Poortmans P, Hensley FW, Krengli M. ESTRO IORT Task Force/ACROP recommendations for intraoperative radiation therapy in borderline-resected pancreatic cancer. Clin Transl Radiat Oncol. 2020;23:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Jia SN, Wen FX, Gong TT, Li X, Wang HJ, Sun YM, Yang ZC. A review on the efficacy and safety of iodine-125 seed implantation in unresectable pancreatic cancers. Int J Radiat Biol. 2020;96:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | Xu KC, Niu LZ, Hu YZ, He WB, He YS, Zuo JS. Cryosurgery with combination of (125)iodine seed implantation for the treatment of locally advanced pancreatic cancer. J Dig Dis. 2008;9:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 138. | Wu Y, Gu Y, Zhang B, Zhou X, Li Y, Qian Z. Laparoscopic ultrasonography-guided cryoablation of locally advanced pancreatic cancer: a preliminary report. Jpn J Radiol. 2022;40:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 139. | Kwon D, McFarland K, Velanovich V, Martin RC 2nd. Borderline and locally advanced pancreatic adenocarcinoma margin accentuation with intraoperative irreversible electroporation. Surgery. 2014;156:910-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 140. | Papoulas M, Abdul-Hamid S, Peddu P, Cotoi C, Heaton N, Menon K. Irreversible electroporation in borderline resectable pancreatic adenocarcinoma for margin accentuation. J Surg Case Rep. 2018;2018:rjy127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 141. | Petty JK, Vetto JT. Beyond doughnuts: tumor board recommendations influence patient care. J Cancer Educ. 2002;17:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 142. | Brauer DG, Strand MS, Sanford DE, Kushnir VM, Lim KH, Mullady DK, Tan BR Jr, Wang-Gillam A, Morton AE, Ruzinova MB, Parikh PJ, Narra VR, Fowler KJ, Doyle MB, Chapman WC, Strasberg SS, Hawkins WG, Fields RC. Utility of a multidisciplinary tumor board in the management of pancreatic and upper gastrointestinal diseases: an observational study. HPB (Oxford). 2017;19:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 143. | Pawlik TM, Laheru D, Hruban RH, Coleman J, Wolfgang CL, Campbell K, Ali S, Fishman EK, Schulick RD, Herman JM; Johns Hopkins Multidisciplinary Pancreas Clinic Team. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081-2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 144. | Syed AR, Carleton NM, Horne Z, Dhawan A, Bedi G, Kochhar G, Morrissey S, Williams H, Atkinson D, Schiffman S, Monga D, Lupetin A, Kirichenko A, Mitre M, Dhawan M, Kulkarni A, Thakkar S. Survival Trends for Resectable Pancreatic Cancer Using a Multidisciplinary Conference: the Impact of Post-operative Chemotherapy. J Gastrointest Cancer. 2020;51:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Hansen MFC, Storkholm JH, Hansen CP. The results of pancreatic operations after the implementation of multidisciplinary team conference (MDT): A quality improvement study. Int J Surg. 2020;77:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 146. | Kirkegård J, Aahlin EK, Al-Saiddi M, Bratlie SO, Coolsen M, de Haas RJ, den Dulk M, Fristrup C, Harrison EM, Mortensen MB, Nijkamp MW, Persson J, Søreide JA, Wigmore SJ, Wik T, Mortensen FV. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br J Surg. 2019;106:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 147. | Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, Schwartz L, Frankel W, Martin R, Conway W, Truty M, Kindler H, Lowy AM, Bekaii-Saab T, Philip P, Talamonti M, Cardin D, LoConte N, Shen P, Hoffman JP, Venook AP. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016;151:e161137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 148. | Nagakawa Y, Hosokawa Y, Nakayama H, Sahara Y, Takishita C, Nakajima T, Hijikata Y, Kasuya K, Katsumata K, Tokuuye K, Tsuchida A. A phase II trial of neoadjuvant chemoradiotherapy with intensity-modulated radiotherapy combined with gemcitabine and S-1 for borderline-resectable pancreatic cancer with arterial involvement. Cancer Chemother Pharmacol. 2017;79:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. | Masui T, Takaori K, Anazawa T, Sato A, Nakano K, Uchida Y, Yogo A, Goto Y, Matsumoto S, Kodama Y, Kanai M, Isoda H, Mizumoto M, Kawaguchi Y, Shibuya K, Itasaka S, Uemoto S. A Prospective Study of Intensity-modified Radiation Therapy in Comparison with Conventional 3D-RT for BR Pancreatic Cancer Patients with Arterial Involvement. Anticancer Res. 2017;37:7023-7030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 150. | Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Faris JE, Zhu AX, Goyal L, Lillemoe KD, DeLaney TF, Fernández-Del Castillo C, Ferrone CR, Hong TS. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 433] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 151. | Tran NH, Sahai V, Griffith KA, Nathan H, Kaza R, Cuneo KC, Shi J, Kim E, Sonnenday CJ, Cho CS, Lawrence TS, Zalupski MM. Phase 2 Trial of Neoadjuvant FOLFIRINOX and Intensity Modulated Radiation Therapy Concurrent With Fixed-Dose Rate-Gemcitabine in Patients With Borderline Resectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2020;106:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 152. | Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Eijck CH, van Tienhoven G; Dutch Pancreatic Cancer Group. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38:1763-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 720] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 153. | Takahashi S, Ohno I, Ikeda M, Konishi M, Kobayashi T, Akimoto T, Kojima M, Morinaga S, Toyama H, Shimizu Y, Miyamoto A, Tomikawa M, Takakura N, Takayama W, Hirano S, Otsubo T, Nagino M, Kimura W, Sugimachi K, Uesaka K. Neoadjuvant S-1 With Concurrent Radiotherapy Followed by Surgery for Borderline Resectable Pancreatic Cancer: A Phase II Open-label Multicenter Prospective Trial (JASPAC05). Ann Surg. 2022;276:e510-e517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 154. | Hayashi T, Nakamura T, Kimura Y, Yoshida M, Someya M, Kawakami H, Sakuhara Y, Katoh N, Takahashi K, Ambo Y, Miura K, Motoya M, Tanaka E, Murakawa K, Yamabuki T, Yamazaki H, Katanuma A, Hirano S; Hokkaido Pancreatic Study Group. Phase 2 Study of Neoadjuvant Treatment of Sequential S-1-Based Concurrent Chemoradiation Therapy Followed by Systemic Chemotherapy with Gemcitabine for Borderline Resectable Pancreatic Adenocarcinoma (HOPS-BR 01). Int J Radiat Oncol Biol Phys. 2019;105:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 155. | Sharp WV, Donovan DL. Retroperitoneal approach to the abdominal aorta: revisited. J Cardiovasc Surg (Torino). 1987;28:270-273. [PubMed] |
| 156. | Yue Y, Osipov A, Fraass B, Sandler H, Zhang X, Nissen N, Hendifar A, Tuli R. Identifying prognostic intratumor heterogeneity using pre- and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J Gastrointest Oncol. 2017;8:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 157. | Chen X, Oshima K, Schott D, Wu H, Hall W, Song Y, Tao Y, Li D, Zheng C, Knechtges P, Erickson B, Li XA. Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: An exploratory study. PLoS One. 2017;12:e0178961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 158. | Kaissis G, Ziegelmayer S, Lohöfer F, Steiger K, Algül H, Muckenhuber A, Yen HY, Rummeny E, Friess H, Schmid R, Weichert W, Siveke JT, Braren R. A machine learning algorithm predicts molecular subtypes in pancreatic ductal adenocarcinoma with differential response to gemcitabine-based vs FOLFIRINOX chemotherapy. PLoS One. 2019;14:e0218642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |