Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2919
Peer-review started: July 29, 2023
First decision: October 29, 2023
Revised: November 12, 2023
Accepted: December 11, 2023
Article in press: December 11, 2023
Published online: December 27, 2023
Processing time: 151 Days and 2.3 Hours
Esophageal atresia (EA) is a life-threatening congenital malformation in newborns, and the traditional repair approaches pose technical challenges and are extremely invasive. Therefore, surgeons have been actively investigating new minimally invasive techniques to address this issue. Magnetic compression anastomosis has been reported in several studies for its potential in repairing EA. In this paper, the primary repair of EA with magnetic compression anastomosis under thoracoscopy was reported.
A full-term male weighing 3500 g was diagnosed with EA Gross type C. The magnetic devices used in this procedure consisted of two magnetic rings and several catheters. Tracheoesophageal fistula ligation and two purse strings were performed. The magnetic compression anastomosis was then completed thoracoscopically. After the primary repair, no additional operation was conducted. A patent anastomosis was observed on the 15th day postoperatively, and the magnets were removed on the 23rd day. No leakage existed when the transoral feeding started.
Thoracoscopic magnetic compression anastomosis may be a promising minimally invasive approach for repairing EA.
Core Tip: Esophageal atresia (EA) is a life-threatening congenital malformation in newborns, and the traditional surgeries pose technical challenges and are extremely invasive. Therefore, surgeons have been actively investigating new minimally invasive techniques to address this issue. In this report, we discussed the primary repair of EA with magnetic compression anastomosis under thoracoscopy. After the primary repair, no additional operation was conducted, and no leakage existed when the transoral feeding started. This case demonstrated that thoracoscopic magnetic compression anastomosis may be a promising minimally invasive approach for repairing EA.
- Citation: Zhang HK, Li XQ, Song HX, Liu SQ, Wang FH, Wen J, Xiao M, Yang AP, Duan XF, Gao ZZ, Hu KL, Zhang W, Lv Y, Zhou XH, Cao ZJ. Primary repair of esophageal atresia Gross type C via thoracoscopic magnetic compression anastomosis: A case report. World J Gastrointest Surg 2023; 15(12): 2919-2925
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2919.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2919
Esophageal atresia (EA) is a life-threatening congenital malformation in newborns, and its repair is the greatest technical challenge in pediatric surgery. The classic approach is through a right laterodorsal extrapleural thoracotomy via the four-interspace transverse incision. However, these open approaches are extremely invasive, and there are many postoperative complications[1]. Pioneering pediatric surgeons have developed some minimally invasive techniques to repair the atresia, such as the thoracoscopic repair[2] and the magnetic compression technique[3]. However, the requirement of high-level operative skills makes thoracoscopic repair difficult to be widely promoted and applied[4]. Therefore, it is necessary to find a minimally invasive procedure that can be easily conducted and does not require a very high level of operative skills. The convenience and efficacy of magnetic compression anastomosis (also known as magnamosis) for treating EA have been shown by several reports[3,5-7]. This report presented a novel minimally invasive approach, i.e., the thoracoscopic esophageal magnetic compression anastomosis.
The primary complaints were drooling of saliva, dyspnea, and vomiting immediately after feeding postnatally for 3 d.
The male in this study was born at gestational week 40 + 6 in a county hospital and weighed 3500 g. Polyhydramnios was found by ultrasonographic examination before birth. Postnatally, drooling of saliva, dyspnea, and vomiting immediately after feeding were observed. The feeding tube could not be passed into the stomach. The diagnosis of EA was confirmed by esophageal angiography. The baby was then transferred to our hospital for further treatment.
No family history of this condition was confirmed.
A gastric tube was placed in the proximal esophageal pouch. Therefore, there were no other positive signs.
The laboratory examinations were normal.
The X-ray film showed that the proximal esophageal pouch was at the fourth thoracic vertebra, and the air in the stomach and intestine indicated a distal tracheoesophageal fistula (Figure 1). With echocardiography, foramen ovale and atrial septal defects were also found.
EA Gross type C.
To avoid the complications associated with open surgery, the baby’s parents signed the written informed consent and authorized the surgeons to perform the thoracoscopic esophageal magnetic compression anastomosis. This study was approved by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University. The operation was performed on the same day the baby arrived at our hospital.
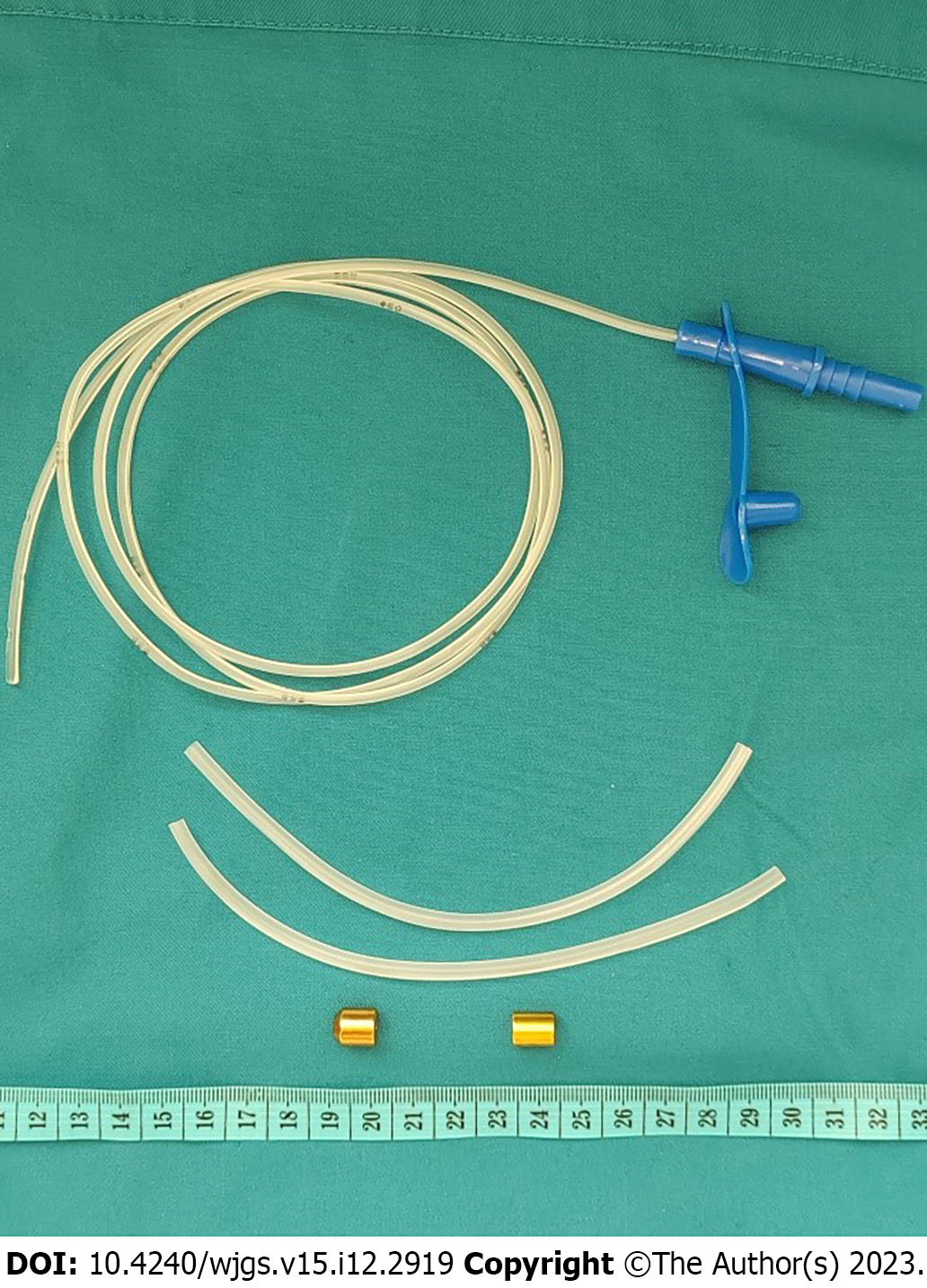
This system consisted of two magnetic rings (NdFeB), a feeding tube, and several catheters (Figure 2). The outer diameters of the magnets used in the proximal pouch and distal esophagus were 9 mm and 7 mm, respectively, having a thickness of 10 mm. The inner diameters of the magnets were 2 mm. The feeding tube (6F) was passed through the magnets to ensure the patency of the esophagus before the anastomosis was formed. The catheters of different outer diameters were utilized for measuring the distal esophageal inner diameter and pushing the magnets. The wall of the catheter was split to facilitate removal after pushing the magnets. The feeding tube was passed through the proximal magnet before insertion into the proximal esophagus.
The neonate was placed in the left lateral decubitus with the right arm fixed over the head under general anesthesia. A tracheabronchoscopic examination was conducted to localize the position of the fistula, which was about 1.5 cm above the carina. Therefore, the endotracheal tube was advanced near the carina, and the right bronchus was blocked via balloon inflating. One-lung ventilation was utilized during the operation. A 3 mm 30° laparoscope and 3 mm instruments were used in this operation.
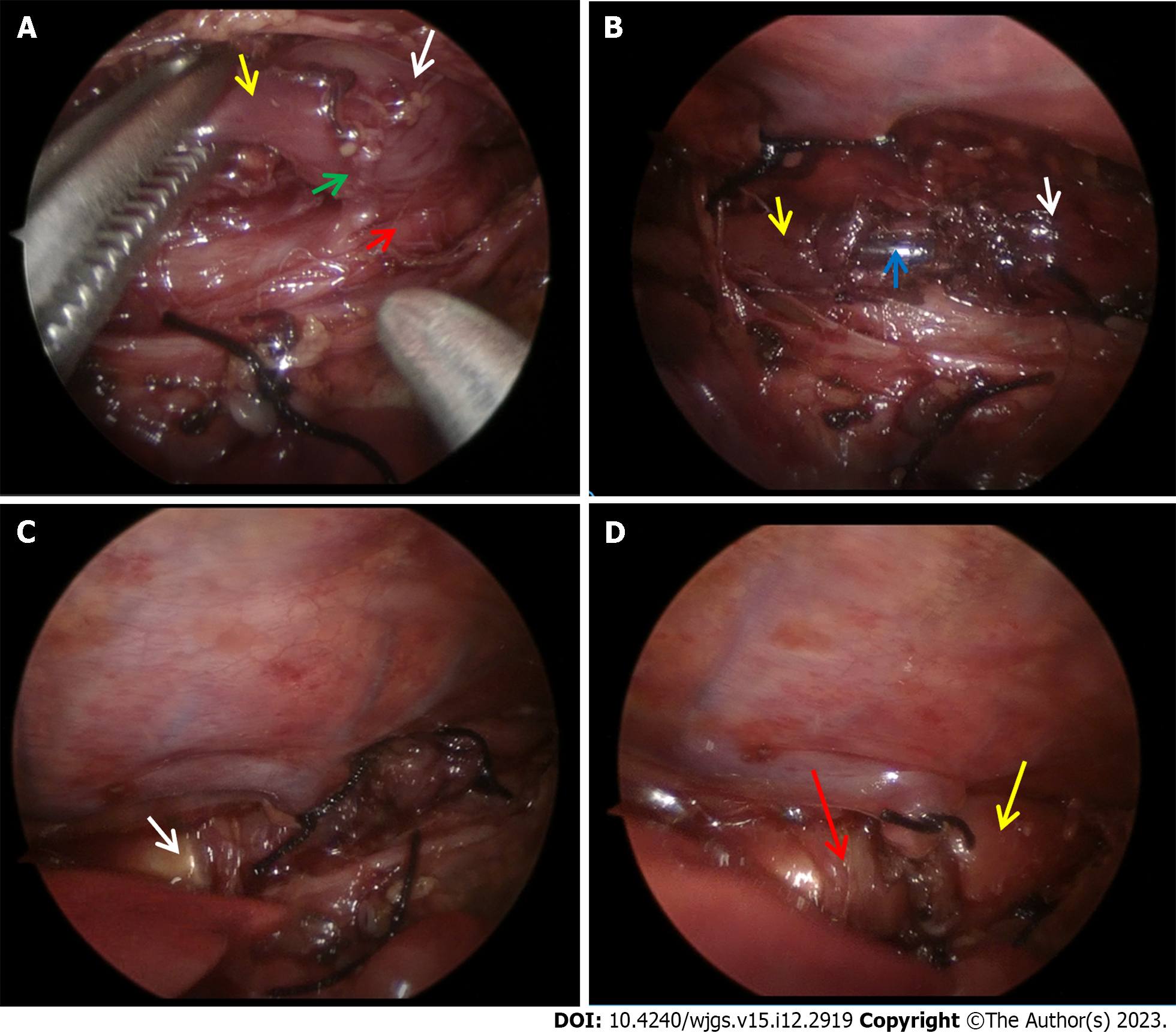
Three trocars were separately placed at the posterior axillary line of the fifth interspace and at the anterior axillary line of the fourth and sixth interspace. The mediastinal pleura was opened at the right anterior to the vertebral column. The distal esophageal fistula was freed after the azygos vein was divided between ties (Figure 3A). The proximal pouch was then adhered to the fistula. The fistula was closed and transected near the trachea by suture ligation. Then the prepared 6F feeding tube was introduced into the esophagus and pushed forward by the anesthetist. The feeding tube was passed out of the proximal pouch through the distal esophagus and eventually into the stomach. Two purse strings with 5-0 Vicryl were separately performed and tied around the feeding tube at the proximal and distal esophageal ends (Figure 3B).
A median upper abdominal transverse incision of about 1.5 cm was made and the stomach was pulled out gently. The feeding tube was pulled out through an incision at the anterior wall of the greater curvature of the stomach. Catheters of different diameters were used to measure the distal esophageal inner diameter from small to the bigger size. Finally, the maximum suitable size was chosen as 7 mm. A magnet with an outer diameter of 7 mm was advanced towards the distal purse string along the feeding tube by pushing with a catheter (Figure 3C). At the same time, the proximal magnet with an outer diameter of 9 mm was advanced towards the proximal pouch along the feeding tube by the anesthetist with another catheter. When these two magnets got close, they assembled together spontaneously to achieve anastomosis (Figure 3D). The catheters were then removed, and a big silk knot was tied over the feeding tube 14 cm from the end. The outer diameter of this knot was larger than the inner diameter of the distal magnet, which prevented the magnets from falling off into the stomach after the esophageal anastomosis formed patently. The feeding tube was then placed back into the stomach. The incision of the stomach was closed with 5-0 Vicryl. A 10 F chest drain tube was placed, and the chest and abdominal incisions were then cosmetically closed with 4-0 Vicryl.
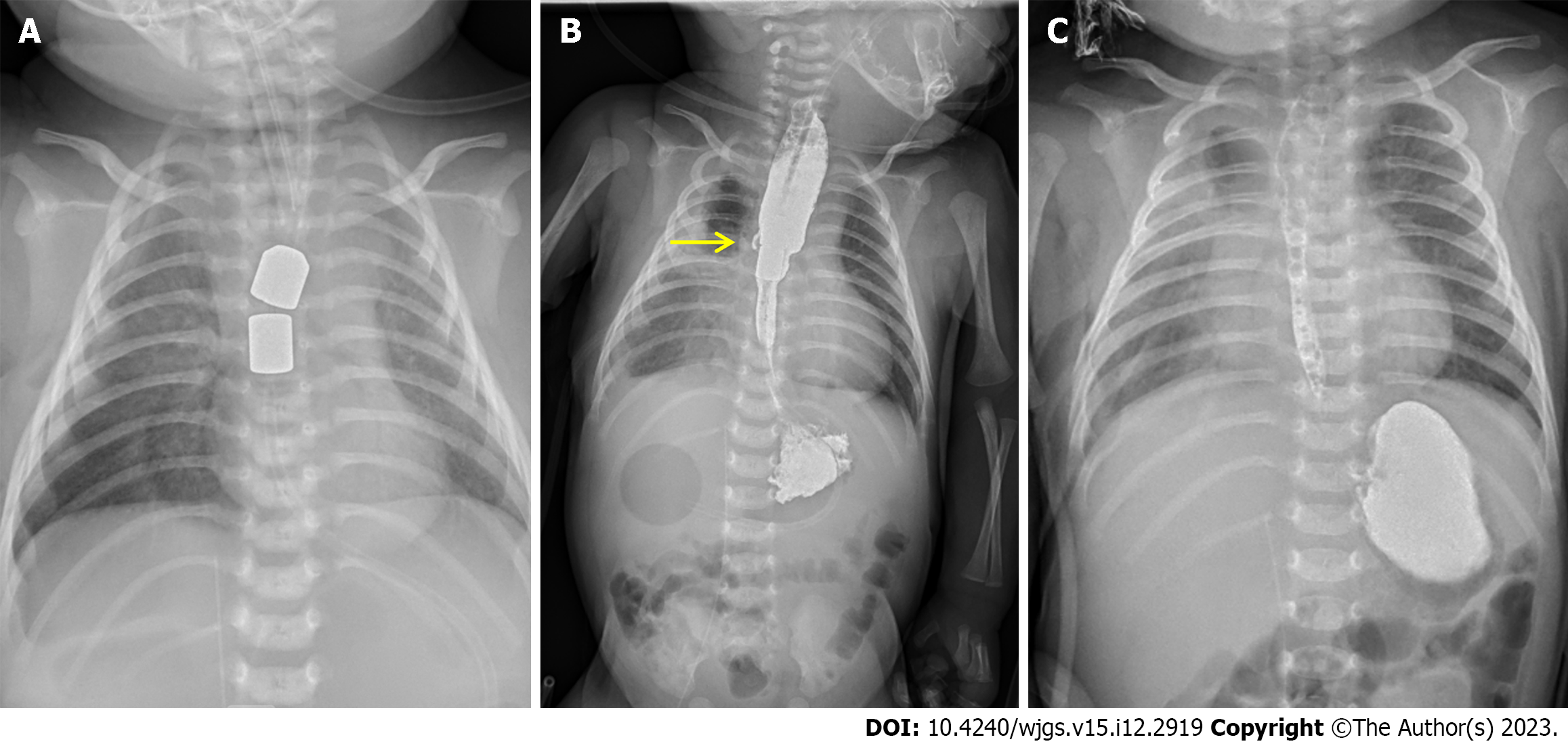
After the operation, the child was admitted to the neonatal intensive care unit. Parenteral nutrition was given until the magnets were removed. Intravenous piperacillin/sulbactam was used as the antibiotic therapy. Another feeding tube was introduced into the proximal esophagus to drain the excessive saliva. An X-ray with a contrast agent was taken to localize the magnets and confirm the anastomotic patency. The magnets assembled well and were located at the sixth thoracic vertebra (Figure 4A). A minor leakage was observed on the 15th postoperative day, and the anastomosis was found to be patent simultaneously (Figure 4B). The anastomotic leakage was conservatively managed to stop spontaneously. The magnets were removed on the 23rd day postoperatively. After that, the esophagographic examination confirmed that no leakage existed (Figure 4C). Transoral feeding was then started on the 23rd day postoperatively.
The surgical repair of EA is not an emergency except the EA affects the neonate negatively, such as combined with respiratory distress requiring ventilatory support[8]. The classic standard approach is the right extrapleural thoracotomy, which is extremely invasive. Since Lobe et al[2] reported the first thoracoscopic repair of EA in 1999, this minimally invasive technique has been gradually accepted and promoted. The perioperative outcomes of thoracoscopic repair have been found to be similar to the open repair except for the longer operative time[1,4]. However, the open conversion rate is dramatically high. Etchill et al[1] reported that the initial thoracoscopic repair was conducted in 133 of 855 neonates, and 70 (53%) cases were converted to thoracotomy. A major cause leading to open conversion was technical difficulties[4]. In addition, the long learning curve also prevents the promotion of thoracoscopic repair[9,10]. Hence, pediatric surgeons have been trying to explore new and simple approaches for EA management.
In 1975, Hendren et al[11] proposed the use of an electromagnetic device to elongate the pouches of the EA with a long gap. Magnetic compression anastomosis using permanent magnets was first reported by Obora et al[2] in 1978 for vascular anastomosis. This technique was earlier used to perform gastroenteric anastomosis or recanalize the biliary stenosis or stricture after a liver transplant[13-16]. In 2009, Zaritzky et al[3] reported their repair experience of esophageal magnetic compression anastomosis in 5 cases. Prototyped catheters and fluoroscopic guidance were used for the placement of the magnets. However, all neonates received a gastrostomy for feeding before the formation of patent anastomosis and the occurrence of anastomosis strictures. Since then, several case reports and case series have been published covering magnetic compression anastomosis combined with endoscopy or thoracotomy[5-7,17,18]. In these previous studies, the patients underwent gastrostomy, thoracotomy, or staged repair, and these extremely invasive procedures might cause more perioperative or postoperative complications such as gastroesophageal reflux, anastomosis stricture, or leakage[8,19].
In this study, the thoracoscopic technique was first combined with magnamosis to achieve primary repair of EA Gross type C. This novel method retained the minimally invasive features of thoracoscopy while avoiding the technical difficulties caused by suturing anastomosis. The feeding tube was passed through the coupled magnets to introduce it into the stomach and used for postoperative feeding and removal of the magnets. The problem of gastrostomy increasing the morbidity of gastroesophageal reflux was avoided in this procedure. At the same time, the distal esophageal inner diameter was measured by the catheters of different diameters. It was helpful to achieve a larger anastomosis with a bigger distal magnet. The magnets before being removed also supported the anastomosis to prevent stricture. The X-ray with the contrast agent demonstrated a wide patent anastomosis after the transoral feeding was given.
Although the postoperative X-ray also found a minor leakage, the contrast agent did not enter the pleural cavity. Most esophageal anastomosis leakages seal spontaneously by conservative methods[20]. Therefore, we did not address this situation. Fortunately, the esophagographic examination showed a patent anastomosis without leakage after the magnets were removed. Another interesting point is that the preoperative examination found that the proximal end was at the fourth thoracic vertebra, whereas the postoperative anastomosis was at the sixth thoracic vertebra. This phenomenon demonstrated that the proximal esophagus was very stretchable. Previous case reports have shown the feasibility of magnetic repair of long-gap EA. Therefore, we believe that our approach can also be extended to primarily repair other subtypes of EA. We look forward to other colleagues using this procedure and accumulating more experience to share.
This report presented the first successful operation of primary repair of EA via thoracoscopic esophageal magnetic compression anastomosis. The procedure is simple, feasible, and effective for treating EA. The favorable outcome demonstrates that it is worth promoting.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martín Del Olmo JC, Spain; Shiryajev YN, Russia S-Editor: Zhang H L-Editor: Filipodia P-Editor: Chen YX
| 1. | Etchill EW, Giuliano KA, Boss EF, Rhee DS, Kunisaki SM. Association of operative approach with outcomes in neonates with esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2021;56:2172-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Lobe TE, Rothenberg S, Waldschmidt J, Stroedter L. Thoracoscopic repair of esophageal atresia in an infant: a surgical first. Pediatric Endosurg Inno Tech. 1999;3:141-148. |
| 3. | Zaritzky M, Ben R, Zylberg GI, Yampolsky B. Magnetic compression anastomosis as a nonsurgical treatment for esophageal atresia. Pediatr Radiol. 2009;39:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Okuyama H, Saka R, Takama Y, Nomura M, Ueno T, Tazuke Y. Thoracoscopic repair of esophageal atresia. Surg Today. 2020;50:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Muensterer OJ, Evans LL, Sterlin A, Sahlabadi M, Aribindi V, Lindner A, König T, Harrison MR. Novel Device for Endoluminal Esophageal Atresia Repair: First-in-Human Experience. Pediatrics. 2021;148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Liu SQ, Lv Y, Fang Y, Luo RX, Zhao JR, Luo RG, Li YM, Zhang J, Zhang PF, Guo JZ, Li QH, Han MX. Magnetic compression for anastomosis in treating an infant born with long-gap oesophageal atresia: A case report. Medicine (Baltimore). 2020;99:e22472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Holler AS, König TT, Chen C, Harrison MR, Muensterer OJ. Esophageal Magnetic Compression Anastomosis in Esophageal Atresia Repair: A PRISMA-Compliant Systematic Review and Comparison with a Novel Approach. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Spitz L. Esophageal atresia. Lessons I have learned in a 40-year experience. J Pediatr Surg. 2006;41:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 9. | Lee S, Lee SK, Seo JM. Thoracoscopic repair of esophageal atresia with tracheoesophageal fistula: overcoming the learning curve. J Pediatr Surg. 2014;49:1570-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ibáñez Pradas V, Couselo Jerez M, Carazo Palacios ME. Thoracoscopic esophageal atresia repair: learning curve analysis using Clavien-Dindo surgical complication classification. Cir Pediatr. 2020;33:166-171. [PubMed] |
| 11. | Hendren WH, Hale JR. Electromagnetic bougienage to lengthen esophageal segments in congenital esophageal atresia. N Engl J Med. 1975;293:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Obora Y, Tamaki N, Matsumoto S. Nonsuture microvascular anastomosis using magnet rings: preliminary report. Surg Neurol. 1978;9:117-120. [PubMed] |
| 13. | Cope C, Clark TW, Ginsberg G, Habecker P. Stent placement of gastroenteric anastomoses formed by magnetic compression. J Vasc Interv Radiol. 1999;10:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Takao S, Matsuo Y, Shinchi H, Nakajima S, Aikou T, Iseji T, Yamanouchi E. Magnetic compression anastomosis for benign obstruction of the common bile duct. Endoscopy. 2001;33:988-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Mimuro A, Tsuchida A, Yamanouchi E, Itoi T, Ozawa T, Ikeda T, Nakamura R, Koyanagi Y, Nakamura K. A novel technique of magnetic compression anastomosis for severe biliary stenosis. Gastrointest Endosc. 2003;58:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Muraoka N, Uematsu H, Yamanouchi E, Kinoshita K, Takeda T, Ihara N, Matsunami H, Itoh H. Yamanouchi magnetic compression anastomosis for bilioenteric anastomotic stricture after living-donor liver transplantation. J Vasc Interv Radiol. 2005;16:1263-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Liu SQ, Lv Y, Luo RX. Endoscopic magnetic compression stricturoplasty for congenital esophageal stenosis: A case report. World J Clin Cases. 2022;10:12313-12318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Ellebaek MBB, Qvist N, Rasmussen L. Magnetic Compression Anastomosis in Long-Gap Esophageal Atresia Gross Type A: A Case Report. European J Pediatr Surg Rep. 2018;6:e37-e39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Comella A, Tan Tanny SP, Hutson JM, Omari TI, Teague WJ, Nataraja RM, King SK. Esophageal morbidity in patients following repair of esophageal atresia: A systematic review. J Pediatr Surg. 2021;56:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Zhao R, Li K, Shen C, Zheng S. The outcome of conservative treatment for anastomotic leakage after surgical repair of esophageal atresia. J Pediatr Surg. 2011;46:2274-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |