Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2907
Peer-review started: September 7, 2023
First decision: September 20, 2023
Revised: September 30, 2023
Accepted: November 14, 2023
Article in press: November 14, 2023
Published online: December 27, 2023
Processing time: 111 Days and 9.6 Hours
Colorectal cancer (CRC) is a prevalent malignant tumor involving adenomas that develop into malignant lesions. Carcinoembryonic antigen (CEA) is a non-specific serum biomarker upregulated in CRC. The concentration of CEA is modulated by tumor stage and grade, tumor site in the colon, ploidy status, and patient smoking status. This study aimed to evaluate current evidence regarding the diagnostic power of CEA levels in the early detection of CRC recurrence in adults.
To evaluate current evidence regarding the diagnostic power of CEA levels in the early detection of CRC recurrence in adults.
A systematic search was performed using four databases: MEDLINE, Cochrane Trials, EMBASE, and the Web of Science. The inclusion criteria were as follows: Adult patients aged ≥ 18 years who had completed CRC curative treatment and were followed up postoperatively; reporting the number of CRC recurrences as an outcome; and randomized, clinical, cohort, and case-control study designs. Studies that were not published in English and animal studies were excluded. The following data were extracted by three independent reviewers: Study design, index tests, follow-up, patient characteristics, and primary outcomes. All statistical analyses were performed using the RevMan 5.4.1.
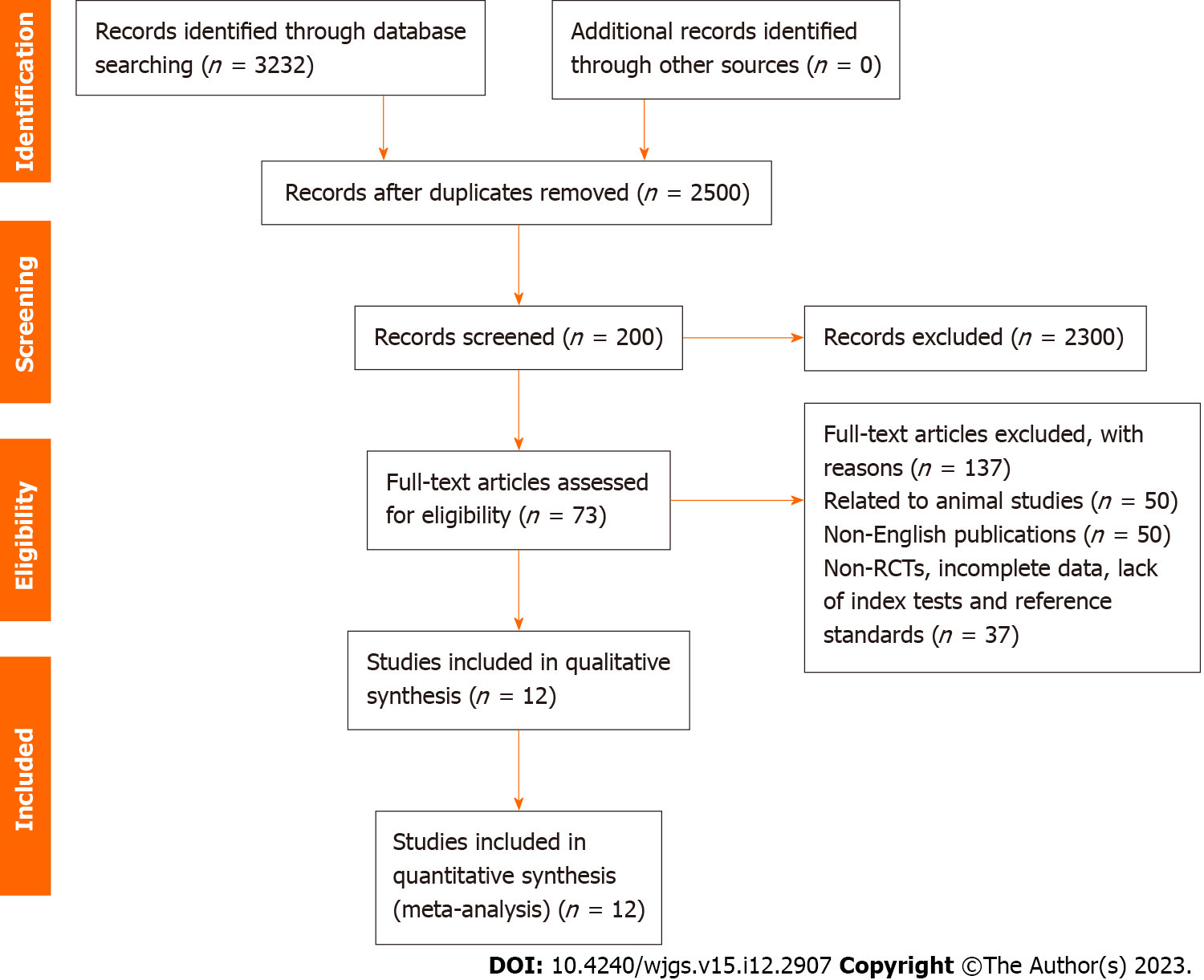
A total of 3232 studies were identified, with 73 remaining following the elimination of duplicates. After screening on predetermined criteria, 12 studies were included in the final analysis. At a reference standard of 5 mg/L, CEA detected only approximately half of recurrent CRCs, with a pooled sensitivity of 59% (range, 33%–83%) and sensitivity of 89% (range, 58%–97%).
CEA is a significant marker for CRC diagnosis. However, it has insufficient sensitivity and specificity to be used as a single biomarker of early CRC recurrence, with an essential proportion of false negatives.
Core Tip: Colorectal cancer (CRC) is a prevalent malignant tumor involving adenomas that develop into malignant lesions. Carcinoembryonic antigen (CEA) is a non-specific serum biomarker upregulated in CRC. The concentration of CEA is modulated by tumor stage and grade, tumor site in the colon, ploidy status, and patient smoking status. Overall, CEA remains an important diagnostic tool for CRC detection and management. When used in combination with other diagnostic tests, such as colonoscopy and imaging examinations, CEA can provide valuable information regarding the presence and progression of CRC and treatment effectiveness.
- Citation: Wang R, Wang Q, Li P. Significance of carcinoembryonic antigen detection in the early diagnosis of colorectal cancer: A systematic review and meta-analysis. World J Gastrointest Surg 2023; 15(12): 2907-2918
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2907.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2907
Colorectal cancer (CRC) is a prevalent malignant tumor involving adenomas that develop into malignant lesions. CRC can be detected through early screening and treated with surgery or colonoscopy, increasing the survival rate by 90%[1]. Carcinoembryonic antigen (CEA), commonly found in epithelial cells in the colon, stomach, prostate, or cervix, is a non-specific serum biomarker upregulated in CRC[2].
CEA is a glycoprotein with a molecular weight of 200 kDa. CEA is generally eliminated from the serum after birth, with small amounts retained in colonic tissues that are associated with chromosome 19q13.2[2]. CEA is a member of the CEACaM immunoglobulin family and is involved in cell adhesion, migration, and proliferation[3]. In normal cells, CEA is primarily located in the endoluminal section of the cell membrane, where it inhibits cell apoptosis and tumor pathogenesis[3].
Controversy surrounds CEA use in practice owing to variability in the measurement of CEA levels. Foremost, enzyme-linked immunosorbent assays, which are often used to test for the presence of CEA, can vary depending on the testing procedures, yielding inconsistent CEA levels. For example, using monoclonal antibodies against reactive isotopes of CEA produces significant errors in patients receiving secondary doses of monoclonal antibodies[2,4]. Moreover, in patients with CRC, the concentration of CEA is modulated by tumor stage and grade, tumor site, ploidy status, and patient smoking status. Because CEA is mainly metabolized in hepatic cells, dysfunction in biliary and hepatic function was associated with false positive detection due to increased levels of CEA[5]. Tumor differentiation also affects CEA levels, with higher levels implying appropriately differentiated tumors and vice versa. Lastly, Duffy[5] suggested that the isolation of CEA from liver metastases leaves a glycoprotein with 60% carbohydrate content and a relatively high molecular mass; therefore, CEA is heterogeneous because of significant variations in its carbohydrate side chains, consisting of mannose, galactose, and sialic acid.
CEA levels increase with CRC disease progression, with significantly differentiated CRCs being associated with higher levels of CEA per gram of protein than poorly differentiated CRCs[4,5]. Therefore, CEA testing has been suggested for CRC diagnosis, particularly for detecting disease recurrence and monitoring the response to therapy. However, the use of CEA in the screening of CRC in asymptomatic individuals remains controversial and, therefore, is not recommended in routine practice[6].
According to Lakemeyer et al[7] CEA testing can help detect early CRC recurrence in patients who have already been diagnosed with CRC. Increasing blood levels of CEA provides a biomarker of CRC recurrence, even before it appears in imaging tests[8]. Early detection of CRC recurrence can allow for early treatment of recurrent disease, improving patient outcomes. In addition to early detection, CEA testing can be used to determine treatment effectiveness and, therefore, the risk for CRC recurrence. Specifically, in patients with high pretreatment CEA levels, a decrease in CEA levels can indicate treatment effectiveness[9]. Conversely, a continued rise in CEA levels after treatment would suggest cancer recurrence. However, considering the inconsistencies in using CEA for CRC detection, this study aimed to evaluate current evidence regarding the diagnostic power of CEA levels in the early detection of CRC recurrence in adults.
A systematic search was performed using three databases: MEDLINE, Cochrane Trials, EMBASE, and the Web of Science. The databases were searched from 1990 to 2022 using keywords such as "recurrence," "carcinoembryonic antigen," "colorectal cancer," "follow-up," and "curative." This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[10,11] and the Cochrane Handbook for Systematic Reviews[12,13]. The evidence from the findings of this study was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation procedure[14].
This study adopted the Participants, Intervention, Comparison, and Outcomes model to determine the inclusion and exclusion criteria. The inclusion criteria were as follows: Adult patients, ≥ 18 years of age, who had undergone curative CRC treatment with postoperative follow-up; reporting of the number of CRC recurrences as an outcome; inclusion of sufficient information to calculate contingency tables for CEA, index tests, physical examinations, and echography; and randomized, clinical, and cohort and case-control study designs. Studies published in languages other than English, involving animal models, and not reporting CEA levels in CRC were excluded.
Our study team included three reviewers who independently reviewed studies identified by our search. The titles and abstracts were screened using our predetermined inclusion and exclusion criteria. The reference list of selected publications was reviewed to identify other eligible studies. A full-text review was performed to confirm eligibility. Differences in opinions between independent reviewers were resolved through discussion and consensus before voting.
The data collection forms outlined by the standard Cochrane Library were used to extract data from eligible studies. The following data were extracted: Study design, index tests, follow-up, patient characteristics, CRC recurrent, and primary outcomes measured in each study.
The Cochrane risk of bias (ROB) tool was used to assess the quality of the selected studies. The risk of blinding evaluators was discarded as a bias criterion as it is impossible to blind medical professionals to the measurement of CEA levels used in the diagnostic process. Therefore, ROB was evaluated based on patient selection, index tests, reference standards, flow, and timing.
Our meta-analysis assessed outcomes based on pooled odds ratios and their 95% confidence intervals using forest plots. All statistical analyses were performed using Review Manager 5.4.1 (RevMan, Copenhagen, The Cochrane Collaboration, 2014). The heterogeneity of studies was analyzed using statistic, with P values < 0.05 considered statistically significant. The effects of heterogeneity on CEA levels were assessed using the I2 statistic, with scores between 0% and 40% regarded as insignificant inconsistencies in the measurement of CEA levels. The Mantel–Haenszel random effects model was used to evaluate the association between CEA levels and CRC recurrence as its assumptions were met. Clinical heterogeneity was examined to determine random effects on the association between CEA levels and CRC recurrence. Heterogeneity was explained based on sample size, sex, methodological quality, and study design. Moreover, differences in CRC subtypes, percentage of recurrences, testing frequency, tumor site, and follow-up parameters were examined. Sensitivity analyses were performed in various domains, including a validated CEA measurement.
In total, 3232 studies were identified, with 73 retained after eliminating duplicate records. After review, based on eligibility criteria, 12 studies were included in the final analysis[15-26] (Figure 1).
The 12 studies included in the final analysis reported on 3223 patients; 60% were men aged 25–95 years (Table 1). Of these, 569 patients were from secondary care facilities. Most patients were diagnosed with stage II or III CRC. Two studies included patients with stage IV CRC (Bhatti et al[16] and Shinkins et al[26])with curative treatment, including resectioning liver metastases. The reported duration of follow-up ranged from 17 to 99 months, with a CRC recurrence rate of 20%.
| Ref. | Year of publication | Study design | Sample size | Age (yr) | Location of cancer | Stage of the tumour | Treatment therapy |
| Augestad et al[15] | 2014 | Randomized controlled trial | 110 | 65.4 ± 8.1; males = 59.1%, females = 40.9% | Colon (110 patients) | I (24), II (55), III (32) | Surgical treatment for all patients |
| Bhatti et al[16] | 2015 | Prospective cohort study | 569 | 70; males = 51%, females = 49% | Colon (336 patients) and rectum (233 patients) | 0 (31), I (33), II (78), III (288), IV (137) | Surgical treatment (569 patients), neoadjuvant chemotherapy (106 patients) and adjuvant chemotherapy (204 patients) |
| Chang et al[17] | 2017 | Prospective cohort study | 357 | 63.8 ± 11.5; males = 57.7%, females = 42.3% | Colon (259 patients) and rectum (98 patients) | I (97), II (125), III (135) | Surgical treatment (357 patients), neoadjuvant radiotherapy and chemotherapy (81 patients) |
| Gilardoni et al[18] | 2015 | Retrospective cohort study | 196 | 70; males = 59.7%, females = 40.3% | Colon (196 patients) | I (65), III (131) | Surgical treatment (196 patients) |
| Guo et al[19] | 2018 | Retrospective cohort study | 178 | 59.7 ±10.6; males = 66.3%; females = 32.7% | Colon (79 patients) and rectum (42 patients) | 0 (2), I (11), II (60), III (105) | Surgical treatment (178 patients) and chemotherapy (66 patients) |
| Hara et al[20] | 2011 | Prospective cohort study | 127 | 63.4 ±9.4; males = 55.1%, females = 44.9% | Colon (85 patients) and rectum (42 patients) | III (127) | Surgical treatment (127 patients), adjuvant chemotherapy (110 patients), adjuvant radiotherapy (1 patient) |
| Jones et al[21] | 2015 | Retrospective cohort study | 118 | N/A; males = 61.9%, females = 38.1% | Colon (66 patients) and rectum (52 patients) | I (26), II (47), III (45) | Surgical treatment (118 patients), adjuvant chemotherapy (73 patients) |
| Kim and Lee[22] | 2013 | Retrospective cohort study | 336 | 60.4 ± 11.1; males = 60.7%, females = 39.3% | Colon (224 patients) and rectum (112 patients) | II (189 patients) and III (147 patients) | Surgical treatment for all patients |
| Moloney et al[23] | 2019 | Retrospective cohort study | 138 | 67; males = 54.3%, females = 45.7% | Colon (90 patients) and rectum (48 patients) | I (58 patients), II (69 patients), III (11 patients) | Surgical treatment for all patients |
| Nicolini et al[24] | 2010 | Prospective cohort study | 108 | 60 | Colon (69 patients) and rectum (39 patients) | I (29 patients), II (41 patients), and III (38 patients) | Surgical treatment for all patients |
| Rodrigues et al[25] | 2017 | Prospective cohort study | 404 | 64.6; males = 59.7%, females = 40.3% | Colon (199 patients) and rectum (205 patients) | II (177 patients) and III (227 patients) | Surgical treatment (404 patients), neoadjuvant chemotherapy in the rectum (175), adjuvant chemotherapy (196 patients) and adjuvant chemotherapy in the colon (86 patients) |
| Shinkins et al[26] | 2017 | Randomized controlled trial | 582 | 65; males = 61.3%, females = 38.7% | N/A | I (110 patients), II (282 patients), III (166 patients) and U (24 patients) | Surgical treatment for all patients |
Our study compared the diagnostic values of follow-up procedures used in detecting recurrence rates of CRC. Nine studies reported CEA as follow-up tests with 2 studies reporting ultrasound and 1 study involving a physical examination. In studies that reported CEA, a cut-off value of 5 micrograms per litre was used in all patients as the standard threshold. The reference standard varied in various studies with histopathological and radiological examinations being widely used (see Table 2)
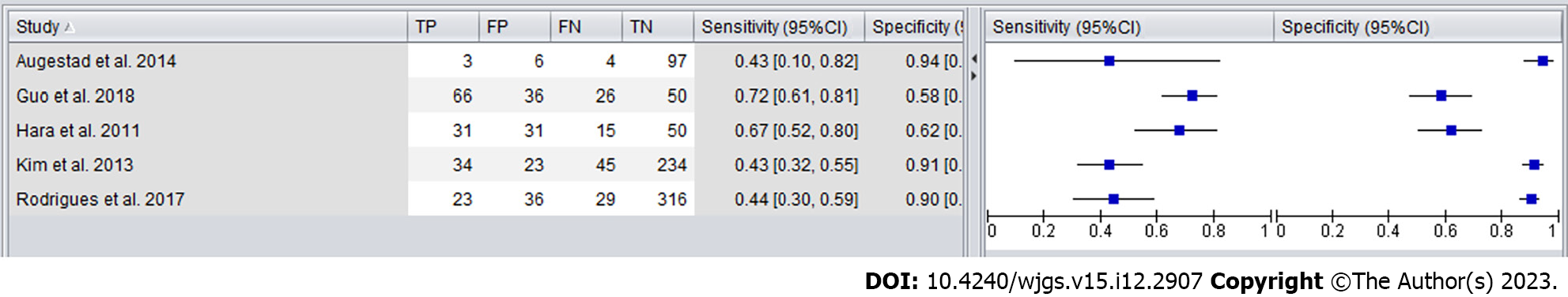
| Ref. | Year of publication | Sample size | Percent recurrence (%) | Duration of follow-up (mo) | Index test | Frequency (mo) | Standard of reference | True positive | False positive | False negative | True negative |
| Augestad et al[15] | 2014 | 110 | 12.7 | 17 | CEA reference threshold of 5 g/L | 6 | Colonoscopy, contrast-enhanced ultrasound, computed tomography scans, PET scans | 3 | 6 | 4 | 97 |
| Bhatti et al[16] | 2015 | 569 | 26.2 | 6-50 | CEA reference threshold of 5 g/L | 6 | CT and PET scans with or without biopsies | 123 | 23 | 26 | 397 |
| Chang et al[17] | 2017 | 357 | 18.8 | 3-64 | CEA reference threshold of 5 g/L | 6 | CT scan | 44 | 59 | 23 | 231 |
| Hara et al[20] | 2011 | 127 | 36.2 | N/A | CEA reference threshold of 5 g/L | 6 | Radiological examination | 31 | 31 | 15 | 50 |
| Guo et al[19] | 2018 | 178 | 51.7 | N/A | CEA reference threshold of 5 g/L | 6 | Radiological examination involving X-rays, CT scans | 66 | 36 | 26 | 50 |
| Kim and Lee[22] | 2013 | 336 | 23.5 | 36-134 | CEA reference threshold of 5 g/L | 6 | Biopsies and radiological examination | 34 | 23 | 45 | 234 |
| Moloney et al[23] | 2019 | 138 | 4.3 | 25 | N/A | 6 | CT scans, colonoscopy and ultrasound | 2 | 5 | 4 | 127 |
| Rodrigues et al[25] | 2017 | 404 | 12.9 | 3-79 | CEA reference threshold of 5 ug/L for smokers and a 3 g/L for non-smokers | 6 | Radiological examinations | 23 | 36 | 29 | 316 |
| Shinkins et al[26] | 2017 | 582 | 17.9 | N/A | CEA reference threshold of 5 g/L | 6 | CT scans, colonoscopy | 51 | 12 | 53 | 466 |
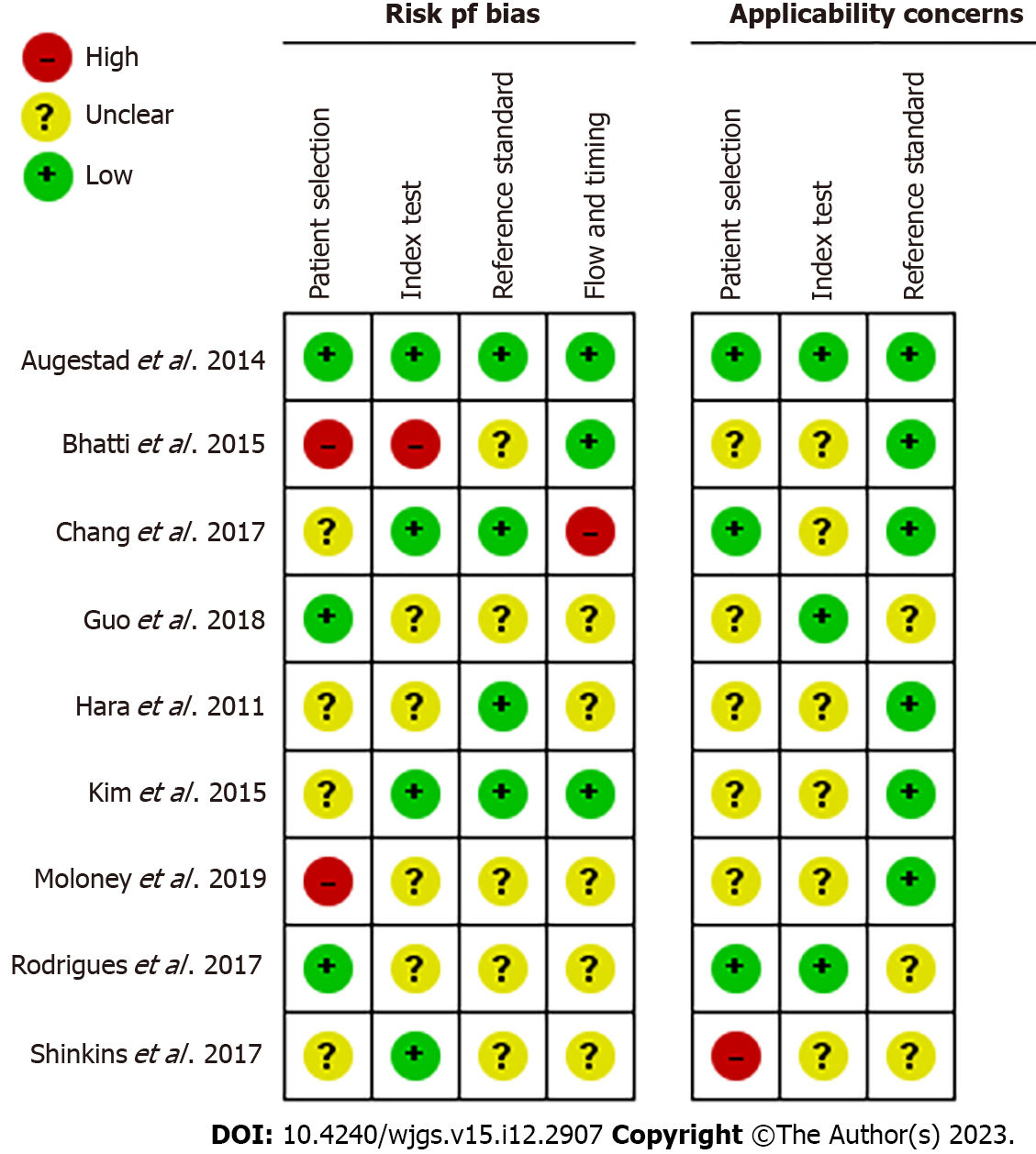
The ROB allowed us to assess the methodological quality of selected studies. We found four studies with a relatively high ROB regarding patient selection, while six studies had a higher ROB regarding reference standards.
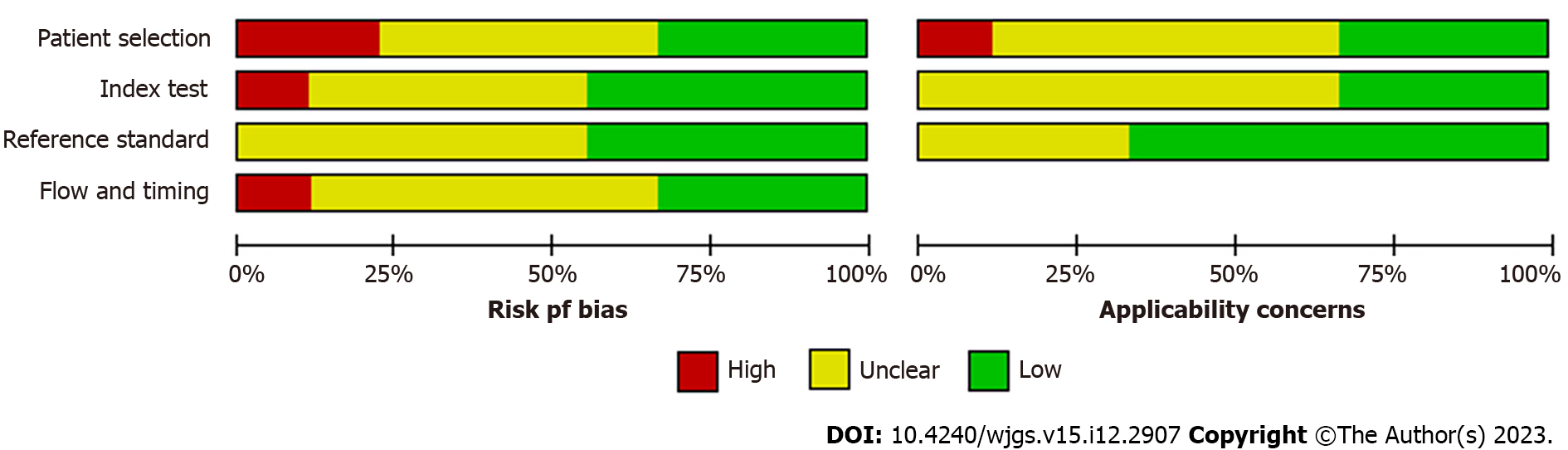
ROB was assessed for patient selection, index tests used, reference standards for CEA levels, timing of CEA testing, and flow of patients through the study (Figure 2). As shown in Figure 3, most studies had an unclear and low ROB across the four domains of patient selection, index test, reference standard, and flow and timing, with a high ROB observed in only four studies on various domains. The ROB for patient selection was unclear-to-low due to a lack of randomization, with patients selected based on specific features that could influence measured outcomes. Bias in index tests used in diagnostic studies can lead to under- or over-estimation of the diagnostic accuracy of a test and, thus, the selection and interpretation of a gold standard was low. The reference standard is often associated with a misclassification bias when it is not sensitive or sufficient to diagnose the desired medical condition. Bias in the timing of the index test and subsequent flow of patients through the study can further contribute to variability in the evaluation of diagnostic accuracy.
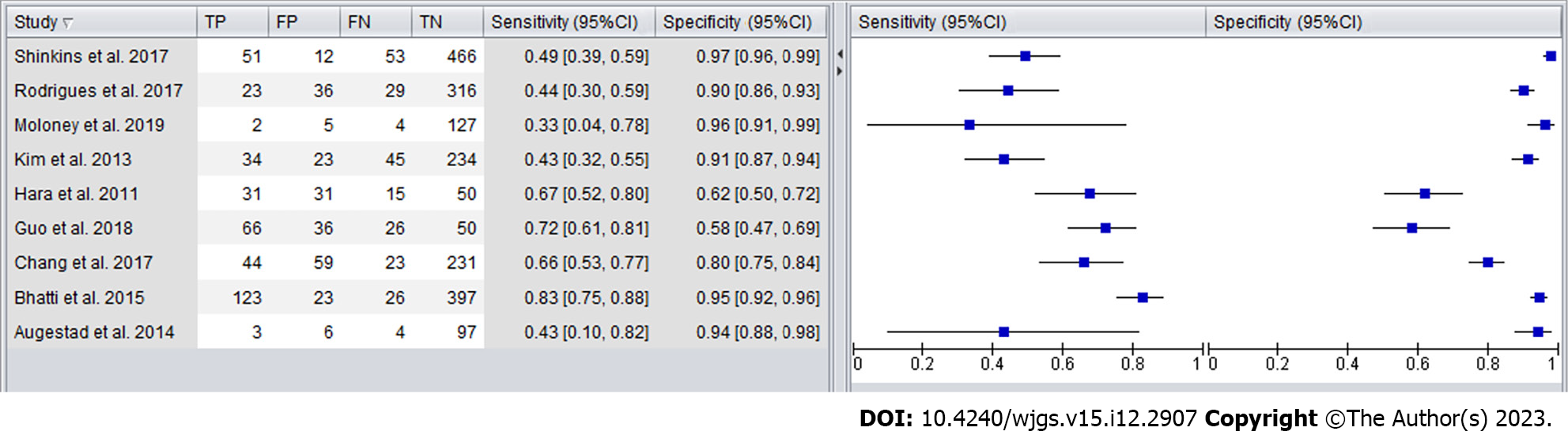
Forest plots were created to compare the diagnostic accuracy of the different follow-up tests (Figures 4 and 5). The sensitivity for detecting CRC recurrence overall ranged between 33% and 83%, with a specificity range from 58% to 97%.
As shown in Figure 6, the sensitivity and specificity of CEA in detecting CRC recurrence in high-quality studies ranged from 43% to 72%, with a specificity range from 58% to 94%.
Our findings revealed that using CEA at a reference level of 5 mg/L detected only approximately half of CRC recurrence, with CEA having neither the sensitivity nor specificity required to be used as a single test clinically. Although CEA can be useful for early identification of CRC recurrence, it must be combined with other tests, such as ultrasound or physical examination.
The recommended reference standard for CEA testing is 5 mg/L, with levels > 5 mg/L considered abnormal. However, it is essential to note that not all patients with CRC have elevated CEA levels and that some patients with other conditions may also have elevated CEA levels. The significance of CEA detection in the early diagnosis of CRC lies in its ability to detect cancer in its early stages before symptoms appear. CEA testing is often used with other diagnostic tests, such as colonoscopy or biopsy, for early CRC diagnosis. This is important as early diagnosis can optimize treatment and, thus, patient outcomes and survival.
Our findings are similar to Hung et al[27], who identified elevated serum CEA levels > 5 mg/L in 63.6% of patients with recurrent CRC. For patients with low serum CEA levels (2 mg/L), suspected CRC recurrence was assessed based on symptomology and radiological examination. Repeated measurement of postoperative CEA levels is recommended to improve the detection of CRC recurrence for early treatment, with monitoring at 3 and 6 mo recommended for five years after initial diagnosis. In addition, annual computed tomography (CT) imaging is recommended to enhance CRC recurrence detection. Before curative treatment, patients with high CEA levels are expected to exhibit decreased or normal levels postoperatively. Persistently high or increased levels during routine postoperative monitoring suggest CRC recurrence and are an indication for follow-up imaging examination, including radiography, CT, and positron-emission tomography (PET)[27]. Therefore, the monitoring of CEA levels should be considered as one component of a comprehensive postoperative follow-up for early diagnosis of CRC recurrence.
Despite advancements in surgical procedures, care, and adjuvant and neoadjuvant chemotherapy[28], postoperative follow-up is recommended for CRC recurrence for early treatment to improve patient outcomes and survival. Our analysis included studies with a postoperative follow-up duration of at least six months for CRC recurrence detection. Our findings indicate the possibility of missed diagnosis of CRC recurrence, including false negatives, when using only CEA levels due to its lower-than-needed diagnostic sensitivity and specificity. Therefore, CEA levels should not be used as a single technique for CRC follow-up.
The incorporation of imaging examinations in CRC follow-up has been inconsistent and lacks predefined standards. Locker et al[29] suggested that shorter follow-up intervals and precise radiological examination improved the detection of CRC recurrence, increasing patient survival by allowing for early intervention. In contrast, Primrose et al[30] suggested that shorter follow-up intervals and detailed procedures after curative CRC treatment did not yield a survival advantage. Moreover, they reported a higher risk of recurrence in patients who underwent CT and CEA measurements than in those who were followed-up only using CEA or CT imaging, but with no statistical difference in overall survival between the two groups. Based on these results, the combination of CEA and CT imaging may be significant only during the early stages of follow-up and not the later stages. Our findings indicated that follow-up should be more intensive during the first three years after curative CRC treatment, with the inclusion of imaging and physical examination recommended considering the 30% CRC recurrence rate within the first three years postoperatively and that > 70% of recurrent CRCs were not detected by CEA levels. In addition, we propose that adoption of PET imaging over other imaging modalities could be significant for detecting metastasis. We recognize that although PET imaging provides high sensitivity and specificity for CRC recurrence compared to CEA, it is relatively expensive, and its availability is limited in some regions of the world[28]. As a rule, medical professionals should incorporate PET or CT as secondary diagnostic tools when an elevation in CEA is detected.
Our findings indicated a higher rate of CRC recurrence among patients with higher preoperative CEA levels. However, frequent radiological examination was associated with a good prognosis and delayed detection of CRC recurrent among patients with low preoperative CEA levels. In their study of 106 patients with CRC recurrence after surgical treatment, Saito et al[31] reported that 56% of patients had normal CEA levels at the time of diagnosis and serum CEA concentrations > 5 mg/L at the time of CRC recurrence. Using a cutoff CEA level of 5 mg/L lowers the sensitivity of detecting recurrent CRC, with 80% of patients with CEA levels > 5 mg/L reporting abnormal symptoms at the time of diagnosis of CRC recurrence. In comparison, among patients with CEA levels < 5 mg/L, 42% of the patients reported abnormal symptoms at the time of diagnosis of recurrent CRC. Therefore, surveillance of recurrent CRC using CEA levels alone is insufficient and risky due to its low diagnostic accuracy for CRC recurrence.
Incorporating other tumor markers, such as carbohydrate antigen 19 and cancer antigen 125, is essential in determining CRC recurrence rates, particularly in patients who do not exhibit elevated levels of CEA before surgery or during follow-up. We propose that adoption of other tumor markers could increase the specificity and precision of early detection of CRC. Several studies have proposed that a CEA cutoff value of 5 mg/L should be adopted for CRC recurrence detection in follow-up studies. However, we propose that this cutoff value should be slightly lowered for patients with low CEA levels to permit the early detection of recurrent CRC, such as 10 µg/dL.
In our analysis, we included studies that adopted colonoscopy[32] as a reference standard for CRC recurrence detection in addition to CEA levels. Colonoscopy refers to medical tests performed inside the bowel to detect abnormalities. It involves a long, flexible tube with a tiny camera placed at the tip of the rectum to visualize any changes. According to the American Cancer Society, individuals with a high risk of CRC should begin screening at the age of 45 years, and individuals with a family history of CRC or predisposition should undergo regular screening. A colonoscopy permits doctors or cancer professionals to analyze the rectum and colon for abnormalities, such as polyps or growth, which can be biopsied for further examination. Colonoscopy is considered the gold standard for CRC detection; combined with CEA testing, it is highly effective for detecting early-stage CRC. In addition to its effectiveness in detecting CRC, polyps and other growths can be removed during the procedure to prevent CRC development[32]. Although colonoscopy is generally a safe and effective procedure, it does carry some risks, such as bleeding or perforation of the colon. However, these risks are rare, and the benefits of early detection and prevention of CRC far outweigh the risks associated with the procedure.
Our analysis included studies that adopted contrast-enhanced ultrasound (CEUS) and CEA for CRC detection. CEUS is an imaging technique that uses contrast agents, such as microbubbles, to improve the clarity and visualization of images in the rectum and colon. It permits the visualization of functional and structural systems of the rectum and colon to offer a complete image of abnormalities or growths[33,34]. Contrast agents represent areas of higher blood flow, indicating the presence of tumors. CEUS is more robust than CT or magnetic resonance imaging (MRI) as it does not involve ionizing radiation and is highly safe for patients who require frequent medical examinations. The high effectiveness of CEUS for early-stage CRC detection has been reported, with a sensitivity of 92% and specificity of 89%[35]. Therefore, CEUS may be a valuable tool for the early detection of treatable CRC. However, CEUS has not been widely adopted in clinical practice, and further research is required to comprehend its effectiveness and limitations compared with other imaging techniques.
CT imaging for CRC detection and staging involves numerous radiographs to create clear and detailed images of the colon and rectum to reveal tumors or enlarged lymph nodes. CT imaging is highly effective for early CRC detection, which improves treatment outcomes and overall survival rates[36,37]. Moreover, CT imaging can be used to monitor disease progression, assess treatment effectiveness, measure tumor size, and monitor change over time. Combining CT images and CEA measurement increases the sensitivity and specificity of detecting early CRC by identifying abnormal growth or lesions within the colon and rectum and determining the cancer stage. CT can also detect the presence of cancerous cells in other parts of the body, such as the liver, indicating whether the cancer has metastasized. However, CT imaging is associated with high exposure to ionizing radiation, which increases cancer risk, particularly in young patients. However, the benefits of early detection and treatment of CRC generally outweigh the risks associated with CT imaging.
Our findings indicate that combined radiological examinations, including CT, MRI, and CEAU, are adequate for early CRC detection. Radiographs can detect abnormalities in the colon and rectum, such as growth or blockage[28,38]. However, radiography alone is insufficient for CRC detection as it does not provide a detailed image of the colon and rectum. In this regard, CT or MRI is recommended over plain radiography. Moreover, because MRI provides detailed images of the colon and rectum, similar to CT, but without radiation exposure, it should be considered over CT.
The diagnostic accuracy of CEA for detecting CRC recurrence is influenced by various factors, such as cancer stage of cancer, CEA test sensitivity and specificity, and other factors that can affect CEA levels, such as smoking and other medical conditions. For example, smoking may have elevated CEA levels even without CRC. Furthermore, inflammatory bowel disease or liver disease can yield an elevation in CEA levels owing to inflammation in the body. Our findings indicate a sensitivity of 40%–70% CEA and a specificity of 80%–90%, suggesting that CEA can correctly identify the presence of CRC in 40%–70% of cases and the absence of CRC in 80–90% of cases.
The limitations of our systematic review and meta-analysis must be acknowledged in applying the findings for practice. First, few studies were identified in which CEA was solely used as the follow-up test, without including imaging examination or colonoscopy. Therefore, further studies should be performed on a larger prospective and randomized basis to examine CEA's diagnostic accuracy in terms of patient outcomes, quality of life, and the cost of care or treatment. Second, we excluded studies not published in English, which may have limited the findings. The presence of patient selection bias and applicability concerns have significant implications for the validity and generalizability of the study findings. For example, studies that only included patients at a particular stage of CRC or those who had undergone a specific type of treatment may not represent the broader population of patients with CRC. This may have led to incorrect conclusions regarding diagnostic accuracy or the effectiveness of specific tests and treatments.
The strengths of our study also need to be specified. First, we adhered to the methodology of the Cochrane Handbook for Systematic Reviews and PRISMA guidelines. Second, we adopted a robust and broad search strategy that retrieved articles from all databases up to 2022. Most of the included studies reported a CEA level of 5 mg/L as the index test alongside other reference standards, such as imaging techniques.
Overall, CEA remains an essential diagnostic tool for CRC detection and management. CEA can provide valuable information regarding the presence and progression of CRC and treatment effectiveness when combined with other diagnostic tests, such as colonoscopy and imaging examinations.
CEA is a significant marker for CRC diagnosis. However, it has insufficient sensitivity and specificity to be used as a single biomarker of early CRC recurrence, with an essential proportion of false negatives.
Colorectal cancer (CRC) is a prevalent malignant tumor involving adenomas that develop into malignant lesions. CRC can be detected through early screening and treated with surgery or colonoscopy, increasing the survival rate by 90%. Therefore, carcinoembryonic antigen (CEA) testing has been suggested for CRC diagnosis, particularly for detecting disease recurrence and monitoring the response to therapy. However, the use of CEA in the screening of CRC in asymptomatic individuals remains controversial and, therefore, is not recommended in routine practice. Controversy surrounds CEA use in practice owing to variability in the measurement of CEA levels.
Controversy surrounds CEA use in practice owing to variability in the measurement of CEA levels. Foremost, enzyme-linked immunosorbent assays, which are often used to test for the presence of CEA, can vary depending on the testing procedures, yielding inconsistent CEA levels.
However, considering the inconsistencies in using CEA for CRC detection, this study aimed to evaluate current evidence regarding the diagnostic power of CEA levels in the early detection of CRC recurrence in adults.
Our research methods were a systematic review and meta-analysis significance of CEA detection in the early diagnosis of CRC.
CEA testing is often used with other diagnostic tests, such as colonoscopy or biopsy, for early CRC diagnosis. This is important as early diagnosis can optimize treatment and, thus, patient outcomes and survival. Therefore, the monitoring of CEA levels should be considered as one component of a comprehensive postoperative follow-up for early diagnosis of CRC recurrence.
We concluded that the CEA detection was significant in the early diagnosis of CRC.
Our research perspectives involved investigating the sensitivity and specificity of CEA as a diagnostic marker for CRC. Also, we sought to examine the possibility of early detection of CRC and how it improves the possibility of early treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chourdakis M, Greece; Inoue H, Japan S-Editor: Lin C L-Editor: A P-Editor: Yu HG
| 1. | Mo S, Dai W, Wang H, Lan X, Ma C, Su Z, Xiang W, Han L, Luo W, Zhang L, Wang R, Zhang Y, Zhang W, Yang L, Lu R, Guo L, Zheng Y, Huang M, Xu Y, Liang L, Cai S, Cai G. Early detection and prognosis prediction for colorectal cancer by circulating tumour DNA methylation haplotypes: A multicentre cohort study. EClinicalMedicine. 2023;55:101717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Kankanala VL, Mukkamalla SKR. Carcinoembryonic Antigen. 2023 Jan 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 3. | Zi-Yang Y, Kaixun Z, Dongling L, Zhou Y, Chengbin Z, Jimei C, Caojin Z. Carcinoembryonic antigen levels are increased with pulmonary output in pulmonary hypertension due to congenital heart disease. J Int Med Res. 2020;48:300060520964378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, Yen CC, Lin TC, Jiang JK, Yang SH, Wang HS, Chen PM. Preoperative carcinoembryonic antigen level as an independent prognostic factor in colorectal cancer: Taiwan experience. Jpn J Clin Oncol. 2000;30:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem. 2001;47:624-630. [PubMed] |
| 6. | Siregar GA, Sibarani H. Comparison of Carcinoembryonic Antigen Levels Among Degree of Differentiation and Colorectal Cancer's Location in Medan. Open Access Maced J Med Sci. 2019;7:3447-3450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Lakemeyer L, Sander S, Wittau M, Henne-Bruns D, Kornmann M, Lemke J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Minato T, Ito S, Li B, Fujimori H, Mochizuki M, Yamaguchi K, Tamai K, Shimada M, Tokunaga H, Shigeta S, Sato I, Shima H, Yamada H, Yaegashi N, Yasuda J. Liquid biopsy with droplet digital PCR targeted to specific mutations in plasma cell-free tumor DNA can detect ovarian cancer recurrence earlier than CA125. Gynecol Oncol Rep. 2021;38:100847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | AL-Nafakh ZM, AL-Dujaili AN, Rudha AR. Assessment of cancer embryonic antigen (CEA) biomarker in women with breast cancer disease. AIP Conference Proceedings. 2020;2290:020042. [DOI] [Full Text] |
| 10. | Selçuk AA. A Guide for Systematic Reviews: PRISMA. Turk Arch Otorhinolaryngol. 2019;57:57-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 11. | Sarkis-Onofre R, Catalá-López F, Aromataris E, Lockwood C. How to properly use the PRISMA Statement. Syst Rev. 2021;10:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 12. | Tarsilla M. Cochrane handbook for systematic reviews of interventions. Journal of Multidisciplinary Evaluation. 2010;6:142-148. [DOI] [Full Text] |
| 13. | Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2910] [Article Influence: 485.0] [Reference Citation Analysis (0)] |
| 14. | Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Augestad KM, Norum J, Rose J, Lindsetmo RO. A prospective analysis of false positive events in a National Colon Cancer Surveillance Program. BMC Health Serv Res. 2014;14:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 16. | Bhatti I, Patel M, Dennison AR, Thomas MW, Garcea G. Utility of postoperative CEA for surveillance of recurrence after resection of primary colorectal cancer. Int J Surg. 2015;16:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Chang YT, Yeh YS, Ma CJ, Huang CW, Tsai HL, Huang MY, Cheng TL, Wang JY. Optimization of a multigene biochip for detection of relapsed and early relapsed colorectal cancer. J Surg Res. 2017;220:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Gilardoni E, Bernasconi DP, Poli S, Garancini M, Luperto M, Zucchini N, Bovo G, Totis M, Bugatti A, Gianotti L. Surveillance for early stages of colon cancer: potentials for optimizing follow-up protocols. World J Surg Oncol. 2015;13:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Guo Y, Chen F, Cui W. Usefulness of plasma D-dimer level for monitoring development of distant organ metastasis in colorectal cancer patients after curative resection. Cancer Manag Res. 2018;10:4203-4216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Hara M, Sato M, Takahashi H, Takayama S, Takeyama H. Accuracy of monitoring serum carcinoembryonic antigen levels in postoperative stage III colorectal cancer patients is limited to only the first postoperative year. Surg Today. 2011;41:1357-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Jones RP, McWhirter D, Fretwell VL, McAvoy A, Hardman JG. Clinical follow-up does not improve survival after resection of stage I-III colorectal cancer: A cohort study. Int J Surg. 2015;17:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Kim HS, Lee MR. Diagnostic Accuracy of Elevated Serum Carcinoembryonic Antigen for Recurrence in Postoperative Stage II Colorectal Cancer Patients: Comparison With Stage III. Ann Coloproctol. 2013;29:155-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Moloney J, Partridge C, Delanty S, Lloyd D, Nguyen MH. High efficacy and patient satisfaction with a nurse-led colorectal cancer surveillance programme with 10-year follow-up. ANZ J Surg. 2019;89:1286-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Nicolini A, Ferrari P, Duffy MJ, Antonelli A, Rossi G, Metelli MR, Fulceri F, Anselmi L, Conte M, Berti P, Miccoli P. Intensive risk-adjusted follow-up with the CEA, TPA, CA19.9, and CA72.4 tumor marker panel and abdominal ultrasonography to diagnose operable colorectal cancer recurrences: effect on survival. Arch Surg. 2010;145:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Rodrigues RV, Pereira da Silva J, Rosa I, Santos I, Pereira N, Soares C, Pereira AD. Intensive Follow-Up After Curative Surgery for Colorectal Cancer. Acta Med Port. 2017;30:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Shinkins B, Nicholson BD, Primrose J, Perera R, James T, Pugh S, Mant D. The diagnostic accuracy of a single CEA blood test in detecting colorectal cancer recurrence: Results from the FACS trial. PLoS One. 2017;12:e0171810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Hung H, You J, Chiang J, Hsieh P, Chiang S, Lai C, Tasi W, Yeh C. Why recurrence was initially suspected during colorectal cancer postoperative surveillance?: A retrospective analysis. Medicine (Baltimore). 2020;99:e22803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Young PE, Womeldorph CM, Johnson EK, Maykel JA, Brucher B, Stojadinovic A, Avital I, Nissan A, Steele SR. Early detection of colorectal cancer recurrence in patients undergoing surgery with curative intent: current status and challenges. J Cancer. 2014;5:262-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1111] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 30. | Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D; FACS Trial Investigators. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 31. | Saito G, Sadahiro S, Kamata H, Miyakita H, Okada K, Tanaka A, Suzuki T. Monitoring of Serum Carcinoembryonic Antigen Levels after Curative Resection of Colon Cancer: Cutoff Values Determined according to Preoperative Levels Enhance the Diagnostic Accuracy for Recurrence. Oncology. 2017;92:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Stark UA, Frese T, Unverzagt S, Bauer A. What is the effectiveness of various invitation methods to a colonoscopy in the early detection and prevention of colorectal cancer? Protocol of a systematic review. Syst Rev. 2020;9:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Hwang M, Back SJ, Didier RA, Lorenz N, Morgan TA, Poznick L, Steffgen L, Sridharan A. Pediatric contrast-enhanced ultrasound: optimization of techniques and dosing. Pediatr Radiol. 2021;51:2147-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Badea R, Socaciu M, Ciobanu L, Hagiu C, Golea A. Contrast-enhanced ultrasonography (CEUS) for the evaluation of the inflammation of the digestive tract wall. J Gastrointestin Liver Dis. 2010;19:439-444. [PubMed] |
| 35. | Barr RG, Huang P, Luo Y, Xie X, Zheng R, Yan K, Jing X, Xu H, Fei X, Lee JM. Contrast-enhanced ultrasound imaging of the liver: a review of the clinical evidence for SonoVue and Sonazoid. Abdom Radiol (NY). 2020;45:3779-3788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 36. | Bruening W, Sullivan N, Paulson EC, Zafar H, Mitchell M, Treadwell J, Schoelles K. Imaging Tests for the Staging of Colorectal Cancer [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Sep-. [PubMed] |
| 37. | Kijima S, Sasaki T, Nagata K, Utano K, Lefor AT, Sugimoto H. Preoperative evaluation of colorectal cancer using CT colonography, MRI, and PET/CT. World J Gastroenterol. 2014;20:16964-16975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Nicholson BD, Shinkins B, Pathiraja I, Roberts NW, James TJ, Mallett S, Perera R, Primrose JN, Mant D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. 2015;2015:CD011134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |