Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2820
Peer-review started: November 3, 2023
First decision: November 16, 2023
Revised: November 22, 2023
Accepted: December 8, 2023
Article in press: December 8, 2023
Published online: December 27, 2023
Processing time: 54 Days and 2.4 Hours
Primary hepatocellular carcinoma (HCC) is a common malignant tumour, and its early symptoms are often not obvious, resulting in many patients experiencing middle- to late-stage disease at the time of diagnosis. The optimal time for surgery is often missed for these patients, and those who do undergo surgery have unsatisfactory long-term outcomes and a high recurrence rate within five years. Therefore, postoperative follow-up treatments, such as transhepatic arterial chemoembolization (TACE), have become critical to improving survival and reducing recurrence rates.
To validate the prophylactic role of TACE after hepatic resection and to assess its impact on patient prognosis.
This study investigated the efficacy of TACE in patients with intermediate-stage HCC after hepatectomy. When the post-treatment results of the observation group and the control group were compared, it was found that the inclusion of TACE significantly improved the clinical efficacy, reduced the levels of tumour markers and did not aggravate the damage to liver function. Thus, this may be an effective and comprehensive treatment strategy for patients with intermediate-stage HCC that helps to improve their quality of life and survival time.
When the baseline data were analysed, no statistical differences were found between the two groups in terms of gender, age, hepatitis B virus, cirrhosis, Child-Pugh grading, number of tumours, maximum tumour diameter and degree of tumour differentiation. The assessment of clinical efficacy showed that the post-treatment overall remission rate of the observation group was significantly higher than that of the control group. In terms of changes in tumour markers, the alpha-fetoprotein and carcinoembryonic antigen levels in the patients in the observation group decreased more significantly after treatment compared with those in the control group. When post-treatment changes in liver function indicators were analysed, no statistical differences were found in the total bilirubin, alanine aminotransferase and aspartate aminotransferase levels between the two groups.
In patients with intermediate-stage HCC, post-hepatectomy TACE significantly improved clinical outcomes, reduced tumour-marker levels and may have improved the prognosis by removing residual lesions. Thus, this may be an effective and comprehensive treatment strategy for patients with intermediate-stage HCC.
Core Tip: This study investigated the efficacy of transhepatic arterial chemoembolization (TACE) in patients with intermediate-stage hepatocellular carcinoma (HCC) after hepatectomy. When the post-treatment results of the observation group and the control group were compared, it was found that the inclusion of TACE significantly improved the clinical efficacy, reduced the levels of tumour markers and did not aggravate the damage to liver function. Thus, this may be an effective and comprehensive treatment strategy for patients with intermediate-stage HCC that helps to improve their quality of life and survival time.
- Citation: Hu YD, Zhang H, Tan W, Li ZK. Impact of hepatectomy and postoperative adjuvant transarterial chemoembolization on serum tumor markers and prognosis in intermediate-stage hepatocellular carcinoma. World J Gastrointest Surg 2023; 15(12): 2820-2830
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2820.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2820
Hepatocellular carcinoma (HCC) is a common malignant tumour in clinical practice, and its high morbidity and mortality rates and low cure rate make it a serious health problem[1]. In recent years, the incidence and mortality rates of HCC have been increasing, and HCC is characterised by an undetectable pathogenesis, high malignancy, rapid disease progression and poor therapeutic outcomes[2]. As a result, HCC has long ranked among the top three malignant tumours in terms of morbidity and mortality. Globally, HCC causes about one million deaths each year, which has resulted in HCC being termed the ‘king of cancers’[3].
In China, the incidence of HCC has been high due to a large number of people being infected with and carrying hepatitis B virus (HBV)[4]. According to statistics, the incidence of HCC in China accounts for about 43% of the global incidence and about 780000 people are diagnosed with HCC each year[5]. According to data from the Chinese National Cancer Centre, the incidence of HCC is the fourth highest among all malignant tumours in China, while its mortality rate is the second highest after that of lung cancer, with more than 500000 deaths due to HCC every year[6]. Although the HBV infection rate is gradually decreasing, the incidence of HCC is still rising due to lifestyle changes[7].
In terms of the clinical management of HCC, surgical approaches are preferred, including conventional surgery, radiofrequency ablation, transhepatic arterial chemoembolization (TACE) and other related therapies[8]. The choice of treatment is often closely related to the tumour stage and the health of the liver. Surgical procedures, particularly liver transplantation, although theoretically very effective, are subject to a number of constraints in their practical applications due to donor shortages and limitations in indications[9]. It is also worth noting that early-stage HCC is difficult to detect, and this is partly because regular health check-ups are not common in some areas and because the rich blood supply provided by the liver to the tumour allows the disease to rapidly progress[10]. As a result, by the time many patients are diagnosed, they have middle- to advanced-stage disease, which greatly reduces the chance of surgery being successful. In addition, the presence of other health conditions, such as cirrhosis or cardiopulmonary dysfunction, can further reduce the surgery and treatment success rates.
TACE is considered the main non-surgical treatment option for HCC. Studies have shown that the structures that supply blood to HCC tumours differ from those that supply blood to other tumour types and that blood is mainly supplied to HCC tumours by the portal system and hepatic arterial system[11]. TACE exploits this feature - the aim is to cut off the tumour’s blood supply through chemoembolization and thus eliminate the tumour cells. According to the Barcelona Clinic Liver Cancer (BCLC) classification, TACE is a recommended method for the treatment of mid-stage liver cancer, and its effectiveness in prolonging survival has also been demonstrated in several studies[12,13]. However, TACE has some limitations. For example, tumour cells may survive the treatment, and this increases the risk of recurrence and metastasis. Multiple TACE treatments may also affect the blood supply to the liver.
In patients with intermediate-stage liver cancer, performing TACE after palliative surgery could not only reduce the blood supply to the tumour but also potentially control or eliminate lesions that were not detected or resected during surgery. However, the combination of hepatic resection and TACE for intermediate-stage HCC is an understudied area. Therefore, in this study, we investigated the effect of hepatic resection combined with TACE vs hepatic resection alone in patients with intermediate-stage HCC who were treated at Lishui Central Hospital from 2016 to 2020. We analysed the relevant influencing factors to provide more evidence-based recommendations for the treatment of intermediate-stage HCC.
A retrospective analysis of 241 patients with HCC who were treated at Lishui Central Hospital from March 2016 to March 2020 was performed. The study was approved by the Medical Ethics Committee of Lishui Central Hospital. The ethical number is Research Ethics Approval (2023) No. (665).
The inclusion criteria were as follows: (1) Diagnosis of HCC by preoperative ultrasound-guided puncture biopsy or postoperative pathology or by clinical criteria[14]; (2) BCLC stage B HCC; (3) Receiving HCC treatment for the first time, with no history of liver cancer surgery, TACE treatment or chemotherapy; and (4) Complete clinical data available.
The exclusion criteria were as follows: (1) Lesions in the liver, of which most exceeded half of the liver, but primary lesions and metastatic lesions could be removed by local surgery; (2) A tumour diameter > 10 cm, without envelope or the envelope had been ruptured; (3) The lesion was found to have invaded the cancer embolus during the preoperative examination or surgery, but the embolus was removable; (4) The tumour was found to be ruptured in the preoperative or intraoperative examination, but the primary lesion could still be surgically removed; (5) Patients with both right and left half of the liver, tumour rupture, macrovascular invasion, lymph node or distant metastasis in patients with advanced HCC; and (6) Patients who underwent surgical resection that fulfilled the conditions for palliative resection.
Among the 241 patients we screened, 108 met the inclusion criteria (and did not meet the exclusion criteria). These patients were divided into two groups: 50 patients underwent hepatectomy as the control group and 58 patients underwent hepatectomy combined with TACE as the observation group.
The patients in the control group underwent hepatic resection only, while the patients in the observation group also underwent TACE after hepatic resection.
Hepatic resection: For single tumours, standard radical resection was performed when the criterion for radical resection (i.e., the distance between the cutting edge and the tumour margin was at least 1 cm) was met. For multiple tumours in the same hepatic lobe, efforts were made to achieve radical resection by ensuring that the distance between the cutting edge and the edge of the tumour was at least 1 cm and that the histological examination of the cutting edge was negative. Palliative resection was performed in patients with giant tumours, multiple foci of disease scattered within the liver or multiple satellite foci around the main tumour. After complete resection of the main tumour, small foci or satellite foci were treated with ethanol curing or microwave curing under ultrasound guidance. When lymphatic metastases were present, perihepatic lymph node dissection was also required.
TACE: The patients in the observation group underwent TACE one month after resection. Using the Seldinger technique, the catheter was inserted via the right femoral artery and placed in the hepatic innominate artery for digital subtraction angiography. When tumour vascular staining was detected, a suspension containing 1 g 5-fluorouracil (Wuhu Shengsheng Zhongren Pharmaceutical Co., Ltd, State Drug Licence H20030345), 150 mg oxaliplatin (Harbin Pharmaceutical Group Bioengineering Co., Ltd, State Drug Licence H20133094), super-liquid iodised oil (Guerbet, France, Imported Drug Registration Certificate no: H20150099) and 50 mg epirubicin [Pfizer Pharmaceuticals (Wuxi) Co., Ltd., State Pharmaceutical Approval No. H20000496] was injected into the blood vessels. If no tumour vascular staining was detected, the drug suspension was slowly injected into the hepatic innominate artery for prophylactic treatment based on super-liquid iodinated oil deposition and vascular casts.
The patients’ clinical data and laboratory parameters were collected from their electronic pathology and outpatient review records. Clinical data were collected on the patients’ gender, age and HBV and cirrhosis status, as well as the Child-Pugh classification, number of tumours, maximum tumour diameter and degree of tumour differentiation. Laboratory parameters included serum alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), total bilirubin (TBIL), alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
All patients were reviewed monthly for six months after surgery, and five-year survival data were recorded. Survival time was defined as the period after surgery until the patient’s death or the follow-up cutoff time.
Two months post-treatment, we objectively evaluated the solid tumours using the World Health Organization’s criteria for the evaluation of solid tumours[15] to assess the clinical efficacy of the respective treatments. The following terms and definitions were used: complete remission (CR) = complete disappearance of all lesions for at least four weeks; partial remission (PR) = more than 50% reduction in the product of the largest pendant diameters of all measurable lesions for at least four weeks; stable disease = no more than 50% reduction in the product of the largest pendant diameters of all measurable lesions and no more than 25% increase in their size for at least four weeks; and progressive disease = an increase in size of any lesion by more than 25% or the appearance of new lesions. The remission rate was derived from the sum of CR and PR.
The main observational index was the clinical efficacy. Cox regression was used to analyse the prognostic factors that affected the patients’ five-year survival. The secondary observational index was the statistical clinical data of the patients in the two groups. Tumour marker and liver function changes in the patients in the two groups were compared before and after treatment (four weeks after treatment).
SPSS 26.00 software (IBM Corporation, Armonk, New York, United States) was used to analyse the data collected in this study, and GraphPad Prism 9 software (GraphPad Software, Inc. San Diego, California, United States) was used to visualise the data. Comparisons between count data were performed using the χ2 test, comparisons of measures between the two groups were performed using independent t-tests and comparisons between the preoperative and postoperative periods within the groups were performed using paired t-tests. Independent prognostic factors that affected the patients were analysed using Cox regression. Kaplan-Meier survival curves were used to analyse the survival rates of the two groups. A statistical difference was indicated when P was < 0.05.
When we compared the clinical data from the patients in the two groups, it was found that there was no statistical difference in the gender, age, HBV, cirrhosis, Child-Pugh classification, number of tumours, maximum tumour diameter and degree of tumour differentiation between the two groups (P > 0.05; Table 1).
| Considerations | Control group (n = 50) | Observation group (n = 58) | χ2 | P value |
| Distinguishing between the sexes | ||||
| Male | 28 | 36 | 0.448 | 0.575 |
| Females | 23 | 22 | ||
| Age | ||||
| ≥ 55 yr | 28 | 35 | 0.329 | 0.565 |
| < 55 yr | 23 | 23 | ||
| HBV | ||||
| Be | 40 | 45 | 0.093 | 0.760 |
| Clogged | 10 | 13 | ||
| Cirrhosis | ||||
| Be | 22 | 30 | 0.423 | 0.642 |
| Clogged | 28 | 28 | ||
| Child-push grading | ||||
| Grade A | 35 | 40 | 0.013 | 0.907 |
| Level B | 15 | 18 | ||
| Number of tumours | ||||
| ≥ 3 | 27 | 38 | 1.486 | 0.222 |
| < 3 | 23 | 20 | ||
| Maximum tumour diameter | ||||
| ≥ 5 cm | 23 | 18 | 2.554 | 0.110 |
| < 5 cm | 27 | 40 | ||
| Degree of tumour differentiation | ||||
| Highly differentiated | 22 | 25 | 2.424 | 0.297 |
| Middle ground | 20 | 20 | ||
| Low polarisation | 8 | 3 |
The clinical efficacy of the two treatment modalities was assessed, and the results showed that the total remission rate in the control group was significantly lower than that in the observation group after treatment, indicating a statistically significant difference in the clinical efficacy of the treatments (P = 0.040; Table 2).
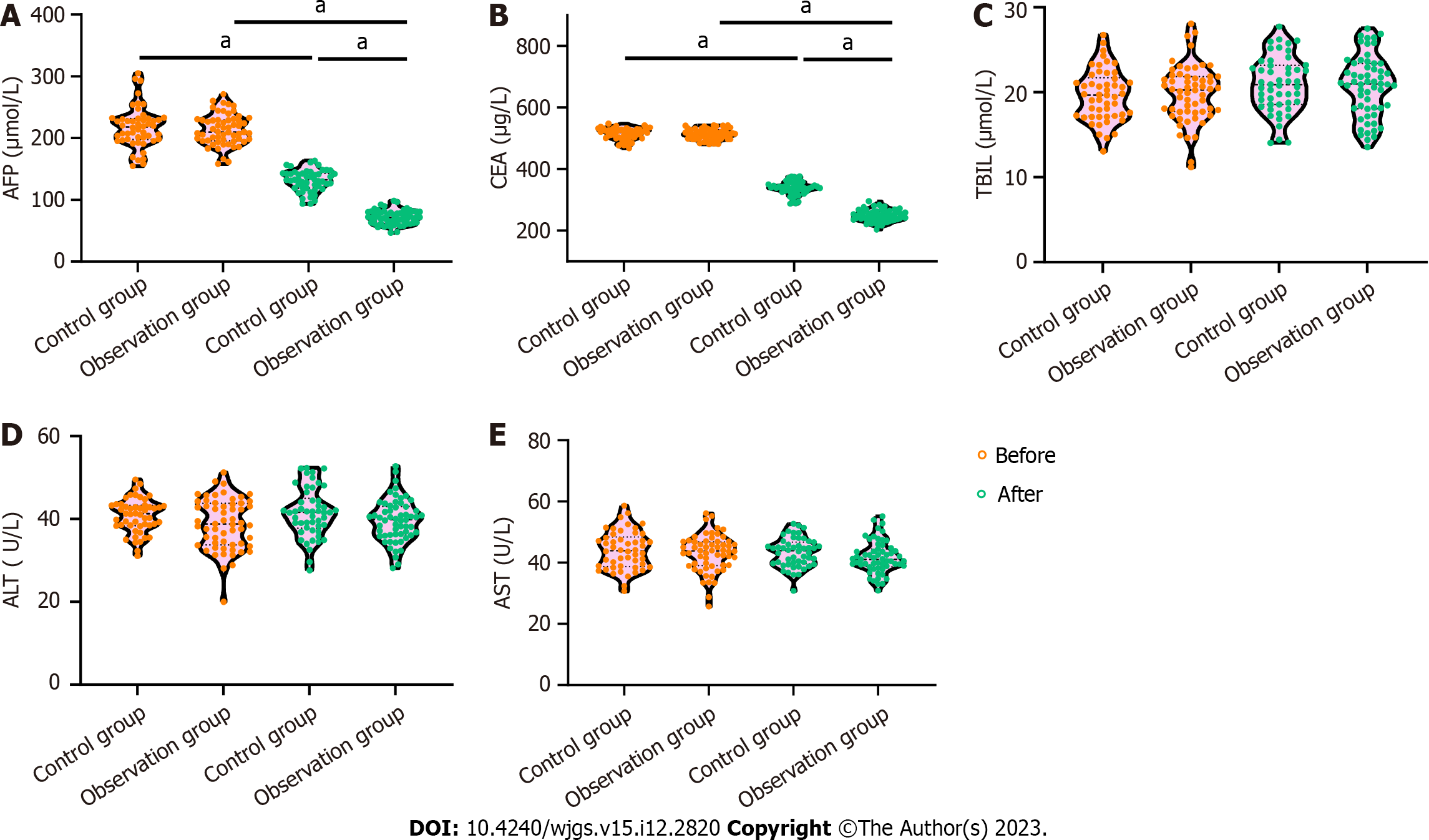
A comparison of the AFP and CEA levels in the two groups before and after the respective treatments revealed that there was no difference in the AFP and CEA levels between the two groups before treatment (P > 0.05) and that the AFP and CEA levels in the two groups were significantly lower after treatment compared with before treatment (P < 0.0001). In addition, the AFP and CEA levels in the observation group decreased more significantly after treatment compared with those in the control group (P < 0.0001; Figures 1A and B).
A comparison of the TBIL, ALT and AST levels in the two groups before and after treatment revealed that there was no difference in the TBIL, ALT and AST levels between the two groups before treatment (P > 0.05), and there was no statistically significant difference in the TBIL, ALT and AST levels between the two groups after treatment compared with those before treatment (P > 0.05). In addition, there was no statistically significant difference between the TBIL, ALT and AST levels in the observation group and the control group after treatment (P < 0.001; Figures 1C-E).
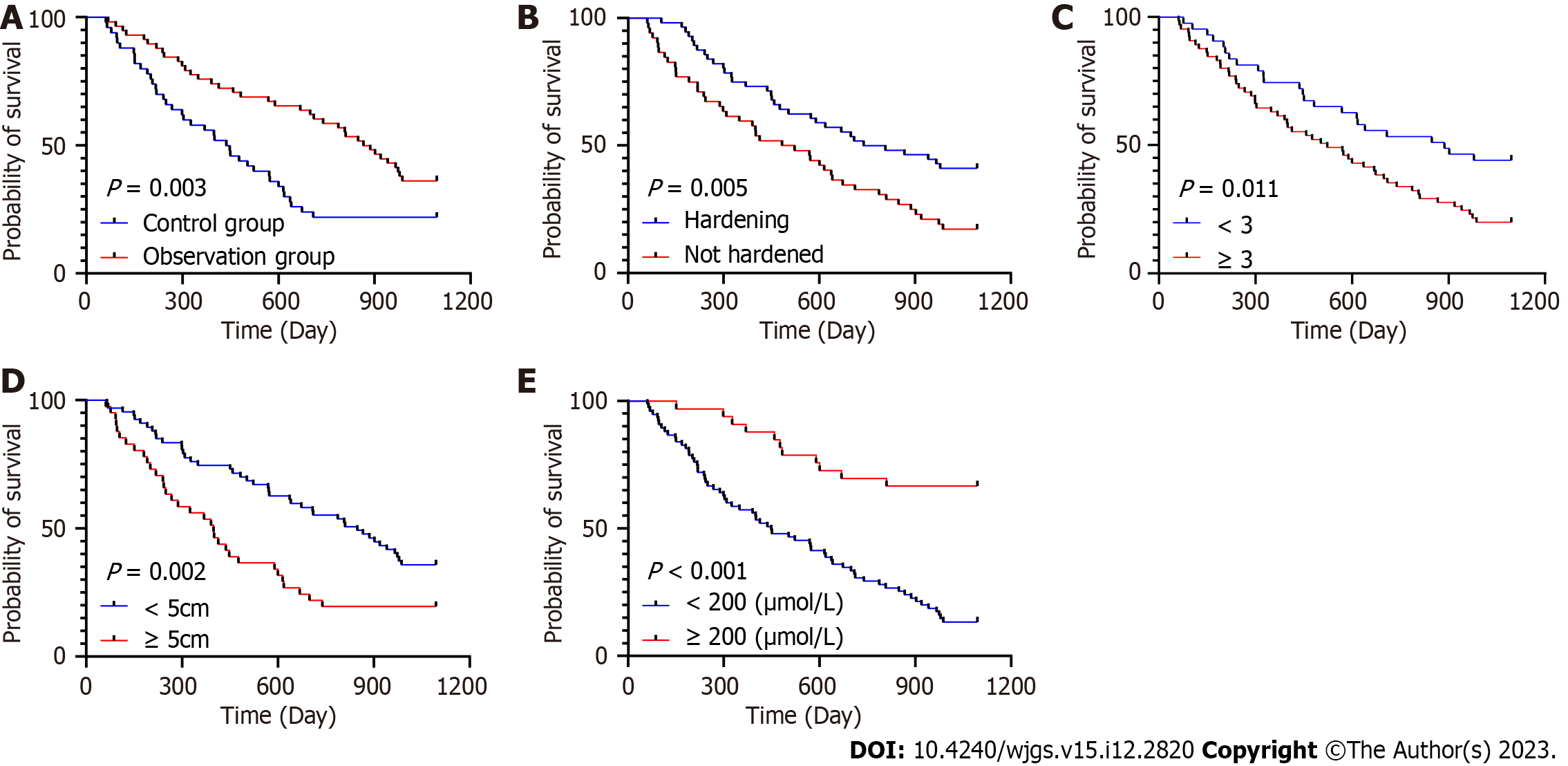
In this study, the level of AFP showed a strong association with the patients’ five-year survival in both unifactorial and multifactorial Cox regression analyses (P < 0.001). In addition, age, cirrhosis, number of tumours, maximum tumour diameter and treatment regimen also showed significant associations in unifactorial analyses, while cirrhosis, number of tumours, maximum tumour diameter and treatment regimen continued to show significant associations in multifactorial analyses (P < 0.05; Tables 3 and 4, Figure 2). These factors may be key variables that influenced the patients’ five-year survival.
| Considerations | Beta value | SE | χ2 value | P value | HR value | 95%CI | |
| Lower limit | Limit | ||||||
| Distinguishing between the sexes | -0.288 | 0.231 | 1.556 | 0.212 | 0.750 | 0.477 | 1.179 |
| Age | 0.608 | 0.243 | 6.263 | 0.012a | 1.837 | 1.141 | 2.958 |
| HBV | 0.179 | 0.288 | 0.386 | 0.534 | 1.196 | 0.680 | 2.105 |
| Cirrhosis | 0.646 | 0.232 | 7.737 | 0.005b | 1.909 | 1.210 | 3.009 |
| Child-push grading | 0.164 | 0.257 | 0.408 | 0.523 | 1.178 | 0.712 | 1.948 |
| Number of tumours | 0.62 | 0.248 | 6.261 | 0.012a | 1.859 | 1.144 | 3.021 |
| Maximum tumour diameter | 0.686 | 0.234 | 8.590 | 0.003b | 1.986 | 1.255 | 3.143 |
| Degree of tumour differentiation | 0.439 | 0.150 | 8.557 | 0.003b | 1.551 | 1.156 | 2.080 |
| AFP | -1.516 | 0.329 | 21.232 | < 0.001c | 0.220 | 0.115 | 0.418 |
| CEA | -0.177 | 0.242 | 0.537 | 0.464 | 0.838 | 0.521 | 1.346 |
| TBIL | 0.104 | 0.231 | 0.201 | 0.654 | 1.109 | 0.705 | 1.744 |
| ALT | 0.372 | 0.265 | 1.969 | 0.161 | 1.451 | 0.863 | 2.441 |
| AST | -0.321 | 0.271 | 1.405 | 0.236 | 0.725 | 0.426 | 1.234 |
| Treatment programme | 0.625 | 0.232 | 7.250 | 0.007b | 1.868 | 1.185 | 2.945 |
| Considerations | Beta value | SE | χ2 value | P value | HR value | 95%CI | |
| Lower limit | Limit | ||||||
| Age | 0.399 | 0.247 | 2.607 | 0.106 | 1.491 | 0.918 | 2.421 |
| Cirrhosis | 0.617 | 0.237 | 6.751 | 0.009b | 1.853 | 1.164 | 2.950 |
| Number of tumours | 0.586 | 0.256 | 5.246 | 0.022a | 1.798 | 1.088 | 2.969 |
| Maximum tumour diameter | 0.678 | 0.249 | 7.443 | 0.006b | 1.970 | 1.210 | 3.207 |
| Degree of tumour differentiation | 0.120 | 0.151 | 0.634 | 0.426 | 1.128 | 0.839 | 1.517 |
| AFP | -1.434 | 0.335 | 18.329 | < 0.001c | 0.238 | 0.124 | 0.460 |
| Treatment programme | 0.642 | 0.248 | 6.703 | 0.010a | 1.900 | 1.169 | 3.088 |
Surgery is the mainstay of treatment for HCC; however, because it is difficult to detect early-stage HCC, many patients are diagnosed when the disease is in the middle to late stages[16]. These patients often miss the optimal time for surgery, and those who undergo surgery often have unsatisfactory long-term results and a high recurrence rate within five years[17]. Therefore, it is critical to administer follow-up treatment after surgery. The aim of this study was to verify the preventive role of postoperative TACE in patients who had undergone hepatic resection.
TACE is an interventional treatment that delivers chemotherapeutic drugs directly to liver tumours via the hepatic artery and thus effectively targets tumour tissue[18]. Despite its efficacy, there are challenges associated with TACE due to the complex arterial supply of HCC tissues. Even after embolization of the main supply vessels, tumours may continue to grow because of collateral circulation, leading to postoperative recurrence[19]. Nevertheless, recent studies have suggested that prophylactic TACE following hepatectomy can enhance survival rates and reduce recurrence in patients with intermediate- and advanced-stage HCC[12,20]. However, the use of TACE in this context is being debated; there are concerns about the potential for TACE to further impair immune and liver functions post-hepatectomy and thus increase the risk of recurrence[21].
In this study, a significant increase in the total effective rate was observed in patients who underwent TACE one month post-surgery compared to those who did not. This improvement is attributed to the capacity of TACE to control and eliminate both evident and undetected residual microscopic lesions that could result in tumour recurrence. Miyayama et al[22] reported similar findings; they showed that while TACE was a secondary treatment option for small HCC tumours, super-selective TACE was particularly effective for tumours smaller than 3 cm, achieving complete embolization in most cases. Additionally, Bai et al[23] revealed that using sorafenib after hepatectomy in patients with intermediate-stage HCC could reduce recurrence rates and improve prognosis. Our findings align with these results, suggesting that postoperative TACE, like the administration of targeted drugs, can produce synergistic effects to reduce recurrence and enhance prognosis. The results from the Miyayama et al[22] and Bai et al[23] studies underscore the value of designing and implementing comprehensive postoperative treatment models. Our study differs in that we observed the effects of different integrated treatment modalities, offering insights into how to optimise postoperative care in HCC treatment.
AFP is a specific diagnostic indicator for HCC. In healthy people, AFP is expressed at very low levels; however, the level of AFP rises significantly with the progression of HCC[24]. AFP inhibits the immune function of the body by inducing apoptosis of lymphocytes and inhibiting the secretion of tumour necrosis factor by lymphocytes. CEA is a non-specific glycoprotein component of cancer cells that is highly expressed in patients with malignant tumours of the digestive and respiratory systems[25]. In this study, we found that the levels of AFP and CEA decreased more significantly in the observation group compared with those in the control group after treatment. This indicates that performing TACE after hepatic resection has a positive therapeutic effect. In addition, the differences in the TBIL, ALT and AST levels between the two groups after treatment suggest that TACE can effectively result in the killing of tumour cells without aggravating the damage to the liver function of patients.
Early symptoms of primary HCC are not obvious, which makes early diagnosis difficult. When a tumour reaches about 6 cm in diameter, most patients will have symptoms such as fatigue, vomiting and jaundice; however, some patients will have no obvious symptoms and miss the optimal treatment time[26]. Therefore, it is particularly important to accurately assess the prognosis of patients with HCC. In this study, cirrhosis, number of tumours, maximum tumour diameter and treatment regimen were found to be independent prognostic factors affecting five-year survival[27,28]. Cirrhosis provides the pathological basis for HCC, increasing tumour number and diameter predict the seriousness of the disease and different treatment regimens have a significant impact on prognosis. For example, comprehensive postoperative treatment can remove residual lesions, reduce recurrence and improve patient prognosis[27,28]. The combination of these factors reflects the severity and progression of the disease and is key to assessing the prognosis. Wu et al[29] also found that AFP, maximum tumour diameter, number of tumours, cirrhosis and treatment regimen were independent factors that affect prognosis. In addition, Wang et al[30] showed that postoperative TACE significantly shortened disease-free survival in patients with intermediate-stage HCC, which is consistent with our findings and emphasises the importance of integrating disease status and treatment efficacy when assessing prognosis.
However, this study has some research limitations. First, the sample size was small, only 108 patients were included, and there was some bias between the groups. Second, this was a retrospective study; hence, we could not completely control the influence of confounding factors. Finally, the results of this study need to be expanded and validated in clinical settings to have a greater impact. Therefore, subsequent studies should include larger sample sizes, and conducting a multicentre, joint study will improve the validity of the results. In addition, prospective randomised controlled studies should be conducted to minimise bias and thus enhance the validity of the findings and to promote translational applications.
The results of this study suggest that performing TACE after hepatic resection in patients with intermediate-stage HCC: (1) Can significantly improve the clinical outcome and reduce the levels of tumour markers; and (2) May improve the prognosis by removing residual lesions. Our findings provide evidence of the value of performing postoperative TACE in patients with intermediate-stage HCC.
Primary liver cancer is a common malignant tumour with inconspicuous early symptoms, resulting in many patients being in the middle to late stage when diagnosed. Follow-up treatment after surgery is the key, in which transhepatic arterial chemoembolization (TACE), as an interventional therapy, has received more and more attention in recent years.
Although TACE is considered an effective treatment, controversy still exists regarding its prophylactic use after hepatic cancer resection. The aim of this study was to verify the prophylactic role of postoperative TACE and its impact on patient prognosis.
To assess the clinical efficacy of TACE after resection of hepatocellular carcinoma (HCC) and its effect on tumour markers and liver function indices. To further explore the prognostic factors affecting patients’ 5-year survival.
This study compared the clinical data and treatment outcomes of two groups of patients with intermediate stage HCC. The observation group was treated with TACE after liver cancer resection, while the control group was not.
The overall effective rate of patients in the observation group was significantly higher than that of the control group. TACE treatment significantly reduced serum alpha-fetoprotein and carcinoembryonic antigen levels without exacerbating the impairment of liver function in patients. Cirrhosis, number of tumours, maximum tumour diameter and treatment regimen were identified as independent prognostic factors affecting patients’ 3-year survival.
TACE after hepatectomy for HCC significantly improves clinical outcomes, reduces tumour marker levels and may improve prognosis by removing residual lesions. This provides an effective comprehensive treatment strategy for patients with intermediate-stage HCC.
Future studies should expand the sample size and conduct prospective randomised controlled studies to further validate the findings of this study. In addition, exploring other possible integrated treatment strategies is also a direction for future research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imai Y, Japan; Mitroulis I, Greece S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023;27:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 231] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 2. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2893] [Article Influence: 482.2] [Reference Citation Analysis (17)] |
| 3. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 4. | Li H, Yan L, Shi Y, Lv D, Shang J, Bai L, Tang H. Hepatitis B Virus Infection: Overview. Adv Exp Med Biol. 2020;1179:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Zhang C, Dai D, Zhang W, Yang W, Guo Y, Wei Q. Role of m6A RNA methylation in the development of hepatitis B virus-associated hepatocellular carcinoma. J Gastroenterol Hepatol. 2022;37:2039-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, Xing B, Sun W, Ren L, Hu B, Li C, Zhang L, Qin G, Zhang M, Chen N, Huang Y, Zhou J, Liu M, Zhu X, Qiu Y, Sun Y, Huang C, Yan M, Wang M, Liu W, Tian F, Xu H, Wu Z, Shi T, Zhu W, Qin J, Xie L, Fan J, Qian X, He F; Chinese Human Proteome Project (CNHPP) Consortium. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 623] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 7. | Chen S, Gao Y, Wang Y, Daemen T. The combined signatures of hypoxia and cellular landscape provides a prognostic and therapeutic biomarker in hepatitis B virus-related hepatocellular carcinoma. Int J Cancer. 2022;151:809-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Zhang Z, Zeng X, Wu Y, Liu Y, Zhang X, Song Z. Cuproptosis-Related Risk Score Predicts Prognosis and Characterizes the Tumor Microenvironment in Hepatocellular Carcinoma. Front Immunol. 2022;13:925618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 162] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 9. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 590] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 10. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 421] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 11. | Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 12. | Brown AM, Kassab I, Massani M, Townsend W, Singal AG, Soydal C, Moreno-Luna L, Roberts LR, Chen VL, Parikh ND. TACE versus TARE for patients with hepatocellular carcinoma: Overall and individual patient level meta analysis. Cancer Med. 2023;12:2590-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Hiraoka A, Kumada T, Tada T, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimura T, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Naganuma A, Tanaka T, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Koizumi Y, Nakamura S, Joko K, Iijima H, Hiasa Y, Kudo M; Real-life Practice Experts for HCC (RELPEC) Study Group; HCC 48 Group (Hepatocellular Carcinoma Experts from 48 Clinics in Japan. Early experience of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma BCLC-B stage patients classified as beyond up to seven criteria - Multicenter analysis. Hepatol Res. 2022;52:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol. 2018;7:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Sato Y, Watanabe H, Sone M, Onaya H, Sakamoto N, Osuga K, Takahashi M, Arai Y; Japan Interventional Radiology in Oncology Study Group-JIVROSG. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118:16-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Gilles H, Garbutt T, Landrum J. Hepatocellular Carcinoma. Crit Care Nurs Clin North Am. 2022;34:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 17. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1219] [Article Influence: 406.3] [Reference Citation Analysis (41)] |
| 18. | Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, Huang F, Tang R, Cheng Y, Huang Z, Liang Y, Fan H, Qiao L, Li F, Zhuang W, Peng B, Wang J, Li J, Kuang M. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol. 2023;41:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 247] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 19. | Kudo M. Atezolizumab plus Bevacizumab Followed by Curative Conversion (ABC Conversion) in Patients with Unresectable, TACE-Unsuitable Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer. 2022;11:399-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Liu J, Zhang J, Wang Y, Shu G, Lou C, Du Z. HAIC versus TACE for patients with unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Medicine (Baltimore). 2022;101:e32390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Sidali S, Trépo E, Sutter O, Nault JC. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J. 2022;10:765-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 22. | Miyayama S, Yamashiro M, Ikeda R, Matsumoto J, Takeuchi K, Sakuragawa N, Ueda T, Sanada T, Notsumata K, Terada T. Efficacy of Superselective Conventional Transarterial Chemoembolization Using Guidance Software for Hepatocellular Carcinoma within Three Lesions Smaller Than 3 cm. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Bai S, Yang P, Liu J, Xue H, Xia Y, Liu F, Yang Z, Zhang L, Wu Y, Shen F, Wang K. Surgical Margin Affects the Long-Term Prognosis of Patients With Hepatocellular Carcinoma Undergoing Radical Hepatectomy Followed by Adjuvant TACE. Oncologist. 2023;28:e633-e644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Yang Q, Li G, Wu X, Lin H, Wu W, Xie X, Zhu Y, Cai W, Shi C, Zhuo S. A novel therapeutic strategy of combined camrelizumab and apatinib for the treatment of advanced hepatocellular carcinoma. Front Oncol. 2023;13:1136366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Zhang T, Cheng S, Li J, Shang Y, Zheng M. Evaluation of the effect of ultrasound interventional injection of cisplatin in the treatment of liver cancer. Am J Transl Res. 2021;13:5603-5609. [PubMed] |
| 26. | Pan G, Wang R, Jia S, Li Y, Jiao Y, Liu N. SLC25A11 serves as a novel prognostic biomarker in liver cancer. Sci Rep. 2020;10:9871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, Himmelsbach V, Schulze K, von Felden J, Fründt TW, Stadler M, Heinzl H, Shmanko K, Spahn S, Radu P, Siebenhüner AR, Mertens JC, Rahbari NN, Kütting F, Waldschmidt DT, Ebert MP, Teufel A, De Dosso S, Pinato DJ, Pressiani T, Meischl T, Balcar L, Müller C, Mandorfer M, Reiberger T, Trauner M, Personeni N, Rimassa L, Bitzer M, Trojan J, Weinmann A, Wege H, Dufour JF, Peck-Radosavljevic M, Vogel A, Pinter M. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2022;76:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 184] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 28. | Piñero F, Anders M, Bermudez C, Demirdjian E, Varón A, Palazzo A, Rodriguez J, Beltrán O, da Fonseca LG, Ridruejo E, Caballini P, Tamagnone N, Reggiardo V, Cheinquer H, Araujo A, Arufe D, Marín JI, Ratusnu N, Manero E, Perez D, Villa M, Orozco F, Murga D, Marciano S, Bessone F, Silva M, Mendizabal M. Liver decompensation is a frequent cause of treatment discontinuation and prognostic factor in intermediate-advanced HCC. Ann Hepatol. 2023;28:101110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Wu Z, Cui L, Qian J, Luo L, Tu S, Cheng F, Yuan L, Zhang W, Lin W, Tang H, Li X, Li H, Zhang Y, Zhu J, Li Y, Xiong Y, Hu Z, Peng P, He Y, Liu L, He K, Shen W. Efficacy of adjuvant TACE on the prognosis of patients with HCC after hepatectomy: a multicenter propensity score matching from China. BMC Cancer. 2023;23:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Wang L, Lin N, Lin K, Xiao C, Wang R, Chen J, Zhou W, Liu J. The Clinical Value of Postoperative Transarterial Chemoembolization for Resectable Patients with Intermediate Hepatocellular Carcinoma After Radical Hepatectomy: a Propensity Score-Matching Study. J Gastrointest Surg. 2021;25:1172-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |