Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2831
Peer-review started: September 14, 2023
First decision: November 2, 2023
Revised: November 15, 2023
Accepted: December 5, 2023
Article in press: December 5, 2023
Published online: December 27, 2023
Processing time: 104 Days and 4 Hours
Radiation enteritis, which often occurs during radiation-induced acute intestinal symptoms (RIAIS), is the most common and important complication during radiotherapy for cervical cancer. RIAIS caused by abdominal and pelvic radiotherapy will affect nutrient intake, digestion, absorption, and metabolism, leading to malnutrition or poorer nutritional status. In patients with malignant tumors, malnutrition can adversely affect the curative effect and response of radiotherapy by reducing radiosensitivity, affecting the precision of radiotherapy placement and increasing the incidence of radiotherapy-related adverse reactions.
To analyze nutritional risk, skeletal muscle depletion, and lipid metabolism phenotype in acute radiation enteritis.
Fifty patients with cervical cancer received external beam radiotherapy, and 15 patients received brachytherapy after external beam radiotherapy. Body weight, body composition parameters, nutritional risk screening (NRS) 2002 score, and blood biochemical indices of patients with cervical cancer during periradiation were tested by a one-way repeated measures analysis of variance. Metabolomics analysis was used to identify characteristic lipid metabolism pathways. Clinical factors that affect linoleic acid changes were screened using the generalized evaluation equation.
Among the 50 patients, 37 had RIAIS, including 34 patients with grade 1-2 RIAIS and 3 patients with grade 3 RIAIS. The NRS 2002 score of patients who underwent cervical cancer radiotherapy continued to increase during the periradiation period, and 42 patients who underwent cancer radiotherapy had nutritional deficits (NRS 2002 score ≥ 3 points) at the end of radiotherapy. Correlation analyses revealed that body weight and body mass index changes were closely associated with body fat content (R2 = 0.64/0.51). The results of the univariate analysis showed that radiotherapy time, percentage reduction of serum albumin, and percentage reduction of serum prealbumin were the key factors affecting skeletal muscle exhaustion (P < 0.05). Metabolomic analysis of fecal supernatants of cervical cancer patients during the periradiation period revealed the involvement of linoleic acid, cholic acid, arachidonic acid, and N-acetyl-L-benzene alanine in the metabolic pathway of linoleic acid.
Cervical cancer radiotherapy patients faced nutritional risks, decreased serum albumin synthesis, and increased risk of skeletal muscle exhaustion. Linoleic acid was a biomarker of high nutritional risk.
Core Tip: The periradiation period of patients with cervical cancer increases with the time of radiotherapy, and the decrease in serum albumin synthesis increases the risk of skeletal muscle exhaustion. Linoleic acid is a biomarker for patients with radiation-induced acute intestinal symptoms at high nutritional risk. Patients undergoing external irradiation for cervical cancer should undergo regular nutritional risk assessments and individualized nutritional interventions.
- Citation: Ma CY, Zhao J, Qian KY, Xu Z, Xu XT, Zhou JY. Analysis of nutritional risk, skeletal muscle depletion, and lipid metabolism phenotype in acute radiation enteritis. World J Gastrointest Surg 2023; 15(12): 2831-2843
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2831.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2831
Organs at risk (OAR) in the target area of abdominal and pelvic radiotherapy included the small intestine, colon, rectum, and bladder. Radiation enteritis (RE) is the most common and important complication during radiotherapy for cervical cancer; it can be divided into acute phase RE and chronic phase RE. Cases with severe RE may present with complications such as intestinal wall ulcers, intestinal wall perforation, and even sinus formation[1]. Acute or chronic RE caused by abdominal and pelvic radiotherapy will affect nutrient intake, digestion, absorption, and metabolism, leading to malnutrition or poorer nutritional status[2].
In patients with malignant tumors, malnutrition can adversely affect the curative effect and response of radiotherapy by reducing radiosensitivity, affecting the accuracy of radiotherapy placement, increasing the incidence of radiotherapy-related adverse reactions, reducing radiotherapy tolerance, and even prolonging the total length of hospital stay, affecting the quality of life of patients[3,4]. Although molecular target research on the nutritional risk induced by RE is currently lacking, it is now a research hotspot, and the intestinal barrier itself and the intestinal environment can be observed and studied intuitively and non-invasively using metabolites in human fecal supernatants.
In this study, we analyzed the nutritional risk and metabolite targets in the fecal supernatant while the patient was experiencing acute radiation-induced acute intestinal symptoms (RIAIS), the changing trends in these aspects, and the relationship between nutritional risk and metabolite targets with RIAIS.
In this study, 51 patients with cervical cancer who received local pelvic radiotherapy in the Department of Radiation Therapy of the First Affiliated Hospital of Soochow University between September 2017 and June 2018 were initially included. After excluding 1 patient whose specimen did not meet the criteria of the stool sample collection process, 50 patients were included.
We included patients who: (1) Were aged 18–80 years; (2) Were pathologically diagnosed with cervical cancer; (3) Had no history of intestinal or metabolic diseases; (4) Voluntarily accepted the nutritional advice of the clinical nutritionist for 3 d before radiotherapy for diet standardization; (5) Had good comprehension and communication skills; and (6) Consented to routine bloods, blood biochemistry, and stool tests to be performed and body composition data to be noted at the start of radiotherapy (T0), the 2nd week (T2), and 4th week (T4) after the start of radiotherapy and at the end of radiotherapy (Tf).
We excluded patients: (1) With severe heart, liver, and kidney insufficiency; (2) Who could not stand independently to complete body composition measurements; (3) Who had a cardiac pacemaker installed (a contraindication to body composition analysis); (4) Whose treatment had to be terminated due to severe complications during or after radiotherapy (e.g., cardiopulmonary complications, liver and kidney failure, severe infection, severe bone marrow suppression, RE above grade 3, massive bleeding, recto-vaginal fistula, and vesico-vaginal fistula); (5) Who did not consent for biological sample collection at the above-mentioned time points; and (6) Who did not consent for participation in this study.
This study was approved by the Hospital Ethics Committee (2016 Ethics Approval No. 100), and all healthy controls and enrolled patients gave written informed consent. We certify that the study was performed according to the 1964 declaration of HELSINKI and later amendments.
Positioning for radiotherapy simulation: The patients were placed in the supine position, in which they were fixed with a vacuum pad. They were instructed to empty the bladder 1 h before being put in this position, drink 800 mL of water, and fill the bladder. All patients underwent simulated computed tomography (CT) positioning using a Philips large-aperture CT machine (Philips, Brilliance CT Big Bore), followed by enhanced CT scanning (slice thickness, 5 mm). The scanned region ranged from the upper border of the 11th thoracic vertebra to 5 cm below the ischial tuberosity. CT positioning images were transmitted to the Swedish Monaco (V5.1.1, Elekta, Sweden) treatment planning system.
Delineation of the target area for radiotherapy: Magnetic resonance imaging, CT, or positron emission tomography-CT examinations are routinely performed pre-radiotherapy evaluation. As described in the following steps, the target area for pelvic external irradiation of cervical cancer and OAR were delineated according to the standards of the American Radiation Therapy Oncology Group and Japan Clinical Oncology Group.
Postoperative assisted pelvic external irradiation target area: Risk factors were clarified according to the 2018 guidelines of the International Federation of Gynecology and Obstetrics before delineation: (1) High risk factors (Peters′ criteria) included lymph node metastasis, parametrial invasion, and positive surgical margin; and (2) Intermediate risk factors (squamous cell carcinoma: Sedlis’ criteria; adenocarcinoma: 4-factor model) included tumor size, lymphovascular space invasion, and cervical deep stromal invasion, among others. The clinical target volume included the pelvic lymphatic drainage area, the vaginal wall, the upper vagina (combined with the invasion situation), and the individualized delineation of the residual positive lymph nodes, suspicious lymphangiocysts, and microscopically positive ligament remnants according to the actual condition.
Radical pelvic external irradiation target volume: This individually included the tumor target volume (gross tumor volume, including primary tumor and pelvic positive lymph nodes), the cervix, uterus, vagina (combined with invasion), parametrium, and pelvic lymphatic drainage area and outlined the extended field lymphatic drainage area according to the actual condition.
OAR: Susceptible organs included the rectum, bladder, spinal cord, bilateral femoral heads, colon, and small intestine, among others, and the delineated area consisted of the upper and lower 3 cm of the planning target volume (PTV). The target area and OAR for all patients were delineated by the same physician and then reviewed by the same chief physician.
Design of the radiotherapy plan: Physicists used the Monaco treatment planning system based on the operation shown in Figure 1. The 7-field inverse dynamic volumetric modulated arc therapy (VMAT) plan design is a unified parameter during operation. The X-ray beam energy was 6 MV. More than 95% of the PTV received the prescribed dose. We ensured that no dose hotspots received ≥ 110% of the prescribed dose outside of the PTV. The dose of OAR was uniformly limited: bladder V40 < 50%; rectum V40 < 40%; colon V40 < 30%; small intestine V40 < 20%; and femoral head V45 < 5%.
Adjuvant VMAT target volume prescription dose after the operation: This dose for medium-risk PTV1 was 45 Gy/25 fractions, and high-risk PTV1 was 50.4 Gy/28 fractions; the prescribed dose of the remaining target area can be implemented according to the radical external irradiation program.
Prescription dose of radical VMAT target area: This dose was 45 Gy/25 fractions for the pelvic lymphatic drainage area (PTV1), 50 Gy/25 fractions for the parametrial region (PTV2), and 56 Gy/28 fractions for the positive lymph node. If there were positive paraaortic lymph nodes, the dose for the paraaortic lymphatic drainage area (PTV3) was 36-40 Gy/20 fractions.
Implementation of the VMAT plan: VMAT was implemented using the Synergy linear accelerator (Elekta, Sweden). All patients were treated five times a week, once a day, and at least once a week. Cone beam CT (Elekta, Sweden) image-guided VMAT was used, and the error margin for each treatment was restricted to within 3 mm.
Image-guided preparation for post-loaded radiotherapy: All patients were required to take a laxative to cleanse the intestines the night before post-load radiotherapy. In the morning, patients continued fasting and engaged in daily bowel movements while lying supine and immobilizing the pelvic area. The simulation positioning machine performed a non-enhanced CT scan, which scanned the area from the third lumbar vertebra to the mid-thigh of the patient, with a scan layer thickness of 0.3 cm. After completion of the scan, the CT images were transferred to the Oncentra system (V 4.5.3, Elekta, Sweden).
Delimitation of the target area for post-load radiotherapy: Following the delineation standards of the Groupe Européen de Curiethérapie and the European Society for Radiotherapy & Oncology, the high-risk clinical target volume, bladder, rectum, sigmoid colon, and contours were analyzed. Using high-risk clinical target volume as a reference, the sigmoid colon and rectal contours were delineated 2 cm below and 2 cm above the upper edge of the uterus, respectively.
Planning for radiotherapy: Based on the Oncentra treatment planning system, reverse planning for intracavitary radiotherapy was designed and optimized. A physicist and a senior radiation oncologist reviewed the plan and reached a consensus.
Dosage for the target area: The target area D90 was ≥ 90% of the prescription. After external beam irradiation, patients underwent two post-load 192Ir high-dose rate treatments per week, with a total treatment of 4-6 times and a frequency of 2-3 times per week.
OAR limits: Bladder D2 cm3, rectum D2 cm3, sigmoid colon D2 cm3, all < 450 cGy.
In this paper, among the 50 cervical cancer patients, 18 patients and 32 patients received concurrent chemoradiotherapy (CCRT) and sequential chemoradiotherapy, respectively. The chemotherapy regimen was administered every 21 d for a median of 3 wk (range, 2-4 wk) and included paclitaxel + carboplatin or cisplatin. The relevant chemotherapy regimen was determined based on the National Comprehensive Care Network guidelines, and all doses were adjusted.
The 2002 nutritional risk screening (NRS) tool (NRS 2002) was used for NRS within 24 h of admission. The patient’s nutritional support method was accordingly personalized and customized regarding nutritional education, oral enteral nutrition preparations, and parenteral nutritional support. The energy target was 25-30 Kcal/(kg·d). According to the patient’s tumor burden, stress state, and RIAIS, it was individualized and dynamically adjusted. During radiotherapy, the patients were advised to take a light, low-residue, high-quality protein diet.
Using the InBody S10 multiproduct bioelectrical impedance analyzer (InBody Co. Ltd., Seoul, Korea), the body composition of the healthy control group was measured on the 4th day after receiving nutritional advice and at T0, T2, T4, and Tf. The patients were asked to fast for 2 h before the test, remain quiet, remove any metal objects worn, and wear a patient’s gown. Then, the instrument was attached to them via four electrodes on both hands and feet, and their body composition was measured using multi-frequency bioelectric impedance methods. Measurements were taken for the following body composition parameters: Body weight; body fat content; skeletal muscle content (the detected fat-free tissue minus water); body mass index (BMI); and phase angle.
Routine blood (including white blood cells, neutrophils, red blood cells, hemoglobin, and platelets) and serum albumin and prealbumin data were collected at T0, T2, T4, and Tf. We then evaluated adverse reactions to radiotherapy at the four time points according to the National Institutes of Health Common Adverse Event Evaluation Criteria 5.0, which included myelosuppression grades (including white blood cells, neutrophils, red blood cells, hemoglobin, and platelets) and RIAIS grades (including diarrhea, abdominal pain, colitis, anal distention, and hematochezia).
Stool sample collection: Stool samples were collected from patients undergoing radiotherapy for cervical cancer at T0, T2, T4, and Tf. Then, 2 g of fecal samples were collected at each time point, aliquoted into cryovials, and stored in liquid nitrogen.
Metabolomics detection of fecal supernatant samples: Metabolomic analysis of the fecal supernatant was entrusted to BioNovoGene, Ltd. (Suzhou, China) for testing and quality control (see the Supplementary materials for detailed experimental steps).
SPSS 20.0 software was used for statistical analyses. Measurement data were expressed as mean ± standard deviation, and counting data were expressed as frequency and percentage. Variables at different time points were analyzed using one-way repeated measures analysis of variance. Univariate and multivariate analyses were performed using generalized evaluation equations to select independent variables that affected the dependent variable. A P value < 0.05 indicated a statistically significant difference.
In the statistical analysis of metabolomics results, the principal component analysis model obtained through unsupervised analysis methods (e.g., principal component analysis) reflected the original state of metabolomics data, which can help master radiotherapy in patients with cervical cancer. The Partial Least-Squares Discriminant Analysis method was used to eliminate systematic variation unrelated to the pathological state, helping to identify the differential metabolites in feces responsible for class separation. Orthogonal-Partial Least-Squares Discriminant Analysis can effectively reduce the model’s complexity and enhance its interpretation ability without reducing its predictive ability. This maximizes the observation of metabolite changes at different time points during radiotherapy. The basic screening conditions for metabolites were a P value ≤ 0.05 and a variable importance projection value of ≥ 1. Considering that this study involved the analysis of metabolites in four time periods, one-dimensional analysis of variance was used to search for differential metabolites further. A P value < 0.05 indicated a statistically significant difference.
In this study, 50 patients who underwent radiotherapy for cervical cancer aged 30-79 years (median age: 51 years) were included. The total dose of local pelvic radiotherapy was 45.0-50.4 Gy, and the total number of radiotherapy sessions was 25-28, administered at a frequency of 5 times a week. There were no unplanned interruptions of radiotherapy; therefore, the completion rate of radiotherapy was 100%. Among these 50 patients, 46 had squamous cell carcinoma, 2 had adenocarcinoma, and 2 had adenosquamous cell carcinoma; moreover, 40 had stage I-II disease, and 10 had stage III-IV disease. Fifty patients with cervical cancer received external beam radiotherapy, and 15 patients received brachytherapy after external beam radiotherapy. Specifically, 14 patients out of these 50 patients received radical radiotherapy, and 36 patients received postoperative adjuvant radiotherapy, among whom 13 received CCRT, and 23 received sequential chemoradiotherapy.
Among the 50 patients, 37 had RIAIS, including 34 patients with grade 1-2 RIAIS and 3 patients with grade 3 RIAIS. None of the patients had grade 4 RIAIS (see Table 1 for details). The main clinical symptoms included diarrhea, abdominal pain, colitis, anal swelling, and hematochezia; 50% had grade 1-2 myelosuppression, and 20% had grade 3 myelosuppression (Figure 2A). The NRS 2002 score of patients undergoing cervical cancer radiotherapy continued to increase in the periradiation period [1.24 ± 0.52 (T0) vs 1.60 ± 0.70 (T2) vs 2.12 ± 0.77 (T4) vs 2.96 ± 0.53 (Tf) (Figure 2B)], and 42 patients undergoing cervical cancer radiotherapy had a nutritional deficit (NRS 2002 score ≥ 3 points) at the end of radiotherapy.
| Adverse reaction | Level 0 | Level 1 | Level 2 | Level 3 |
| Diarrhea | 13 (26) | 29 (58) | 5 (10) | 3 (6) |
| Abdominal pain | 29 (58) | 18 (36) | 3 (6) | 0 (0) |
| Colitis | 38 (76) | 12 (24) | 0 (0) | 0 (0) |
| Anal swelling | 33 (66) | 11 (22) | 6 (12) | 0 (0) |
| Hematochezia | 35 (70) | 15 (30) | 0 (0) | 0 (0) |
| Myelosuppression | 15 (30) | 13 (26) | 12 (24) | 10 (20) |
Changes in body weight and body composition of patients with cervical cancer during the periradiation period are shown in Figures 2C-I (see Supplementary Table 1 for details). In the cervical cancer radiotherapy group, increasing the dose of radiotherapy was associated with decreases in body weight, skeletal muscle content, BMI, phase angle, serum albumin levels, and serum prealbumin levels, and their values were the lowest at the end of radiotherapy. Except for the reduction in body fat content, the differences for all parameters between different time points during the treatment were statistically significant for all combinations (all P < 0.05, Figure 2C-I). Correlation analyses revealed that body weight and BMI changes were closely associated with body fat content (R2 = 0.64/0.51; Figure 2J).
The dependent variable was the percentage of skeletal muscle exhaustion. Age, tumor stage, radiotherapy time, body weight, BMI, body fat content, the percent decrease in serum albumin, and the percent decrease in serum prealbumin were used as independent variables. Univariate analysis was performed using generalized evaluation equations. The results of the univariate analysis showed that radiotherapy time, the percentage reduction of serum albumin, and the percentage reduction of serum prealbumin were the key factors affecting skeletal muscle exhaustion (P < 0.05, Table 2).
| Project | B | SE | Exp (B) | 95%CI | Wald χ2 | P value | |
| Lower limit | Upper limit | ||||||
| Time of radiotherapy | |||||||
| T2 | Reference | ||||||
| T4 | 0.405 | 0.381 | 1.013 | 1.001 | 1.518 | 1.834 | 0.042 |
| Tf | 1.023 | 0.538 | 1.023 | 1.004 | 1.238 | 17.394 | 0.015 |
| Weight | 0.294 | 0.345 | 0.925 | 0.681 | 3.019 | 2.736 | 0.045 |
| Percentage reduction of serum albumin | 1.281 | 0.326 | 3.602 | 1.902 | 6.823 | 15.461 | < 0.001 |
| Percentage reduction of serum prealbumin | 0.892 | 0.411 | 2.491 | 1.138 | 2.831 | 12.028 | < 0.001 |
| Tumor staging | |||||||
| I–II | Reference | ||||||
| III–IV | 1.029 | 0.024 | 3.092 | 0.001 | 3.861 | 0.492 | 0.689 |
| BMI | 0.836 | 0.277 | 1.029 | 0.308 | 3.892 | 0.108 | 0.588 |
| Body fat content | 0.424 | 0.011 | 1.281 | 0.891 | 1.782 | 0.347 | 0.052 |
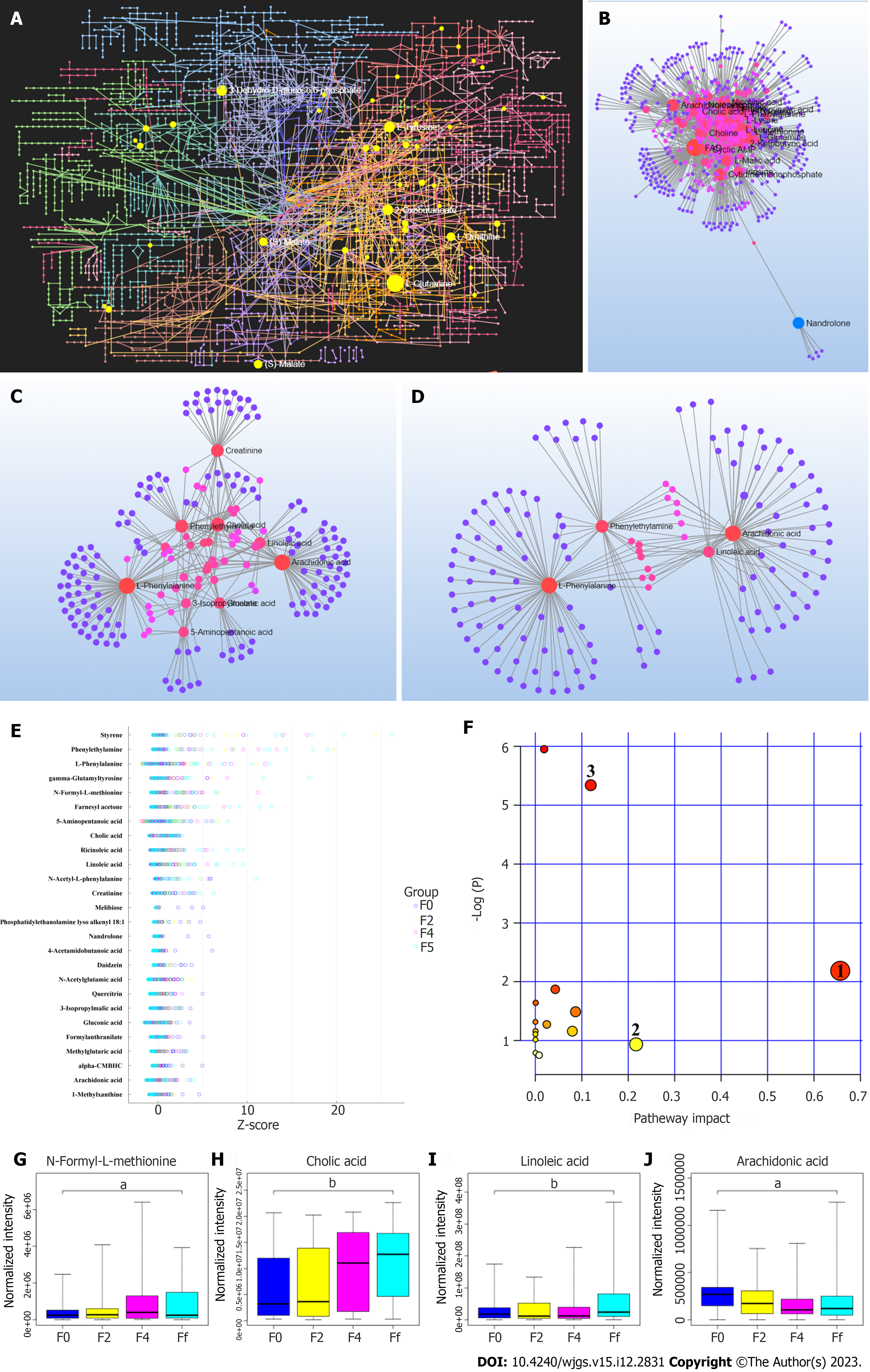
Metabolomics analysis was performed on the fecal supernatant samples (Figure 1A), and 1-21 metabolites (see Supplementary Table 2 for details) with significant changes were screened (Figure 1B). Time-variable factors were also included to screen for co-metabolites with significant changes in the fecal supernatant. Consequently, 26 co-differential metabolites were identified, namely 1-methylxanthine, cholic acid, phenylethylamine, styrene, farnesyl acetone, 5-aminopentanoic acid, N-acetylglutamic acid, phosphatidylethanolamine lyso alkenyl 18: 1, linoleic acid, gamma-glutamyltyrosine, l-phenylalanine, quercitrin, alpha-carboxymethylbutyl hydroxychroman, methylglutaric acid, creatinine, ricinoleic acid, N-acetyl-l-phenylalanine, 4-acetamidobutanoic acid, N-formyl-l-methionine, gluconic acid, nandrolone, arachidonic acid, 3-isopropylmalic acid, melibiose, daidzein, and formylanthranilate (Figure 1). These selected 26 metabolites were clustered and summarized (Figure 1E). Four molecules were identified to be involved in the linoleic acid metabolic pathway, namely linoleic acid, cholic acid, arachidonic acid, and N-acetyl-L-benzene alanine. Furthermore, levels of linoleic acid, cholic acid, and N-formyl-L-methionine were found to increase significantly in the fecal supernatant, while the expression of arachidonic acid reached its lowest before rebounding in the 4th week of radiotherapy. The changes in expression are shown in Figure 1G-H.
The change in linoleic acid content in the fecal supernatant was used as the dependent variable. Age, tumor stage, radiotherapy time, radiotherapy method, body weight, BMI, percentage of skeletal muscle exhaustion, body fat content, phase angle, percentage of serum albumin reduction, percentage of serum prealbumin reduction, and NRS 2002 scores of inpatients were used as independent variables. Univariate analysis was performed using generalized evaluation equations. The results showed statistically significant differences in radiotherapy time, percentage of skeletal muscle exhaustion, phase angle, percentage reduction of serum albumin, percentage reduction of serum prealbumin, and the NRS 2002 score (P < 0.05). Factors identified as statistically significant in the univariate analysis (P < 0.05) were included in the multivariate analysis, and the results showed that the time of radiotherapy, the percentage of skeletal muscle exhaustion, and the NRS 2002 score were associated with the linoleic acid content in the fecal supernatant. A higher NRS 2002 score was associated with a higher linoleic acid content in the fecal supernatant (odds ratio: 1.104), indicating that for every 1-point increase in the nutritional risk score, the linoleic acid content in the fecal supernatant increased by 10.4% relative to the original (Table 3).
| Project | B | SE | Exp (B) | 95%CI | Wald χ2 | P value | |
| Lower limit | Upper limit | ||||||
| Time of radiotherapy | |||||||
| T2 | Reference | ||||||
| T4 | 0.701 | 0.402 | 1.194 | 1.042 | 1.598 | 3.928 | 0.038 |
| Tf | 1.638 | 0.618 | 1.284 | 1.403 | 1.902 | 5.392 | 0.019 |
| Percentage of skeletal muscle exhaustion | 0.361 | 0.203 | 1.021 | 1.081 | 2.214 | 7.027 | 0.011 |
| NRS 2002 score | 1.029 | 0.682 | 1.104 | 1.002 | 2.482 | 12.382 | < 0.001 |
| Percentage reduction of serum albumin | 0.792 | 0.134 | 1.294 | 0.293 | 2.521 | 0.782 | 0.363 |
| Percentage reduction of serum prealbumin | 1.385 | 0.382 | 0.821 | 0.564 | 1.905 | 0.556 | 0.892 |
| Phase angle | 0.729 | 0.103 | 0.892 | 0.361 | 1.028 | 0.992 | 0.059 |
Gastrointestinal toxicity is an adverse effect commonly encountered in patients undergoing radiotherapy for cervical cancer. Folkert et al[5] found that in cervical cancer patients who received adjuvant postoperative radiotherapy and chemotherapy, acute gastrointestinal toxicity occurred in 23.5% of the cases, grade 3 RIAIS occurred in 2.9% of the cases, and chronic gastrointestinal toxicity occurred in 14.7% of the cases (all grade 1). However, due to incomplete medical history records or insufficient assessment, mild toxic reactions can often be underestimated[6].
In terms of the incidence of acute gastrointestinal reactions in patients with cervical cancer who received radical radiotherapy and chemotherapy, Dang et al[7] found that grade 1-2 RE occurred in 78.4% of the cases, and grade 3 RE occurred in 8.1% of the cases. In a review article, Laan et al[8] analyzed 515 patients with locally advanced cervical cancer who received radical radiotherapy and chemotherapy; consequently, they reported that the incidence of late RE of grade 3 or above was 11.5%. In the present study, 50 patients received intensity-modulated radiation therapy and the dose limits for surrounding normal tissues (including the small intestine, colon, rectum, and bladder) all met the standard requirements. Furthermore, the incidence rates of grade 1-2 RIAIS and grade 3 RIAIS were 68% and 6%, respectively, which were lower than the corresponding historical data reported by Dang et al[7] and Laan et al[8]. This indicates that even in the era of intensity-modulated radiotherapy, which is more effective than three-dimensional conformal radiotherapy in protecting normal tissues, RE is still worthy of attention as one of the toxic reactions during cervical cancer radiotherapy.
Patients who undergo radiotherapy for malignant tumors go through three stages during the entire radiotherapy process, which is collectively called the periradiotherapy period[9]. Accumulating evidence shows that whole-course nutritional management rather than prevention or intervention independent of a certain stage is needed for patients in this period[10,11]. The European Society for Clinical Nutrition and Metabolism[12] and the Chinese Society for Parenteral and Enteral Nutrition[13] recommend using the NRS 2002 score to screen for nutritional risk in patients with general adult cancer. An NRS 2002 total score of ≥ 3 indicates a nutritional risk. In such patients, further nutritional assessment is required. Based on the findings of the patient’s subjective overall assessment [the patient-generated subjective global assessment (PG-SGA) score], nutritional therapy pathways are selected.
Tian et al[14] used NRS 2002 with PG-SGA to evaluate the nutritional status of patients undergoing cervical cancer surgery. They found that NRS 2002 and PG-SGA are suitable for preoperative NRS for patients undergoing cervical cancer surgery. However, the positive rate of PG-SGA was higher than that of NRS 2002, and its time requirement was challenging. Thus, NRS 2002 was identified as more suitable for quick and convenient clinical evaluation. In this study, overall, 42 patients undergoing radiotherapy for cervical cancer had nutritional risk (NRS 2002 score ≥ 3 points) at the end of radiotherapy, which reflects the prevalence, commonality, and universality of nutritional risk in patients undergoing radiotherapy for malignant tumors in the periradiation period.
Weight loss is one of the main manifestations of malnutrition in patients with cervical cancer who undergo radiotherapy[15,16], particularly in cases where the radiotherapy target area covers the digestive system, or radiotherapy is administered concurrently with cisplatin chemotherapy. Lakomy et al[17] found that for patients undergoing radiotherapy for cervical cancer with the radiotherapy target area involving the abdominal aorta, limiting the duodenal dose to V55 < 15 cm3 and V60 < 2 cm3 can maximize a satisfactory local control rate and minimize duodenal toxicity, while grade 3 duodenal toxicity was associated with weight loss. A study by Ohno et al[18] confirmed that the low-dose (20-30 mg/m2 cisplatin) CCRT group had significantly higher weight loss at 3-4 wk after the start of radiotherapy than the radiotherapy alone group. Here, all patients undergoing radiotherapy for cervical cancer experienced weight loss, which was positively correlated with the dose of radiotherapy (P < 0.05); however, no adverse events such as radiotherapy intolerance occurred, which may be attributed to intensity-modulated radiotherapy reducing the severity of toxicity to normal tissues around the target area.
Body composition changes during cancer treatment are associated with poorer outcomes and increased morbidity and mortality[19]. Lee et al[20] analyzed the data on the skeletal muscle index, skeletal muscle density, and the total adipose tissue index of 278 locally advanced cervical cancer patients who received chemotherapy and radiotherapy between 2004 and 2017. They found that muscle loss after chemotherapy and radiotherapy was prominent in locally advanced cervical cancer. Kumar et al[21] confirmed that skeletal muscle depletion was significantly associated with RE during chemotherapy and radiotherapy in patients with cervical cancer.
In another clinical study, Basile et al[22] reported muscle content loss as a predictor of adverse clinical outcomes in pancreatic cancer. In animal experiments, Han et al[23] and Kim et al[24] confirmed that the muscles exhibit acute atrophy after exposure to radiation, which was attributed to a decrease in the myosin content and changes in the proportion of the myosin heavy chain. This atrophy was found to be radiation dose dependent. This shows that radiation markedly affects protein metabolism in the body. Glass et al[25] showed that an imbalance of protein synthesis and hydrolysis processes led to decreased protein synthesis and increased hydrolysis, which consequently induced muscle atrophy, leading to fatigue and limited mobility.
In the present study, we found increased radiotherapy time, decreased percentages of serum albumin and serum prealbumin, and increased percentage reduction in skeletal muscle content (suggesting that radiotherapy affects patients’ protein metabolism and skeletal muscles) in the periradiotherapy period of patients with cervical cancer undergoing radiotherapy. The goal of nutritional risk intervention should be to promote protein synthesis and absorption, slow skeletal muscle breakdown, and maintain a relatively stable body weight with the aim of improving radiotherapy accuracy, improving patient tolerance, reducing weight loss, and targeting deformation induced by reduction in body anteroposterior diameter.
Malnutrition is prevalent in cancer patients, possibly due to inflammation and altered fatty acid metabolism. Linoleic acid is a precursor of polyunsaturated fatty acids involved in the biosynthesis of prostaglandins and cell membranes. The linoleic acid metabolic pathway is reported to participate in the intestinal inflammation cascade reaction by influencing cholesterol synthesis and lipid metabolism[26,27]. Cellular lipid peroxidation processes were observed in high-dose irradiation models[28,29]. Increased levels of intracellular linoleic acid can stimulate reactive oxygen species production and activate redox-sensitive factors, such as NK-κB and AP-1[30].
Conversely, linoleic acid can cause the deposition of Ca2+ in mitochondria, leading to temporary hyperpolarization of the mitochondrial membrane, reduced consumption of oxygen, increased release of cytochrome C, and consequential apoptosis. In addition, linoleic acid is also reported to increase the expression and activity of the activated peroxisome proliferator-activated receptor-γ in adipocytes, skeletal muscle, colonic mucosa, and macrophages, thus reducing the activity of NF-κB, inhibiting arachidonic acid production, and consequently slowing the inflammation response[31,32].
In the present study, the expression of linoleic acid, cholic acid, and N-formyl-L-methionine was significantly upregulated in the fecal supernatant, and the process was time/dose-dependent; however, the expression of arachidonic acid reached its lowest before rebounding in the 4th week of radiotherapy, indicating that the intestinal mucosa still has the self-regulation effect of inflammation 4 wk before radiotherapy. However, when the prescribed dose in the target area is more than 40 Gy, the inhibitory effect of arachidonic acid is lost, suggesting that the negative feedback regulation mechanism mentioned above is facing difficulties.
At this point in time, the patients developed RIAIS-related myelosuppression, hematochezia, anal swelling, colitis, abdominal pain, and diarrhea, indicating the dose-effect relationship of RIAIS. Furthermore, when the nutritional risk score increased by 1 point in the present study, the linoleic acid content in the fecal supernatant increased by 10.4% compared to the original. Some studies suggested that the level of inflammatory factors in the body can be reduced by reducing linoleic acid when there is a certain degree of inflammatory response in the body, and the underlying mechanism probably involves inhibition of the interaction of fatty acids and their derivatives with proinflammatory cytokines[33,34]. Therefore, an appropriate nutritional intervention at this time can significantly influence the occurrence, development, and outcome of RIAIS.
In this article, we preliminarily discussed changes in body weight, body composition, and influencing factors in the periradiotherapy period of patients receiving external irradiation for cervical cancer. Cancer radiotherapy patients were identified to have nutritional risk (NRS 2002 score ≥ 3 points), but body weight, skeletal muscle content, BMI, phase angle, serum albumin levels, and serum prealbumin levels were significantly reduced in all patients. Further statistical analysis revealed that radiotherapy time, percentage reduction of serum albumin, and percentage reduction in serum prealbumin as the key factors affecting skeletal muscle exhaustion. This suggests that: (1) Decreased serum albumin synthesis increases the risk of skeletal muscle exhaustion; (2) Patients with cervical cancer should pay more attention to nutritional support during the period of radiotherapy; and (3) Nutritional risk assessment and individualized nutritional interventions should be performed regularly. Furthermore, metabolomics analysis identified the lipid metabolism pathway in the fecal supernatant as the metabolic phenotype pathway of RIAIS. Further analyses revealed that the increased nutritional risk increased the linoleic acid content in the fecal supernatant, suggesting that linoleic acid is a biomarker in RIAIS patients with high nutritional risk.
Radiation enteritis, which often occurs during radiation-induced acute intestinal symptoms (RIAIS), is the most common and important complication during radiotherapy for cervical cancer. RIAIS caused by abdominal and pelvic radiotherapy will affect nutrient intake, digestion, absorption, and metabolism, leading to malnutrition or poorer nutritional status. In patients with malignant tumors, malnutrition can adversely affect the curative effect and response of radiotherapy by reducing radiosensitivity, affecting the accuracy of radiotherapy placement, and increasing the incidence of radiotherapy-related adverse reactions.
To verify the correlation between malnutrition caused by RIAIS and intestinal lipid metabolism disorders.
To investigate the changes in nutritional risk, skeletal muscle exhaustion, and lipid metabolism phenotype and their relationships during RIAIS.
Fifty patients with cervical cancer received external beam radiotherapy, and 15 patients received brachytherapy after external beam radiotherapy. The body weight, body composition parameters, nutritional risk screening (NRS) 2002 score, and blood biochemical indices of patients with cervical cancer during periradiation were tested by one-way repeated measures analysis of variance. Metabolomics analysis was used to identify the characteristic lipid metabolism pathways. The clinical factors affecting linoleic acid changes were screened using the generalized evaluation equation.
Of the 50 patients, 37 had RIAIS, including 34 patients with grade 1-2 RIAIS and 3 patients with grade 3 RIAIS. The NRS 2002 score of patients undergoing radiotherapy for cervical cancer continued to increase in the periradiation period, and 42 patients undergoing radiotherapy for cervical cancer had nutritional deficits (NRS 2002 score ≥ 3 points) by the end of radiotherapy. Correlation analyses revealed that body weight and body mass index changes were closely associated with body fat content (R2 = 0.64/0.51). The results of the univariate analysis showed that time of radiotherapy, percentage reduction of serum albumin, and percentage reduction of serum prealbumin were the key factors affecting skeletal muscle exhaustion (P < 0.05). Metabolomics analysis of fecal supernatant during the periradiation period of patients with cervical cancer was identified to be involved in the linoleic acid metabolic pathway, namely linoleic acid, cholic acid, arachidonic acid, and N-acetyl-L-benzene alanine.
Cervical cancer radiotherapy patients faced nutritional risks, and decreased serum albumin synthesis increased the risk of skeletal muscle exhaustion. Linoleic acid was a biomarker of high nutritional risk.
Further analyses revealed that the increased nutritional risk increased the linoleic acid content in the fecal supernatant, suggesting that linoleic acid is a biomarker in RIAIS patients with high nutritional risk.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moradi L, Iran; Yadav BS, India S-Editor: Lin C L-Editor: Filipodia P-Editor: Cai YX
| 1. | Wang Y, Kong W, Lv N, Li F, Chen J, Jiao S, Ding D, Zhao H, Song D. Incidence of radiation enteritis in cervical cancer patients treated with definitive radiotherapy versus adjuvant radiotherapy. J Cancer Res Ther. 2018;14:S120-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Ma CY, Zhao J, Gan GH, He XL, Xu XT, Qin SB, Wang LL, Li L, Zhou JY. Establishment of a prediction model for severe acute radiation enteritis associated with cervical cancer radiotherapy. World J Gastroenterol. 2023;29:1344-1358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 3. | Wang Y, Qiang WM, Li JQ, Shen AM, Chen XC, Li XF, Zhang BZ, Xie J, Yan R, Li XH, Zhang ZL, Wang CL, Li LY. The effect of chronoradiotherapy on cervical cancer patients: A multicenter randomized controlled study. Front Oncol. 2022;12:1021453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Feng T, Shou HF, Yuan SH, Tang HR, Lyu XJ, Yin ZM, Lou HM, Ni J. [Treatment and prognosis analysis of 488 patients with FIGO 2018 stage Ⅲc squamous cervical cancer]. Zhonghua Fu Chan Ke Za Zhi. 2023;58:359-367. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Folkert MR, Shih KK, Abu-Rustum NR, Jewell E, Kollmeier MA, Makker V, Barakat RR, Alektiar KM. Postoperative pelvic intensity-modulated radiotherapy and concurrent chemotherapy in intermediate- and high-risk cervical cancer. Gynecol Oncol. 2013;128:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Wang W, Zhang F, Hu K, Hou X. Image-guided, intensity-modulated radiation therapy in definitive radiotherapy for 1433 patients with cervical cancer. Gynecol Oncol. 2018;151:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Dang YZ, Li P, Li JP, Bai F, Zhang Y, Mu YF, Li WW, Wei LC, Shi M. The Efficacy and Late Toxicities of Computed Tomography-based Brachytherapy with Intracavitary and Interstitial Technique in Advanced Cervical Cancer. J Cancer. 2018;9:1635-1641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Laan JJ, van Lonkhuijzen LRCW, van Os RM, Tytgat KM, Dávila Fajardo R, Pieters BR, Stalpers LJA, Westerveld GH. Socioeconomic status as an independent risk factor for severe late bowel toxicity after primary radiotherapy for cervical cancer. Gynecol Oncol. 2017;147:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Zhang X, Liu J, Yu H, Su X, Chen H, He Y, Liu Z, Hu X. Weight Change Trajectory in Patients With Locally Advanced Nasopharyngeal Carcinoma During the Peri-Radiation Therapy Period. Oncol Nurs Forum. 2021;48:65-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, de van der Schueren MA, Singer P. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015;34:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1171] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 11. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale R, Waitzberg DL, Bischoff SC, Singer P. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr. 2017;36:623-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 1060] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 12. | Guiráo TN, de Oliveira STP, Bezerra AE, Françoso BS, Dos Santos BD, Sicchieri JMF, Chiarello PG. Development of a nutritional risk screening tool for cancer patients undergoing outpatient treatment. Clin Nutr ESPEN. 2022;52:240-244. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 13. | Zhang Z, Wan Z, Zhu Y, Zhang L, Wan H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: A multi-center study. Nutrition. 2021;83:111072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Tian M, Fu H, Du J. Application value of NRS2002 and PG-SGA in nutritional assessment for patients with cervical cancer surgery. Am J Transl Res. 2021;13:7186-7192. [PubMed] |
| 15. | Jou J, Coulter E, Roberts T, Binder P, Saenz C, McHale M, Plaxe S, Mayadev J, Eskander RN. Assessment of malnutrition by unintentional weight loss and its implications on oncologic outcomes in patient with locally advanced cervical cancer receiving primary chemoradiation. Gynecol Oncol. 2021;160:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Lee J, Chang CL, Lin JB, Wu MH, Sun FJ, Wu CJ, Tai HC, Hsu SM, Chen YJ. The Effect of Body Mass Index and Weight Change on Late Gastrointestinal Toxicity in Locally Advanced Cervical Cancer Treated With Intensity-modulated Radiotherapy. Int J Gynecol Cancer. 2018;28:1377-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Lakomy DS, Wu J, Chapman BV, Yu ZH, Lee B, Klopp AH, Jhingran A, Eifel PJ, Lin LL. Use of Specific Duodenal Dose Constraints During Treatment Planning Reduces Toxicity After Definitive Paraaortic Radiation Therapy for Cervical Cancer. Pract Radiat Oncol. 2022;12:e207-e215. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Ohno T, Kato S, Wakatsuki M, Noda SE, Murakami C, Nakamura M, Tsujii H. Incidence and temporal pattern of anorexia, diarrhea, weight loss, and leukopenia in patients with cervical cancer treated with concurrent radiation therapy and weekly cisplatin: comparison with radiation therapy alone. Gynecol Oncol. 2006;103:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Clifford B, Koizumi S, Wewege MA, Leake HB, Ha L, Macdonald E, Fairman CM, Hagstrom AD. The Effect of Resistance Training on Body Composition During and After Cancer Treatment: A Systematic Review and Meta-Analysis. Sports Med. 2021;51:2527-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Lee J, Lin JB, Wu MH, Chang CL, Jan YT, Chen YJ. Muscle Loss after Chemoradiotherapy as a Biomarker of Distant Failures in Locally Advanced Cervical Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Kumar SA, Gururajachar MJ, Martin VP. Correlation of skeletal muscle depletion with acute toxicities for cervical cancer patients undergoing concurrent chemoradiation: A prospective study. J Cancer Res Ther. 2022;18:1525-1529. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Basile D, Corvaja C, Caccialanza R, Aprile G. Sarcopenia: looking to muscle mass to better manage pancreatic cancer patients. Curr Opin Support Palliat Care. 2019;13:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Han X, Pires L, Browne JD, Sullivan CA, Zhao W, Feng X. Increased Expression of MuRF1 Is Associated with Radiation-induced Laryngeal Muscle Atrophy. Anticancer Res. 2015;35:6049-6056. [PubMed] |
| 24. | Kim EJ, Lee M, Kim DY, Kim KI, Yi JY. Mechanisms of Energy Metabolism in Skeletal Muscle Mitochondria Following Radiation Exposure. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Glass DJ, Campbell KP, Rudnicki MA. Skeletal muscle's 3rd year anniversary. Skelet Muscle. 2014;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Gabrielson DK, Brezden-Masley C, Keith M, Bazinet RP, Sykes J, Darling PB. Evaluation of Nutritional, Inflammatory, and Fatty Acid Status in Patients with Gastric and Colorectal Cancer Receiving Chemotherapy. Nutr Cancer. 2021;73:420-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Calder PC, Waitzberg DL, Klek S, Martindale RG. Lipids in Parenteral Nutrition: Biological Aspects. JPEN J Parenter Enteral Nutr. 2020;44 Suppl 1:S21-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Boldyreva LV, Morozova MV, Saydakova SS, Kozhevnikova EN. Fat of the Gut: Epithelial Phospholipids in Inflammatory Bowel Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | He F, Huang X, Wei G, Lin X, Zhang W, Zhuang W, He W, Zhan T, Hu H, Yang H. Regulation of ACSL4-Catalyzed Lipid Peroxidation Process Resists Cisplatin Ototoxicity. Oxid Med Cell Longev. 2022;2022:3080263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Zhou H, Zhou YL, Mao JA, Tang LF, Xu J, Wang ZX, He Y, Li M. NCOA4-mediated ferritinophagy is involved in ionizing radiation-induced ferroptosis of intestinal epithelial cells. Redox Biol. 2022;55:102413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 31. | Hatanaka E, Levada-Pires AC, Pithon-Curi TC, Curi R. Systematic study on ROS production induced by oleic, linoleic, and gamma-linolenic acids in human and rat neutrophils. Free Radic Biol Med. 2006;41:1124-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Lee MH, Lee JH, Kim WJ, Kim SH, Kim SY, Kim HS, Kim TJ. Linoleic Acid Attenuates Denervation-Induced Skeletal Muscle Atrophy in Mice through Regulation of Reactive Oxygen Species-Dependent Signaling. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Haghighatdoost F, Nobakht M Gh BF. Effect of conjugated linoleic acid on blood inflammatory markers: a systematic review and meta-analysis on randomized controlled trials. Eur J Clin Nutr. 2018;72:1071-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Viladomiu M, Hontecillas R, Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur J Pharmacol. 2016;785:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |