Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2890
Peer-review started: August 2, 2023
First decision: October 20, 2023
Revised: November 3, 2023
Accepted: November 28, 2023
Article in press: November 28, 2023
Published online: December 27, 2023
Processing time: 147 Days and 10.5 Hours
Carcinoembryonic antigen (CEA) is a broad-spectrum tumor marker for differential diagnosis, monitoring, and response assessment of a variety of mali
To evaluate whether serum CEA could predict the prognosis in patients with colorectal cancer liver metastasis (CRCLM) before and after liver resection (LR).
PubMed, Embase, Cochrane, and Web of Science were systematically searched to retrieve literature, with a search cut-off date of February 27, 2023. Articles were strictly screened for inclusion according to pre-specified inclusion and exclusion criteria. Data were pooled and analyzed using Stata 16.0.
This meta-analysis included 36 studies involving a total of 11143 CRCLM patients. The results showed that a high pre-LR serum CEA level was correlated with poor overall survival (OS) [hazard ratio (HR) = 1.61, 95% confidence interval (CI): 1.49-1.75, P < 0.001] and recurrence-free survival (HR = 1.27, 95%CI: 1.11-1.45, P < 0.001) in CRCLM patients. A high post-LR serum CEA level predicted poor OS (HR = 2.66, 95%CI: 2.10-3.38, P < 0.001). A comparison by treatment modality, analysis modality, patient source, and cutoff-value showed that overall, high preoperative and postoperative serum CEA levels remained correlated with a poor prognosis.
This study concluded that high pre-LR and post-LR serum CEA levels were significantly correlated with a poor prognosis in CRCLM patients.
Core Tip: Carcinoembryonic antigen (CEA) is a broad-spectrum tumor marker for differential diagnosis, monitoring, and response assessment of a variety of malignancies. This meta-analysis was aimed at evaluating whether serum CEA could predict the prognosis in patients with colorectal cancer liver metastasis before and after liver resection. Articles were strictly screened for inclusion according to pre-specified inclusion and exclusion criteria.
- Citation: Tang F, Huang CW, Tang ZH, Lu SL, Bai T, Huang Q, Li XZ, Zhang B, Wu FX. Prognostic role of serum carcinoembryonic antigen in patients receiving liver resection for colorectal cancer liver metastasis: A meta-analysis. World J Gastrointest Surg 2023; 15(12): 2890-2906
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2890.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2890
Cancer is a major public health problem worldwide, and colorectal cancer (CRC), one of the malignancies of the digestive system[1]. Globally, there are more than 1.85 million new cases of CRC and 850000 deaths each year[2]. Recurrence and metastasis are inherent characteristics of cancer. Metastasis and infiltration can cause changes in the affected organs, thereby resulting in increased difficulty in treatment and a poor prognosis[3,4]. The liver is the major target organ of hematogenous metastasis in patients with CRC in the intermediate to advanced stages, and hematogenous liver metastasis is the leading cause of a poor prognosis or even death[5,6]. The main treatments for CRC liver metastasis (CRCLM) are surgical resection, local therapy, and chemotherapy, in which liver resection (LR) is considered the preferred curative treatment for CRCLM[7,8]. Compared with many other abdominal surgeries, LR is a complex procedure with inherent risks, such as long operative time, increased risk for bleeding, pulmonary complications, post-hepatectomy liver failure and kidney failure[9]. The incidence and mortality at 30 d after LR were reported to be 14%-55% and 0%-11.9%, respectively, with a 5-year survival rate of only about 30%-50% and a recurrence rate of up to 60%[10-12], so it will be valuable to find appropriate prognostic markers to predict the outcome in CRCLM patients after LR.
The main prognostic markers of CRC that are widely used currently in clinical practice are carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA). The serum CA19-9 level is often affected by a variety of neoplastic disorders of the gastrointestinal tract and benign biliary diseases (such as primary sclerosing cholangitis or bile duct obstruction due to common bile duct stones), which means that CA19-9 may not be an ideal marker for CRC patients[13]. CEA, also known as CD66e, is a glycoprotein consisting of about 100 amino acid residues, produced and secreted by gastrointestinal epithelial cells, and it is one of the most widely used tumor markers worldwide[14]. It had been reported that the initial level of CEA was closely related to the prognosis of CRC patients after LR[15]. It was shown that the serum CEA level was closely related to the proliferation, growth and degree of infiltration of tumor cells, and of certain value in the diagnosis of CRCLM[16,17], so it can be used as an indicator for early diagnosis and prognosis of CRCLM[18,19]. The CEA level has been a long-established tumor marker, recommended by the American Society of Clinical Oncology as a marker of CRC[20], and included in the tumor-node-metastasis system (so-called stage C) to provide additional prognostic information[21]. Most clinical guidelines recommend measuring preoperative and postoperative serum CEA levels to predict the prognosis of CRC[22-25]. It was also shown that postoperative CEA was an important prognostic factor for CRC[26-28]. In addition, it was confirmed that preoperative and postoperative serum CEA levels were correlated with the outcome in CRC patients, and an increased postoperative CEA level was possibly of more significant prognostic value than an increased preoperative CEA level[29].
Therefore, this paper explored whether serum CEA in CRCLM patients receiving LR played a significant predictive and prognostic role before and after surgery by summarizing currently available research data, so as to provide a scientific basis for further improving the prognosis in CRCLM patients.
PubMed, Embase, Cochrane and Web of Science were searched with a time frame until February 27, 2023. The keywords mainly included “Carcinoembryonic Antigen”, “Colorectal Neoplasms” and “liver metastasis”, and search was based on a combination of subject headings and free-text words. The search strategy is detailed in Supplementary Table 1.
Studies meeting all of the following criteria were included: (1) Study subjects: Adult patients definitively diagnosed with CRC with liver metastasis according to pathological histology who have received LR; (2) Exposure: Clearly reported pre-LR and post-LR serum CEA levels; (3) Reported the effect of serum CEA levels on at least one of the following outcome measures: Overall survival (OS) (time from the start of randomization grouping to death due to any cause), disease-free survival (DFS) (time from the start of randomization until disease recurrence or death of the patient due to disease progression), and recurrence-free survival (RFS) (time from randomization grouping to evidence of disease recurrence); and (4) Study type: Cohort studies.
Studies meeting any of the following criteria were excluded: (1) Duplicate studies using the same populations or overlapping databases; (2) Meta-analyses, systematic reviews, reviews, letters, responses, conference abstracts, case reports, guidelines, consensuses; and (3) Animal or in vitro experiments.
The retrieved studies were imported into Endnote X9, and then, duplicate references were excluded automatically by the software and manually. Subsequently, initial screening was performed by reading the titles and abstracts, next, the full texts of studies that passed initial screening were downloaded and then read for re-screening to select the original studies that finally met the inclusion criteria for a meta-analysis. The literature screening process was carried out independently by two investigators (Tang F and Huang CW), and then, the studies included by them were cross-checked. Any disputes were resolved with the assistance of a third investigator (Wu FX).
After literature screening was completed, an Excel data extraction form specific to this study was developed to summarize the information on the included articles as follows: (1) General information: The first author, year of publication, country, study type, age and gender in a study group; and (2) Study characteristics: Interventions, exposure levels, the analysis modality, risk ratios for outcome measures with 95% confidence intervals (95%CIs).
For studies with incomplete data, attempts were made to contact the corresponding authors of the studies. Two appraisers (Tang ZH and Lu SL) independently extracted information from the eligible studies, and any disagreements between them were resolved through a third person (Bai T).
Two reviewers (Li XZ and Zhang B) independently assessed the methodological quality of each included cohort study using eight items from three modules of the Newcastle-Ottawa Scale (NOS)[30]. An assessment consisted of three main parts: selection of study populations (0-4), comparability of groups (0-2), and outcome measures (0-3). Studies with total scores ≥ 6 were considered of high quality. Any disagreements that arose were resolved through discussion or, if necessary, arbitration by a third person (Huang Q).
A meta-analysis was performed using Stata16.0. Hazard ratios (HRs) and 95%CIs for serum CEA levels as a prognostic indicator were extracted directly from the included articles, or univariate data were estimated for some articles using Engauge 11.3 and Excel tables for calculating HRs and 95%CIs according to the methods illustrated by Parmar et al[31] and Tierney et al[32], if multiple estimates were reported in the same article, the results of multivariate analysis adjusted for confounding factors would be selected. Heterogeneity among the included studies was assessed using I2[33]. A fixed-effects model was used if I2 < 50% (low heterogeneity), and a random-effects model was used if I2 ≥ 50% (high heterogeneity). To investigate the sources of heterogeneity, subgroup analysis was performed by patient source, treatment modality, analysis modality, and cut-off value > 5 ng/mL or not, and further sensitivity analysis was performed to investigate the stability of the study results. Sensitivity analysis was aimed at assessing the effect of individual studies on the overall outcome by excluding 1 study at a time. Publication bias was assessed by Begg’s and Egger’s tests. If bias existed, correction would be performed using the trim-and-fill method. All P values were two-sided, and P < 0.05 was set to indicate a statistically significant difference.
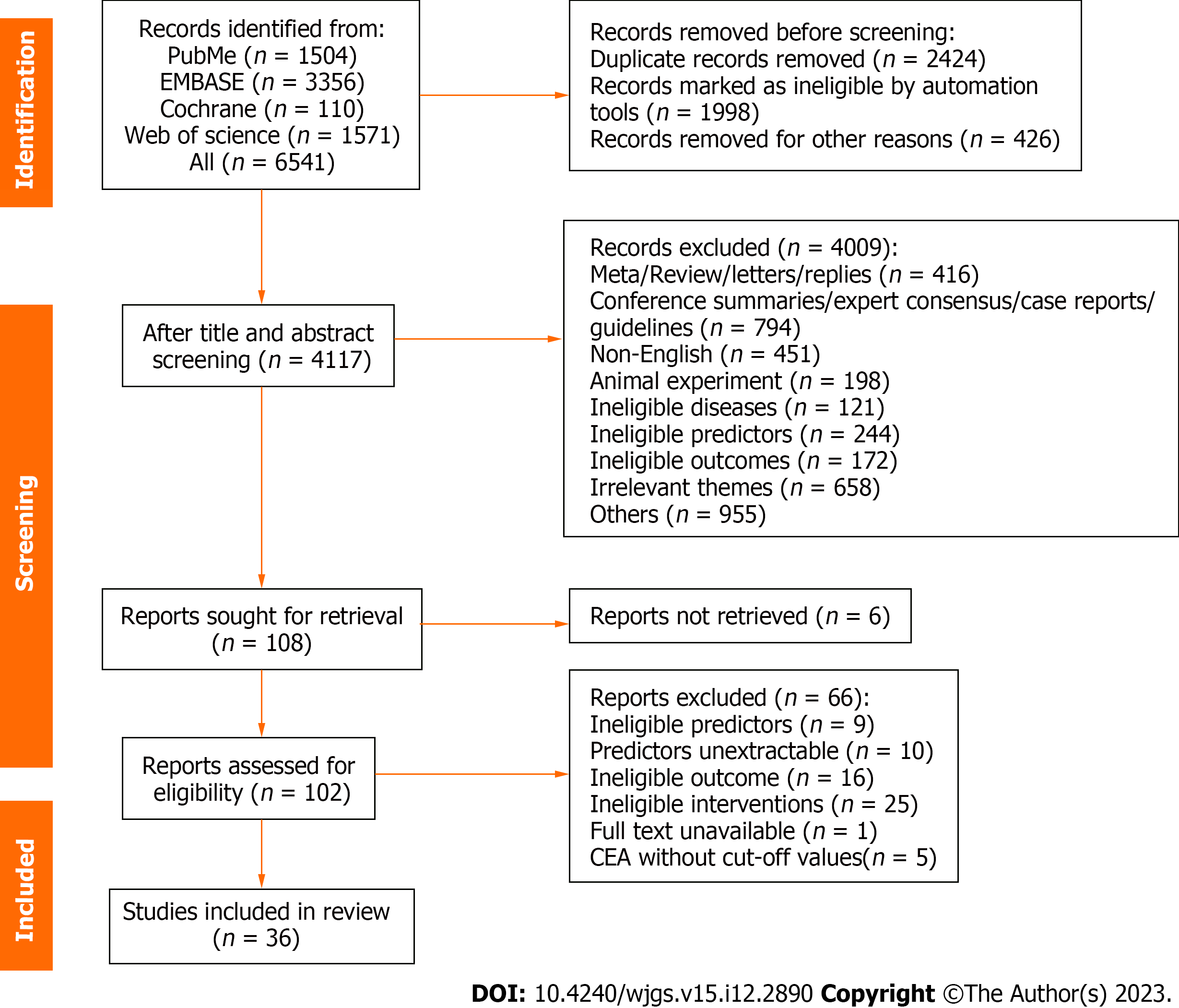
A total of 6541 studies were collected by searching the four databases. A total of 2424 duplicates were excluded automatically and manually, and 4009 of the remaining 4117 studies were excluded after reading the titles and abstracts. The full texts of the remaining 108 articles were read and re-screened. Finally, 36 articles met all inclusion criteria and were included. The specific reasons for exclusion and the literature search process are shown in Figure 1.
Table 1 summarizes the general characteristics of the included studies. A total of 36 studies were included, involving 11143 subjects, composed of 6769 men and 4374 women. Of these studies, 19 were from Asia[34-52], 11 from Europe[53-63], 4 from North America[64-67], and one study each from Oceania[68] and South America[69]. The years of publication ranged from 1994 to 2023. A total of 31 studies discussed pre-LR CEA levels, and 9 studies investigated the effect of post-LR CEA levels on prognosis in CRCLM patients. The cut-off values for CEA ranged from 4.9 ng/mL to 200 ng/mL. The results of quality assessment based on the NOS are detailed in Supplementary Table 2, with all studies scored more than 6 and being of high quality.
| No. | Ref. | Country | Study type | Treatment | Univariate/multivariate analysis | Cut-off (ng/mL) | Sample size | Age | Male/female | Outcomes | Nos |
| 1 | Meng et al[34], 2021 | China | Cohort study | Hepatectomy + chemotherapy | Multivariate | 100 | 234 | Range (n): < 65, 164; ≥ 65, 70 | 126/108 | OS | 9 |
| 2 | Okimoto et al[35], 2017 | Japan | Cohort study | Hepatectomy + chemotherapy | Multivariate | 10 | 134 | Median (range): 63 (30-87) | 90/44 | OS | 8 |
| 3 | Kamphues et al[53], 2021 | Germany | Cohort Study | Hepatectomy | Multivariate | 6.15 | 1643 | Median (range): 62 (18-90) | 1018/625 | OS | 7 |
| 4 | Kawahara et al[36], 2018 | Japan | Cohort study | Hepatectomy | Univariate | 5/50 | 66 | Median (range): 65.2 (31-80) | 45/21 | RFS | 7 |
| 5 | Hof et al[54], 2016 | The Netherlands | Cohort study | Hepatectomy | Multivariate | 200 | 431 | mean ± SD: 62.9 ± 9.4 | 264/167 | OS | 8 |
| 6 | Chiang et al[37], 2019 | China | Cohort study | Hepatectomy + chemotherapy | Multivariate | 5 | 490 | Median (range): 60.3 (28.8-88.0) | 332/158 | RFS, OS | 8 |
| 7 | Peltonen et al[55], 2018 | Finland | Cohort study | Hepatectomy + chemotherapy | Univariate | 5 | 168 | Median (range): 64.3 (36.3-81.5) | 101/67 | OS, DFS | 8 |
| 8 | Lu et al[38], 2016 | China | Cohort study | Hepatectomy + chemotherapy | Univariate | 5 | 141 | Median (range): 60 (20-82) | 92/49 | OS | 7 |
| 9 | John et al[56], 2013 | United Kingdom | Cohort study | Hepatectomy + chemotherapy | Univariate | 200 | 432 | Median (range): 64.5 (29-85) | 289/143 | OS | 7 |
| 10 | Yi et al[39], 2013 | Korea | Cohort study | Hepatectomy + chemotherapy | Multivariate | 5 | 76 | Median (range): 57(31-75) | 47/29 | DFS | 8 |
| 11 | Gervaz et al[57], 2000 | Switzerland | Cohort study | Hepatectomy | Univariate | 4 | 49 | ≥ 18 | 35/14 | OS | 7 |
| 12 | Sasaki et al[40], 2005 | Japan | Cohort study | Hepatectomy | Multivariate | 10 | 103 | Range (n): ≤ 32, 164; > 60, 71 | 56/47 | OS | 9 |
| 13 | Wang et al[41], 2017 | China | Cohort study | Hepatectomy + chemotherapy | Multivariate | 5 | 159 | Median (range): Non-targeted therapy group 52 (35-83); bevacizumab combined treatment group 43 (32-71); cetuximab combined treatment group 59 (28-76) | 101/58 | RFS, OS | 8 |
| 14 | Takamizawa et al[42], 2022 | Japan | Cohort study | Hepatectomy | Multivariate | 5 | 554 | Median (range): 62 (21-88) | 358/196 | RFS, OS | 9 |
| 15 | Masuda et al[64], 2018 | United States | Cohort study | Hepatectomy + RFA | Multivariate | 30 | 116 | mean ± SD: tumors ≥ 4, 58.7 ± 10.6; tumors < 4, 57.6 ± 12.0 | 74/42 | OS | 7 |
| 16 | Zhang et al[43], 2017 | China | Cohort study | Hepatectomy | Multivariate | 100 | 102 | Range (n): < 60, 66; ≥ 60, 36 | 63/39 | OS | 9 |
| 17 | Ishii et al[44], 2022 | Japan | Cohort study | Hepatectomy | Univariate | 5 | 90 | Median (range): High CII 65 (31-87); low CII 65 (32-82) | 58/32 | RFS, OS | 7 |
| 18 | Tanaka et al[45], 2008 | Japan | Cohort study | Hepatectomy + chemotherapy | Multivariate | 12 | 79 | Range (n): ≥ 60, 46; < 60, 33 | 49/30 | OS | 9 |
| 19 | Sasaki et al[65], 2016 | United States | Cohort study | Hepatectomy + RFA | Multivariate | 30 | 485 | Median (IQR): 58.5 (49.1-66.5) | 290/195 | OS | 8 |
| 20 | Chen et al[46], 2020 | China | Cohort study | Hepatectomy + chemotherapy | Univariate | 10 | 141 | Median (IQR): 55 (49.0-62.0) | 92/49 | PFS, OS | 7 |
| 21 | Montalti et al[58], 2015 | Belgium | Cohort study | Hepatectomy + chemotherapy | Multivariate | 10 | 114 | mean ± SD: 66.4 ± 0.89 | 78/36 | OS, RFS | 6 |
| 22 | Reddy et al[67], 2009 | United States | Cohort study | Hepatectomy + chemotherapy | Multivariate | 200 | 499 | Median (range): 57(49-66) | 294/205 | OS | 6 |
| 23 | Reddy et al[66], 2009 | United States | Cohort study | Hepatectomy + chemotherapy | Multivariate | 10 | 230 | Median (range): 61(33-83) | 137/93 | OS | 7 |
| 24 | Li et al[47], 2023 | China | Cohort study | Hepatectomy + chemotherapy | Univariate | 200 | 431 | Median (IQR): 59 (49-68) | 282/149 | RFS, OS | 7 |
| 25 | Niu et al[68], 2007 | Australia | Cohort study | Hepatectomy | Multivariate | 5 | 315 | mean ± SD: 62 ± 11 | 239/176 | OS | 6 |
| 26 | Polivka et al[59], 2020 | The Czech Republic | Cohort study | Hepatectomy | Multivariate | 4.9 | 71 | Median (range): 62.7 (29-77) | 29/42 | DFS, OS | 6 |
| 27 | Takeda et al[48], 2022 | Japan | Cohort study | Hepatectomy + chemotherapy | Multivariate | 10 | 238 | Range (n): < 60, 123; ≥ 60, 115 | 184/54 | OS | 8 |
| 28 | Dumarco et al[69], 2023 | Brazil | Cohort study | Hepatectomy | Multivariate | 20 | 137 | Median (range): 58.2 (23-87) | 75/62 | OS, DFS | 8 |
| 29 | Kim et al[49], 2019 | Korea | Cohort study | Hepatectomy + chemotherapy | Multivariate | 100 | 83 | mean ± SD: 59.5 ± 10.0 | 62/21 | OS | 8 |
| 30 | Peng et al[50], 2017 | China | Cohort study | Hepatectomy | Univariate | 200 | 150 | Median (range): 58 (20-82) | 97/53 | RFS, OS | 7 |
| 31 | Imai et al[60], 2016 | France | Cohort study | Hepatectomy | Multivariate | 50 | 846 | Median (range): 61 (28-89) | 502/344 | OS | 8 |
| 32 | Pawlik et al[61], 2005 | Switzerland | Cohort study | Hepatectomy | Multivariate | 200 | 566 | Median 60 | 221/345 | OS | 6 |
| 33 | Miki et al[51], 2018 | Japan | Cohort study | Hepatectomy | Multivariate | 5 | 73 | Median (range): 59 (27-82) | 49/24 | DFS | 8 |
| 34 | Hohenberger et al[62], 1994 | Germany | Cohort study | Hepatectomy | Univariate | 5 | 166 | Median (range): 59 (30-79) | 99/67 | OS | 7 |
| 35 | Arru et al[65], 2008 | Italy | Cohort study | Hepatectomy | Multivariate | 5/200 | 297 | Range (n): < 65, 177; ≥ 65, 120 | 171/126 | OS | 8 |
| 36 | Yoshino et al[52], 2022 | Japan | Cohort study | Hepatectomy | Multivariate | 5 | 633 | Median (range): 64.0 (27.0-92.0) | 414/219 | RFS, OS | 8 |
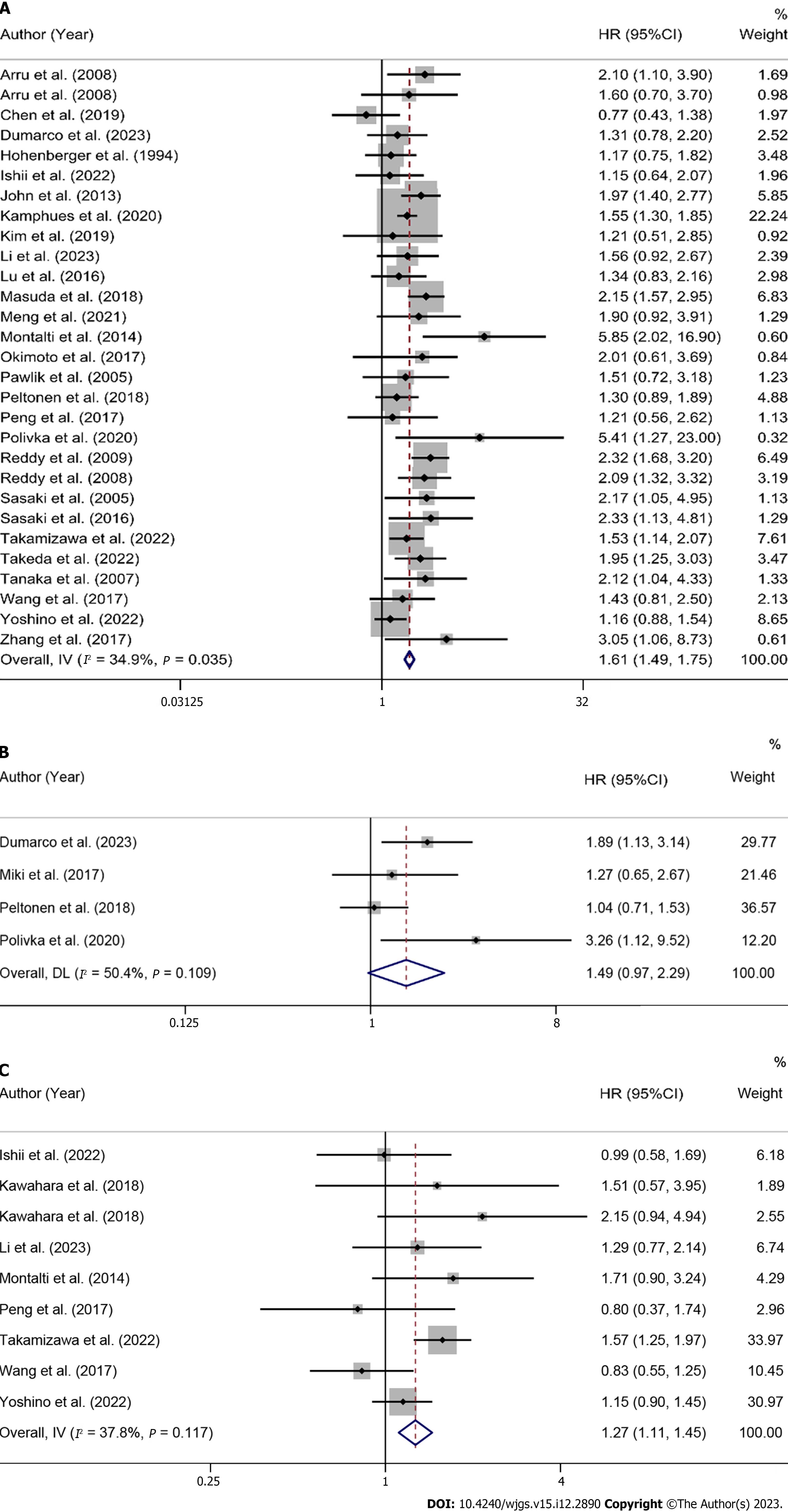
Overall and subgroup analysis of OS: A total of 28 studies reported pre-LR serum CEA levels to predict OS in patients[34,35,38,40-50,53,55,56,58,59,61-67,69]. The heterogeneity test results showed I2 = 34.9%, so a fixed-effects model was used for analysis. The results showed that high CEA levels before treatment were correlated with poor OS, and the differences were statistically significant (HR = 1.61, 95%CI: 1.49-1.75, P < 0.001) in Figure 2A and Table 2.
| Outcome | Number of studies | Model | Hazard ratio (95%CI) | P value | Heterogeneity (P value, I2) |
| Pre-LR | |||||
| OS | 28 | Fixed | 1.61 (1.49-1.75) | < 0.001 | 0.035, 34.9% |
| Region | |||||
| Europe | 8 | Fixed | 1.60 (1.41-1.82) | < 0.001 | 0.086, 42.3% |
| Asia | 15 | Fixed | 1.43 (1.25-1.63) | < 0.001 | 0.377, 6.8% |
| South America | 1 | Fixed | 1.31 (0.78-2.20) | 0.307 | / |
| North America | 4 | Fixed | 2.21 (1.82-2.69) | < 0.001 | 0.980, 0.0% |
| Treatment modality | |||||
| LR alone | 12 | Fixed | 1.46 (1.31-1.64) | < 0.001 | 0.405, 4.1% |
| LR + chemotherapy | 14 | Fixed | 1.74 (1.52-1.98) | < 0.001 | 0.053, 41.4% |
| LR + radiofrequency ablation | 2 | Fixed | 2.18 (1.63-2.91) | < 0.001 | 0.844, 0.0% |
| Analysis modality | |||||
| Multivariate | 20 | Fixed | 1.70 (1.55-1.87) | < 0.001 | 0.099, 29.8% |
| Univariate | 6 | Fixed | 1.23 (1.00-1.52) | 0.053 | 0.618, 0.0% |
| Survival curve | 2 | Fixed | 1.62 (1.24-2.13) | < 0.001 | 0.067, 70.2% |
| Cut-off | |||||
| ≤ 5 ng/mL | 9 | Fixed | 0.32 (0.21-0.43) | < 0.001 | 0.570, 0.0% |
| 5-50 ng/mL | 11 | Fixed | 0.54 (0.42-0.66) | < 0.001 | 0.034, 48.7% |
| 100 ng/mL | 3 | Fixed | 0.60 (0.11-1.09) | 0.017 | 0.407, 0.0% |
| 200 ng/mL | 6 | Fixed | 0.65 (0.45-0.84) | < 0.001 | 0.565, 0.0% |
| DFS | 4 | Random | 1.49 (0.97-2.29) | 0.067 | 0.109, 50.4% |
| Region | |||||
| South America | 1 | Random | 1.89 (1.13-3.15) | 0.015 | / |
| Asia | 1 | Random | 1.27 (0.63-2.57) | 0.507 | / |
| Europe | 2 | Random | 1.65 (0.55-4.93) | 0.373 | 0.049, 74.2% |
| Treatment modality | |||||
| LR alone | 3 | Random | 1.81 (1.20-2.72) | 0.005 | 0.340, 7.4% |
| LR + chemotherapy | 1 | Random | 1.04 (0.71-1.53) | 0.833 | / |
| Analysis modality | |||||
| Multivariate | 3 | Random | 1.81 (1.20-2.72) | 0.005 | 0.340, 7.4% |
| Univariate | 1 | Random | 1.04 (0.71-1.53) | 0.833 | / |
| Cut-off | |||||
| > 5 ng/mL | 1 | Random | 1.89 (1.13-3.15) | 0.015 | / |
| ≤ 5 ng/mL | 3 | Random | 1.37 (0.80-2.35) | 0.255 | 0.142, 48.7% |
| RFS | 8 | Fixed | 1.27 (1.11-1.45) | < 0.001 | 0.117, 37.8% |
| Region | |||||
| Asia | 7 | Fixed | 1.25 (1.09-1.43) | 0.001 | 0.101, 41.6% |
| Europe | 1 | Fixed | 1.71 (0.90-3.24) | 0.101 | / |
| Treatment modality | |||||
| LR alone | 5 | Fixed | 1.32 (1.14-1.53) | < 0.001 | 0.176, 34.8% |
| LR + chemotherapy | 3 | Fixed | 1.10 (0.83-1.47) | 0.509 | 0.135, 50.1% |
| Analysis modality | |||||
| Univariate | 3 | Fixed | 1.07 (0.76-1.49) | 0.709 | 0.569, 0.0% |
| Survival curve | 1 | Fixed | 1.85 (0.99-3.47) | 0.055 | / |
| Multivariate | 4 | Fixed | 1.29 (1.11-1.49) | 0.001 | 0.030, 66.5% |
| Cut-off | |||||
| ≤ 5 ng/mL | 5 | Fixed | 0.22 (0.08-0.37) | 0.003 | 0.060, 55.7% |
| 5-50 ng/mL | 2 | Fixed | 0.62 (0.12-1.13) | 0.016 | 0.669, 0.0% |
| 200 ng/mL | 2 | Fixed | 0.11 (-0.32 to 0.54) | 0.615 | 0.313, 1.9% |
| Post-LR | |||||
| OS | 8 | Random | 2.66 (2.10-3.38) | < 0.001 | 0.002, 68.7% |
| Region | |||||
| Asia | 2 | Random | 2.63 (1.87-3.70) | < 0.001 | 0.040, 76.2% |
| Europe | 5 | Random | 3.04 (2.10-4.40) | < 0.001 | 0.022, 65.1% |
| Oceania | 1 | Random | 1.63 (1.09-2.42) | 0.016 | / |
| Treatment modality | |||||
| LR + chemotherapy | 2 | Random | 2.75 (1.70-4.44) | < 0.001 | 0.046, 74.8% |
| LR alone | 6 | Random | 2.66 (1.91-3.69) | < 0.001 | 0.003, 71.9% |
| Analysis modality | |||||
| Multivariate | 5 | Random | 2.23 (1.79-2.78) | < 0.001 | 0.061, 55.7% |
| Survival curve | 2 | Random | 4.40 (2.58-7.51) | < 0.001 | 0.215, 35.0% |
| Univariate | 1 | Random | 3.68 (2.35-5.79) | < 0.001 | / |
| Cut-off | |||||
| ≤ 5 ng/mL | 6 | Random | 1.07 (0.78-1.37) | < 0.001 | 0.001, 74.9% |
| 5-50 ng/mL | 1 | Random | 0.74 (0.36-1.13) | < 0.001 | / |
| 200 ng/mL | 1 | Random | 0.64 (0.10-1.17) | 0.019 | / |
| DFS | 2 | Fixed | 3.23 (2.20-4.75) | < 0.001 | 0.182, 43.8% |
| RFS | 2 | Fixed | 2.38 (2.05-2.77) | < 0.001 | 0.160, 49.3% |
Subgroup analysis was performed by patient source, treatment modality, analysis modality, and cut-off value of ≤ 5 ng/mL, 5-50 ng/mL, 100 ng/mL, 200 ng/mL. Subgroup analysis showed that high preoperative serum CEA levels remained a predictor of poor OS in CRCLM patients, regardless of whether they were from Asian (HR = 1.43, 95%CI: 1.25-1.63, P < 0.001), European (HR = 1.60, 95%CI: 1.41-1.82, P < 0.001), or North American (HR = 2.21, 95%CI: 1.82-2.69, P < 0.001) populations, as shown in Supplementary Figure 1 and Table 2.
High preoperative serum CEA levels remained a predictor of poor OS in CRCLM patients, regardless of whether they received LR alone (HR = 1.46, 95%CI: 1.31-1.64, P < 0.001), LR + chemotherapy (HR = 1.74, 95%CI: 1.52-1.98, P < 0.001), or LR + radiofrequency ablation (HR = 2.18, 95%CI: 1.63-2.91, P < 0.001), as shown in Supplementary Figure 2 and Table 2.
High preoperative serum CEA levels remained correlated with poor OS in CRCLM patients in both multivariate analysis (HR = 1.70, 95%CI: 1.55-1.87, P < 0.001) and survival curves (HR = 1.62, 95%CI: 1.24-2.13, P < 0.001), and the difference was statistically significant, whereas in univariate analysis, such correlation was not found (HR = 1.23, 95%CI: 1.00-1.52, P = 0.053), as shown in Supplementary Figure 3 and Table 2.
The results of subgroup analysis by cut-off values of ≤ 5 ng/mL (HR = 0.32, 95%CI: 0.21-0.43, P < 0.001), 5-50 ng/mL (HR = 0.54, 95%CI: 0.42-0.66, P < 0.001), 100 ng/mL (HR = 0.60, 95%CI: 0.11-1.09, P = 0.017), 200 ng/mL (HR = 0.65, 95%CI: 0.45-0.84, P < 0.001) also showed that high preoperative serum CEA levels were a predictor of poor OS in CRCLM patients, as shown in Supplementary Figure 4 and Table 2.
Overall and subgroup analysis of DFS: A total of 4 studies reported pre-LR serum CEA levels to predict DFS in patients[51,55,59,69]. The heterogeneity test results showed I2 = 50.4%, so a fixed-effects model was used for analysis. Overall results showed no statistically significant correlation between pre-LR serum CEA and DFS in patients (HR = 1.49, 95%CI: 0.97-2.29, P = 0.067) (Figure 2B and Table 2). To investigate the sources of heterogeneity, subgroup analysis was performed by patient source, treatment modality, analysis modality, and cut-off value > 5 ng/mL or not. The results showed that treatment modality, analysis modality, and cut-off value classification possibly caused heterogeneity. High preoperative serum CEA levels in patients receiving LR alone were potentially correlated with shorter DFS (HR = 1.81, 95%CI: 1.20-2.72, P = 0.005). Multivariate analysis also showed that high preoperative CEA levels were correlated with poorer DFS (HR = 1.81, 95%CI: 1.20-2.72, P = 0.005). The results of subgroup analysis of the European patient group (HR = 1.65, 95%CI: 0.55-4.93, P = 0.373) and the cut-off values ≤ 5 ng/mL (HR = 1.37, 95%CI: 0.80-2.35, P = 0.255) suggested no such prognostic significance, as detailed in Supplementary Figures 5-8 and Table 2.
Overall and subgroup analysis of RFS: A total of 8 studies reported pre-LR serum CEA levels to predict RFS in patients[36,41,42,44,47,50,52,58]. The heterogeneity test results showed I2 = 37.9%, so a fixed-effects model was used for analysis. The results showed that high preoperative serum CEA levels were correlated with poor RFS, and the difference was statistically significant (HR = 1.27, 95%CI: 1.11-1.45, P < 0.001), as shown in Figure 2C and Table 2.
Subgroup analysis was performed by patient source, treatment modality, analysis modality, and cut-off value of ≤ 5 ng/mL, 5-50 ng/mL, 200 ng/mL. The results of subgroup analysis showed that in the Asian patient population (HR = 1.25, 95%CI: 1.09-1.43, P = 0.001), high preoperative serum CEA levels predicted shorter RFS in the subgroup of patients receiving LR only (HR = 1.32, 95%CI: 1.14-1.53, P < 0.001), the subgroup of multivariate analysis (HR = 1.29, 95%CI: 1.11-1.49, P = 0.001), and the subgroup of cut-off values of ≤ 5 ng/mL (HR = 0.22, 95%CI: 0.08-0.37, P = 0.003) and 5-50 ng/mL (HR = 0.62, 95%CI: 0.12-1.13, P = 0.016). In contrast, such predictive ability was not significant in the subgroup receiving LR + chemotherapy (HR = 1.10, 95%CI: 0.83-1.47, P = 0.509), the subgroup of in univariate analysis (HR = 1.07. 95%CI: 0.76-1.49, P = 0.709), and the subgroup of cut-off values of 200 ng/mL (HR = 0.11, 95%CI: -0.32 to 0.54, P = 0.615), as shown in Supplementary Figures 9-12 and Table 2.
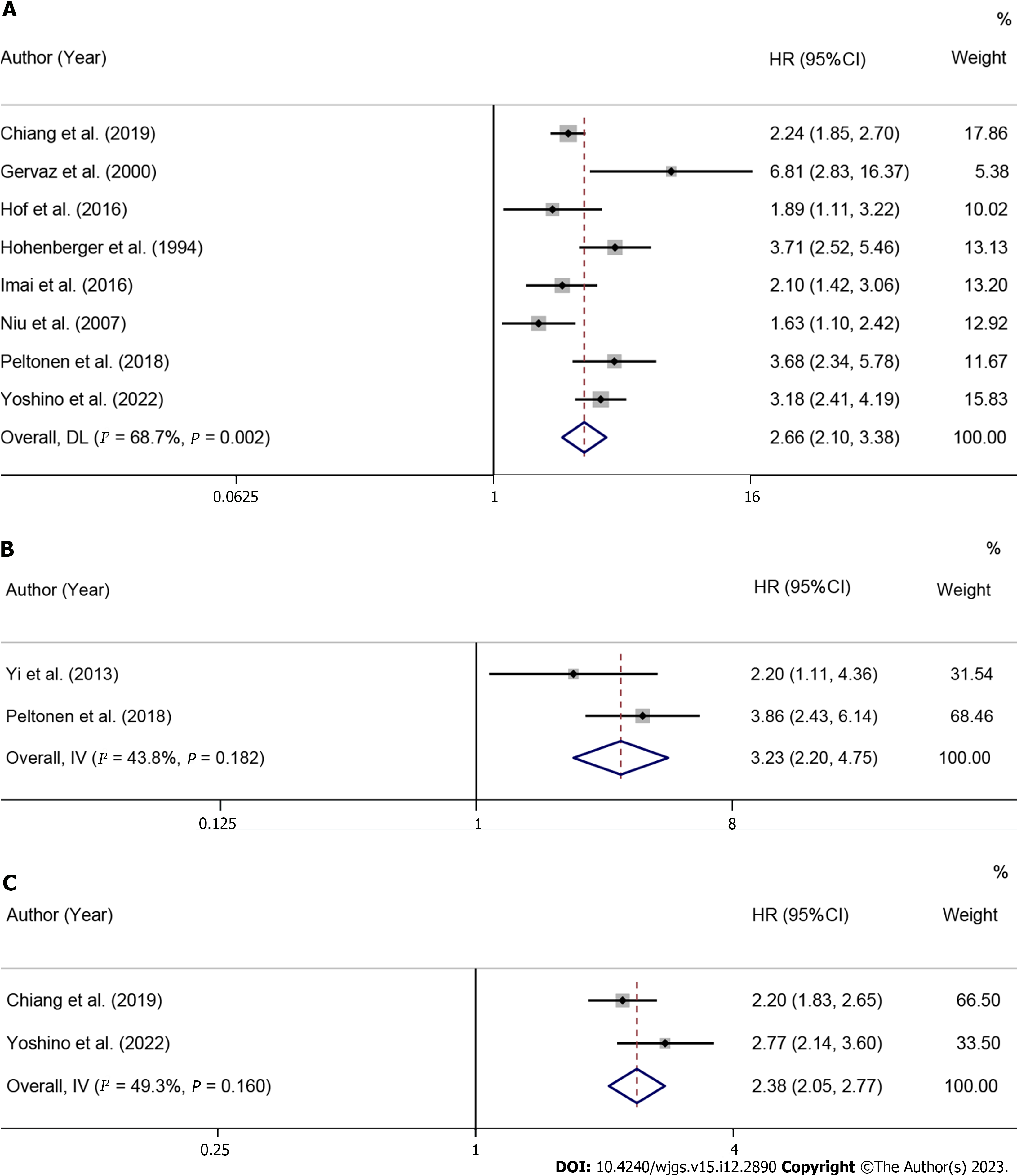
Overall and subgroup analysis of OS: A total of 8 studies reported post-LR serum CEA levels to predict OS in patients[37,52,54,55,57,60,62,68]. The heterogeneity test results showed I2 = 68.7%, so a fixed-effects model was used for analysis. The results showed that high post-LR CEA levels were correlated with poor OS, and the difference was statistically significant (HR = 2.66, 95%CI: 2.10-3.38, P < 0.001), as shown in Figure 3A and Table 2. To investigate the sources of heterogeneity, subgroup analysis was performed by patient source, treatment modality, analysis modality, and cut-off values of ≤ 5 ng/mL, 5-50 ng/mL, 200 ng/mL. The results showed that high postoperative serum CEA levels were a predictor of poor OS in CRCLM patients from Asia (HR = 2.63, 95%CI: 1.87-3.70, P < 0.001) and Europe (HR = 3.04, 95%CI: 2.10-4.40, P < 0.001), as shown in Supplementary Figure 13 and Table 2.
High postoperative serum CEA levels were a predictor of poor OS in CRCLM patients receiving LR alone (HR = 2.66, 95%CI: 1.91-3.69, P < 0.001) and those receiving LR + chemotherapy (HR = 2.75, 95%CI: 1.70-4.44, P < 0.001), as shown in Supplementary Figure 14 and Table 2. Heterogeneity (I2) test results showed that the analysis modality might partially cause heterogeneity (I2 = 55.7% in multivariate analysis; I2 = 35.0% in the survival curve). Subgroup analysis showed that high postoperative serum CEA levels were correlated with poor OS in CRCLM patients, with statistically significant differences in both multivariate analysis (HR = 2.23, 95%CI: 1.79-2.78, P < 0.001) and survival curves (HR = 4.40, 95%CI: 2.58-7.51, P < 0.001), as shown in Supplementary Figure 15 and Table 2.
The results of subgroup analysis showed that high postoperative serum CEA levels were a predictor of poor OS in CRCLM patients regardless of cut-off values ≤ 5 ng/mL (HR = 1.07, 95%CI: 0.78-1.37, P < 0.001), 5-50 ng/mL (HR = 0.74, 95%CI: 0.36-1.13, P < 0.001) or 200 ng/mL (HR = 0.64, 95%CI: 0.10-1.17, P = 0.019), as shown in Supplementary Figure 16 and Table 2.
DFS: A total of 2 studies reported post-LR CEA levels to predict DFS in patients[39,55]. The heterogeneity test results showed I2 = 43.8%, so a fixed-effects model was used for analysis. The results showed that high postoperative CEA levels were correlated with poor DFS, and the difference was statistically significant (HR = 3.23, 95%CI: 2.20-4.75, P < 0.001), as shown in Figure 3B and Table 2.
RFS: A total of 2 studies reported post-LR CEA levels to predict RFS in patients[37,52]. The heterogeneity test results showed I2 = 49.3%, so a fixed-effects model was used for analysis. The results showed that high postoperative CEA levels were correlated with poor RFS, and the difference was statistically significant (HR = 2.38, 95%CI: 2.05-2.77, P < 0.001), as shown in Figure 3C and Table 2.
Sensitivity analysis was performed by excluding each article one by one to investigate the stability of the merged HRs of preoperative OS, DFS, RFS, and postoperative OS. The results suggested that the results were stable with low sensitivity, as shown in Supplementary Figures 17-20.
Publication bias was assessed by Begg’s and Egger’s tests. Begg’s test results showed P (preoperative OS) = 0.209, P (preoperative RFS) = 0.754, and P (postoperative OS) = 0.536, all more than 0.05, as shown in Supplemen
Previous meta-analyses confirmed that increased preoperative CEA was correlated with the occurrence, progression, and prognosis of multiple cancers[70-72]. Although more original studies have reported the effect of CEA levels on prognosis in CRCLM patients, currently there is still no meta-analysis of the correlation between serum CEA and prognosis of CRCLM. Therefore, this was the first meta-analysis to investigate the effect of pre- and post-LR serum CEA levels on prognosis in CRCLM patients. A retrospective analysis of clinical data and experimental results from 36 studies involving 11143 CRCLM patients found that high levels of preoperative or postoperative serum CEA in patients who had received LR were mostly indicative of a poor prognosis. Firstly, CRCLM patients with higher levels of pre- or post-LR serum CEA had poor OS and were not affected by patient source, treatment modality, or cut-off values. Secondly, high pre-LR serum CEA levels also predicted shorter RFS (P < 0.001), which was also confirmed by the results of subgroups of the Asian population, patients receiving LR alone, multivariate analysis, the ≤ 5 ng/mL, and the 5-50 ng/mL. Because of high heterogeneity of the summary results of the meta-analysis in the DFS group, subgroup analysis was performed, and the results showed that high pre-LR serum CEA levels were significantly correlated with poor DFS in the group receiving LR alone (P < 0.05) and the group included in multivariate analysis (P < 0.05). Finally, regarding postoperative serum CEA predicting DFS and RFS, there were only two studies on each of them, so the evidence obtained so far in this study can only suggest that there may be potential for postoperative serum CEA to predict DFS and RFS in CRCLM patients, which needs to be confirmed by more future studies. CEA is a highly glycosylated cell surface protein that is an immunoglobulin superfamily cell adhesion molecule. Different glycosylation patterns result in different molecular weights in normal and cancer cells. CEA is attached to the cell membrane surface via a glycosylphosphatidylinositol (GPI) anchor, which can be cleaved by GPI-specific phospholipase D to release CEA from the membrane to cause it to be shed[73]. CEA can inhibit the death of circulating tumor cells: On the one hand, it protects circulating colon cancer cells from death in the blood or prevents circulating cell death as a general inhibitor of anoikis[74,75]; on the other hand, it binds to the heterogeneous nuclear RNA binding protein M4, a receptor protein in macrophages (Kupffer cells) that protect the liver, and activates Kupffer cells to secrete various cytokines that alter the liver microenvironment to facilitate cancer cell survival[76]. Subsequently, CEA upregulates cell adhesion molecules for metastasis, such as promoting migration of CRC cells, especially to the liver, which can be measured in the serum of cancer patients[77]. Therefore, increased CEA may mean that cancer cells are occurring or developing. Serum CEA levels are therefore measured to predict the occurrence, development, and prognosis of cancer.
In this study, the effect of preoperative and postoperative serum CEA levels on prognosis in CRCLM patients receiving LR was evaluated, and subgroup analysis was performed in terms of several potential influencing factors. Regarding preoperative results, it was found that the analysis modality (univariate/multivariate/survival curve) was the only factor contributing to a difference in OS results in this study, which might be due to the fact that univariate analysis did not exclude the effect of confounding factors that caused the difference in the results. For DFS, only 4 studies were included, and the small sample size was possibly the most influential factor on heterogeneity and results; in addition, the analysis modality, treatment modality and cut-off values might partially contribute to high heterogeneity and had potential effects on the results. For RFS, CRCLM itself is a highly malignant disease prone to recur, so there might be more factors influencing RFS, and at least the number of studies, treatment modality, analysis modality and cut-off values were found to potentially affect both outcomes and heterogeneity. Regarding postoperative results, the OS group had high heterogeneity, which was possibly due to the analysis modality. The preoperative and postoperative predictive outcomes remained basically consistent except for DFS, which was possibly due to the inclusion of too few studies. Finally, the use of neoadjuvant or adjuvant chemotherapy drugs before or after LR may alter CEA levels, thereby affecting the prognosis of patients with CRCLM. Relevant studies have shown that neoadjuvant chemotherapy can reduce CEA levels by eliminating potential tumor micrometastases; additionally, CEA levels can temporarily increase during adjuvant chemotherapy, possibly because cancer cells are effectively killed, leading to the release of CEA into the blood[78,79].
Since its discovery, CEA has been gradually shown to be overexpressed in most human cancers[80]. It was initially considered a tumor marker specific for colon/rectal cancer, and later increased CEA was also found in lung/breast/thyroid cancers and other cancers. Currently, CEA is often used as a serum tumor marker for the diagnosis of CRC or CRCLM[81]. The prognostic value of CEA in CRCLM patients has been confirmed in a variety of treatment modalities besides LR. Peng et al[82] reported that CEA levels might be a valuable prognostic factor for early recurrence in CRCLM patients after microwave ablation. Weiner et al[83] found that lower CEA levels were independently correlated with a higher survival rate in CRCLM patients treated with yttrium-90 radioembolization. Recent studies have found that CEA could predict preoperative lymph node metastasis in patients with thyroid cancer, preoperative progression-free survival and OS in patients with lung cancer, and postoperative OS in patients with esophageal cancer[84-86]. Currently prediction of outcome in CRCLM patients after LR remains a major challenge, so identification of preoperative and postoperative levels of the marker of poor prognosis may improve prognosis in CRCLM patients to some extent and facilitate better management and treatment.
However, potential limitations of this study have to be considered. Firstly, CEA was reported to be correlated with clinicopathological parameters[87], and some of the univariate studies we included did not eliminate the effect of confounding factors, which may lead to overestimated effect sizes. Secondly, the cut-off values of serum CEA in the included studies ranged from 4-200 ng/mL, which may be due to different approaches to measurement but may have a certain impact on the effect value. Therefore, further research is needed to standardize the cut-off value. Thirdly, it is a pity that there were few original studies on postoperative serum CEA to predict patient prognosis. Despite the limitations, our study has certain implications for clinical practice.
High pre-LR and post-LR serum CEA levels were significantly correlated with a poor prognosis in CRCLM patients, especially poor OS.
Carcinoembryonic antigen (CEA), also known as CD66e, is a glycoprotein consisting of about 100 amino acid residues, produced and secreted by gastrointestinal epithelial cells, and it is one of the most widely used tumor markers worldwide. It had been reported that the initial level of CEA was closely related to the prognosis of colorectal cancer (CRC) patients after liver resection (LR).
This paper explored whether serum CEA in CRC liver metastasis (CRCLM) patients receiving LR played a significant predictive and prognostic role before and after surgery by summarizing currently available research data, so as to provide a scientific basis for further improving the prognosis in CRCLM patients.
This object of this paper is to explore whether serum CEA in CRCLM patients receiving LR played a significant predictive and prognostic role before and after surgery by summarizing currently available research data, so as to provide a scientific basis for further improving the prognosis in CRCLM patients.
PubMed, Embase, Cochrane and Web of Science were searched with a time frame until February 27, 2023. The retrieved studies were imported into Endnote X9, and then, duplicate references were excluded automatically by the software and manually. After literature screening was completed, an Excel data extraction form specific to this study was developed to summarize the information on the included articles. The data were pooled and analyzed using Stata 16.0.
This study included 36 studies involving a total of 11143 CRCLM patients. The results showed that a high pre-LR serum CEA level was correlated with poor overall survival (OS) [hazard ratio (HR) = 1.61, 95% confidence interval (CI): 1.49-1.75, P < 0.001] and recurrence-free survival (RFS) (HR = 1.27, 95%CI: 1.11-1.45, P < 0.001) in CRCLM patients. A high post-LR serum CEA level predicted poor OS (HR = 2.66, 95%CI: 2.10-3.38, P < 0.001).
High serum CEA levels in CRCLM patients were significantly associated with poor OS before and after LR surgery.
Regarding postoperative serum CEA predicting disease-free survival (DFS) and RFS, there were only two studies on each of them, so the evidence obtained so far in this study can only suggest that there may be potential for postoperative serum CEA to predict DFS and RFS in CRCLM patients, which needs to be confirmed by more future studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H, Japan; Cerwenka H, Austria S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 384] [Article Influence: 128.0] [Reference Citation Analysis (6)] |
| 2. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1425] [Article Influence: 356.3] [Reference Citation Analysis (0)] |
| 3. | Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: Metastases to a single organ. World J Gastroenterol. 2015;21:11767-11776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 232] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (2)] |
| 4. | Eng C, Jácome AA, Agarwal R, Hayat MH, Byndloss MX, Holowatyj AN, Bailey C, Lieu CH. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol. 2022;23:e116-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 5. | Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Zhang L, Wang H, Chen L, Sui G. Diagnostic performance of contrast-enhanced ultrasound and magnetic resonance imaging for detecting colorectal liver metastases: A systematic review and meta-analysis. Dig Liver Dis. 2019;51:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1032] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 8. | Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 9. | Agarwal V, Divatia JV. Enhanced recovery after surgery in liver resection: current concepts and controversies. Korean J Anesthesiol. 2019;72:119-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Egger ME, Ohlendorf JM, Scoggins CR, McMasters KM, Martin RC 2nd. Assessment of the reporting of quality and outcome measures in hepatic resections: a call for 90-day reporting in all hepatectomy series. HPB (Oxford). 2015;17:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 12. | Assumpcao L, Choti MA, Gleisner AL, Schulick RD, Swartz M, Herman J, Gearhart SL, Pawlik TM. Patterns of recurrence following liver resection for colorectal metastases: effect of primary rectal tumor site. Arch Surg. 2008;143:743-9; discussion 749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Folseraas T, Boberg KM. Cancer Risk and Surveillance in Primary Sclerosing Cholangitis. Clin Liver Dis. 2016;20:79-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem. 2001;47:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 479] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Dexiang Z, Li R, Ye W, Haifu W, Yunshi Z, Qinghai Y, Shenyong Z, Bo X, Li L, Xiangou P, Haohao L, Lechi Y, Tianshu L, Jia F, Xinyu Q, Jianmin X. Outcome of patients with colorectal liver metastasis: analysis of 1,613 consecutive cases. Ann Surg Oncol. 2012;19:2860-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Yamashita S, Chun YS, Kopetz SE, Vauthey JN. Biomarkers in colorectal liver metastases. Br J Surg. 2018;105:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D; ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 798] [Article Influence: 159.6] [Reference Citation Analysis (0)] |
| 18. | Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1105] [Cited by in RCA: 1118] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 19. | D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1111] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 21. | Thirunavukarasu P, Sukumar S, Sathaiah M, Mahan M, Pragatheeshwar KD, Pingpank JF, Zeh H 3rd, Bartels CJ, Lee KK, Bartlett DL. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer Version 2 2020. [cited 15 May 2023]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428. |
| 23. | NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Rectal Cancer Version 3 2020. [cited 15 May 2023]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1461. |
| 24. | Diagnosis And Treatment Guidelines For Colorectal Cancer Working Group CSOCOC. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version). Chin J Cancer Res. 2019;31:117-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 25. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1194] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 26. | You W, Yan L, Cai Z, Xie L, Sheng N, Wang G, Wu X, Wang Z. Clinical Significances of Positive Postoperative Serum CEA and Post-preoperative CEA Increment in Stage II and III Colorectal Cancer: A Multicenter Retrospective Study. Front Oncol. 2020;10:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann Surg Oncol. 2009;16:3087-3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Fan J, Liu Y, Cai X, Wang J, Guo R, Ji Y, Li C, Xu Y, Li X, Zhang C, Zhang R, Zhu J, Cai S. A Novel Prognostic Model Incorporating Carcinoembryonic Antigen in 3-Week or Longer Postoperative Period for Stage III Colon Cancer: A Multicenter Retrospective Study. Front Oncol. 2020;10:566784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Lin JK, Lin CC, Yang SH, Wang HS, Jiang JK, Lan YT, Lin TC, Li AF, Chen WS, Chang SC. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. 2011;26:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12615] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 31. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 32. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4949] [Article Influence: 274.9] [Reference Citation Analysis (0)] |
| 33. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25761] [Article Influence: 1120.0] [Reference Citation Analysis (0)] |
| 34. | Meng Q, Zheng N, Wen R, Sui J, Zhang W. Preoperative nomogram to predict survival following colorectal cancer liver metastasis simultaneous resection. J Gastrointest Oncol. 2021;12:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Okimoto S, Kobayashi T, Tashiro H, Kuroda S, Ishiyama K, Ide K, Abe T, Hashimoto M, Iwako H, Hamaoka M, Honmyo N, Yamaguchi M, Ohdan H. Significance of the Glasgow Prognostic Score for patients with colorectal liver metastasis. Int J Surg. 2017;42:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Kawahara H, Yoshida S, Tohyama Y, Yanagisawa S, Misawa T, Yanaga K. Serum Carcinoembryonic Antigen Levels Before the First Curative Hepatectomy for Metastatic Colorectal Cancer Is a Predictor of Recurrence. Anticancer Res. 2018;38:5351-5355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Chiang JM, Hung HY, You JF, Chiang SF, Lee CF, Chou HS, Lee WC, Chan KM. Applicability of postoperative carcinoembryonic antigen levels in determining post-liver-resection adjuvant chemotherapy regimens for colorectal cancer hepatic metastasis. Medicine (Baltimore). 2019;98:e17696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Lu Z, Peng J, Wang Z, Pan Z, Yuan Y, Wan D, Li B. High preoperative serum CA19-9 level is predictive of poor prognosis for patients with colorectal liver oligometastases undergoing hepatic resection. Med Oncol. 2016;33:121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Yi JH, Kim H, Jung M, Shin SJ, Choi JS, Choi GH, Baik SH, Min BS, Kim NK, Ahn JB. Prognostic factors for disease-free survival after preoperative chemotherapy followed by curative resection in patients with colorectal cancer harboring hepatic metastasis: a single-institute, retrospective analysis in Asia. Oncology. 2013;85:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Analysis of preoperative prognostic factors for long-term survival after hepatic resection of liver metastasis of colorectal carcinoma. J Gastrointest Surg. 2005;9:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Wang Y, Yuan YF, Lin HC, Li BK, Wang FH, Wang ZQ, Ding PR, Chen G, Wu XJ, Lu ZH, Pan ZZ, Wan DS, Sun P, Yan SM, Xu RH, Li YH. Pathologic response after preoperative therapy predicts prognosis of Chinese colorectal cancer patients with liver metastases. Chin J Cancer. 2017;36:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Takamizawa Y, Inoue M, Moritani K, Tsukamoto S, Esaki M, Shimada K, Kanemitsu Y. Prognostic impact of conversion hepatectomy for initially unresectable colorectal liver metastasis. Langenbecks Arch Surg. 2022;407:2893-2903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 43. | Zhang YF, Mao R, Chen X, Zhao JJ, Bi XY, Li ZY, Zhou JG, Zhao H, Huang Z, Sun YK, Cai JQ. Prognostic Analysis of 102 Patients with Synchronous Colorectal Cancer and Liver Metastases Treated with Simultaneous Resection. Chin Med J (Engl). 2017;130:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Ishii M, Tominaga T, Nonaka T, Oyama S, Moriyama M, Maruyama K, Sawai T, Nagayasu T. Colon inflammatory index as a useful prognostic marker after R0 resection in patients with colorectal cancer liver metastasis. PLoS One. 2022;17:e0273167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 45. | Tanaka K, Shimada H, Ueda M, Matsuo K, Endo I, Togo S. Role of hepatectomy in treating multiple bilobar colorectal cancer metastases. Surgery. 2008;143:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Chen Q, Wu C, Zhao H, Wu J, Zhao J, Bi X, Li Z, Huang Z, Zhang Y, Zhou J, Cai J. Neo-adjuvant Chemotherapy-Induced Neutropenia Is Associated with Histological Responses and Outcomes after the Resection of Colorectal Liver Metastases. J Gastrointest Surg. 2020;24:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Li YT, Wang XY, Zhang B, Tao BR, Chen ZM, Ma XC, Han JH, Zhang C, Zhang R, Chen JH. The prognostic significance of clinicopathological characteristics in early-onset versus late-onset colorectal cancer liver metastases. Int J Colorectal Dis. 2023;38:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Takeda K, Sawada Y, Yabushita Y, Honma Y, Kumamoto T, Watanabe J, Matsuyama R, Kunisaki C, Misumi T, Endo I. Efficacy of neoadjuvant chemotherapy for initially resectable colorectal liver metastases: A retrospective cohort study. World J Gastrointest Oncol. 2022;14:1281-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Kim H, Jung HI, Kwon SH, Bae SH, Kim HC, Baek MJ, Lee MS. Preoperative neutrophil-lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis. Ann Surg Treat Res. 2019;96:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Peng J, Li H, Ou Q, Lin J, Wu X, Lu Z, Yuan Y, Wan D, Fang Y, Pan Z. Preoperative lymphocyte-to-monocyte ratio represents a superior predictor compared with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for colorectal liver-only metastases survival. Onco Targets Ther. 2017;10:3789-3799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Miki H, Akiyoshi T, Ogura A, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S, Noma H, Saiura A, Fukunaga Y, Ueno M. Pretreatment Serum Carbohydrate Antigen 19-9 Concentration Is a Predictor of Survival of Patients Who Have Undergone Curative Resection of Stage IV Rectal Cancer. Dig Surg. 2018;35:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Yoshino K, Osumi H, Ito H, Kamiimabeppu D, Ooki A, Wakatsuki T, Shimozaki K, Nakayama I, Ogura M, Takahari D, Chin K, Oba A, Ono Y, Sato T, Inoue Y, Takahashi Y, Yamaguchi K, Shinozaki E. Clinical Usefulness of Postoperative Serum Carcinoembryonic Antigen in Patients with Colorectal Cancer with Liver Metastases. Ann Surg Oncol. 2022;29:8385-8393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Kamphues C, Andreatos N, Kruppa J, Buettner S, Wang J, Sasaki K, Wagner D, Morioka D, Fitschek F, Løes IM, Imai K, Sun J, Poultsides G, Kaczirek K, Lønning PE, Endo I, Baba H, Kornprat P, Aucejo FN, Wolfgang CL, Kreis ME, Weiss MJ, Margonis GA. The optimal cut-off values for tumor size, number of lesions, and CEA levels in patients with surgically treated colorectal cancer liver metastases: An international, multi-institutional study. J Surg Oncol. 2021;123:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Hof J, Wertenbroek MW, Peeters PM, Widder J, Sieders E, de Jong KP. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg. 2016;103:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 55. | Peltonen R, Österlund P, Lempinen M, Nordin A, Stenman UH, Isoniemi H. Postoperative CEA is a better prognostic marker than CA19-9, hCGβ or TATI after resection of colorectal liver metastases. Tumour Biol. 2018;40:1010428317752944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | John SK, Robinson SM, Rehman S, Harrison B, Vallance A, French JJ, Jaques BC, Charnley RM, Manas DM, White SA. Prognostic factors and survival after resection of colorectal liver metastasis in the era of preoperative chemotherapy: an 11-year single-centre study. Dig Surg. 2013;30:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Gervaz P, Blanchard A, Pampallona S, Mach JP, Fontolliet C, Gillet M. Prognostic value of postoperative carcinoembryonic antigen concentration and extent of invasion of resection margins after hepatic resection for colorectal metastases. Eur J Surg. 2000;166:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Montalti R, Tomassini F, Laurent S, Smeets P, De Man M, Geboes K, Libbrecht LJ, Troisi RI. Impact of surgical margins on overall and recurrence-free survival in parenchymal-sparing laparoscopic liver resections of colorectal metastases. Surg Endosc. 2015;29:2736-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Polivka J, Windrichova J, Pesta M, Houfkova K, Rezackova H, Macanova T, Vycital O, Kucera R, Slouka D, Topolcan O. The Level of Preoperative Plasma KRAS Mutations and CEA Predict Survival of Patients Undergoing Surgery for Colorectal Cancer Liver Metastases. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Imai K, Allard MA, Benitez CC, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Bismuth H, Baba H, Adam R. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist. 2016;21:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 61. | Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715-722, discussion 722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 811] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 62. | Hohenberger P, Schlag PM, Gerneth T, Herfarth C. Pre- and postoperative carcinoembryonic antigen determinations in hepatic resection for colorectal metastases. Predictive value and implications for adjuvant treatment based on multivariate analysis. Ann Surg. 1994;219:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, Pulitanò C, Gavazzi F, Ferla G, Di Carlo V, Staudacher C. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 64. | Masuda T, Margonis GA, Andreatos N, Wang J, Warner S, Mirza MB, Angelou A, Damaskos C, Garmpis N, Sasaki K, He J, Imai K, Yamashita YI, Wolfgang CL, Baba H, Weiss MJ. Combined Hepatic Resection and Radio-frequency Ablation for Patients with Colorectal Cancer Liver Metastasis: A Viable Option for Patients with a Large Number of Tumors. Anticancer Res. 2018;38:6353-6360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Sasaki K, Margonis GA, Andreatos N, Kim Y, Wilson A, Gani F, Amini N, Pawlik TM. Combined resection and RFA in colorectal liver metastases: stratification of long-term outcomes. J Surg Res. 2016;206:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Reddy SK, Zorzi D, Lum YW, Barbas AS, Pawlik TM, Ribero D, Abdalla EK, Choti MA, Kemp C, Vauthey JN, Morse MA, White RR, Clary BM. Timing of multimodality therapy for resectable synchronous colorectal liver metastases: a retrospective multi-institutional analysis. Ann Surg Oncol. 2009;16:1809-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Reddy SK, Broadwater G, Niedzwiecki D, Barbas AS, Hurwitz HI, Bendell JC, Morse MA, Clary BM. Multiagent chemotherapy for isolated colorectal liver metastases: a single-centered retrospective study. J Gastrointest Surg. 2009;13:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Niu R, Yan TD, Zhu JC, Black D, Chu F, Morris DL. Recurrence and survival outcomes after hepatic resection with or without cryotherapy for liver metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:2078-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Dumarco RB, Fonseca GM, Coelho FF, Jeismann VB, Makdissi FF, Kruger JAP, Nahas SC, Herman P. Multiple colorectal liver metastases resection can offer long-term survival: The concept of a chronic neoplastic disease. Surgery. 2023;173:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Nasralla A, Lee J, Dang J, Turner S. Elevated preoperative CEA is associated with subclinical nodal involvement and worse survival in stage I non-small cell lung cancer: a systematic review and meta-analysis. J Cardiothorac Surg. 2020;15:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 71. | Li X, Dai D, Chen B, Tang H, Xie X, Wei W. Clinicopathological and Prognostic Significance of Cancer Antigen 15-3 and Carcinoembryonic Antigen in Breast Cancer: A Meta-Analysis including 12,993 Patients. Dis Markers. 2018;2018:9863092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 72. | Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:e0124151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 73. | Yamamoto Y, Hirakawa E, Mori S, Hamada Y, Kawaguchi N, Matsuura N. Cleavage of carcinoembryonic antigen induces metastatic potential in colorectal carcinoma. Biochem Biophys Res Commun. 2005;333:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Wirth T, Soeth E, Czubayko F, Juhl H. Inhibition of endogenous carcinoembryonic antigen (CEA) increases the apoptotic rate of colon cancer cells and inhibits metastatic tumor growth. Clin Exp Metastasis. 2002;19:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Ordoñez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000;60:3419-3424. [PubMed] |
| 76. | Bajenova OV, Zimmer R, Stolper E, Salisbury-Rowswell J, Nanji A, Thomas P. Heterogeneous RNA-binding protein M4 is a receptor for carcinoembryonic antigen in Kupffer cells. J Biol Chem. 2001;276:31067-31073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Zhou H, Fuks A, Alcaraz G, Bolling TJ, Stanners CP. Homophilic adhesion between Ig superfamily carcinoembryonic antigen molecules involves double reciprocal bonds. J Cell Biol. 1993;122:951-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Tang XH, Wu XL, Gan XJ, Wang YD, Jia FZ, Wang YX, Zhang Y, Gao XY, Li ZY. Using Normalized Carcinoembryonic Antigen and Carbohydrate Antigen 19 to Predict and Monitor the Efficacy of Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 79. | Lehtomäki K, Heervä E, Kellokumpu-Lehtinen PL, Mustonen H, Salminen T, Joensuu H, Hermunen K, Boisen MK, Johansen JS, Haglund C, Osterlund P. Transient Changes in Serum CEA, CA19-9, CRP, YKL-40, and IL-6 during Adjuvant Chemotherapy and Survival of Patients with Colorectal Cancer. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 80. | Blumenthal RD, Leon E, Hansen HJ, Goldenberg DM. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer. 2007;7:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 81. | Lee JH, Lee SW. The Roles of Carcinoembryonic Antigen in Liver Metastasis and Therapeutic Approaches. Gastroenterol Res Pract. 2017;2017:7521987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Peng S, Huang P, Yu H, Wen Y, Luo Y, Wang X, Zhou J, Qin S, Li T, Chen Y, Liu G, Huang M. Prognostic value of carcinoembryonic antigen level in patients with colorectal cancer liver metastasis treated with percutaneous microwave ablation under ultrasound guidance. Medicine (Baltimore). 2018;97:e0044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Weiner AA, Gui B, Newman NB, Nosher JL, Yousseff F, Lu SE, Foltz GM, Carpizo D, Lowenthal J, Zuckerman DA, Benson B, Olsen JR, Jabbour SK, Parikh PJ. Predictors of Survival after Yttrium-90 Radioembolization for Colorectal Cancer Liver Metastases. J Vasc Interv Radiol. 2018;29:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Huang J, Xiao Y, Zhou Y, Deng H, Yuan Z, Dong L, Lan J, Li X, Liu G, Hu H, Huang S, Yang X. Baseline serum tumor markers predict the survival of patients with advanced non-small cell lung cancer receiving first-line immunotherapy: a multicenter retrospective study. BMC Cancer. 2023;23:812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 85. | Huang Y, Liu F, Xu R, Zhou F, Yang W, He Y, Liu Z, Hou B, Liang L, Zhang L, Liu M, Pan Y, Liu Y, He Z, Ke Y. Postoperative serum squamous cell carcinoma antigen and carcinoembryonic antigen predict overall survival in surgical patients with esophageal squamous cell carcinoma. Front Oncol. 2023;13:1263990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | Lin X, Huo J, Su H, Zhu C, Xu Y, Zhang F. Risk factors for cervical lymph node metastasis in the central or lateral cervical region in medullary thyroid carcinoma: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | Agrawal AK, Jelen M, Rudnicki J, Grzebieniak Z, Zyśko D, Kielan W, Słonina J, Marek G. The importance of preoperative elevated serum levels of CEA and CA15-3 in patients with breast cancer in predicting its histological type. Folia Histochem Cytobiol. 2010;48:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |