Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2855
Peer-review started: September 13, 2023
First decision: September 28, 2023
Revised: October 18, 2023
Accepted: November 21, 2023
Article in press: November 21, 2023
Published online: December 27, 2023
Processing time: 105 Days and 3.9 Hours
Gastric cancer (GC) is a deadly tumor with the fifth highest occurrence and highest global mortality rates. Owing to its heterogeneity, the underlying me
To investigate the clinical outcomes of TP53 and CDH1 mutations in GC.
In this study, 202 gastric adenocarcinoma tumor tissues and their corresponding normal tissues were collected. A total of 490 genes were identified using target capture. Through t-test and Wilcoxon rank-sum test, somatic mutations, mi
The mutation rates of 32 genes, including TP53, SPEN, FAT1, and CDH1 exceeded 10%. TP53 mutations had a slightly lower overall occurrence rate (33%). The TP53 mutation rate was significantly higher in advanced stages (stage III/IV) than that in early stages (stage I/II) (P < 0.05). In contrast, CDH1 mutations were sig
Different somatic mutation patterns in TP53 and CDH1 indicate two major mechanisms of GC.
Core Tip: Mutational separation of TP53 and CDH1 in gastric cancer (GC) reveals their distinct mechanisms. TP53 mutations are associated with advanced-stage tumors and poor prognoses, whereas CDH1 mutations are associated with diffuse GC. This study highlights the heterogeneity of GC and provides insights into potential targeted therapies based on specific mutation patterns. Understanding the mutational landscape of TP53 and CDH1 can contribute to personalized treatment approaches for patients with GC.
- Citation: Liu HL, Peng H, Huang CH, Zhou HY, Ge J. Mutational separation and clinical outcomes of TP53 and CDH1 in gastric cancer. World J Gastrointest Surg 2023; 15(12): 2855-2865
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2855.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2855
Gastric cancer (GC) is one of the most severe malignancies globally, with the fifth leading incidence and highest mortality rates[1]. Global Cancer Statistics in 2018 revealed that GC was the second most prevalent malignant tumor in China, with high morbidity and mortality rates and the third leading cause of cancer-related deaths globally after lung cancer[2,3]. Although it remains unclear, the pathogenesis of GC is caused by several factors, including genetic background and the external environment[4]. Although standardized treatment for GC is continually improving, its overall incidence and mortality rates remain high. The poor prognosis of patients with GC is attributed to limited therapeutic interventions[5,6]. However, detecting hidden symptoms in the early stages is difficult; hence, most patients are diagnosed at advanced stages[7,8]. The current treatment for GC is primarily surgical resection combined with preoperative or postoperative adjuvant chemotherapy or radiochemotherapy[5,9]. Chemotherapy remains the primary method for postoperative treatment of advanced GC. D2 gastrectomy is the recommended treatment for GC, followed by postoperative adjuvant chemotherapy[10]. However, the tumor response rate to postoperative chemotherapy is low, and patients respond differently to chemotherapy[11,12]. This difference in the response to chemotherapy among patients occurs because GC is a heterogeneous disease that can manifest as differences in gene expression, biological features, and drug sensitivity[13]. Studies on the pathogenesis, biological markers, targeted sequencing, and treatment of GC are advancing given the rapid developments in molecular biology, genomics, bioinformatics, and high-throughput next-generation sequencing. Specifically, advancements in individualized treatment and precision medicine for tumors underscore the need to understand the biological characteristics of GC.
The activation of oncogenes or inactivation of tumor suppressor genes caused by somatic gene mutations modulate the development of malignant tumors, as shown by in depth research on the molecular basis of GC[14]. Therefore, identifying potential driver genes and mutations associated with GC is key to understanding the mechanism of GC occurrence and development, as well as in formulating a follow-up treatment scheme. In this study, target-capture sequencing was used to sequence 490 genes from 202 gastric adenocarcinoma (GAC) cases and adjacent tissue samples to detect somatic mutations.
This study enrolled 202 patients with GAC comprising 135 and 67 male and female patients, respectively, who underwent surgery at the Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, China, between January 1, 2014, and December 31, 2015. Primary GAC tumor tissues and matched non-cancerous (NC) tissues located at least 5 cm away from the tumor core were obtained after surgical resection, immediately processed, and stored for subsequent use. None of the recruited patients received chemotherapy or radiotherapy before surgery. Histopathological diagnosis was performed preoperatively and confirmed surgically based on the World Health Organization Classification of Tumors[15]. Tumor stage was defined according to the eighth IASLC (international association for the study of lung cancer)/AJCC (American joint committee on cancer) staging system[16]. Written informed consent was obtained from each patient prior to surgery. This study was approved by the Research Ethics Committee of Central South University (NO. 2023087), China. All specimens were handled and anonymized according to ethical and legal guidelines.
DNA was extracted from the cancer and NC tissues using a customized panel from Roche NimbleGen, Inc. The customized panel included the exons and hotspots of 490 genes, with a total length of 1 Mb. An × 10 sequencer (Illumina Inc.) was used for sequencing in the PE150 mode. All patients underwent curative resection; 27 IA-stage patients did not receive comprehensive treatment, 22 IB-stage patients received S1 chemotherapy, and patients with stage II and above received SOX chemotherapy. After treatment, the patient follow-ups were conducted via phone calls and online contact.
Mapping and somatic mutation calling: quality control was performed on raw sequencing data using FastQC[17]; and the sequences were trimmed for adapters and low-quality bases using Trimmomatic, version 0.38, HEADCROP:3 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36[18]. The trimmed reads were then aligned to GRCh37/hg19 using BWA-MEM version 0.7.17[19]. PICARD[20] was used to add read groups and mark the duplicates. The Genome Analysis Toolkit version 3.8[21] was used for realignment of the indel area and base quality recalibration. The Genome Analysis Toolkit was also used for germline and somatic variant calling with Haplotype Caller and MuTect2, respectively. The variants were annotated using ANNOVAR[22].
Microsatellite instability (MSI) detection: Five commonly used MSI sites, BAT25, BAT26, NR21, NR24, and MONO27, were used for detection. MSI-high (MSI-H) and MSI-low (MSI-L) were selected if at least two loci between the cancer and NC tissues was correspondingly unstable.
All statistical analyses were performed using the SPSS software package (version 23.0 (SPSS Inc., Chicago, IL, United States) and R [R Core Team (2018). R: Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/]. Quantitative data are presented as mean ± SD. Pearson's Chi-squared test was used to compare the difference among ranked data, whereas the one way analysis of variance test was performed to compare the differences among quantitative data. Survival analyses were performed using the Kaplan–Meier method and compared using the Wilcoxon log-rank test. P < 0.05 indicated statistically significant differences. For survival analysis, overall survival (OS) was defined as the period from the date of pathological diagnosis to the date of death or last follow-up. The cause of death in this study was the aggravation of GC.
The average age of the cohort was 55.53 ± 10.25 years, range 26–82 years. Tumor diameters were < 5 cm in 158 patients and ≥ 5 cm in 44 patients. There were 39 and 163 cases in the medium-to-high and low differentiation groups, respectively. In terms of TNM stage, 94 and 108 patients were classified with stage I/II and III/IV disease, respectively. Local lymph node metastasis was detected in 127 patients, and no metastasis was observed in 75 patients.
In all tumor and NC samples, the average sequencing base was 2.17 Gb and 1.19 Gb, and the mean sequencing depths were 829 × and 457 × respectively. In the target area, each pair of samples exhibited a mean somatic mutation of 23.1. Among all mutations, point mutations constitute the majority[23], of which missense mutations account for the largest fraction[24]. Small indels primarily comprise of frameshift mutations. Among the point mutations, the order of mutation type sorted by proportion was C > T, followed by T > C, and T > G. Simultaneously, the ratio of C > T mutations is related to age. Older patients had a larger ratio of C > T mutations, possibly because of somatic methylation and lifespan. All sites were stable between tissues and the samples were microsatellite-stable (MSS).
Among the 202 samples, nine MSI-H, 19 MSI-L, and 172 MSS were detected, whereas the MSI states of the remaining two samples could not be determined[25-28]. Two MSS samples were filtered out for all single-nucleotide variants under standard criteria (variation quality: PASS, location: exon, and mutation frequency > 0.01); thus, 200 samples were used for mutation-related analysis.
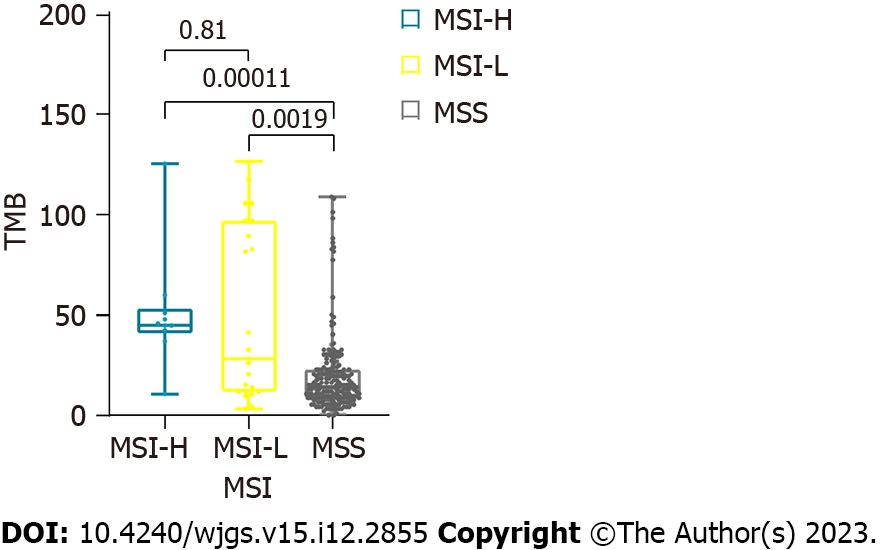
The tumor mutation burden (TMB) was calculated as the total number of somatic mutations divided by the capture size in Mb[29]. The TMB values in the MSI-H and MSI-L samples were significantly higher than those in the MSS samples (Wilcoxon rank-sum test, both P < 0.01) (Figure 1). The TMB values were 19.0 and 55.0 in the MSS and MSI samples, respectively, with an average of 52.5 and 56.1 for MSI-H and MSI-L, respectively. Nevertheless, no significant difference was noted in TMB values between the MSI-H and MSI-L groups[30,31]. The proportion of somatic point mutations in MSI samples was significantly higher than that in MSS samples, which is consistent with previous findings[32]. The increase in somatic mutations caused by MSI was not statistically significant according to pathological classification (Lauren classification) or clinical stage (TNM stage).
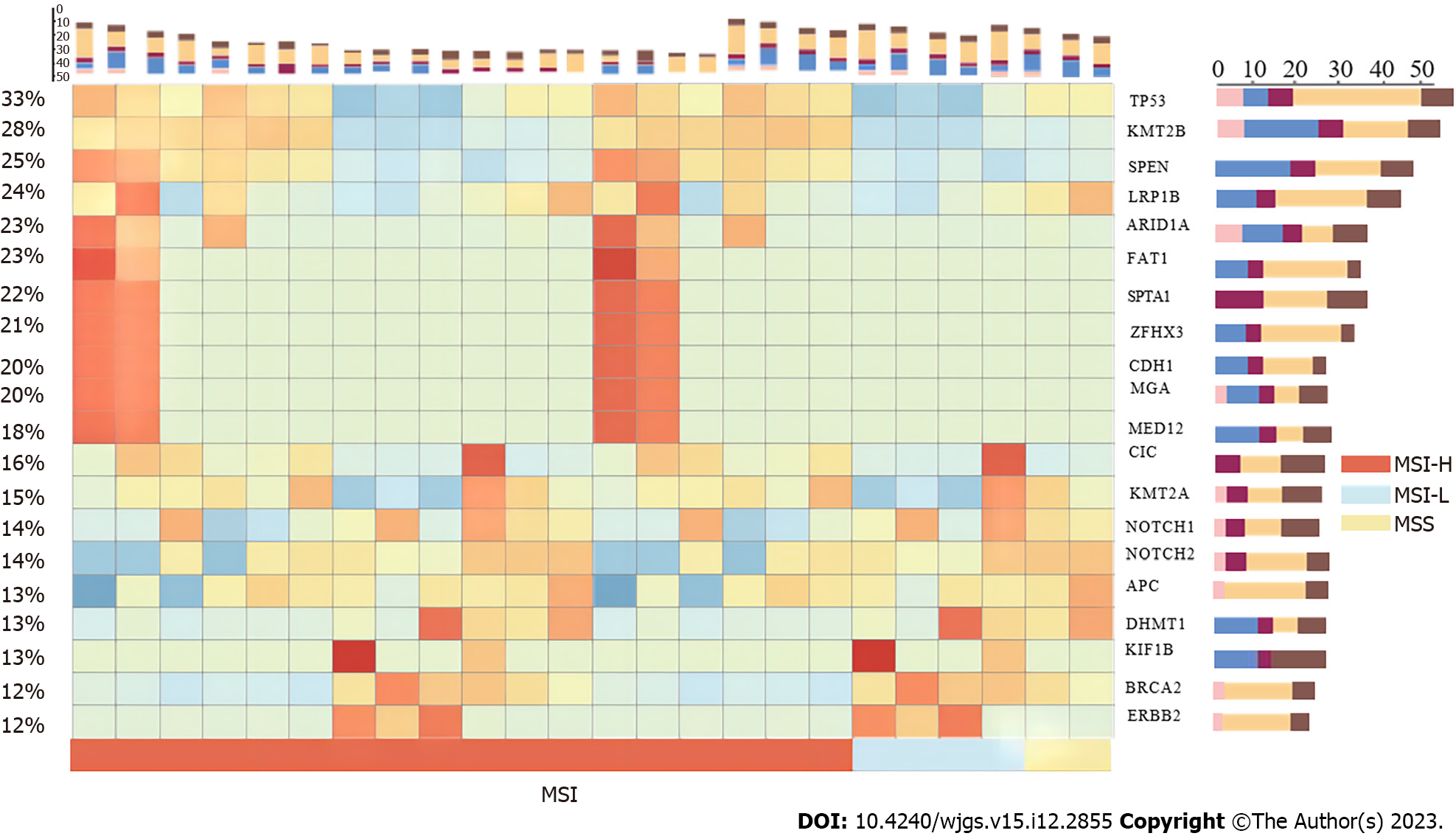
Nearly all patients harbored somatic mutations in the target area. The most commonly mutated gene was TP53, as previously discovered; however, the mutation rate of TP53 was 33%, which is far lesser than that reported in other studies[23,26,27,33,34]. In total, 32 genes had mutation rates > 10%, and 10 of these genes (including KMT2B, SPEN, and LRP1B) had mutation rates > 20% (Figure 2). Among the MSS and MSI samples, 31.8% (54/170) and 42.8% (12/28), respectively, had somatic mutations in TP53, whereas the difference was not significant between the groups (P < 0.3). Moreover, no obvious differences in the ratio of gene mutations were noted in the tumor differentiation levels or pathological types.
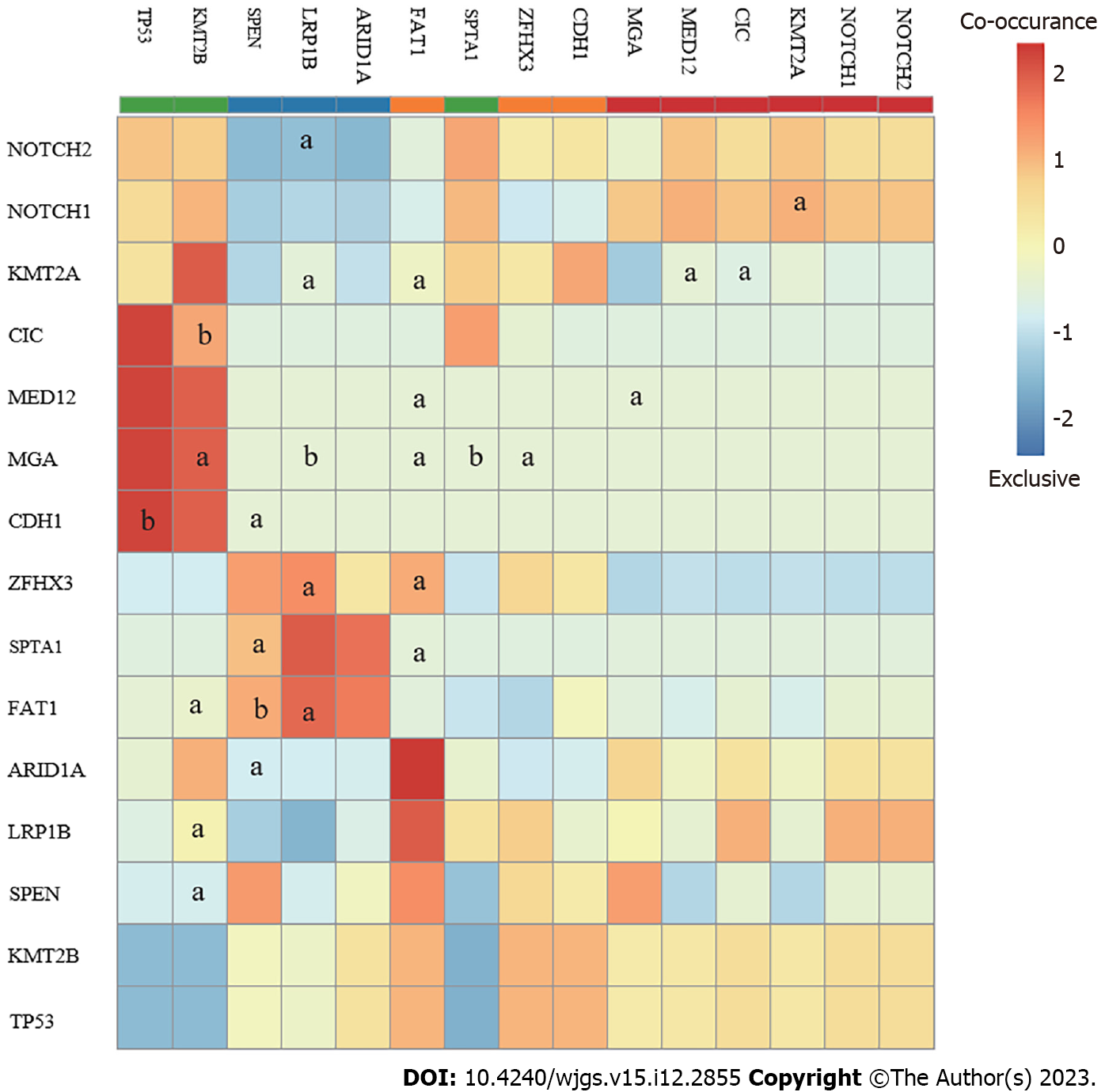
After co-analyzing the top 15 somatically mutated genes, TP53 did not co-mutate with other genes but was exclusively mutated with CDH1. TP53 was the most frequently mutated somatic protein in the GAC and modulated tumorigenesis. Co-analysis results suggested that TP53 mutations may be a special molecular type that does not interact with other genes during GC occurrence (Figure 3). CDH1 is an important gene associated with GAC. Therefore, further investigations were necessary because mutated TP53 and CDH1 may indicate two distinct patterns in the pathogenesis of GAC. Additionally, all genes, including FAT1, MGA, and ZFHX3, were co-mutated, except for TP53 and CDH1. Mutations in TP53 and CDH1 may present different patterns (Figure 4).
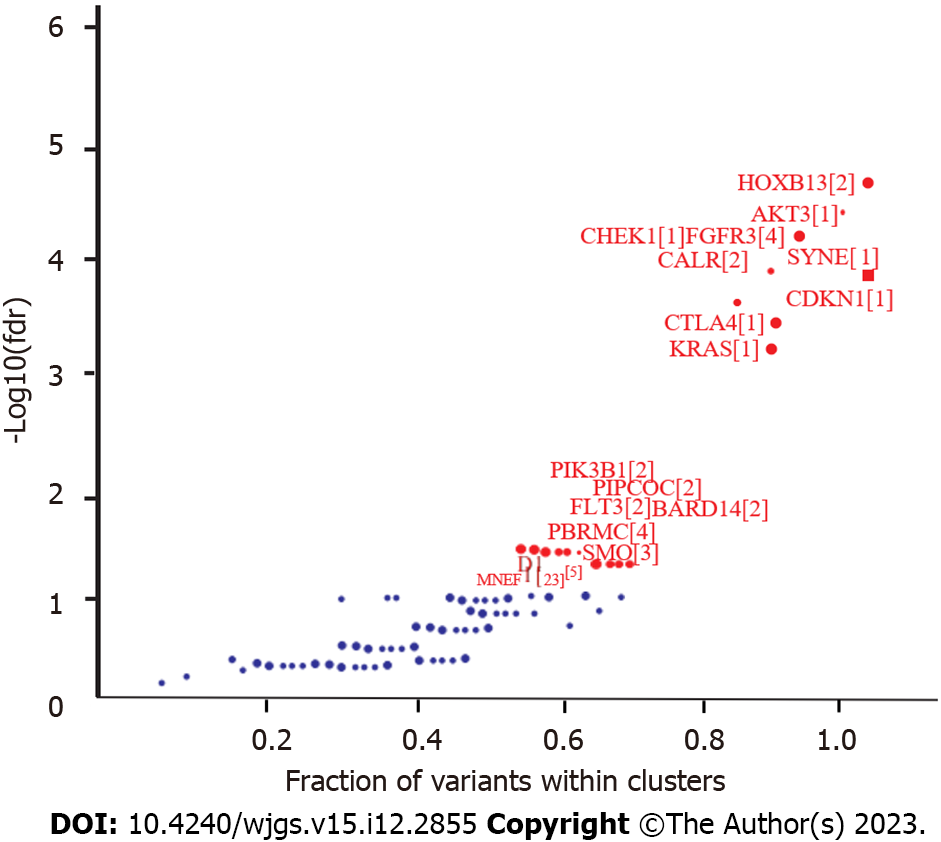
The driver genes were identified using OncodriveCLUST in maftools[35], followed by strict additional filtering criteria to focus on top driver genes (Figure 3). A total of 59 genes (false discovery rate < 0.05) were detected, of which only seven genes, including KMT2B, SPEN, FAT1, MGA, MED12, KIF1B, and ERBB2, overlapped with the top 20 somatically mutated genes. Most of the top driver genes, including HOXB13, AKT3, CHEK1, FGFR3, and CALR, were not included in the list of somatically mutated genes. Meanwhile, the top mutated genes, including TP53 and CDH1 were not important in the driver gene clusters because more regional clusters were identified based on the positions of the gene mutations. Low HOXB13 expression is responsible for poor tumor differentiation, metastasis[36], and poor prognosis of GC. AKT3, which has a somatic mutation frequency of 7.5%, is an important driver gene. AKT3 is an isoserine/threonine protein kinase that regulates TP53 activity through acetylation. CHEK1 (also known as CHK1), a gene involved in the DNA damage checkpoint pathway, cooperates with mismatch repair (MMR) deficiency to trigger chromosomal instability in MMR-deficient colorectal cancer cells[37]. Among the eight samples with CHEK1 mutations, the numbers of MSI-H, MSI-L, and MSS were two, two, and four, respectively, which were significantly different from those in the whole cohort (P < 0.05). High CALR expression was observed in 20 of 30 patients with GC and was responsible for positive serosal invasion, lymph node metastasis, perineural invasion, and poor survival[38,39]; it is a good biomarker of prognosis in GC[40].
The OS of patients with MSS tumors was not significantly better than that of patients with MSI tumors (P = 0.215), whereas in patients with early GC (pT1), the OS of MSS was significantly longer than that of MSI (P = 0.034). The non-significant difference in OS between patients with MSI tumors and those with MSS tumors conflicts with the findings of previous reports. Several GC studies have suggested a positive relationship between MSI-H phenotype, mismatch repair deficiency, and better prognosis[41-43]. Although the outcome was not statistically significant, the survival analysis of 27 patients with stage IA suggested that patients with MSI tumors had a better prognosis than those with MSS tumors, whereas the MSS group had a better prognosis than patients with stage IB disease. Retrospective Asian studies confirmed the hypothesis that patients with MSS tumors benefit from adjuvant 5-fluorouracil-based chemotherapy, whereas those with MSI-H stage II or III GC do not[44-47]. Postoperative adjuvant chemotherapy was administered to patients with stage IB and advanced stages. Therefore, we speculated that adjuvant chemotherapy contributed to the differences in OS between patients with MSS and those with MSI at different stages.
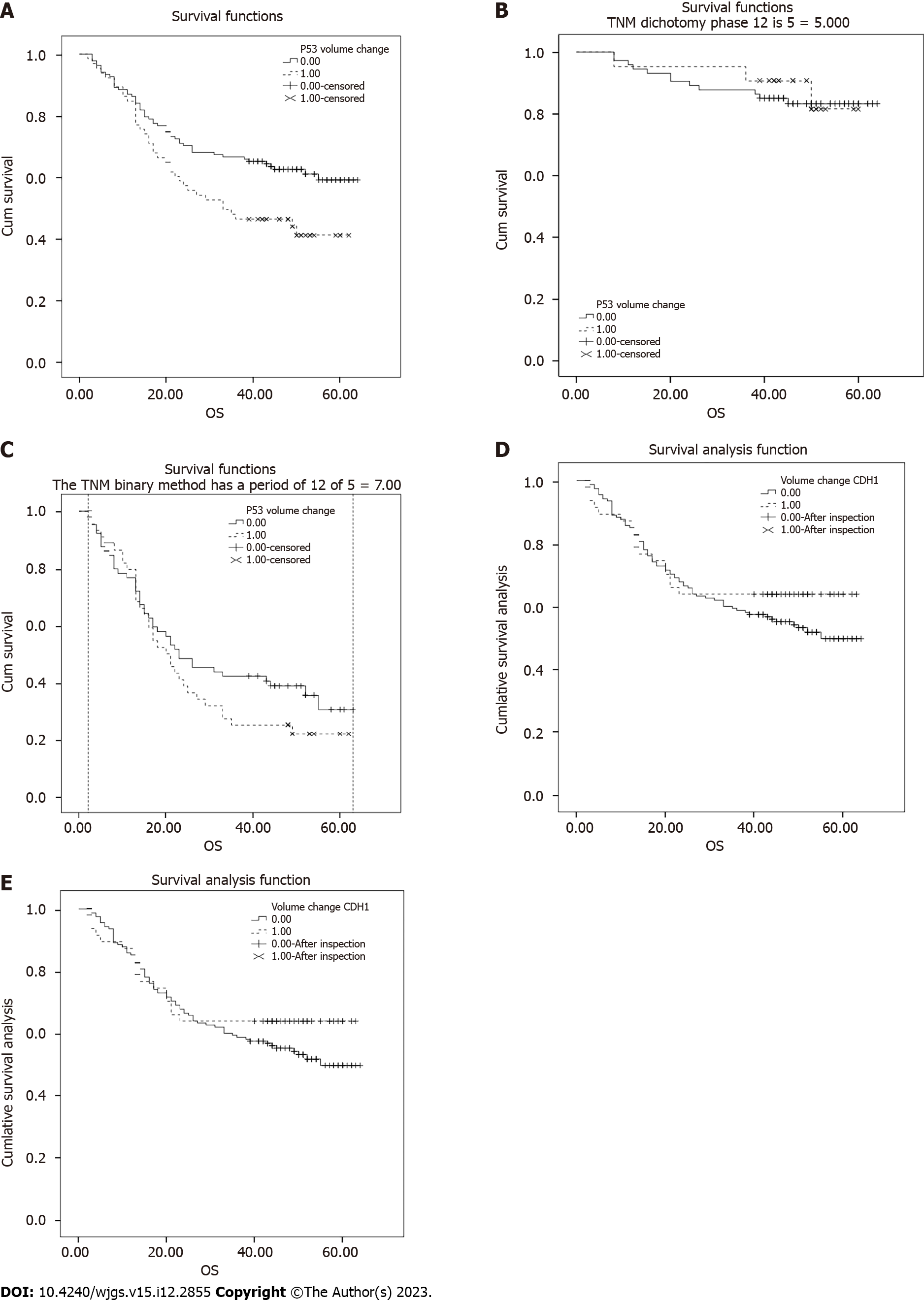
Survival analysis revealed that somatic mutations in TP53 were significantly correlated with a 5-year OS rate of 52.34% and a median survival time of 60.00 mo. Specifically, samples with TP53 somatic mutations had a significantly lower 5-year OS rate than those without TP53 somatic mutations (39% vs 58%, P = 0.01; Figure 5A). The samples were successfully classified based on pathological stage, and TP53 did not affect the OS rate in the early (I/II) or middle-late (III/IV) stages (Figure 5B and C). Thus, the TP53 mutation ratio was not associated with the pathological type, and a decrease in the OS rate was associated with a high mutation rate of TP53 in middle-late stage cases. The mean OS rate of samples with TP53 mutations was lower in diffuse GC than in those without TP53 mutations (30.87 vs 37.49, Wilcoxon P = 0.064). In contrast, CDH1 somatic mutations were not significantly associated with OS (63.83% vs 49.36%, P = 0.33; Figure 5D). Among patients without TP53 mutations, those with CDH1 mutations appeared to have higher survival rates; however, this difference was not significant. Moreover, those with both TP53 and CDH1 mutations had the worst 5-year OS rates (P = 0.02; Figure 5E). Nine patients had both TP53 and CDH1 mutations, of which one case had the highly differentiated intestinal type, whereas the other eight cases had Lauren diffuse GC.
Further investigation of the poorly differentiated diffuse cases led to the identification of 38 TP53 and 36 CDH1 mutations. Low-differentiation and diffuse GC had a higher percentage of CDH1 somatic mutations, which was significantly different from other types of GC. Survival analysis revealed an overall poor prognosis in patients with poorly differentiated diffuse GC; the presence of P53 mutations resulted in the worst prognosis, whereas that of CDH1 had no significant effect.
This study analyzed surgical cases between January 2014 and December 2015 at Xiangya Hospital and evaluated survival time after treatment. These results are similar to those of other epidemiological studies of GC in China. However, there is a lack of large-scale molecular genetic research on GC in China, because most existing studies have obtained specimens from Europe, America, Korea, and Vietnam. Our study revealed approximately 24.1 somatic mutations on average in the 1M capture area of GC samples, corroborating previous studies[23,26,27,33,34]. MSI is associated with a number of somatic mutations but may not be directly related to the pathological type and prognosis. The mutation frequencies of 32 genes, including TP53, KMT2B, SPEN, FAT1, and CDH1, in GC exceeded 10%. Our study provides molecular genetic data on Chinese patients with GC.
Our analysis of driver genes differed from that of previous studies in that TP53 and CDH1 were not identified as important driver genes. Driver gene mutations are typically increase the net cell growth under specific microenvironmental conditions in cells in vivo. TP53 and CDH1 did not co-mutate with other genes, showing unique biological features in GC samples.
Other studies have suggested that TP53, a crucial gene associated with GC development, has a somatic mutation rate of approximately 50%[27,33,34]. In this study, the overall mutation rate of TP53 was 33%; however, it was significantly higher in stages III/IV (41%) than in stages I/II (23%; P < 0.01). The mutation type of TP53 was consistent with that reported in previous studies[25-27], mainly single nucleotide mutations, such as C/T and T/C. Mutations located in exon 5 accounted for approximately 36% of all TP53 mutations, consistent with other studies, hence the overall lower TP53 mutation rate may be due to the composition of the clinical samples. Somatic mutations occur exclusively in TP53 and CDH1. TP53 did not co-mutate with other genes. Therefore, TP53 mutations may represent a unique type of GC tumorigenesis. Notably, the number and probability of lymph node metastases in patients with P53 mutations have increased, which may promote a high incidence of TP53 mutations in stage III/IV GC. Consequently, TP53 mutations modulate lymph node metastasis.
CDH1 is closely associated with GC and is the causative gene of diffuse hereditary lung cancer. In diffuse GC, the CDH1 mutation rate (25%) was higher than that in the non-diffuse type (11%; P < 0.05), which was consistent with previous findings. In contrast to TP53 mutations, CDH1 mutations play an important role in the development of diffuse GC through distinct mechanisms. As there is a clear connection between TP53 and tumor development, tumors with poor prognosis may be more likely to be in advanced stages. Although they have no direct effect on prognosis, the CDH1 mutation is a substantial contributor to diffuse GC. TP53 and CDH1 mutations may indicate two different types of GC at the molecular level, which warrants further investigation. The Cancer Genome Atlas had 23.5 mutations in this area[23]. Moreover, the types and composition of mutations were similar to those reported in previous studies[26,27]. the results reported in previous studies[48,49].
GC is the third most common malignant tumor in China, and its incidence is much higher than that in Western countries. This study provides crucial molecular data on GC in Chinese patients because large-scale Chinese genetic evidence is lacking. This study revealed that TP53 and CDH1 mutations affect two important pathways in the occurrence and development of GC. The pathogenesis of GC in the Han Chinese population (in the middle and lower reaches of the Yangtze River), as well as the diagnosis and treatment of GC, would benefit from our findings. The proportion of MSI samples was consistent with that in previous research[30]. The prevalence of MSI-H GC in Asians is commonly < 10% of all GC cases[31], which is lower than most of the occurrence rates reported in Western studies.
Gastric cancer (GC) is the third most common malignant tumor in China, and its incidence is much higher than that in Western countries. This study provides crucial molecular data on GC in Chinese patients because large-scale Chinese genetic evidence is lacking. This study revealed that TP53 and CDH1 mutations affect two important pathways in the occurrence and development of GC. The pathogenesis of GC in the Han Chinese population (in the middle and lower reaches of the Yangtze River), as well as the diagnosis and treatment of GC, would benefit from our findings.
One of the challenges to the design of effective treatments for GC is heterogeneity, its poses an obstacle for the uniform therapy plan irrespective of specific subtypes of tumors in clinical practice.
TP53 and CDH1 have been reported to be closely related to GC; therefore, we aimed to investigate the clinical outcomes of TP53 and CDH1 mutations in GC.
Two hundred and two primary GC tissues and matched non-cancerous (NC) tissues were sampled via surgery. After DNA extraction for cancer tissue and NC tissue, DNA was captured using customized panel from Roche NimbleGen Inc. The customized panel included the exons and hotspots of 490 genes with a total length of 1 Mb 10 sequencer (Illumina, Inc).
The mutation rates of 32 genes exceeded 10% including TP53, SPEN, FAT1, and CDH1 etc. We found that TP53 mutations had a slightly lower overall occurrence rate (33%), whereas the mutation type was similar to that reported in other studies. The TP53 mutation rate was significantly higher in the advanced stages (stage III/IV) than that in the early stages (stage I/II) (P < 0.05). In contrast, we also found that CDH1 mutation is significantly related to diffuse GC. TP53 is related to the poor prognosis of advanced-stage tumors; nevertheless, CDH1 corresponds to a diffuse type of cancer. Moreover, TP53 was exclusively mutated to CDH1, which is the major reason for the two different GC mechanisms.
Different somatic mutation patterns of TP53 and CDH1 indicate two major mechanisms underlying GC.
Understanding the mutational landscape of TP53 and CDH1 would positively affect the pathogenesis of GC in the Han Chinese population (in the middle and lower reaches of the Yangtze River), as well as guiding the diagnosis and treatment of GC.
We are sincerely grateful to Yin-Ming Han and Zhi-Liang Fu for their assistance with data sequencing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hauters P, Belgium; Sabzikarian M, Iran S-Editor: Lin C L-Editor: A P-Editor: Yu HG
| 1. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 726] [Article Influence: 103.7] [Reference Citation Analysis (1)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 3. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1122] [Article Influence: 187.0] [Reference Citation Analysis (1)] |
| 4. | Yusefi AR, Bagheri Lankarani K, Bastani P, Radinmanesh M, Kavosi Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac J Cancer Prev. 2018;19:591-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 161] [Reference Citation Analysis (0)] |
| 5. | Dikken JL, van de Velde CJ, Coit DG, Shah MA, Verheij M, Cats A. Treatment of resectable gastric cancer. Therap Adv Gastroenterol. 2012;5:49-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Yu B, Xie J. Identifying therapeutic targets in gastric cancer: the current status and future direction. Acta Biochim Biophys Sin (Shanghai). 2016;48:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Maconi G, Manes G, Porro GB. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol. 2008;14:1149-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: current status of diagnosis and treatment. Cancers (Basel). 2013;5:48-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Kilic L, Ordu C, Yildiz I, Sen F, Keskin S, Ciftci R, Pilanci KN. Current adjuvant treatment modalities for gastric cancer: From history to the future. World J Gastrointest Oncol. 2016;8:439-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 11. | Zhao JH, Gao P, Song YX, Sun JX, Chen XW, Ma B, Yang YC, Wang ZN. Which is better for gastric cancer patients, perioperative or adjuvant chemotherapy: a meta-analysis. BMC Cancer. 2016;16:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Li X, Cai H, Zheng W, Tong M, Li H, Ao L, Li J, Hong G, Li M, Guan Q, Yang S, Yang D, Lin X, Guo Z. An individualized prognostic signature for gastric cancer patients treated with 5-Fluorouracil-based chemotherapy and distinct multi-omics characteristics of prognostic groups. Oncotarget. 2016;7:8743-8755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, Tan SH, Wu J, Lee MH, Ooi CH, Rha SY, Wong WK, Boussioutas A, Yeoh KG, So J, Yong WP, Tsuburaya A, Grabsch H, Toh HC, Rozen S, Cheong JH, Noh SH, Wan WK, Ajani JA, Lee JS, Tellez MS, Tan P. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476-485, 485.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 14. | Bracken-Clarke D, Kapoor D, Baird AM, Buchanan PJ, Gately K, Cuffe S, Finn SP. Vaping and lung cancer - A review of current data and recommendations. Lung Cancer. 2021;153:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumors of the digestive system. Geneva: World Health Organization, 2010. |
| 16. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4397] [Article Influence: 549.6] [Reference Citation Analysis (4)] |
| 17. | Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc. |
| 18. | Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114-2120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30322] [Cited by in RCA: 41063] [Article Influence: 3733.0] [Reference Citation Analysis (1)] |
| 19. | Li H. Aligning sequence reads, clone sequences, and assembly contigs with BWA-MEM; 2013 [cited DATE]. Database: figshare [Internet]. Available from: https://doi.org/10.6084/M9.FIGSHARE.963153.V1. |
| 20. | Lin D, Zou Y, Li X, Wang J, Xiao Q, Gao X, Lin F, Zhang N, Jiao M, Guo Y, Teng Z, Li S, Wei Y, Zhou F, Yin R, Zhang S, Xing L, Xu W, Wu X, Yang B, Xiao K, Wu C, Tao Y, Yang X, Zhang J, Hu S, Dong S, Ye S, Hong Z, Pan Y, Yang Y, Sun H, Cao G. MGA-seq: robust identification of extrachromosomal DNA and genetic variants using multiple genetic abnormality sequencing. Genome Biol. 2023;24:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16037] [Cited by in RCA: 18942] [Article Influence: 1262.8] [Reference Citation Analysis (0)] |
| 22. | Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7976] [Cited by in RCA: 10438] [Article Influence: 695.9] [Reference Citation Analysis (0)] |
| 23. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4848] [Article Influence: 440.7] [Reference Citation Analysis (2)] |
| 24. | Chen K, Yang D, Li X, Sun B, Song F, Cao W, Brat DJ, Gao Z, Li H, Liang H, Zhao Y, Zheng H, Li M, Buckner J, Patterson SD, Ye X, Reinhard C, Bhathena A, Joshi D, Mischel PS, Croce CM, Wang YM, Raghavakaimal S, Lu X, Pan Y, Chang H, Ba S, Luo L, Cavenee WK, Zhang W, Hao X. Mutational landscape of gastric adenocarcinoma in Chinese: implications for prognosis and therapy. Proc Natl Acad Sci U S A. 2015;112:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 25. | Yang Q, Zhu C, Zhang Y, Wang Y, Zhu L, Yang X, Li J, Nie H, Jiang S, Zhang X, Cao X, Li Q, Tian G, Hu L, Zhao G, Zhang Z. Molecular analysis of gastric cancer identifies genomic markers of drug sensitivity in Asian gastric cancer. J Cancer. 2018;9:2973-2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Tahara T, Shibata T, Okamoto Y, Yamazaki J, Kawamura T, Horiguchi N, Okubo M, Nakano N, Ishizuka T, Nagasaka M, Nakagawa Y, Ohmiya N. Mutation spectrum of TP53 gene predicts clinicopathological features and survival of gastric cancer. Oncotarget. 2016;7:42252-42260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Pan X, Ji X, Zhang R, Zhou Z, Zhong Y, Peng W, Sun N, Xu X, Xia L, Li P, Lu J, Tu J. Landscape of somatic mutations in gastric cancer assessed using next-generation sequencing analysis. Oncol Lett. 2018;16:4863-4870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Podolskiy DI, Lobanov AV, Kryukov GV, Gladyshev VN. Analysis of cancer genomes reveals basic features of human aging and its role in cancer development. Nat Commun. 2016;7:12157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Takeshima H, Ushijima T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis Oncol. 2019;3:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 30. | Zhu L, Li Z, Wang Y, Zhang C, Liu Y, Qu X. Microsatellite instability and survival in gastric cancer: A systematic review and meta-analysis. Mol Clin Oncol. 2015;3:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Kim JY, Shin NR, Kim A, Lee HJ, Park WY, Kim JY, Lee CH, Huh GY, Park DY. Microsatellite instability status in gastric cancer: a reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J Pathol. 2013;47:28-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Park J, Yoo HM, Jang W, Shin S, Kim M, Kim Y, Lee SW, Kim JG. Distribution of somatic mutations of cancer-related genes according to microsatellite instability status in Korean gastric cancer. Medicine (Baltimore). 2017;96:e7224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Tan P, Yeoh KG. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology. 2015;149:1153-1162.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 34. | Li X, Wu WK, Xing R, Wong SH, Liu Y, Fang X, Zhang Y, Wang M, Wang J, Li L, Zhou Y, Tang S, Peng S, Qiu K, Chen L, Chen K, Yang H, Zhang W, Chan MT, Lu Y, Sung JJ, Yu J. Distinct Subtypes of Gastric Cancer Defined by Molecular Characterization Include Novel Mutational Signatures with Prognostic Capability. Cancer Res. 2016;76:1724-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747-1756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1228] [Cited by in RCA: 3099] [Article Influence: 442.7] [Reference Citation Analysis (0)] |
| 36. | Sui BQ, Zhang CD, Liu JC, Wang L, Dai DQ. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Oncol Lett. 2018;15:8833-8840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Jardim MJ, Wang Q, Furumai R, Wakeman T, Goodman BK, Wang XF. Reduced ATR or Chk1 expression leads to chromosome instability and chemosensitization of mismatch repair-deficient colorectal cancer cells. Mol Biol Cell. 2009;20:3801-3809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Sun J, Mu H, Dai K, Yi L. Calreticulin: a potential anti-cancer therapeutic target. Pharmazie. 2017;72:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 39. | Chen CN, Chang CC, Su TE, Hsu WM, Jeng YM, Ho MC, Hsieh FJ, Lee PH, Kuo ML, Lee H, Chang KJ. Identification of calreticulin as a prognosis marker and angiogenic regulator in human gastric cancer. Ann Surg Oncol. 2009;16:524-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Han Y, Liao Q, Wang H, Rao S, Yi P, Tang L, Tian Y, Oyang L, Shi Y, Zhou Y. High expression of calreticulin indicates poor prognosis and modulates cell migration and invasion via activating Stat3 in nasopharyngeal carcinoma. J Cancer. 2019;10:5460-5468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Lin JT, Wu MS, Shun CT, Lee WJ, Wang JT, Wang TH, Sheu JC. Microsatellite instability in gastric carcinoma with special references to histopathology and cancer stages. Eur J Cancer. 1995;31A:1879-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Polom K, Marano L, Marrelli D, De Luca R, Roviello G, Savelli V, Tan P, Roviello F. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg. 2018;105:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 43. | Mathiak M, Warneke VS, Behrens HM, Haag J, Böger C, Krüger S, Röcken C. Clinicopathologic Characteristics of Microsatellite Instable Gastric Carcinomas Revisited: Urgent Need for Standardization. Appl Immunohistochem Mol Morphol. 2017;25:12-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 44. | An JY, Kim H, Cheong JH, Hyung WJ, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | Fang WL, Chang SC, Lan YT, Huang KH, Chen JH, Lo SS, Hsieh MC, Li AF, Wu CW, Chiou SH. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J Surg. 2012;36:2131-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, Fassan M, Rugge M, Valeri N, Okines A, Hewish M, Allum W, Stenning S, Nankivell M, Langley R, Cunningham D. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017;3:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 391] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 47. | Kim SY, Choi YY, An JY, Shin HB, Jo A, Choi H, Seo SH, Bang HJ, Cheong JH, Hyung WJ, Noh SH. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: Results from a large cohort with subgroup analyses. Int J Cancer. 2015;137:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 48. | Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, Li X, Babur O, Hsu TK, Lichtarge O, Weinstein JN, Akbani R, Wheeler DA. Integrated Analysis of TP53 Gene and Pathway Alterations in The Cancer Genome Atlas. Cell Rep. 2019;28:1370-1384.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 49. | Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, Santos TA, Claro I, Carvalho J, Nielsen C, Padilla S, Lum A, Talhouk A, Baker-Lange K, Richardson S, Lewis I, Lindor NM, Pennell E, MacMillan A, Fernandez B, Keller G, Lynch H, Shah SP, Guilford P, Gallinger S, Corso G, Roviello F, Caldas C, Oliveira C, Pharoah PD, Huntsman DG. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015;1:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |