Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2866
Peer-review started: September 8, 2023
First decision: October 24, 2023
Revised: November 1, 2023
Accepted: November 17, 2023
Article in press: November 17, 2023
Published online: December 27, 2023
Processing time: 110 Days and 8.6 Hours
Rapid regeneration of the residual liver is one of the key determinants of successful partial hepatectomy (PHx). At present, there is a lack of recognized safe, effective, and stable drugs to promote liver regeneration. It has been reported that vagus nerve signaling is beneficial to liver regeneration, but the potential mechanism at play here is not fully understood.
To explore the effect and mechanism of hepatic vagus nerve in liver regeneration after PHx.
A PHx plus hepatic vagotomy (Hv) mouse model was established. The effect of Hv on liver regeneration after PHx was determined by comparing the liver regeneration levels of the PHx-Hv group and the PHx-sham group mice. In order to further investigate the role of interleukin (IL)-22 in liver regeneration inhibition mediated by Hv, the levels of IL-22 in the PHx-Hv group and the PHx-sham group was measured. The degree of liver injury in the PHx-Hv group and the PHx-sham group mice was detected to determine the role of the hepatic vagus nerve in liver injury after PHx.
Compared to control-group mice, Hv mice showed severe liver injury and weakened liver regeneration after PHx. Further research found that Hv downregulates the production of IL-22 induced by PHx and blocks activation of the signal transducer and activator of transcription 3 (STAT3) pathway then reduces the expression of various mitogenic and anti-apoptotic proteins after PHx. Exogenous IL-22 reverses the inhibition of liver regeneration induced by Hv and alleviates liver injury, while treatment with IL-22 binding protein (an inhibitor of IL-22 signaling) reduce the concentration of IL-22 induced by PHx, inhibits the activation of the STAT3 signaling pathway in the liver after PHx, thereby hindering liver regeneration and aggravating liver injury in PHx-sham mice.
Hv attenuates liver regeneration after hepatectomy, and the mechanism may be related to the fact that Hv downregulates the production of IL-22, then blocks activation of the STAT3 pathway.
Core Tip: In this study, we investigated the role and mechanism of the vagus nerve in liver regeneration using a partial hepatectomy (PHx) plus hepatic vagotomy (Hv) mouse model. We found that Hv attenuates liver regeneration after hepatectomy, the mechanism may be related to the fact that Hv downregulates the production of interleukin-22, then blocks activation of the signal transducer and activator of transcription 3 pathway after PHx. These results provide a theoretical basis for the development of drugs that promote liver regeneration with the vagus nerve as a new target.
- Citation: Zhou H, Xu JL, Huang SX, He Y, He XW, Lu S, Yao B. Hepatic vagotomy blunts liver regeneration after hepatectomy by downregulating the expression of interleukin-22. World J Gastrointest Surg 2023; 15(12): 2866-2878
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2866.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2866
The liver has an enormous capacity for self-regeneration. Results from rodents show that the residual liver will recover its original quality within two weeks after partial hepatectomy (PHx)[1,2]. Nevertheless, in the face of chronic liver injury induced by chronic hepatitis, alcohol abuse, and a high-fat diet, the liver’s ability to regenerate itself enough to maintain normal organ function is challenged[3-5]. Moreover, the incidence of hepatocellular carcinoma is increasing due to long-term chronic liver injury[6,7]. The risk of critical liver failure increases with the reduction in residual liver volume after hepatectomy, which greatly limits the proportion of patients receiving surgical treatment, resulting in adverse outcomes for many of them[8-10]. Therefore, improving the ability of the liver to regenerate is important for enriching the toolbox of liver surgical treatment. Unfortunately, there are no drugs generally recognized as safe and effective for clinicians to promote liver regeneration.
As a new CD4+Th cytokine discovered in 2000, the role of interleukin (IL)-22 in promoting liver regeneration has been widely reported[11-13]. Although the role of chemokines and cytokines in liver regeneration has been reported widely, some studies have identified the role of the vagal nerve in liver regeneration after hepatectomy[14-16]. Wang et al[14] found that hepatic vagotomy (Hv) can delay liver regeneration after PHx in mice, with the peak proliferation postponed for two days. In mice treated with exogenous netrin-1 (a critical axon-guiding protein), the inhibition of liver regeneration induced by Hv was reversed as netrin-1 promoted the repair and regeneration of the vagus nerve. An earlier study from another team also observed adverse effects of Hv on liver regeneration and confirmed the core role of IL-6 in this process[15]. Hv downregulates the expression of FoxM1 in hepatocytes by inhibiting the production of IL-6 in liver macrophages after PHx, finally inhibiting liver regeneration. Our previous study found that Hv aggravates liver ischemia–reperfusion injury by downregulating the expression of IL-22 in animals[17]. Several research teams have also noticed a possible role of the vagus nerve in liver regeneration; unfortunately, this process involves a variety of cytokines, chemokines, and signaling pathways, which we know little about[14-16].
Using a PHx-Hv mouse model, we investigated the role and potential mechanism of the vagus nerve in liver regeneration. We found that excision of the hepatic branch of the vagus nerve can inhibit liver regeneration at an early stage and aggravate liver injury significantly after PHx. Further experiments showed that Hv could downregulate the expression of IL-22 induced by PHx significantly, thereby inhibiting the activation of the signal transducer and activator of transcription 3 (STAT3) signaling pathway. To clarify the correlation between the downregulation of IL-22 (caused by Hv) and the inhibition of liver regeneration, PHx-Hv group mice were treated with exogenous IL-22, while mice in the PHx group were treated with IL-22 binding protein (IL-22BP, an inhibitor of IL-22 signaling). As expected, the administration of IL-22 fusion protein reversed the inhibition of liver regeneration caused by Hv in the early stage of hepatectomy, while mice treated with IL-22BP showed more severe liver injury and inhibition of liver regeneration, indicating that the inhibition of liver regeneration caused by Hv relates to the downregulation of IL-22 expression in the liver. These results provide a theoretical basis for the development of drugs that promote liver regeneration with the vagus nerve as a new target.
Recombinant mouse IL-22 was purchased from Novoprotein Scientific Inc. (Shanghai, China). Anti-FoxM1 antibody was obtained from Proteintech Group, Inc. (Rosemont, IL, United States). Mouse anti–IL-22 antibody and recombinant mouse IL-22BP protein were obtained from R&D Systems (Minneapolis, MN, United States). Anti-STAT3, anti–phospho-STAT3 (Tyr 705), anti-CyclinD1, and anti-PCNA antibodies were purchased from Cell Signaling Technology (Danvers, MA, United States). A BrdU in situ detection kit was obtained from Becton, Dickinson, and Company (Franklin Lakes, NJ, United States) and a mouse IL-22 enzyme-linked immunosorbent assay (ELISA) kit was obtained from Beijing 4A Biotech Co., Ltd. (Beijing, China).
Animal experiments follow the principle of minimizing pain or discomfort. The C57BL/6 male mice aged 8-10 wk used for this study were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice could drink freely but were forbidden from eating anything 12 h before the operation. Pentobarbital sodium was used as an anesthetic before the operation. Mice were randomly divided into the following six groups: The SP group (sham operation for PHx), the Hv group, the PHx-sham group, the PHx-Hv group, the PHx-Hv-IL-22 group and the PHx-IL-22BP group. The specific surgical (PHx and Hv) procedures are described in detail below. Mice in the PHx-Hv-IL-22 group received 125 μg/kg of IL-22 intravenous therapy 30 min before PHx, and the PHx-IL-22BP mice were treated with 5 μg of IL-22BP delivered intraperitoneally 2 h before the abdominal incision. Mice were euthanized at 6, 24, and 48 h after surgery, and liver and serum samples were collected for analysis.
The specific operation of PHx was as described previously[18]. In brief, about 70% of the liver lobes (including the left posterior lobe, left anterior lobe, and right anterior lobe) were ligated and resected after a midline laparotomy was performed in the mice.
We performed hepatic branch vagotomy as previously described[17]. First, we gently pulled down the esophagus and stomach after fully exposing the abdominal cavity, then dissociated the liver carefully and pulled up the right and front lobes of the liver in order to separate the hepatic branch from the abdominal branch (a few millimeters above the cardia) of the vagus nerve. In animals receiving Hv, the vagus nerve of the hepatic branch was gently transected with fine forceps.
Animals were intraperitoneally injected with 5-bromo-2’-deoxyuridine (50 μg/g of body weight; Sigma-Aldrich, St. Louis, MO, United States) 2 h before euthanasia. The liver samples obtained were stained according to the instructions of the BrdU in situ Detection Kit, and BrdU-positive cells were considered to be regenerated hepatocytes. We determined the number of BrdU+ hepatocytes and total hepatocytes in four to six microscope fields (200 ×) to calculate the BrdU+ hepatocyte/total hepatocyte ratio, which directly reflects the level of hepatocyte regeneration.
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in addition to liver hematoxylin and eosin (H&E) staining were used as indicators of liver injury. Both serum ALT and AST activity were measured using an automatic chemical analyzer (Hitachi 3100; Hitachi Ltd., Tokyo, Japan). In previous studies, we found that mice undergoing 70% PHx showed varying degrees of residual liver damage after surgery, manifested as liver parenchymal hemorrhage and necrosis[18]. H&E staining of liver tissue was commonly used in many of our studies, which has been described in detail in previous articles[17].
Serum and liver tissue homogenate of mice at different time points after PHx were obtained for the detection of IL-22 level. The quantification of IL-22 level was performed using a commercial mouse ELISA kit according to the manufacturer’s instructions. In short, add standards and samples to the microplate strips, then sequentially add antibodies, IL-22 conjugate, substrate solution, and stop solution. Finally, measure the optical density values of each well at a wavelength of 450 nm within 30 min.
TRIzol reagent (Invitrogen, Carlsbad, CA, United States) was used to extract total RNA from mouse livers; then, we used the PrimeScript reverse transcription kit (Takara Biotechnology Co., Ltd., Kusatsu, Japan) to reverse-transcribe RNA into complementary DNA. We use SYBR Green PCR Master Mix (CoWin Biosciences, Cambridge, MA, United States) to perform quantitative real-time polymerase chain reaction (qPCR) using the 7500 system (Applied Biosystems, Waltham, MA, United States). We used 18sRNA as a housekeeping gene. The sequence of IL-22 oligonucleotide used in this study was as follows: forward (5’-GCTCAGCTCCTGTCACATCA-3’) and reverse (5’-CAGTTCCCCAATCGCCTTGA-3’).
Western blot analysis of animal liver tissue to measure the expression level of related proteins was reported in detail in our previous article[17]. The protein was extracted from hepatic tissue, separated by sodium-dodecyl sulfate gel electrophoresis, and transferred to polyvinylidene fluoride membrane. After incubated with primary and secondary antibodies, the protein bands were visualized using a luminescent reagent kit. The dilution ratios of all primary antibodies used in this study were determined according to the manufacturer’s protocols. We visualized protein bands using a hypersensitive chemiluminescence kit using ImageJ 1.6.0 (United States National Institutes of Health, Bethesda, MD, United States).
Comparisons between two groups were performed with Student’s t-test; for more than two groups, one-way analysis of variance followed by Tukey’s post hoc test was used. All data in this paper are expressed as mean ± SD values. SPSS software (version 19.0; IBM Corporation, Armonk, NY, United States) we used for statistical analysis, and the difference was considered statistically significant when P < 0.05.
BrdU staining of liver tissue showed that a significant increase in BrdU+ hepatocytes could be seen at 48 h after PHx, while almost no positive hepatocytes were seen at earlier observation points (6 or 24 h) (Figure 1A). The proportion of BrdU+ hepatocytes in the PHx-sham group at 48 h was greater than that in the PHx-Hv group, suggesting that Hv can hinder the process of liver regeneration after PHx (Figures 1A and B). The liver weight/body weight ratio in the PHx-sham group was higher than that in the PHx-Hv group at 48 h after PHx, suggesting that liver regeneration in the former group was faster than that in the latter group, which confirmed the BrdU staining results (Figure 1C).
H&E staining results showed that liver tissue of mice in the PHx-Hv group was more severely damaged than that in the PHx-sham group, showing a wider range of necrosis (Figure 1D). The activities of serum ALT and AST in mice increased significantly and reached a peak value at 24 h (within our observation time period) after PHx. Compared to the activities of ALT and AST in the PHx-sham group, those in the PHx-Hv group were higher at 24 h and 48 h after PHx, and the difference in AST was more significant at 24 h (Figures 1E and F). All these results indicate that Hv may aggravate hepatic injury after PHx.
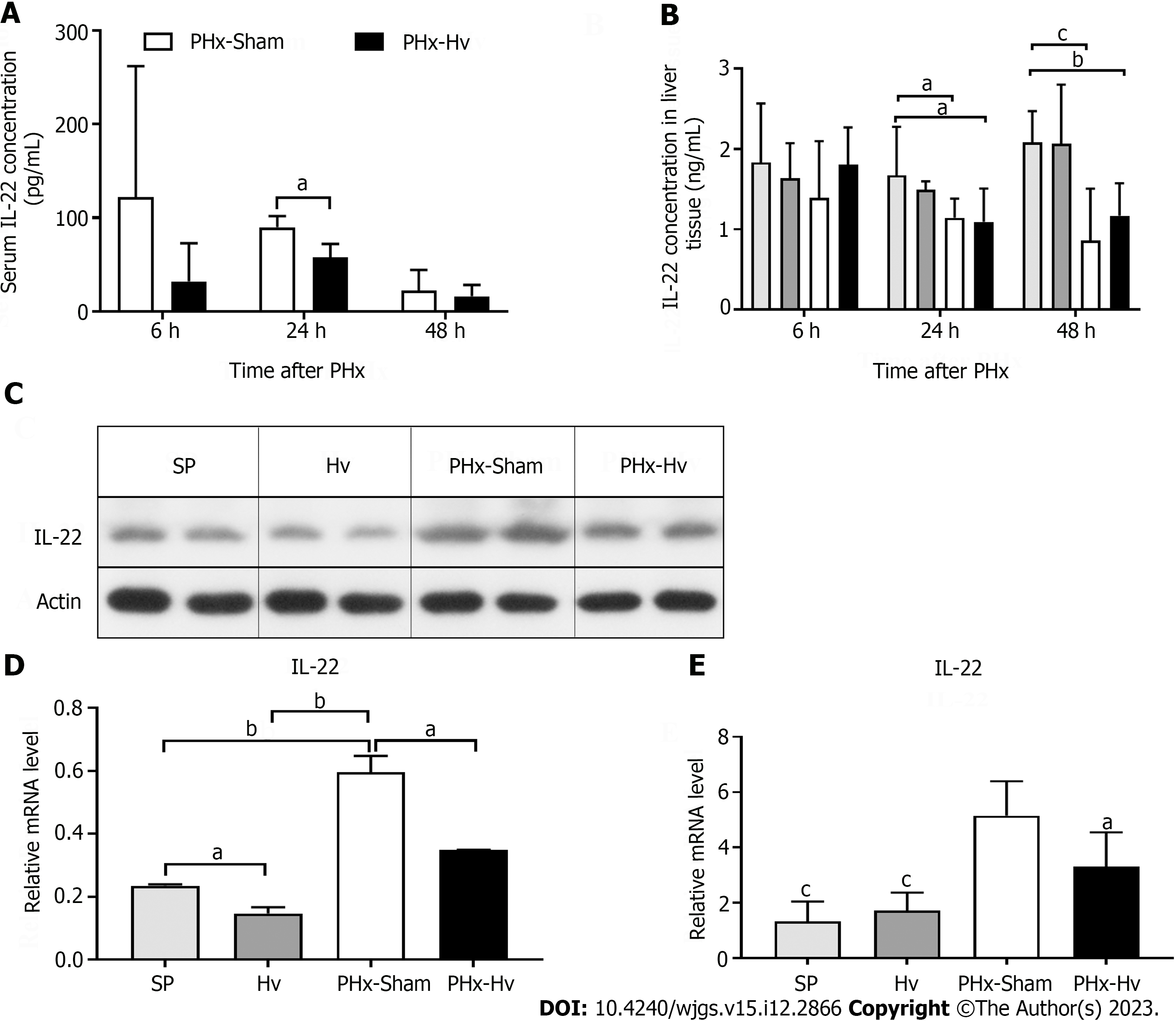
We observed that the IL-22 concentration in the serum of mice receiving PHx (i.e., mice in the PHx-sham and PHx-Hv groups) significantly increased after surgery, while the serum IL-22 concentration was too low to be detected in mice not receiving PHx (i.e., those in the SP and Hv groups). Compared to the serum IL-22 level in the PHx-Hv group, that in the PHx-sham group was higher at 6 and 24 h after PHx, and the difference at 24 h was statistically significant (Figure 2A). Similarly, the concentration of IL-22 in the liver tissue homogenate was highest at 6 h after PHx, then gradually decreased; however, there was no significant difference between the PHx-sham group and the PHx-Hv group at any time point (Figure 2B). Western blotting and qPCR technology were used to further determine the difference in IL-22 expression in liver tissues of the PHx-sham group and the PHx-Hv group. As expected, compared to the level of IL-22 protein in liver tissue from the SP and Hv groups, that from the PHx-sham and PHx-Hv groups significantly increased at 6 h after PHx, especially in the PHx-sham group (Figures 2C and D). Similarly, the expression of IL-22 mRNA in liver tissue of the PHx-sham group was significantly higher than that in the PHx-Hv group at 6 h after PHx (Figure 2E). In conclusion, these results suggest that Hv reduces IL-22 production induced by PHx.
It is well known that IL-22 effectively promotes liver regeneration, and the mechanism, which is related to the activation of the hepatic STAT3 signaling pathway, promotes the expression of a variety of downstream mitogenic and anti-apoptotic proteins. Our previous study found that vagotomy of the hepatic branch could inhibit the expression of IL-22 after PHx. Here, we tested the STAT3 pathway and related mitogenic and anti-apoptotic protein expression levels using western blotting (Figure 3). The results showed that the expression levels of STAT3, P-STAT3, CyclinD1, and PCNA proteins in livers from the PHx-Hv group decreased significantly compared to those from the PHx-sham group, which was consistent with our expected result. As mentioned above, FoxM1 is a key proliferation-promoting transcription factor necessary for normal cell proliferation. Some researchers found that one of the mechanisms of liver regeneration after PHx is the activation of the hepatic STAT3 signaling pathway, which induces the expression of FoxM1. Our research confirms this conclusion. Compared to the activation of the STAT3 pathway in the PHx-sham group, that in the PHx-Hv group was inhibited, and the expression of hepatic FoxM1 protein was muted.
To further explore the effects of IL-22 on liver regeneration in PHx-Hv mice and then confirm the correlation between the decrease in IL-22 expression caused by Hv and the delay of liver regeneration, PHx-Hv mice were treated with exogenous IL-22 (0.125 μg/g of body weight). Results showed that, compared to the proportion of BrdU+ hepatocytes in liver tissue from the PHx-Hv group, that from the PHx-Hv-IL-22 group significantly increased at 48 h after PHx, and a significant increased liver weight/body weight ratio was observed in the latter group, suggesting that exogenous IL-22 treatment can reverse liver regeneration inhibited by Hv (Figures 4A and C). At different time points after PHx, lower activities of ALT and AST in serum were observed in the IL-22 treatment group, confirming the expected protective effect of IL-22 on liver injury in PHx-Hv mice (Figures 4D and E). Western blot analysis of liver tissue showed that the expression of P-STAT3, CyclinD1, and PCNA protein in the PHx-Hv-IL-22 group were higher than those in the PHx-Hv group, suggesting that the treatment of IL-22 will further activate the STAT3 pathway and its downstream proteins inhibited by Hv (Figures 4F and G).
As a soluble IL-22 receptor, the specific role of IL-22BP in different types of liver injury is controversial, which may be related to the concentration ratio of IL-22BP/IL-22 and the internal environment. Here, we use this model to study the specific role of IL-22BP in liver regeneration after PHx. The concentration of IL-22 in serum (at 6 h) and liver tissue (at 24 h) in the PHx-IL-22BP group after surgery was lower than in the PHx-sham group (Figures 5A and B). Moreover, liver regeneration was significantly higher and more BrdU-positive hepatocytes were seen in the PHx-Sham group than in the PHx-IL-22BP group at 48 h after PHx (Figures 5C and E). The activities of ALT and AST in the PHx-sham group were lower than those in the PHx-IL-22BP group at 48 h (Figures 5F and G), indicating that IL-22BP increased liver injury after PHx.
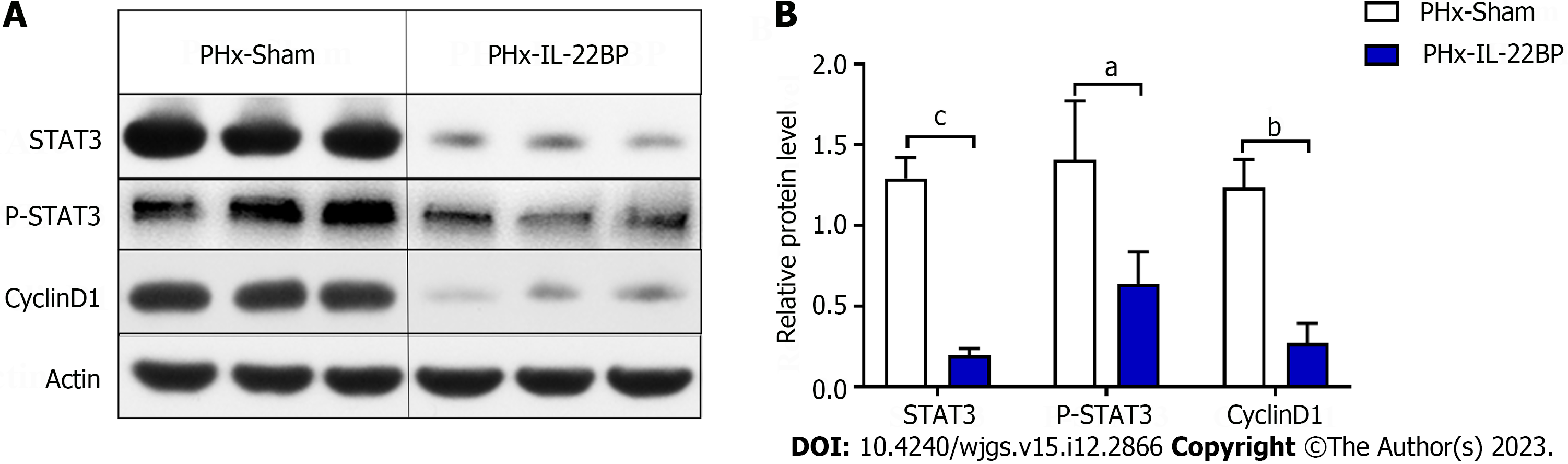
Compared to the expression of STAT3, P-STAT3, and CyclinD1 proteins in the livers of the PHx-sham group, expression of these proteins in the livers of the PHx-IL-22BP group decreased significantly 6 h after PHx (Figure 6), indicating that IL-22BP treatment before PHx would inhibit activation of the STAT3 signaling pathway in the early postoperative period, which may be one of the factors causing the slow rate of liver regeneration after PHx.
Rapid regeneration of the residual liver is one of the key determinants of the success of PHx. Failed regeneration means failure of the liver, which brings great risk to the lives of patients undergoing hepatectomy[14,19,20]. However, compared to the research on cytokines and chemokines, that on the role and potential mechanism of the vagal nerve in liver regeneration is very insufficient. Using a PHx-Hv animal model, we found that hepatic branch vagotomy can aggravate liver injury and inhibit liver regeneration after PHx. The mechanism in this context may be related to the reduction of IL-22 production and the inhibition of the STAT3 signaling pathway activation caused by Hv. These results suggest that the hepatic vagus nerve may play an active role in liver regeneration through the IL-22-STAT3 axis.
The vagus nerve is an important regulatory component of the gastrointestinal-related immune system[21-23]. The liver plays a key role in immune monitoring with a large number of resident macrophages among the organs in the abdominal cavity[24,25]. Therefore, it is generally believed that the liver is the main target of vagal nerve fibers[26]. Nevertheless, we still know little about the role of the vagus nerve in liver injury, liver immunity, and liver regeneration. Research shows that the role of the vagus nerve in the liver immune response includes two aspects: A fast response and a long-term response[26]. The fast response means that the vagal nerve signal can activate and optimize the phagocytic activity of Kupffer cells, while the long-term response means that the vagal nerve produces effects over a long time by regulating the production of various cytokines[26,27]. Using a PHx rat model, scientists discovered the delayed effect of Hv on liver regeneration more than 30 years ago[28]. In recent years, researchers have made more in-depth discoveries to clarify the core role of the vagus-macrophage-hepatocyte link in liver regeneration[15]. We established a PHx-Hv mouse model to clarify the role of the hepatic vagus nerve in liver regeneration and further study its potential mechanism. Our results were as expected; the liver index (liver weight/body weight) decreased significantly and the proportion of BrdU-positive cells in liver tissue was lower in mice in the PHx-Hv group than in those in the PHx-Sham group, indicating that Hv hindered liver regeneration.
The present study showed that an increase in liver IL-22 was observed in mice treated with CCL4, PHx, or liver ischemia/reperfusion injury (IRI), indicating that the injury itself may be one of the factors inducing the release of IL-22[17,29-31]. Data from human patients showed that, compared to serum IL-22 concentration in a healthy control group, serum IL-22 concentration in patients with liver cirrhosis in the compensatory or stable decompensated period did not increase; however, it significantly increased in patients in the acute decompensated period or with acute-on-chronic liver failure, suggesting that the release of IL-22 after liver injury seems to relate to the type (acute/chronic) and degree of injury[32]. Our study found that the concentration of IL-22 in the serum of normal mice was low enough to be difficult to detect. However, the concentration of IL-22 in both serum and liver tissue homogenate increased significantly and reached the peak at 6 h after hepatectomy. These data are similar to those of previous reports, indicating that PHx can stimulate the release of IL-22.
The regulatory role of the nervous system in the expression of IL-22 is still poorly understood. Recently, Liu et al[30] found that environmental eustress acts on type 1 innate lymphoid cells (ILC1s) through sympathetic nerve signals to promote the production of IL-22, then promoting liver regeneration after PHx. Moreover, our previous study found that the vagus nerve plays an important role in the expression of IL-22 induced by IRI in the liver. Hv significantly increases the liver injury caused by ischemia and reperfusion due to the sudden decrease in IL-22 release[17]. In this study, the concentration of IL-22 in the serum and liver homogenate of mice significantly decreased after surgery in the PHx-Hv group but not in the PHx-sham group. These data prove that Hv inhibits the expression of IL-22 once again.
The potential mechanism of the vagus nerve in liver regeneration is not completely clear at present. Izumi et al[15] found the role of the vagus-macrophage-hepatocyte link in liver regeneration after PHx. This study found that vagus-derived cholinergic signals stimulate the production of IL-6 by macrophages in the liver, promote the expression of FoxM1 in liver cells, then promote liver regeneration, indicating that IL-6 plays a central role in this process[15]. Another study found that the neuronal guidance protein netrin-1 plays an important role in vagus signal-mediated liver regeneration as Netrin-1 promotes the repair and regeneration of the vagus nerve after PHx in mice[14]. We found that the release of IL-22 was significantly inhibited after vagotomy. The fact that IL-22 promotes liver regeneration by activating the STAT3 signaling pathway has been widely reported; therefore, IL-22 may be a cytokine that the vagus nerve regulates during liver regeneration. In addition, consistent with previous reports, we confirmed that the expression of FoxM1 protein in liver tissue of mice in the PHx-Hv group decreased (compared to that in the PHx-sham group). Unfortunately, we have not yet confirmed whether this change is only caused by the downregulation of IL-6 or is related to both IL-6 and IL-22.
There are some limitations to this research. Although we have observed the effect of Hv on the expression of IL-22 after PHx, it is not clear which links in the production of IL-22 have been blocked. There are many factors regulating the production of IL-22, including both positive (e.g., IL-7, IL-1 β, and IL-23) and negative (e.g., IL-27, transforming growth factor-β, and IL-22BP) factors[33-38]. Additionally, there are many kinds of lymphoid cells that produce IL-22, including NKT cells, ILCs, γδ T-cells, and αβ T-cells, but which cell is the target of vagal nerve signaling has not been determined[39,40]. The deepening understanding of liver regeneration is one of the research hotspots in recent years. The study of neuromodulation in liver regeneration has become meaningful as it is difficult for patients undergoing large-scale hepatectomy or liver transplantation to avoid the risk of partial loss of innervation of organs[27,41]. In general, this experiment provides data support for studying the role of neuroregulation in liver regeneration, and we will study these questions in the near future.
Our investigation confirmed that Hv aggravates liver injury and hinders liver regeneration after hepatectomy. The mechanism may be related to the downregulation of IL-22 production, which inhibits the activation of the STAT3 pathway caused by Hv in the liver.
There is a lack of recognized safe, effective, and stable drugs to promote liver regeneration at present, which greatly limits the progress of liver surgery. It has been reported that vagus nerve signaling is beneficial to liver regeneration, but the potential mechanism is not fully understood.
In previous studies, we found that blocking the hepatic vagus nerve would exacerbate liver injury caused by ischemia-reperfusion, indicating that the vagus nerve may play a protective role in liver injury. Unfortunately, we cannot determine the effect and mechanism of the vagus nerve in liver regeneration after hepatectomy so far.
Exploring the role and mechanism of hepatic vagus nerve in liver regeneration after partial hepatectomy (PHx).
Establishing a PHx+ hepatic vagotomy (Hv) mice model. The effect of vagus on liver regeneration was determined by comparing the liver regeneration levels of the PHx-Hv group and the PHx-sham group mice. Quantikine enzyme-linked immunosorbent assay kit and molecular biology techniques (western blot, polymerase chain reaction, etc.) have been used to further investigate the potential mechanism of Hv on liver regeneration.
Hv mice showed severe liver injury and weakened liver regeneration after PHx compared to control-group mice. Hv downregulates the production of interleukin-22 (IL-22) induced by PHx, inhibiting the activation of the signal transducer and activator of transcription 3 (STAT3) pathway in the liver. Exogenous IL-22 supplementation reverse the inhibitory effect on liver regeneration induced by Hv, while IL-22 binding protein inhibits the activation of liver STAT3 signaling pathway after PHx, then hindering liver regeneration in PHx-sham mice.
Hv inhibits the regeneration of residual liver after surgery by downregulating the production of IL-22 induced by PHx.
We are currently planning to investigate whether drugs that protect the vagus nerve play a positive role in liver regeneration after PHx.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goja S, India; Pisani LF, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Tao J, Chen Y, Zhuang Y, Wei R, Getachew A, Pan T, Yang F, Li Y. Inhibition of Hedgehog Delays Liver Regeneration through Disrupting the Cell Cycle. Curr Issues Mol Biol. 2022;44:470-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Di-Iacovo N, Pieroni S, Piobbico D, Castelli M, Scopetti D, Ferracchiato S, Della-Fazia MA, Servillo G. Liver Regeneration and Immunity: A Tale to Tell. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Tian Z. HBV-Induced Immune Imbalance in the Development of HCC. Front Immunol. 2019;10:2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 4. | Zhang L, Ma XJ, Fei YY, Han HT, Xu J, Cheng L, Li X. Stem cell therapy in liver regeneration: Focus on mesenchymal stem cells and induced pluripotent stem cells. Pharmacol Ther. 2022;232:108004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Ibrahim S, Weiss TS. Augmenter of liver regeneration: Essential for growth and beyond. Cytokine Growth Factor Rev. 2019;45:65-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023;27:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 229] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 7. | Golabi P, Rhea L, Henry L, Younossi ZM. Hepatocellular carcinoma and non-alcoholic fatty liver disease. Hepatol Int. 2019;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Cui B, Yang L, Zhao Y, Lu X, Song M, Liu C, Yang C. HOXA13 promotes liver regeneration through regulation of BMP-7. Biochem Biophys Res Commun. 2022;623:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Van Haele M, Snoeck J, Roskams T. Human Liver Regeneration: An Etiology Dependent Process. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Huang W, Han N, Du L, Wang M, Chen L, Tang H. A narrative review of liver regeneration-from models to molecular basis. Ann Transl Med. 2021;9:1705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Kudira R, Malinka T, Kohler A, Dosch M, de Agüero MG, Melin N, Haegele S, Starlinger P, Maharjan N, Saxena S, Keogh A, Stroka D, Candinas D, Beldi G. P2X1-regulated IL-22 secretion by innate lymphoid cells is required for efficient liver regeneration. Hepatology. 2016;63:2004-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Xiang X, Feng D, Hwang S, Ren T, Wang X, Trojnar E, Matyas C, Mo R, Shang D, He Y, Seo W, Shah VH, Pacher P, Xie Q, Gao B. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J Hepatol. 2020;72:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 13. | Wu Y, Min J, Ge C, Shu J, Tian D, Yuan Y, Zhou D. Interleukin 22 in Liver Injury, Inflammation and Cancer. Int J Biol Sci. 2020;16:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Wang Z, Jiang T, Aji T, Aimulajiang K, Liu Y, Lv G, Wen H. Netrin-1 promotes liver regeneration possibly by facilitating vagal nerve repair after partial hepatectomy in mice. Cell Signal. 2022;91:110227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Izumi T, Imai J, Yamamoto J, Kawana Y, Endo A, Sugawara H, Kohata M, Asai Y, Takahashi K, Kodama S, Kaneko K, Gao J, Uno K, Sawada S, Kalinichenko VV, Ishigaki Y, Yamada T, Katagiri H. Vagus-macrophage-hepatocyte link promotes post-injury liver regeneration and whole-body survival through hepatic FoxM1 activation. Nat Commun. 2018;9:5300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Xu C, Zhang X, Wang G, Chang C, Zhang L, Cheng Q, Lu A. Role of the autonomic nervous system in rat liver regeneration. Cell Mol Neurobiol. 2011;31:527-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Zhou H, Xu J, Huang S, He Y, He X, Guo L, Yin S, Lu S. Blocking the Hepatic Branch of the Vagus Aggravates Hepatic Ischemia-Reperfusion Injury via Inhibiting the Expression of IL-22 in the Liver. J Immunol Res. 2021;2021:6666428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Zhou H, Xie G, Mao Y, Zhou K, Ren R, Zhao Q, Wang H, Yin S. Enhanced Regeneration and Hepatoprotective Effects of Interleukin 22 Fusion Protein on a Predamaged Liver Undergoing Partial Hepatectomy. J Immunol Res. 2018;2018:5241526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Yagi S, Hirata M, Miyachi Y, Uemoto S. Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 20. | Starlinger P, Luyendyk JP, Groeneveld DJ. Hemostasis and Liver Regeneration. Semin Thromb Hemost. 2020;46:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Lei W, Duan Z. Advances in the Treatment of Cholinergic Anti-Inflammatory Pathways in Gastrointestinal Diseases by Electrical Stimulation of Vagus Nerve. Digestion. 2021;102:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Fülling C, Dinan TG, Cryan JF. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron. 2019;101:998-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 365] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 23. | Berthoud HR, Albaugh VL, Neuhuber WL. Gut-brain communication and obesity: understanding functions of the vagus nerve. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 24. | Guilliams M, Scott CL. Liver macrophages in health and disease. Immunity. 2022;55:1515-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 25. | Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 433] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 26. | Fonseca RC, Bassi GS, Brito CC, Rosa LB, David BA, Araújo AM, Nóbrega N, Diniz AB, Jesus ICG, Barcelos LS, Fontes MAP, Bonaventura D, Kanashiro A, Cunha TM, Guatimosim S, Cardoso VN, Fernandes SOA, Menezes GB, de Lartigue G, Oliveira AG. Vagus nerve regulates the phagocytic and secretory activity of resident macrophages in the liver. Brain Behav Immun. 2019;81:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Li Y, Xu Z, Yu Y, Yuan H, Xu H, Zhu Q, Wang C, Shi X. The vagus nerve attenuates fulminant hepatitis by activating the Src kinase in Kuppfer cells. Scand J Immunol. 2014;79:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Tanaka K, Ohkawa S, Nishino T, Niijima A, Inoue S. Role of the hepatic branch of the vagus nerve in liver regeneration in rats. Am J Physiol. 1987;253:G439-G444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Stülb H, Bachmann M, Gonther S, Mühl H. Acetaminophen-Induced Liver Injury Exposes Murine IL-22 as Sex-Related Gene Product. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Liu T, Li J, Li Q, Liang Y, Gao J, Meng Z, Li P, Yao M, Gu J, Tu H, Gan Y. Environmental eustress promotes liver regeneration through the sympathetic regulation of type 1 innate lymphoid cells to increase IL-22 in mice. Hepatology. 2023;78:136-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74-G80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Schwarzkopf K, Rüschenbaum S, Barat S, Cai C, Mücke MM, Fitting D, Weigert A, Brüne B, Zeuzem S, Welsch C, Lange CM. IL-22 and IL-22-Binding Protein Are Associated With Development of and Mortality From Acute-on-Chronic Liver Failure. Hepatol Commun. 2019;3:392-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Zhu Y, Shi T, Lu X, Xu Z, Qu J, Zhang Z, Shi G, Shen S, Hou Y, Chen Y, Wang T. Fungal-induced glycolysis in macrophages promotes colon cancer by enhancing innate lymphoid cell secretion of IL-22. EMBO J. 2021;40:e105320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 34. | Rae J, Hackney J, Huang K, Keir M, Herman A. Identification of an IL-22-Dependent Gene Signature as a Pharmacodynamic Biomarker. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 35. | Fatkhullina AR, Peshkova IO, Dzutsev A, Aghayev T, McCulloch JA, Thovarai V, Badger JH, Vats R, Sundd P, Tang HY, Kossenkov AV, Hazen SL, Trinchieri G, Grivennikov SI, Koltsova EK. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity. 2018;49:943-957.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 36. | Wang H, Li Z, Yang B, Yu S, Wu C. IL-27 suppresses the production of IL-22 in human CD4(+) T cells by inducing the expression of SOCS1. Immunol Lett. 2013;152:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Kärner J, Wawrzyniak M, Tankov S, Runnel T, Aints A, Kisand K, Altraja A, Kingo K, Akdis CA, Akdis M, Rebane A. Increased microRNA-323-3p in IL-22/IL-17-producing T cells and asthma: a role in the regulation of the TGF-β pathway and IL-22 production. Allergy. 2017;72:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Zenewicz LA. IL-22 Binding Protein (IL-22BP) in the Regulation of IL-22 Biology. Front Immunol. 2021;12:766586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 690] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 40. | Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1417] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 41. | Kandilis AN, Papadopoulou IP, Koskinas J, Sotiropoulos G, Tiniakos DG. Liver innervation and hepatic function: new insights. J Surg Res. 2015;194:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |