Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2783
Peer-review started: October 26, 2023
First decision: November 8, 2023
Revised: November 17, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 27, 2023
Processing time: 62 Days and 9.6 Hours
Primary hepatic carcinoma (PHC) has an insidious onset and is usually diagnosed in the middle and late stages. Although transcatheter arterial chemoembolization (TACE) is the preferred option for treating middle- and advanced-stage PHC, it has limited efficacy in killing tumor cells and poor long-term efficacy. TACE plus percutaneous microwave coagulation therapy (PMCT) is more effective than interventional therapy alone and can improve survival time. However, there are few reports on the effects of TACE and PMCT on serum marker levels and the prognosis of patients with advanced PHC.
To investigate the effect of PMCT + TACE on serum tumor markers and the prognosis of middle-late PHC.
This retrospective study included 150 patients with middle-late PHC admitted to Zhongshan People’s Hospital between March 2018 and February 2021. Patients were divided into a single group (treated with TACE, n = 75) and a combined group (treated with TACE + PMCT, n = 75). Before and after treatment, the clinical efficacy and serum tumor marker levels [carbohydrate antigen 19-9 (CA19-9), alpha-fetoprotein (AFP), and carcinoembryonic antigen (CEA)] of both groups were observed. The 1-year survival rates and prognostic factors of the two groups were analyzed.
The combined group had 21 and 35 cases of complete remission (CR) and partial remission (PR), respectively. The single group had 13 and 25 cases of CR and PR, respectively. After 4 wk of treatment, the serum CA19-9, CEA, and AFP levels in the single and combined groups decreased, with the decrease in the combined group being more significant (P < 0.05). The 1-year survival rate of the combined group (80.00%) was higher than that of the single group (60.00%) (P < 0.05). The average survival time within 1 year in the combined group was 299.38 ± 61.13 d, longer than that in the single group (214.41 ± 72.97 d, P < 0.05). COX analysis revealed that tumor diameter, tumor number, and the treatment method were prognostic factors for patients with middle-late PHC (P < 0.05).
TACE + PMCT is effective in treating patients with mid-late PHC. It reduces the levels of tumor markers, prolongs survival, and improves prognosis.
Core Tip: Middle- and late-stage primary hepatic carcinoma (PHC) has reached a phase of comprehensive treatment. Compared to single interventional therapy, transcatheter arterial chemoembolization (TACE) plus percutaneous microwave coagulation therapy (PMCT) can effectively improve prognosis and prolong patient survival. This study reveals that TACE + PMCT in the management of patients with middle-late PHC enhances tumor marker levels and prolongs survival time. Furthermore, we identify tumor diameter, tumor number, and treatment method as pivotal factors influencing prognosis.
- Citation: Lin ZP, Huang DB, Zou XG, Chen Y, Li XQ, Zhang J. Percutaneous microwave ablation and transcatheter arterial chemoembolization for serum tumor markers and prognostics of middle-late primary hepatic carcinoma. World J Gastrointest Surg 2023; 15(12): 2783-2791
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2783.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2783
Primary hepatic carcinoma (PHC) is a common histological form of liver cancer, accounting for 70.0%–85.0% of total liver cancer cases, with a markedly poor prognosis[1]. Currently, the primary treatment methods for PHC include radical surgical resection, targeted therapy (such as sorafenib, lenvatinib, cabozantinib, etc.), interventional and immunotherapy[2]. Surgical resection can achieve significant efficacy in early PHC cases; however, due to the hidden onset of the disease, that is, the absence of obvious symptoms and signs in the early stages, only 20%–30% of patients undergo surgical resection in the middle to late stages[3]. Interventional therapy is the primary option for middle-aged patients who miss the optimal surgery window[4]. Transcatheter arterial chemoembolization (TACE) is a common type of interventional therapy for patients with middle-to-late stage PHC[5]. Normally, the liver receives blood supply through two routes: the portal vein supplies 70%–75%, and the hepatic artery supplies 20%–25%. However, the liver cancer tissue supplies 90%–95% of the latter[6]. TACE takes advantage of these features. On the one hand, embolization is performed on the target artery supplying blood to the tumor, resulting in tumor shrinkage and ischemic necrosis. On the other hand, it selectively injects chemotherapy drugs into the target lesion, thus achieving the goal of treating PHC with minimal damage to normal liver tissue[7,8]. However, TACE cannot completely eliminate the target lesion and is prone to tumor recurrence. The above drawbacks are major factors for patients undergoing multiple treatments and adjusting their treatment plans[9]. With the continuous development of new hyperthermia technologies, studies have shown that TACE plus percutaneous microwave coagulation therapy (PMCT) can improve the clinical curative effect in patients with liver cancer and prolong survival time[10,11]. In addition, PMCT can effectively improve anti-tumor immune responses in the body[12]. However, there are few reports on the effects of TACE and PMCT on serum marker levels and the prognosis of patients with advanced PHC. This study retrospectively analyzed the effects of PMCT combined with TACE on serum tumor marker levels and prognosis in patients with mid-late PHC to provide a reference for clinical management.
This study involved 150 patients with mid-late PHC admitted to Zhongshan People’s Hospital between March 2018 and February 2021. The inclusion criteria were as follows: (1) PHC diagnosis confirmed by medical imaging, serum tumor marker detection, and pathological test; (2) Tumor TNM stage Ⅱ–IV, with no indication for surgery or surgery cannot be performed; and (3) Complete clinical data and follow-up information. The exclusion criteria were as follows: (1) Significant organ dysfunction (such as kidney, heart, lung, etc.) or severe illnesses; (2) combined with coagulation dysfunction; (3) Contraindications to TACE and PMCT; (4) Presence of other malignant tumors; (5) History of past PMCT or TACE treatment; (6) Recurrence of lung cancer; and (7) Patients with mental illness and inability to cooperate.
Based on the different treatments, the patients were separated into a single group (n = 75) and a combined group (n = 75). No statistically significant differences were observed in the basic data between the single and combined groups (P > 0.05), as shown in Table 1.
| Group | Gender | Age (yr) | Tumor number (cases) | Tumor diameter (cm) | ||
| Male | Female | Single | Multiple | |||
| Combined group (n = 75) | 41 (54.67) | 34 (45.33) | 53.05 ± 13.43 | 47 (62.67) | 28 (37.33) | 5.11 ± 1.27 |
| Single group (n = 75) | 43 (57.33) | 32 (42.67) | 52.51 ± 12.06 | 49 (65.33) | 26 (34.67) | 5.49 ± 1.23 |
| χ2/t value | 0.108 | -0.558 | 0.262 | -1.900 | ||
| P value | 0.742 | 0.580 | 0.793 | 0.059 | ||
The single group was treated under local anesthesia with TACE alone, guided by digital subtraction angiography, and percutaneous puncture of the femoral artery to the mesentery and the common hepatic artery. The catheter was placed into the main supplying artery of the lesion, and oxaliplatin (100 mg) and liquefied iodide (10 mL) were injected through the catheter. The main feeding artery was embolized with a gelatin sponge particle, and the effect of the embolization was again observed on angiography. Depending on the patient's condition before and after the first TACE treatment, the treatment was repeated 1 to 3 times, and the interval between the two TACE treatments was not less than 4 wk.
The combined group underwent a treatment regimen involving TACE + PMCT. Specifically, this group received 1–2 rounds of TACE treatment followed by 2–4 wk of combined PMCT treatment. In contrast, the single group received only TACE treatment. Before initiating microwave ablation (MWA) treatment, imaging studies were conducted on all patients to select the needle entry path. Special care was taken to minimize the risk of puncturing important organs such as the gallbladder, large blood vessels, and gastrointestinal tract.
First, preoperative preparation for conventional MWA was performed. Guided by a spiral CT scan, 1–2 MWA needles were inserted percutaneously into the tumor tissue, and a combined multisite ablation was performed by adjusting the depth and angle of the microwave antenna. The ablation power and time were selected according to the patient's lesion size and tolerance, as well as surrounding organs at risk. Postoperatively, the two groups of patients were treated with liver-protective, antiemetic, and antipyretic agents. Imaging studies were performed in both groups 2–4 wk after surgery, and the therapeutic effect was evaluated.
Clinical efficacy: Four weeks after the last treatment, the clinical efficacy in the enrolled patients was evaluated according to the mRECIST (modified response evaluation criteria in solid tumors) evaluation criteria[13]. Complete remission (CR) was defined as complete tumor disappearance sustained for at least 4 wk. Partial remission (PR) was defined as tumor shrinkage ≥ 30%, sustained for at least 4 wk. The tumor: Stable with a reduction of < 30% or an increase but an increase of < 20%; Disease progression was characterized by tumor enlargement ≥ 25%, new tumors, or metastatic lesions. Objective response rate (ORR) = (PR + CR) cases/total cases × 100%.
Serum tumor markers: Precisely, 5.0 mL of venous blood was collected from the enrolled patients before treatment and 4 wk after treatment. The serum of the collected blood was separated using centrifugation and stored in a specially insulated refrigerator for drugs at 2–6℃. The serum levels of carbohydrate antigen 19-9 (CA19-9), alpha-fetoprotein (AFP), and carcinoembryonic antigen (CEA) in the two groups were measured using an i2000 automatic microparticle chemiluminescence immunoanalyzer (Abbott Laboratories, United States).
Survival: The patients were followed up for 1 year after treatment (until January 2022). The recurrence, metastasis, and death rates in the single and combined groups were recorded, and prognostic factors related to the patients with mid-late PHC were analyzed.
Data were analyzed using IBM SPSS software (version 26.0). Counting variables are expressed as [n (%)], and continuous variables are reported as mean ± SD. These variables were tested using t-tests (also known as Student's t test) and χ2 tests, respectively. The survival rate was analyzed using the Kaplan–Meier method, while prognostic factors were assessed using the COX method. Inspection level as the α = 0.05. Statistical significance was set at P < 0.05.
The combined group had 21 and 35 cases of CR and PR, respectively. The single group had 13 and 24 cases of CR and PR, respectively. The ORR of the combined group was higher (74.67%, P < 0.05) than that of the single group (49.33%), as shown in Table 2.
Before treatment, the levels of serum tumor markers in the single and combined groups were compared (all P > 0.05). After 4 wk of treatment, the serum CA19-9, CEA, and AFP levels in the single and combined groups decreased. The decrease in the combined group was more significant (P < 0.05) (Table 3).
| Group | AFP (g/L) | CEA (g/mg) | CA19-9 (U/mL) | |||
| Pre-treatment | After 4 wk of treatment | Pre-treatment | After 4 wk of treatment | Pre-treatment | After 4 wk of treatment | |
| Combined group (n = 75) | 364.17 ± 50.46 | 212.52 ± 39.522 | 19.44 ± 2.05 | 11.02 ± 2.182 | 239.87 ± 40.16 | 170.25 ± 28.281 |
| Single group (n = 75) | 361.74 ± 51.78 | 256.23 ± 42.11 | 19.32 ± 2.11 | 13.56 ± 2.40 | 240.56 ± 40.22 | 189.54 ± 38.84 |
| t value | -0.290 | -6.553 | 0.325 | -6.770 | -0.105 | -3.477 |
| P value | 0.772 | < 0.001 | 0.746 | < 0.001 | 0.916 | 0.001 |
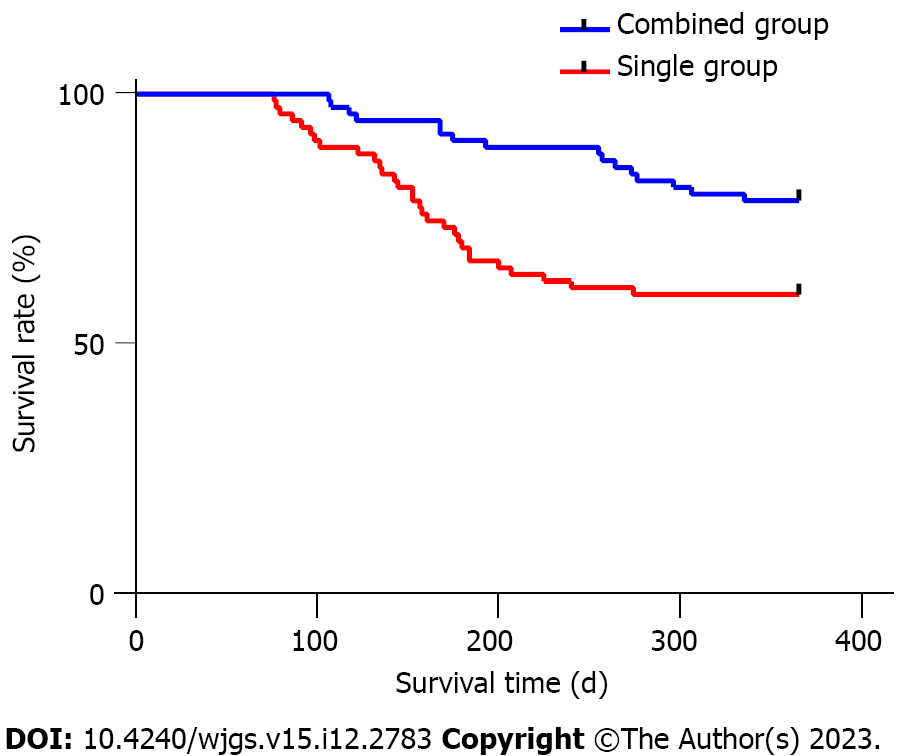
By the end of the follow-up period, the recurrence and metastasis rates in both the combined and single groups yielded P values greater than 0.05. The 1-year survival rate was significantly higher in the combined group (80.00%) compared to the single group (60.00%, P < 0.05), as shown in Table 4. Additionally, the average survival time in the combined group (299.38 ± 61.13 d) was longer than that in the single group (214.41 ± 72.97 d, P < 0.05, Figure 1).
The patients were divided into survival (n = 104) and death (n = 46) groups. Univariate analysis indicated a significant relationship between prognosis and tumor diameter, tumor number, and treatment method (P < 0.05), as shown in Table 5.
| Item | Death group (n = 46) | Survival group (n = 104) | χ2 | P value |
| Gender (male/female) | 21/25 | 63/41 | 2.883 | 0.090 |
| Age (yr) | 0.403 | 0.836 | ||
| ≥ 60 | 16 (34.78) | 38 (36.54) | ||
| < 60 | 30 (65.22) | 66 (63.46) | ||
| Tumor diameter (cm) | 17.559 | < 0.0012 | ||
| ≥ 6 | 31 (67.39) | 32 (30.77) | ||
| < 6 | 15 (32.61) | 72 (69.23) | ||
| Tumor number (cases) | 23.588 | < 0.0012 | ||
| Single | 17 (36.96) | 81 (77.88) | ||
| Multiple | 29 (63.04) | 23 (22.12) | ||
| Treatment method (cases) | 6.145 | 0.0131 | ||
| Single TACE | 30 (65.22) | 45 (43.27) | ||
| TACE + PMCT | 16 (34.78) | 59 (56.73) |
The COX proportional risk model was used to analyze and assign the study variables (Table 6). The results showed that the factors influencing the prognosis of patients with middle and advanced PHC were tumor diameter, tumor number, and treatment methods (P < 0.05, Table 7).
| Variable | |
| Gender | Male = 1, female = 0 |
| Age | ≥ 60 yr = 1, < 60 yr = 0 |
| Tumor diameter | ≥ 6 cm =1, < 6 cm = 0 |
| Tumor number | Single = 1, Multiple = 0 |
| Treatment method | Single TACE = 1, TACE + PMCT = 0 |
| Item | B | SE | Wald | P value | OR | 95%CI |
| Tumor diameter | 0.989 | 0.339 | 8.495 | 0.004 | 2.689 | 1.383-5.230 |
| Tumor number | 1.076 | 0.330 | 10.659 | 0.001 | 2.934 | 1.537-5.598 |
| Treatment method | 1.006 | 0.329 | 9.336 | 0.002 | 1.735 | 1.434-5.215 |
Owing to the hidden onset and rapid progression of PHC, most patients are diagnosed at the mid-late stage, and the opportunity for surgery is lost. Local intervention, thermal ablation, and chemotherapy have become primary treatments. As the preferred treatment for middle-late PHC, TACE can achieve a 1-year survival rate of more than 80%[14]. However, it has been reported in relevant literature that TACE treatment cannot improve the survival rate of patients with tumor diameter > 5 cm, and that the local hypoxia caused by a single TACE treatment may promote the regeneration of tumor blood vessels, resulting in high recurrence and metastasis rates for advanced liver cancer[15,16]. Therefore, combining TACE therapy with other treatment options has become the consensus for treating patients with PHC, especially those with mid-late disease who cannot be treated with surgery[17].
PMCT is a commonly used thermal ablation therapy for tumors. The basic principle is that the polar molecules within a substance can vibrate violently under the action of a rapidly changing electromagnetic field, generating a large amount of heat. Cancer cells stop proliferating when the lesion site is heated to an appropriate temperature, and tumor tissues begin to coagulate and necrose[12,18]. Studies have shown that PMCT has good clinical value because it offers advantages such as a good anti-heat sink effect, a wide MWA range, rapid local lesion warming, short surgical operation time, and no intraoperative current generation[19]. In addition, TACE can induce ischemia and hypoxia in tumor tissues, enhance thermal sensitivity, and enhance the high-temperature killing effect of PMCT on tumor cells[20].
Li et al[21] recently published a study in patients with hepatocellular carcinoma (HCC) treated with TACE combined with MWA, which showed that the CR of TACE + MWA was higher compared to TACE alone, (65% vs 46%). Simultaneously, progression-free survival was significantly prolonged in the TACE + MWA treatment group (29 vs 19 mo), and conventional TACE + MWA did not increase the risk of adverse events. A meta-analysis by Wang et al[22] showed that the survival of patients treated with TACE + MWA was significantly better than that of patients treated with TACE alone, especially in patients with tumor diameters > 5 cm. This study found that, compared to the single group, the combined group had a higher CR (28% vs 17.33%) and a higher ORR (74.67% vs 50.67%). After 1 year of follow-up, there were no statistically significant differences in the recurrence and metastasis rates between the combined and single groups (P > 0.05). The survival rate in the combined group (80.00%) was higher than that in the single group. The average survival time of the combined group (299.38 ± 61.13 d) was longer than that of the single group (214.41 ± 72.97 d). These results suggest that TACE combined with PMCT can effectively treat middle-late PHC and improve patient survival rates.
Tumor markers are important indicators of tumor progression and are related to biological behaviors. As a new type of digestive system-specific antigen, CEA can comprehensively reflect the growth and ablation of malignant tumor cells[23,24]. AFP is a protein produced by embryonic stem cells and is mainly derived from undifferentiated hepatocytes and the fetal yolk sac. Related studies have reported that more than 70% of TACE patients have elevated serum AFP levels, and the more severe the patient's condition, the higher the serum AFP level[25,26]. CA19-9 is an oligosaccharide antigen secreted by digestive tract tumor cells, and its expression is upregulated in patients with liver cancer[27]. This study found that after 4 wk of treatment, the serum levels of CA19-9, AFP, and CEA in the single and combined groups decreased. However, the decrease was more obvious in the combined group, suggesting that combining TACE with PMCT can effectively reduce serum levels of CA19-9, AFP, and CEA in patients with advanced PHC.
This study further explored the factors affecting the prognosis of patients with middle-late PHC. We found that tumor diameter, tumor number, and treatment method (P < 0.05) are factors affecting the prognosis of patients with middle-late PHC. The rationale behind this analysis is that large tumors are not easily inactivated as a whole during treatment, which can easily cause residual tumors and recurrence. In addition, patients with large tumors have a low liver reserve compensatory function, which can easily cause liver failure and death during treatment[28,29]. The number of tumors predicts the degree of intrahepatic spread; the higher the number of tumors, the higher the chance of recurrence[30]. This study proved that the combination of TACE and PMCT can improve patient prognosis.
This was a retrospective study, which was limited by the sample size and follow-up time. Therefore, to further demonstrate the role of combination therapy in patients with mid-late PHC, prospective randomized controlled studies of this nature should be conducted.
TACE + PMCT has good clinical efficacy in the treatment of patients with middle-late PHC and can effectively improve the survival rate of these patients. Tumor diameter, tumor number, and treatment methods are factors associated with the prognosis of patients with middle-late PHC.
Patients with middle-late primary hepatic carcinoma (PHC) exhibit distinct characteristics, such as large tumors, multiple tumor lesions, and satellite lesions. Achieving a good embolization effect after multiple transcatheter arterial chemoembolization (TACE) treatments can often be challenging. percutaneous microwave coagulation therapy (PMCT) can accurately reach the tumor site, heat the tumor locally, and cause coagulation necrosis. Currently, TACE plus PMCT is more effective than interventional therapy alone and can improve survival time. However, there are few reports on the effects of TACE and PMCT on serum markers and the prognosis of patients with advanced PHC.
Compared to single TACE therapy, TACE + PMCT has a better effect in clinical applications. However, most studies have focused on efficacy and safety, and there are few reports on the factors affecting the prognosis of patients with middle-late PHC and the improvement of tumor markers in patients treated with TACE + PMCT.
To explore the effect of TACE + PMCT on tumor markers and prognosis in patients with mid-late PHC.
Patients were divided into a single group (TACE treatment) and a combination group (TACE + PMCT treatment), according to the treatment methods. Serum tumor markers [alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9)] and the clinical efficacy of the single and combined groups were observed before treatment and 4 wk after treatment. The 1-year survival rates and prognostic factors of the two groups were analyzed.
The objective response rate in the combined group was 74.67%, higher than that in the single group (50.67%) (P < 0.05). After 4 wk of treatment, the serum AFP, CEA, and CA19-9 Levels in the single and combined groups decreased, with the decrease in the combined group being more significant (P < 0.05). The 1-year survival rate at the end of the follow-up period was 80.00% in the combined group and 60.00% in the single group (P < 0.05). The average survival time in the combined group was 299.38 ± 61.13 days, longer than that in the single group (214.41 ± 72.97 d, P < 0.05). COX analysis revealed the effect of tumor diameter, the number of tumors, and the treatment method on the prognosis of patients with middle-to-the newest PHC (P < 0.05).
TACE + PMCT has good clinical efficacy in the treatment of mid-late PHC and can effectively improve the survival rate of patients. Tumor diameter, number, and treatment method were related to the prognosis of patients with mid-late PHC.
This study provides a reference for the clinical management of patients with mid-late PHC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nozaki Y, Japan; Takehara T, Japan S-Editor: Lin C L-Editor: A P-Editor: Lin C
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64446] [Article Influence: 16111.5] [Reference Citation Analysis (176)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13206] [Article Influence: 1467.3] [Reference Citation Analysis (3)] |
| 3. | Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK, Are C. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg. 2016;263:1112-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 4. | Ozakyol A. Global Epidemiology of Hepatocellular Carcinoma (HCC Epidemiology). J Gastrointest Cancer. 2017;48:238-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Hatanaka T, Naganuma A, Kakizaki S. Lenvatinib for Hepatocellular Carcinoma: A Literature Review. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Wu Z, Gao J, Zhuang W, Yang J, Guo W. Efficacy and safety of transcatheter arterial chemoembolization plus hepatic arterial infusion chemotherapy in the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis in the main trunk. J Cancer Res Ther. 2022;18:345-351. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Wang YB, Ma R, Wang ZB, Shi QL, Zhang L, Chen WZ, Gong JP, Bai J. Transcatheter Arterial Chemoembolization in Combination With High-Intensity Focused Ultrasound for Intermediate and Advanced Hepatocellular Carcinoma: A Meta-Analysis. Front Oncol. 2022;12:797349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Fang C, Luo R, Zhang Y, Wang J, Feng K, Liu S, Chen C, Yao R, Shi H, Zhong C. Hepatectomy versus transcatheter arterial chemoembolization for resectable BCLC stage A/B hepatocellular carcinoma beyond Milan criteria: A randomized clinical trial. Front Oncol. 2023;13:1101162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Ma K, Liu J, Wang Y, Zhong Y, Wu Z, Fan R, Guo S. Relationship between plasma cell-free DNA (cfDNA) and prognosis of TACE for primary hepatocellular carcinoma. J Gastrointest Oncol. 2020;11:1350-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Xu LF, Sun HL, Chen YT, Ni JY, Chen D, Luo JH, Zhou JX, Hu RM, Tan QY. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Ge Y, Jeong S, Luo GJ, Ren YB, Zhang BH, Zhang YJ, Shen F, Cheng QB, Sui CJ, Wang HY, Xia Q, Chen L. Transarterial chemoembolization versus percutaneous microwave coagulation therapy for recurrent unresectable intrahepatic cholangiocarcinoma: Development of a prognostic nomogram. Hepatobiliary Pancreat Dis Int. 2020;19:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Guo H, Chen B, Li W, Wang H, Zhao S, Chen P, Jiang M, Zhao L, Xu K, Sun H, He Y, Zhou C. Percutaneous Microwave Coagulation Therapy: A Promising Therapeutic Method for Breaking the Barrier of the Intertumor Heterogeneity. J Healthc Eng. 2021;2021:7773163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3293] [Article Influence: 219.5] [Reference Citation Analysis (36)] |
| 14. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 518] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 15. | Wu Z, Cui L, Qian J, Luo L, Tu S, Cheng F, Yuan L, Zhang W, Lin W, Tang H, Li X, Li H, Zhang Y, Zhu J, Li Y, Xiong Y, Hu Z, Peng P, He Y, Liu L, He K, Shen W. Efficacy of adjuvant TACE on the prognosis of patients with HCC after hepatectomy: a multicenter propensity score matching from China. BMC Cancer. 2023;23:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Herber S, Schneider J, Brecher B, Höhler T, Thelen M, Otto G, Pitton MB. [TACE: therapy of the HCC before liver transplantation--experiences]. Rofo. 2005;177:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Wei Y, Dai F, Zhao T, Tao C, Wang L, Ye W, Zhao W. Transcatheter arterial chemoembolization monotherapy vs combined transcatheter arterial chemoembolization-percutaneous microwave coagulation therapy for massive hepatocellular carcinoma (≥10 cm). Cancer Manag Res. 2018;10:5273-5282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Shen X, Ma S, Tang X, Wang T, Qi X, Chi J, Wang Z, Cui D, Zhang Y, Li P, Zhai B. Clinical outcome in elderly Chinese patients with primary hepatocellular carcinoma treated with percutaneous microwave coagulation therapy (PMCT): A Strobe-compliant observational study. Medicine (Baltimore). 2018;97:e11618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Chu C, Yi X, Sun J, Zhang X, Liu S, Zhang N, Wang J. Comparison of anesthetic effects of dexmedetomidine and tramadol, respectively, combined with propofol in percutaneous microwave coagulation therapy for hepatocellular carcinoma. Oncol Lett. 2019;18:3599-3604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Hu Q, Zeng Z, Zhang Y, Fan X. Study of ultrasound-guided percutaneous microwave ablation combined with portal vein embolization for rapid future liver remnant increase of planned hepatectomy. Front Oncol. 2022;12:926810. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Li Z, Jiao D, Han X, Si G, Li Y, Liu J, Xu Y, Zheng B, Zhang X. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided microwave ablation in the treatment of small hepatocellular carcinoma. Cancer Imaging. 2020;20:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Wang L, Ke Q, Lin N, Huang Q, Zeng Y, Liu J. The efficacy of transarterial chemoembolization combined with microwave ablation for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:1288-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Zhang Q, Bian SQ, Lv W, Kou D, Hu HL, Guo SS, Cao ZS. Observation of efficacy of TACE combined with HIFU on patients with middle-advanced liver cancer. Eur Rev Med Pharmacol Sci. 2019;23:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Szemitko M, Golubinska-Szemitko E, Sienko J, Falkowski A, Wiernicki I. Efficacy of Liver Chemoembolization after Prior Cetuximab Monotherapy in Patients with Metastatic Colorectal Cancer. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Chen W, Peng J, Ye J, Dai W, Li G, He Y. Aberrant AFP expression characterizes a subset of hepatocellular carcinoma with distinct gene expression patterns and inferior prognosis. J Cancer. 2020;11:403-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Hu S, Gan W, Qiao L, Ye C, Wu D, Liao B, Yang X, Jiang X. A New Prognostic Algorithm Predicting HCC Recurrence in Patients With Barcelona Clinic Liver Cancer Stage B Who Received PA-TACE. Front Oncol. 2021;11:742630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Lu H, Zheng C, Fan L, Xiong B. Efficacy and Safety of TACE Combined with Regorafenib versus TACE in the Third-Line Treatment of Colorectal Liver Metastases. J Oncol. 2022;2022:5366011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Mack MG, Straub R, Eichler K, Söllner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: laser-induced interstitial thermotherapy--local tumor control rate and survival data. Radiology. 2004;233:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, Yang T, Yan Z, Lei Z, Si A, Wan X, Zhang H, Gao C, Cheng Z, Pawlik TM, Wang H, Lau WY, Wu M, Shen F. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2020;6:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 30. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 472] [Article Influence: 67.4] [Reference Citation Analysis (0)] |