Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2809
Peer-review started: October 7, 2023
First decision: October 24, 2023
Revised: November 6, 2023
Accepted: December 6, 2023
Article in press: December 6, 2023
Published online: December 27, 2023
Processing time: 81 Days and 4.8 Hours
Significant correlation between lymphatic, microvascular, and perineural invasion (LMPI) and the prognosis of pancreatic neuroendocrine tumors (PENTs) was confirmed by previous studies. There was no previous study reported the rela
To determine the feasibility of using preoperative MRI of the pancreas to predict LMPI in patients with non-functioning PENTs (NFPNETs).
A total of 61 patients with NFPNETs who underwent MRI scans and lymphadenectomy from May 2011 to June 2018 were included in this retrospective study. The patients were divided into group 1 (n = 34, LMPI negative) and group 2 (n = 27, LMPI positive). The clinical characteristics and qualitative MRI features were collected. In order to predict LMPI status in NF-PNETs, a multivariate logistic regression model was constructed. Diagnostic performance was evaluated by calculating the receiver operator characteristic (ROC) curve with area under ROC, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy.
There were significant differences in the lymph node metastasis stage, tumor grade, neuron-specific enolase levels, tumor margin, main pancreatic ductal dilatation, common bile duct dilatation, enhancement pattern, vascular and adjacent tissue involvement, synchronous liver metastases, the long axis of the largest lymph node, the short axis of the largest lymph node, number of the lymph nodes with short axis > 5 or 10 mm, and tumor volume between two groups (P < 0.05). Multivariate analysis showed that tumor margin (odds ratio = 11.523, P < 0.001) was a predictive factor for LMPI of NF-PNETs. The area under the receiver value for the predictive performance of combined predictive factors was 0.855. The sensitivity, specificity, PPV, NPV and accuracy of the model were 48.1% (14/27), 97.1% (33/34), 97.1% (13/14), 70.2% (33/47) and 0.754, respectively.
Using preoperative MRI, ill-defined tumor margins can effectively predict LMPI in patients with NF-PNETs.
Core Tip: The correlation between comprehensive magnetic resonance imaging features and lymphatic, microvascular, and perineural invasion (LMPI) of non-functioning pancreatic neuroendocrine tumors (NF-PNETs) were analyzed. A multivariate model was constructed for predicting LMPI in NF-PNETs. Ill-defined tumor margins resulted as an independent risk factor for LMPI in patients with NF-PNETs.
- Citation: Liu YL, Zhu HB, Chen ML, Sun W, Li XT, Sun YS. Prediction of the lymphatic, microvascular, and perineural invasion of pancreatic neuroendocrine tumors using preoperative magnetic resonance imaging. World J Gastrointest Surg 2023; 15(12): 2809-2819
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2809.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2809
Pancreatic neuroendocrine tumors (PNETs), which arise from the pancreatic neuroendocrine cells, are comparatively rare neoplasms that account for approximately 2%-3% of all pancreatic tumors[1-3]. Non-functioning PNETs (NF-PNETs) comprise approximately 70%-90% of all PNETs and are much more common than functioning PNETs[4]. The clinical outcomes tend to vary, with the 5-year survival rates ranging from 30%-66%[5].
Surgical resection is still the most effective treatment for PNENs[6,7]. The clinical outcomes tend to vary, with 5 years recurrence rate after curative surgery ranging from 10% to 40%[8-10]. World Health Organization (WHO) grading[11], lymph node metastasis (LNM), liver metastasis, and some immune-inflammatory markers have been clearly proven as significant prognostic factors with disease relapse after surgery in patients with PNETs[12-17]. Moreover, identifying additional prognostic factors may assist in the stratification of patients for the risk of tumor recurrence or optimization of operation plan.
Lymphatic, microvascular and perineural invasion (LMPI) greatly impact gastroenterological cancers or gastroenterological neuroendocrine tumors[18,19]. In recent years, LMPI has been confirmed as an independent prognostic factor of PENTs[20-22]. However, the identification of lymphatic, microvascular and perineural invasion of PNETs can only be based on the postoperative pathological diagnosis. As a non-invasive imaging modality, magnetic resonance imaging (MRI) has been widely used to evaluate PNETs, mainly in terms of detecting and grading[23-26]. Previous MRI studies mainly focused on differential diagnosis, tumor grading, and recurrence[27-29]. Thus far, no previous study has reported a correlation between MRI parameters and LMPI. Therefore, this study aims to evaluate the ability of preoperative clinical indicators and MRI parameters to predict lymphatic, microvascular and perineural invasion in NF-PNETs.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board of our hospital. The requirement for informed consent was waived. The medical records were searched from May 2011 to June 2018. All resected PNETs with definite pathologically confirmed lymphatic, microvascular and perineural invasion status were enrolled, and the patients were excluded according to the following exclusion criteria: (1) No MRI available or insufficient MR images to do the analysis; (2) The time interval between MRI and surgery was more than two weeks; and (3) Patient received local or systemic treatment before surgery.
Preoperative parameters included gender, age, body mass index, symptom (present or absent), total bilirubin, alanine aminotransferase, aspartate aminotransferase, fasting blood glucose, total lymphocyte count, total neutrophil count, neutrophil-lymphocyte ratio = lymphocyte count/neutrophil count, alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 199, carbohydrate antigen 724, and neuron-specific enolase (NSE). Pathological analysis was based on the WHO 2019 classification, including the tumor grade (according to mitotic count and Ki-67 index), lymphatic invasion, vascular invasion, neural invasion, and lymph node status.
All examinations were performed on 1.5 T (n = 10) or 3.0 T (n = 51) MRI scanners, using an 8-channel phased array body coil with the patients in the supine position. The MRI sequences included T2-weighted single-shot fast spin echo, FSE T1-weighted imaging (T1WI), and diffusion-weighted imaging (DWI). DWI was performed with single-shot echo-planar imaging sequence prior to contrast administration with at least b value of 0 and 1000 s/mm2. Dynamic contrast-enhanced MRI was performed using a breath-hold fat-suppressed 3D T1-weighted LAVA-Flex sequence before and after intravenous administration of Gd- DTPA (Magnevist, Bayer Schering Pharma, Berlin, Germany) at a dose of 0.1 mmol/kg and 2 mL/s, followed by a 20 mL of saline solution flush performed using a power injector. Images were acquired in the arterial phase (20-35 s), portal phase (60-80 s), and delayed phase (180-240 s), respectively.
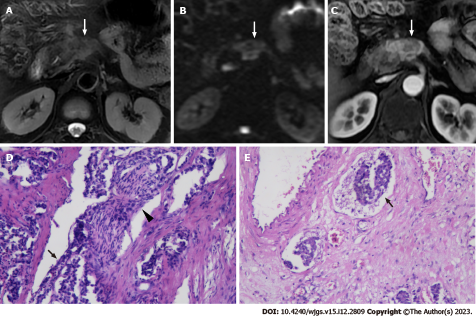
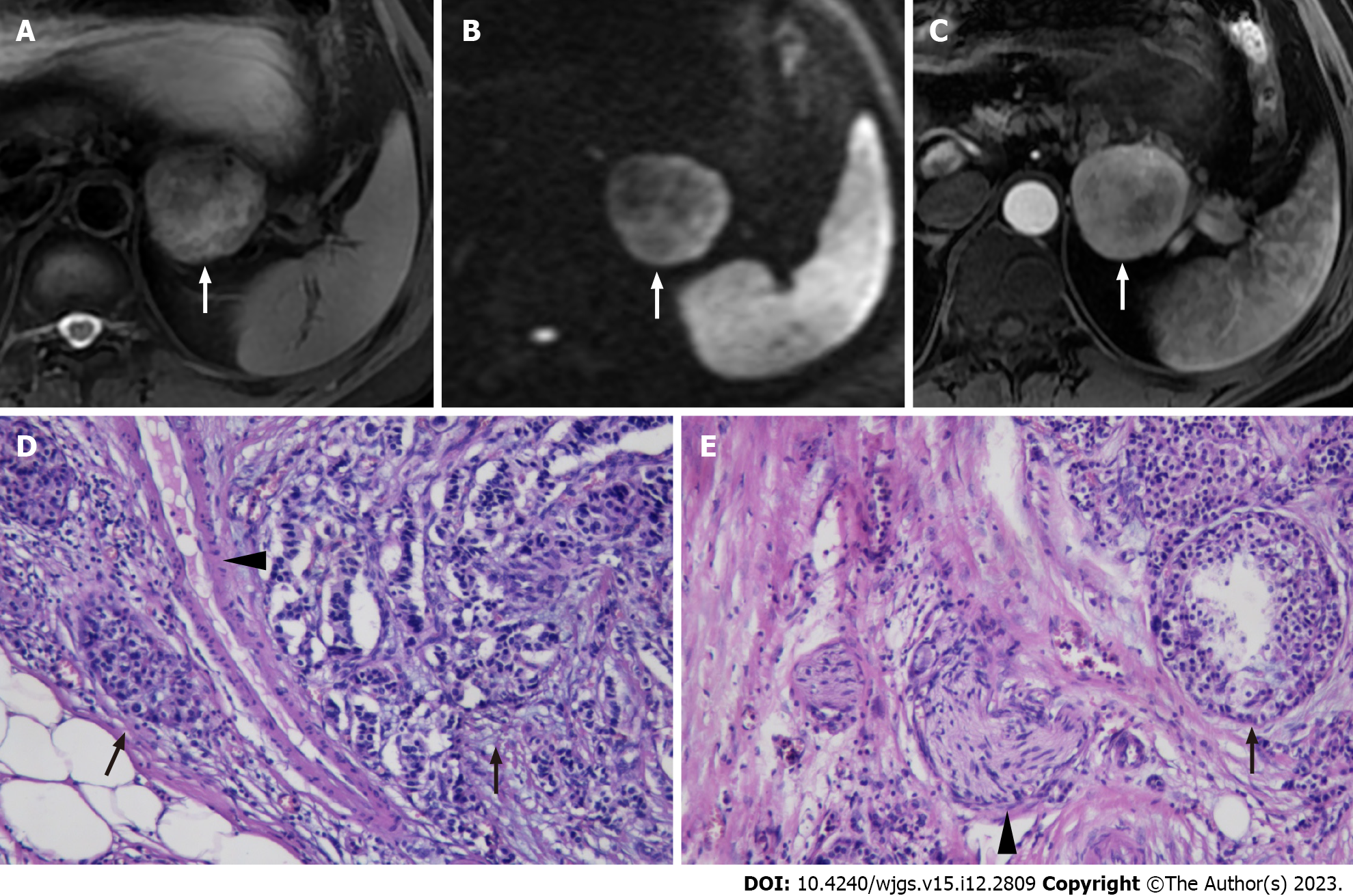
The patients were randomly grouped, and the reviewers were blinded to the clinical information and the pathological reports. The following qualitative features were evaluated: (1) Tumor location (pancreatic head/neck, body or tail); (2) Signal intensity (SI) on T2WI (hypointense, isointense, or hyperintense relative to the surrounding pancreatic parenchyma); (3) Size (maximum diameter of the tumor); (4) Tumor margin (regular or irregular); (5) Exophytic growth (present or absent); (6) Presence of upstream main pancreatic ductal dilatation (MPDD) and/or common bile duct dilatation (CBDD) due to tumor compression; (7) Hyperenhancement at arterial phase (present or absent), presence of vascular and adjacent organs invasion; (8) Enhancement pattern (homogeneous or heterogeneous); (9) Vascular and adjacent tissue involvement (present or absent); and (10) Presence of synchronous liver metastases. The distal main pancreatic duct of the tumor was considered dilated when its diameter was ≥ 5 mm, while CBDD was defined as a diameter of ≥ 10 mm. Vascular invasion was defined as the tumor directly invading adjacent vessels with the results of lumen obstruction or occlusion, abutted > 90° of major peri-pancreatic arteries, or abutted > 180° of the adjacent vein. A regular margin was defined as a round or oval shape with clear demarcation (Figure 1). Otherwise, the tumor with extra-nodular growth and confluent multi-nodular growth was defined as an irregular margin (Figure 2)[30,31].
If the tumor was located in the pancreatic head/neck, regional nodes included those along the common bile duct, common hepatic artery, portal vein, the anterior and posterior surfaces of the pancreatic head, and the superior mesenteric artery. If the tumor was located in the pancreatic body/tail, regional nodes included those along the common hepatic artery, splenic and superior mesenteric artery[27]. All visible regional lymph nodes in the field of the scan were analyzed. The size of the largest lymph node (the long and short axes) was measured, and the short/long ratio was subsequently calculated. The number of lymph nodes with a short axis > 5 mm, > 10 mm detected on the DWI sequence was also recorded. Moreover, morphological involvement of LNM was reported when the lymph node with abnormal round morphology or central necrosis was observed.
The presence of cancer cells and cancer cell nests in the interstitial space was indicative of lymphatic invasion. A space filled with lymph and lymphocytes was especially likely to be a lymphatic vessel. When endothelial cells were identified around the space, the space was considered a lymphatic vessel. When it was difficult to evaluate lymphatic vessels, D2-40 immuno-histochemical staining was applied. The microvascular invasion was highly likely when a circular, semicircular, or oblong cancer cell nest with regular margins was located in the vicinity of vessels and distant from the main lesion. If such a cancer cell nest was surrounded by venous wall structures (such as an internal elastic membrane or perivascular smooth muscle), it was considered to represent microvascular invasion. When it was difficult to identify vessels, Victoria blue staining was applied to elucidate elastic fibers in vessel walls. Perineural invasion was detected by the finding of cancer cells in the perineural space and nerve fiber bundles. An independent board-certified pathologist (TF) updated the data on the prior diagnoses made by previous pathologists using the abovementioned unified definition[20].
One radiologist manually placed regions of interest (ROIs) on the DWI images with a b value of 1000 s/mm2. DCE-MRI and T2WI images were used as a reference for ROI segmentation. ROIs were also drawn along the primary pancreatic tumor’s outer border on every slice, carefully avoiding vascular structures, the biliary duct, the pancreatic duct, and normal pancreatic tissue. ADC values from whole slices of the lesion were averaged as the ADCmean. The tumor’s maximum (ADCmax) and minimum (ADCmin) ADC values were also recorded. Tumor volume was multiplied by the slice thickness.
The differences in clinical factors and MRI features between groups were compared by using the independent t-test or Mann-Whitney test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. The univariate analysis included 39 variables according to LMPI status. A multivariate logistic regression model was established by substituting potentially significant variables from univariate analysis into an equation. Diagnostic performance was evaluated by calculating the receiver operator characteristic (ROC) curve with area under ROC (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy. All statistical analyses were performed with IBM SPSS (Version 22.0; IBM Corp., New York, United States) and R package 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P-value < 0.05 was considered as statistical significance.
A total of 61 patients who met the inclusion criteria were enrolled in the study. The clinical, pathological and MRI features of these patients are shown in Table 1. Their median age was 63 years, 27 (44.3%) patients were male and 34 (55.7%) patients were female (Table 1). The pathologic findings showed LMPI in 27 cases (44.3%) and no LMPI in 34 (55.7%) cases. Patients were divided into two groups according to the LMPI state.
| Factors | Lymphatic, microvascular, and perineural invasion | P value | |
| LMPI (-) (n = 34) | LMPI (+) (n = 27) | ||
| LNM | 0.001 | ||
| Absent | 31 | 14 | |
| Present | 3 | 13 | |
| Grade | < 0.001 | ||
| 1 | 19 | 1 | |
| 2 | 10 | 17 | |
| 3 | 5 | 9 | |
| Gender | 0.622 | ||
| Male | 16 | 11 | |
| Female | 18 | 16 | |
| Age, year | 52.59 ± 13.31 | 55.78 ± 11.26 | 0.324 |
| BMI (kg/m2) | 24.93 ± 3.63 | 24.36 ± 4.72 | 0.593 |
| Symptom | 0.264 | ||
| Absent | 14 | 15 | |
| Present | 20 | 12 | |
| TB, μmol/L | 0.738 | ||
| < 21 | 29 | 22 | |
| ≥ 21 | 5 | 5 | |
| ALT, IU/L | > 0.999 | ||
| < 40 | 30 | 23 | |
| ≥ 40 | 4 | 4 | |
| AST, IU/L | 0.123 | ||
| < 40 | 32 | 21 | |
| ≥ 40 | 2 | 6 | |
| FBG, mmol/L | 0.095 | ||
| < 6.1 | 24 | 14 | |
| ≥ 6.1 | 9 | 13 | |
| TLC, 109/L | 1.77 ± 0.55 | 1.62 ± 0.53 | 0.286 |
| TNC, 109/L | 3.46 ± 1.46 | 3.91 ± 2.17 | 0.338 |
| NLR | 2.05 ± 0.87 | 2.56 ± 1.55 | 0.107 |
| AFP, ng/mL | |||
| < 10.9 | 34 | 27 | |
| ≥ 10.9 | 0 | 0 | |
| CEA, ng/mL | 0.673 | ||
| < 5 | 26 | 25 | |
| ≥ 5 | 4 | 2 | |
| CA199, U/mL | 0.238 | ||
| < 37 | 28 | 22 | |
| ≥ 37 | 2 | 5 | |
| CA724, U/mL | 0.488 | ||
| < 5.9 | 20 | 22 | |
| ≥ 5.9 | 1 | 0 | |
| NSE, ng/mL | 0.023 | ||
| < 16.3 | 19 | 10 | |
| ≥ 16.3 | 4 | 10 | |
| Tumor location | 0.911 | ||
| Head or neck | 17 | 15 | |
| Body | 10 | 7 | |
| Tail | 7 | 5 | |
| SI on T2WI | 0.697 | ||
| Hypointense | 1 | 2 | |
| Isointense | 24 | 19 | |
| Hyperintense | 9 | 6 | |
| Maximum diameter of the tumor | 31.44 ± 16.53 | 45.03 ± 32.21 | 0.11 |
| Tumor margin | < 0.001 | ||
| Defined | 27 | 8 | |
| Illdefined | 7 | 19 | |
| Exophytic growth | 0.666 | ||
| Absent | 17 | 12 | |
| Present | 17 | 15 | |
| MPDD | 0.041 | ||
| Absent | 30 | 18 | |
| Present | 4 | 9 | |
| CBDD | 0.037 | ||
| Absent | 33 | 21 | |
| Present | 1 | 6 | |
| Hyperenhancement at the arterial phase | 0.062 | ||
| Present | 22 | 11 | |
| Absent | 12 | 16 | |
| Enhancement pattern | 0.011 | ||
| Homogeneous | 27 | 13 | |
| Heterogeneous | 7 | 14 | |
| Vascular and adjacent tissue involvement | 0.048 | ||
| Absent | 31 | 19 | |
| Present | 3 | 8 | |
| Synchronous liver metastases | 0.003 | ||
| Absent | 31 | 16 | |
| Present | 3 | 11 | |
| Long axis of the largest lymph node, mm | 7.59 ± 6.41 | 11.63 ± 7.42 | 0.026 |
| Short axis of the largest lymph node, mm | 4.15 ± 3.29 | 7.26 ± 5.27 | 0.006 |
| Ratio of the long/short axis of the largest lymph node | 0.74 ± 0.90 | 1.37 ± 1.18 | 0.266 |
| Shape of the largest lymph node | 0.092 | ||
| Normal | 31 | 20 | |
| Abnormal | 3 | 7 | |
| Number of lymph nodes with the short axis > 5 mm | 0.74 ± 0.88 | 1.37 ± 1.18 | 0.026 |
| Number of lymph nodes with the short axis > 10 mm | 0.09 ± 0.38 | 0.52 ± 0.98 | 0.014 |
| ADCmean, (× 10-3 mm2/s) | 1444.97 ± 360.98 | 1552.26 ± 654.46 | 0.427 |
| ADCmax, (× 10-3 mm2/s) | 2338.36 ± 604.50 | 2813.16 ± 1610.93 | 0.124 |
| ADCmin, (× 10-3 mm2/s) | 562.43 ± 672.51 | 487.88 ± 647.09 | 0.699 |
| Tumor volume, mm3 | 17788.45 ± 22520.13 | 47201.46 ± 85657.55 | 0.029 |
Significant differences were found in tumor margin, enhancement pattern and size of the largest lymph node between the two groups. Irregular margin (P < 0.001) and heterogeneous enhancement pattern (P = 0.011) were more likely to be seen in a patient with LMPI. The long axis of the largest lymph node was significantly larger (7.26 ± 5.27 vs 4.15 ± 3.29, P = 0.006) in patients with LMPI.
Other factors that were identified by univariate analysis to differ between the two groups included lymph node status, tumor grade, NSE level, CBDD, MPDD, synchronous liver metastases, presence of vascular and adjacent organs invasions, a number of lymph nodes, and tumor volume (all P < 0.05).
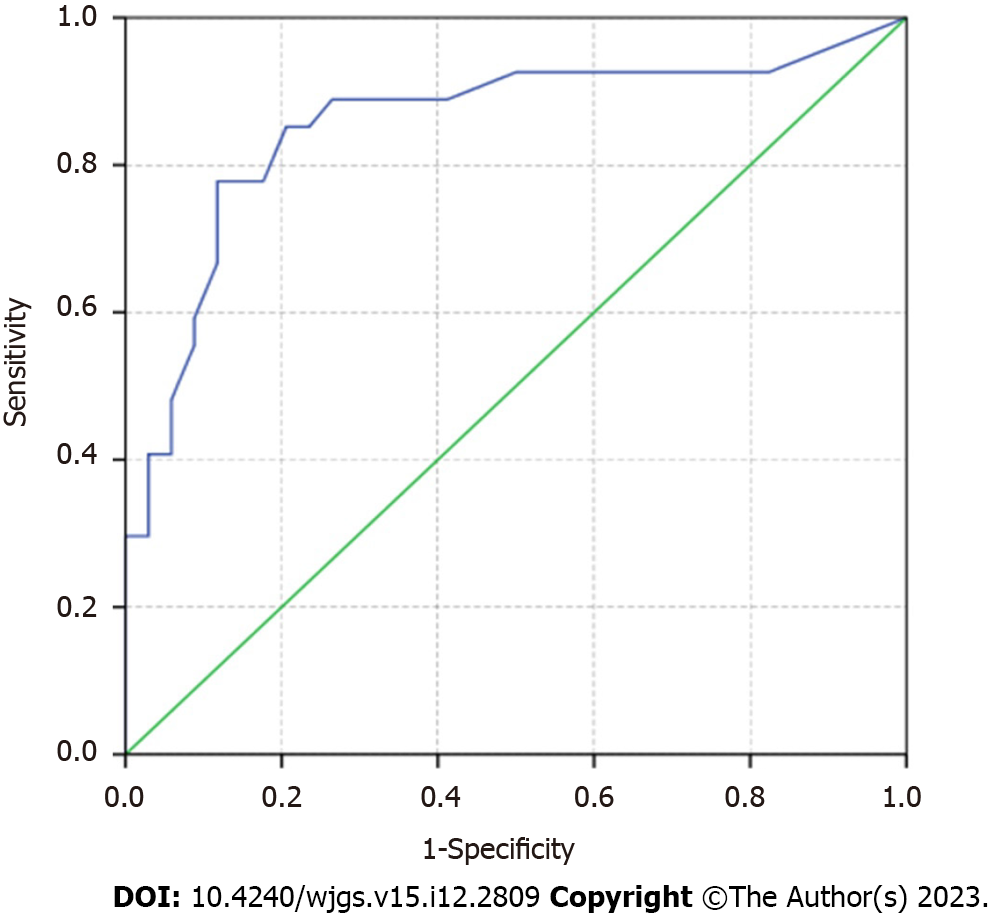
According to the multivariate logistic regression analysis, tumor margin [odds ratio (OR) = 11.523; 95% confidence interval (CI): 2.966-44.761, P < 0.001] was an independent factor associated with LMPI of NF-PNETs. The enhancement pattern and size of the largest lymph node, with P-value of 0.056 (OR = 3.833; 95%CI: 0.907-16.197) and 0.066 (OR = 1.1660; 95%CI: 990-1.374), were included in our predicting model. The diagnostic performance of the multivariate logistic regression model was shown in Figure 3, with an AUC = 0.855 (95%CI: 0.750-0.960). The sensitivity, specificity, PPV, NPV and accuracy of the model were 48.1% (14/27), 97.1% (33/34), 97.1% (13/14), 70.2% (33/47) and 0.754, respectively. The optimal cut-off point of the probability of our multivariate model for predicting LMPI was 43%.
Tumor cells can metastatic to regional lymph nodes or distant organs through vascular and lymphatic vessels and nerves, which may represent the agressiveness of the tumor[9]. LMPI, an independent risk factor for postoperative recurrence of NF-PNETs[20-22], has an important role in lymph node involvement and tumor staging to some extent. Moreover, the combination of WHO grades and LMPI may assist in stratifying the patients at risk of tumor recurrence or optimizing the operation procedure. LMPI is of great significance for deciding whether further cleaning or extended resection is required in gastrointestinal cancer and gastrointestinal neuroendocrine tumors[18,19]. However, LMPI of gastrointestinal cancer can be obtained before endoscopy, while LMPI of PNETs can only be obtained through postoperative pathological examination. Previous researches reported that MRI features could be employed to predict patient’s prognosis, which might guide surgeons’ surgical plan and individualized treatment of PNETs patients[32]. Consequently, this study analyzed the relationship between pre-operative clinical and MRI features and LMPI.
We found that the tumor margin of NF-PNETs was an independent predictor for LMPI (OR = 11.523; 95%CI: 2.966-44.761, P < 0.001). Our results showed that NFPNETs with an ill-defined margin of the tumor were more aggressive, which was consistent with former researches. In their study, it reveal that there was significant correlation between ill-defined margin and clinical variables relevant to disease progressions and also poor overall survival[30]. Likewise, De Robertis et al[33] found that ill-defined margins were more likely to be seen in G2-3 than in G1 tumors, and also more common in stage III-IV PNETs than in low-stage tumors. Our current results revealed that LNM was more common in tumors with irregular margins than those with well-defined margins, which further validated the relevance between ill-defined margins and tumor aggressiveness in NF-PNETs. According to our multivariate model, the best cut-off value of the probability for predicting LMPI was 43%, and to the best of our knowledge, the cut-of value of the probability for the prediction of LMPI has not been reported for NF-PNETs.
Moreover, univariate analysis of this study showed that G2-3 tumors were more likely to develop LMPI (P < 0.001), similar to a previous study[12]. Other factors that were significantly different according to the LMPI state LMPI (P < 0.05) included tumor margin, enhancement pattern, the long axis and the short axis of the largest lymph node of the largest lymph node status, NSE level, CBDD, MPDD, synchronous liver metastases, presence of vascular and adjacent organs invasion, and a number of the lymph node and tumor volume. Most of these factors reflect the imaging performance of aggressiveness of the tumor to some extent. De Robertis et al[33] reported that heterogeneous enhancement is a reliable predictor of G2-3 tuners (specificity, 71%). Oba et al[31] reported that G2-3 tumors are significantly larger than G1 tumors, while Hyodo et al[34] found that heterogeneous enhancement was correlated with tumor size. Nevertheless, our results showed no significant difference in tumor size between the two groups, which could be due to two following reasons: First, we did not group the tumor by tumor sizes, such as 10 mm and 20 mm. Second, we did not further analyze the lymphatic, microvascular and perineural invasions, respectively.
The information on the relationship between ADC values and lymphatic, microvascular and perineural invasion is relatively rare. In their study, Harimoto et al[35] reported that venous, neural invasion and lower mean ADC values ≤ 1458 × 10-6 mm2/s were independent impact factors of LNM. Although our study showed that patients with LMPI were significantly associated with LN status, there was no significant difference in ADC values between the two groups (P > 0.05). This may be because the clinical behavior and histopathologic appearances of NF-PNETs widely vary, which may further lead to the overlap between the ADC values. Partelli et al[36] found that the ADCmean value of PNETs was significantly higher than that in G1-PNETs. But, Hwang et al[26] reported that there was no significant difference of ADC values between the G1 and G2+3 tumors.
First, this study did not analyze lymphatic, microvascular, and perineural invasion respectively. Previous studies revealed that microvascular invasion, microvascular, and perineural invasion were prognostic factors of postoperative recurrence[20-22]. Further studies are needed to evaluate the correlation between MRI features and lymphatic invasion, microvascular invasion and perineural invasion respectively. Second, different MR scanners were used due to the retrospective design; however, different scanners and protocols did not obviously affect the morphological classification and measurement. Thirdly, other information, such as DCE sequence and intravoxel incoherent motion, were not included and should be investigated by future studies.
Herein, we elaborated on the relationship between MRI features and LMPI of NF-PNETs. As one of the MRI features, the tumor margin has an important role in predicting LMPI in patients with NF-PNETs.
Pancreatic neuroendocrine tumors (PNETs) are comparatively rare neoplasms. Lymphatic, microvascular, and perineural invasion (LMPI) was significantly correlated with the prognosis of PENTs which was confirmed by previous studies. There was no previous study reported the relationship between magnetic resonance imaging (MRI) parameters and LMPI.
The key problem is whether preoperative MRI of the pancreas can predict LMPI in patients with non-functioning NF-PNETs.
The main objective is to determine the feasibility to predict lymphatic, microvascular and perineural invasion in patients with non-functioning PENTs (NF-PNETs) by using preoperative MRI of the pancreas. MRI is a non-invasive imaging modality, and there will be more broad application prospects.
The comprehensive clinical indicators and MRI parameters of patients with NF-PNETs were collected. A multivariate logistic regression model was established and the diagnostic performance was evaluated.
Patients were divided into two groups according to the LMPI state. Irregular margin (P < 0.001) and heterogeneous enhancement pattern (P = 0.011) were more likely to be seen in a patient with LMPI. The long axis of the largest lymph node was significantly larger (7.26 ± 5.27 vs 4.15 ± 3.29, P = 0.006) in patients with LMPI. According to the multivariate logistic regression analysis, tumor margin (odds ratio = 11.523; 95% confidence interval: 2.966-44.761, P < 0.001) was an independent factor associated with LMPI of NF-PNETs.
The relationship between MRI features and LMPI of NF-PNETs was elaborated. The tumor margin, which is one of the MRI features, has an important role in predicting LMPI in patients with NF-PNETs.
This study evaluated the relationship between preoperative clinical indicators, MRI parameters and LMPI in NF-PNETs. To evaluate the correlation between MRI features and lymphatic invasion, microvascular invasion and perineural invasion respectively.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sokolova O, Germany; Tse J, Australia S-Editor: Wang JJ L-Editor: A P-Editor: Wu RR
| 1. | Tanaka M, Heckler M, Mihaljevic AL, Probst P, Klaiber U, Heger U, Schimmack S, Büchler MW, Hackert T. Systematic Review and Metaanalysis of Lymph Node Metastases of Resected Pancreatic Neuroendocrine Tumors. Ann Surg Oncol. 2021;28:1614-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3242] [Article Influence: 190.7] [Reference Citation Analysis (0)] |
| 3. | Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol. 2013;20:2815-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, Takayanagi R, Shimatsu A. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Zheng-Pywell R, Fang A, AlKashash A, Awad S, Reddy S, Vickers S, Heslin M, Dudeja V, Chen H, Rose JB. Prognostic Impact of Tumor Size on Pancreatic Neuroendocrine Tumor Recurrence May Have Racial Variance. Pancreas. 2021;50:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, Tseng JF. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Doi R. Determinants of surgical resection for pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci. 2015;22:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Souche R, Hobeika C, Hain E, Gaujoux S. Surgical Management of Neuroendocrine Tumours of the Pancreas. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Pulvirenti A, Pea A, Chang DK, Jamieson NB. Clinical and Molecular Risk Factors for Recurrence Following Radical Surgery of Well-Differentiated Pancreatic Neuroendocrine Tumors. Front Med (Lausanne). 2020;7:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Singh S, Chan DL, Moody L, Liu N, Fischer HD, Austin PC, Segelov E. Recurrence in Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA Oncol. 2018;4:583-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. WHO Classification of Tumours, 4th Edition. Geneva: World Health Organization, 2017. |
| 12. | Izumo W, Higuchi R, Furukawa T, Yazawa T, Uemura S, Shiihara M, Yamamoto M. Evaluation of the Site and Frequency of Lymph Node Metastasis with Non-Functioning Pancreatic Neuroendocrine Tumor. Eur Surg Res. 2019;60:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Song KB, Kim SC, Kim JH, Hong SM, Park KM, Hwang DW, Lee JH, Lee YJ. Prognostic factors in 151 patients with surgically resected non-functioning pancreatic neuroendocrine tumours. ANZ J Surg. 2016;86:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A, Doglioni C, Delle Fave G, Fischer L, Fusai G, de Herder WW, Jann H, Komminoth P, de Krijger RR, La Rosa S, Luong TV, Pape U, Perren A, Ruszniewski P, Scarpa A, Schmitt A, Solcia E, Wiedenmann B. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 15. | Fischer L, Bergmann F, Schimmack S, Hinz U, Prieß S, Müller-Stich BP, Werner J, Hackert T, Büchler MW. Outcome of surgery for pancreatic neuroendocrine neoplasms. Br J Surg. 2014;101:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Miura T, Ohtsuka H, Aoki T, Aoki S, Hata T, Takadate T, Maeda S, Ariake K, Kawaguchi K, Masuda K, Ishida M, Mizuma M, Nakagawa K, Morikawa T, Fujishima F, Kamei T, Sasano H, Unno M. Increased neutrophil-lymphocyte ratio predicts recurrence in patients with well-differentiated pancreatic neuroendocrine neoplasm based on the 2017 World Health Organization classification. BMC Surg. 2021;21:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Harimoto N, Hoshino K, Muranushi R, Hagiwara K, Yamanaka T, Ishii N, Tsukagoshi M, Igarashi T, Tanaka H, Watanabe A, Kubo N, Araki K, Hosouchi Y, Suzuki H, Arakawa K, Hirai K, Fukazawa T, Ikota H, Shirabe K. Prognostic significance of neutrophil-lymphocyte ratio in resectable pancreatic neuroendocrine tumors with special reference to tumor-associated macrophages. Pancreatology. 2019;19:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 603] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1335] [Article Influence: 333.8] [Reference Citation Analysis (2)] |
| 20. | Izumo W, Higuchi R, Furukawa T, Yazawa T, Uemura S, Matsunaga Y, Shiihara M, Takayama Y, Tahara J, Shimizu K, Tokushige K, Yamamoto M. Evaluation of the Significance of Lymphatic, Microvascular and Perineural Invasion in Patients With Pancreatic Neuroendocrine Neoplasms. Cancer Diagn Progn. 2022;2:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Andreasi V, Ricci C, Partelli S, Guarneri G, Ingaldi C, Muffatti F, Crippa S, Casadei R, Falconi M. Predictors of disease recurrence after curative surgery for nonfunctioning pancreatic neuroendocrine neoplasms (NF-PanNENs): a systematic review and meta-analysis. J Endocrinol Invest. 2022;45:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Nanno Y, Toyama H, Otani K, Asari S, Goto T, Terai S, Ajiki T, Zen Y, Fukumoto T, Ku Y. Microscopic venous invasion in patients with pancreatic neuroendocrine tumor as a potential predictor of postoperative recurrence. Pancreatology. 2016;16:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Lo GC, Kambadakone A. MR Imaging of Pancreatic Neuroendocrine Tumors. Magn Reson Imaging Clin N Am. 2018;26:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Chen ZE, Yaghmai V, Nikolaidis P, McCarthy RJ, Merrick L, Miller FH. Diffusion-weighted MR imaging in pancreatic endocrine tumors correlated with histopathologic characteristics. J Magn Reson Imaging. 2011;33:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Sun HT, Zhang SL, Liu K, Zhou JJ, Wang XX, Shen TT, Song XH, Guo YL, Wang XL. MRI-based nomogram estimates the risk of recurrence of primary nonmetastatic pancreatic neuroendocrine tumors after curative resection. J Magn Reson Imaging. 2019;50:397-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Hwang EJ, Lee JM, Yoon JH, Kim JH, Han JK, Choi BI, Lee KB, Jang JY, Kim SW, Nickel MD, Kiefer B. Intravoxel incoherent motion diffusion-weighted imaging of pancreatic neuroendocrine tumors: prediction of the histologic grade using pure diffusion coefficient and tumor size. Invest Radiol. 2014;49:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Han S, Kim JH, Yoo J, Jang S. Prediction of recurrence after surgery based on preoperative MRI features in patients with pancreatic neuroendocrine tumors. Eur Radiol. 2022;32:2506-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 28. | Canellas R, Lo G, Bhowmik S, Ferrone C, Sahani D. Pancreatic neuroendocrine tumor: Correlations between MRI features, tumor biology, and clinical outcome after surgery. J Magn Reson Imaging. 2018;47:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Lotfalizadeh E, Ronot M, Wagner M, Cros J, Couvelard A, Vullierme MP, Allaham W, Hentic O, Ruzniewski P, Vilgrain V. Prediction of pancreatic neuroendocrine tumour grade with MR imaging features: added value of diffusion-weighted imaging. Eur Radiol. 2017;27:1748-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Okabe H, Hashimoto D, Chikamoto A, Yoshida M, Taki K, Arima K, Imai K, Tamura Y, Ikeda O, Ishiko T, Uchiyama H, Ikegami T, Harimoto N, Itoh S, Yamashita YI, Yoshizumi T, Beppu T, Yamashita Y, Baba H, Maehara Y. Shape and Enhancement Characteristics of Pancreatic Neuroendocrine Tumor on Preoperative Contrast-enhanced Computed Tomography May be Prognostic Indicators. Ann Surg Oncol. 2017;24:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Oba A, Kudo A, Akahoshi K, Kishino M, Akashi T, Katsuta E, Iwao Y, Ono H, Mitsunori Y, Ban D, Tanaka S, Eishi Y, Tateishi U, Tanabe M. A simple morphological classification to estimate the malignant potential of pancreatic neuroendocrine tumors. J Gastroenterol. 2017;52:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Zhu HB, Nie P, Jiang L, Hu J, Zhang XY, Li XT, Lu M, Sun YS. Preoperative prediction of lymph node metastasis in nonfunctioning pancreatic neuroendocrine tumors from clinical and MRI features: a multicenter study. Insights Imaging. 2022;13:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | De Robertis R, Cingarlini S, Tinazzi Martini P, Ortolani S, Butturini G, Landoni L, Regi P, Girelli R, Capelli P, Gobbo S, Tortora G, Scarpa A, Pederzoli P, D'Onofrio M. Pancreatic neuroendocrine neoplasms: Magnetic resonance imaging features according to grade and stage. World J Gastroenterol. 2017;23:275-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Hyodo R, Suzuki K, Ogawa H, Komada T, Naganawa S. Pancreatic neuroendocrine tumors containing areas of iso- or hypoattenuation in dynamic contrast-enhanced computed tomography: Spectrum of imaging findings and pathological grading. Eur J Radiol. 2015;84:2103-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Harimoto N, Araki K, Hoshino K, Muranushi R, Hagiwara K, Ishii N, Tsukagoshi M, Igarashi T, Watanabe A, Kubo N, Tomonaga H, Higuchi T, Tsushima Y, Ikota H, Shirabe K. Diffusion-Weighted MRI Predicts Lymph Node Metastasis and Tumor Aggressiveness in Resectable Pancreatic Neuroendocrine Tumors. World J Surg. 2020;44:4136-4141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Partelli S, Gaujoux S, Boninsegna L, Cherif R, Crippa S, Couvelard A, Scarpa A, Ruszniewski P, Sauvanet A, Falconi M. Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). JAMA Surg. 2013;148:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |