Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2765
Peer-review started: September 27, 2023
First decision: October 24, 2023
Revised: November 10, 2023
Accepted: November 24, 2023
Article in press: November 24, 2023
Published online: December 27, 2023
Processing time: 91 Days and 0.1 Hours
Low anterior resection syndrome (LARS) is one of the common postoperative complications in patients with rectal cancer, which seriously affects their postoperative recovery and quality of life (QoL). Electroacupuncture therapy is one of the characteristic therapies of traditional Chinese medicine. There are few reports on the prevention and treatment of LARS by electroacupuncture therapy.
To explore the clinical effectiveness of electroacupuncture in managing rectal cancer patients with postoperative LARS.
A total of 50 patients with LARS after rectal cancer surgery were retrospectively selected as the research subjects. According to the treatment methods, they were divided into an observation group (n = 25) and a control group (n = 25). During the four-week treatment period, the control group received standard defecation function training, while the observation group received electroacupuncture care and traditional defecation function training. The anal pressure index (which includes anal resting pressure, anal systolic pressure, and maximum tolerable volume), European Organization of Research and Treatment of Cancer (EORTC) QoL C30 (QLQ-C30) score, LARS Scale (LARSS) score, Wexner anal incontinence scale score, Xu Zhongfa five-item 10-point scale score, and the occurrence of adverse reactions were compared between the two groups before and after treatment.
The experimental group showed considerably enhanced LARSS scores compared to those in the control group after four weeks of treatment. In the first week, second week, and fourth week, the LARSS score and Wexner anal incontinence scale score decreased, and the Xu Zhong method five-item 10-point scale score increased, with significant differences (P < 0.05). The experimental group showed substantial improvements in anal resting pressure, anal systolic pressure, and maximum tolerance volume after undergoing 4 wk of therapy in the untreated group (P < 0.05). The experimental group's QLQ-C30 score on the EORTC QoL questionnaire was higher than that of the control group during the 1st, 2nd, and 4th wk (P < 0.05). No significant variation between the groups in the frequency of adverse reactions (P > 0.05) was observed.
Electroacupuncture positively impacted LARS following rectal cancer surgery, effectively improving clinical symptoms and anal pressure indicators and patients’ standard of life.
Core Tip: Low anterior resection syndrome is a group of clinical syndromes that often occur in patients with rectal cancer after anus-preserving surgery, which seriously affects the postoperative rehabilitation effect of patients. Electroacupuncture therapy has the effects of improving immune function and regulating intestinal flora balance, but the prevention and treatment effect of anterior resection syndrome has not been reported in relevant literature. This study mainly analyzes the prevention and treatment effect of electroacupuncture on anterior resection syndrome, and provides a reference for clinical reduction of the incidence of anterior resection syndrome.
- Citation: Xu LL, Xiang NJ, Cheng TC, Li YX, Chen P, Jiang ZW, Liu XX. Application of electroacupuncture in the prevention of low anterior resection syndrome after rectal cancer surgery. World J Gastrointest Surg 2023; 15(12): 2765-2773
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2765.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2765
In China, rectal cancer is a prevalent digestive tract cancer. However, the development of numerous anal preservation procedures has significantly increased the chance of survival in rectal cancer patients in recent years[1]. However, after receiving anal preservation surgery, some patients experience a series of symptoms, such as urgency, frequency of stool, fecal incontinence, and functional stomach evacuation disturbance, which are collectively called low anterior resection syndrome (LARS), with an incidence of up to 56.0%[2], seriously affecting postoperative rehabilitation and patient quality of life (QoL). The specific pathophysiology of LARS after rectal cancer surgery remains unclear and may be related to increased neorectum motility, anal sphincter dysfunction, and other factors[3]. No specific treatments for this condition have been found, and Western medicine mainly adopts symptomatic treatment measures such as diet, drugs, and biofeedback[4]. In traditional Chinese medicine, there is no disease called LARS, but it can be classified into constipation and diarrhea according to its clinical symptoms. Traditional Chinese medicine includes internal and external methods for LARS treatments; as patients do not readily accept Chinese herbs because of their exceptional bitter taste, external methods include electroacupuncture, external application, and moxibustion. Some studies have found[5] that electroacupuncture therapy can improve immune function and regulate the balance of intestinal flora. There is no published research on the use of electroacupuncture in LARS after rectal cancer surgery. This study investigated the application value of electroacupuncture therapy for LARS in 50 patients to provide a reference for clinical treatment.
A total of 50 patients with LARS after rectal cancer surgery admitted to our hospital from May 2022 to May 2023 were retrospectively selected. The following were the criteria for inclusion: The patients received a pathological diagnosis of rectal cancer, met the surgical indications, and received surgical treatment; in line with the diagnostic criteria for LARS defined by the international consensus in 2020; age from 18 to 80 years; LARS Scale (LARSS) score ≥ 21 points; an expected survival > 6 mo; Karnofsky functional status score ≥ 60 points; willing agreement to take part in the research and provision of their signature on the informed consent document. The following criteria determined exclusion: Symptoms such as urgency, frequency of stool and fecal incontinence caused by infection, irritable bowel syndrome, and radiation enteritis; metastatic rectal cancer; fistula; local tumor recurrence; previous history of anal or pelvic surgery; contraindications to electroacupuncture; and participation in other clinical trials. Fifty patients were divided into an observation group (n = 25) and a control group (n = 25) according to the different treatment methods. The experimental group comprised 7 females and 18 males, aged 36 to 69 (average age = 66.21 ± 5.62) years. The distance between the tumor and the anal margin was 3-14 cm, averaging 7.92 ± 1.33 cm. In comparison, the control group consisted of 16 males and 9 females, whose ages ranged from 37-84 years, with an average age of 66.07 ± 5.84 years and a distance between the tumor and anal margin of 5-14 cm and an average of 7.62 ± 1.47 cm. The two groups had no appreciable differences in overall data (P > 0.05).
The control group received routine defecation function training, including anal lifting exercise, anal contraction exercise, and defecation reflex training. For the anal lifting exercise, which was performed 3-4 times a day, the protocol mainly involved a squatting-standing-squatting sequential exercise, standing to shrink the anus, squatting to relax the anus, with 30 repetitions each time. For the anal contraction exercise, which was performed 2 times a day, mild and moderate contractions were performed, and then a diastole exercise was performed 10 times, each lasting 5-10 min. For the defecation reflex training, patients were advised to develop regular defecation habits. Based on the training given to the control group, the observational group received electroacupuncture treatment. Acupoint selection was made based on the brain-gut axis theory. Acupoint group 1 included Baihui, Yintang, Tianshu, Qihai, Guanyuan, Zusanli, and Shangjuxu. Acupoint group 2 included Shenshu, Pangguangshu, Ciliao, Zhongliao, and Huiyang. Hwato acupuncture needles (specification of 0.35 mm × 40 mm, Suzhou Medical Supplies Factory Co., LTD., Suxiezhunzhu 20162270970) and a Nanjing Suko electric therapy instrument (XS998) were selected. For the operation method, the patient was placed in the supine and prone positions first. The skin at the patient's acupoints and the operator’s fingers were disinfected with 75% alcohol cotton balls.
After determining the acupoint again, the skin was fixed with the operator’s left hand, and the needle was held in the right hand. The acupuncture point was pierced with the clamping needle method and pierced 1 inch straight. After manipulating the needle and feeling the Qi, the needle was connected to the Nanjing Suko electric therapy instrument. The method of electroacupuncture was to set the adjacent acupoints on the same side into a group, with a total of 4 groups. The waveform stimulation index was set as a dilatational wave (2/100 Hz), and the current intensity was 2 mA (depending on the patient’s tolerance). The needle was kept in place for 20 min. After the needle was removed, the acupoint was pressed locally with a dry cotton ball. The treatment frequency was twice a week for 4 consecutive weeks. A scale evaluation was performed before treatment and at 1 wk, 2 wk, and 4 wk, and the anal resting pressure, rectal defecation threshold, and maximum tolerance volume were monitored during treatment.
The LARSS score, Wexner anal incontinence scale score, Xu Zhongfa 5-item 10-point scale score, and European Organization of Research and Treatment of Cancer (EORTC) QoL C30 (QLQ-C30) score were compared in both groups before and following 1, 2, and 4 wk of treatment. Anal pressure indices were evaluated between the experimental and control groups before and after treatment for 4 wk. The occurrence of adverse responses during treatment was recorded in both groups. LARSS scoring[6]. The scoring content included five aspects: Exhaust incontinence, loose stool incontinence, defecation times, frequent defecation, and urgency. The sum of all scores was the overall score, and the overall score was graded, with 0-20 as zero, 21-29 as mild, and 30-42 as severe. Wexner anal incontinence scale score[7]. The scoring content included the ability to control stool in different traits, the use of sanitary pads, and lifestyle changes. According to the anal incontinence frequency of “never”, “< 1 time/mo”, “≥ 1 time/mo”, “≥ 1 time/wk”, and “≥ 1 times/d”, the score was rated as 0, 1, 2, 3, and 4 points, respectively, and a higher score suggested worse anal function. Xu Zhong used a 5-item 10-point scale. The scoring included the intention of excretion, ability to control defecation, frequency of defecation, sensory function, and defecation time. According to the severity, 0, 1, and 2 points were given, respectively, and a lower score indicated worse anal function. Anal pressure indicators: The anal resting pressure, rectal defecation threshold, and the patient’s maximum tolerated volume in the two groups before and after therapy were detected by a DRIVE manometer produced by the Korea Libo Company. QoL: EORTC QLQ-C30[8] scoring included body, cognition, role, emotion, and social function aspects, with an overall rating of 100, where a higher number indicated an improved standard of life.
SPSS v.25.0 was used to analyze and manage the data that were acquired. The mean ± SD was employed to express measurement data that followed a normal distribution, and the t test was utilized for data comparison. The chi-square test assessed the data after they had been tallied and expressed them as either occurrences or percentages. Statistical significance is indicated by a P value lower than 0.05.
Before treatment, no significant change in the LARSS grading within either group (P > 0.05) was observed. However, after four weeks of administration, the observation group showed significantly better LARSS grades than the control group (P < 0.05, Table 1).
| Groups | Before treatment | Following 1 wk of treatment | Following 2 wk of treatment | Following 4 wk of treatment | ||||||||
| No | Mild | Severe | No | Mild | Severe | No | Mild | Severe | No | Mild | Severe | |
| Observational group | 0 | 6 | 19 | 1 | 7 | 17 | 6 | 9 | 10 | 11 | 10 | 4 |
| Control group | 0 | 8 | 17 | 0 | 9 | 16 | 1 | 10 | 14 | 5 | 8 | 12 |
| χ2 | 0.397 | 1.280 | 4.291 | 6.472 | ||||||||
| P value | 0.529 | 0.527 | 0.117 | 0.039 | ||||||||
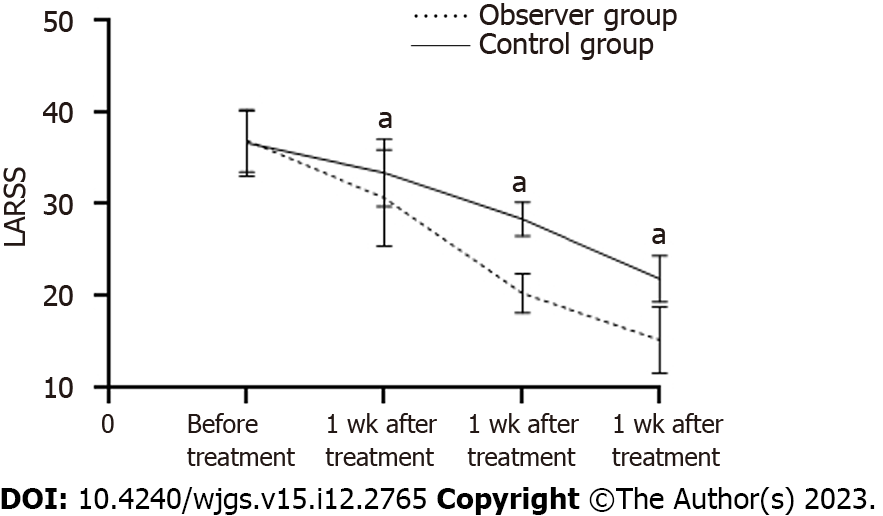
The results revealed no statistically substantial variance in LARSS scores among the observational and control groups before treatment (P > 0.05). The LARSS scores of both groups decreased after therapy (P < 0.05). The observation group exhibited substantially lower scores after 1, 2, and 4 wk of treatment (P < 0.05) than the control group (Table 2, Figure 1).
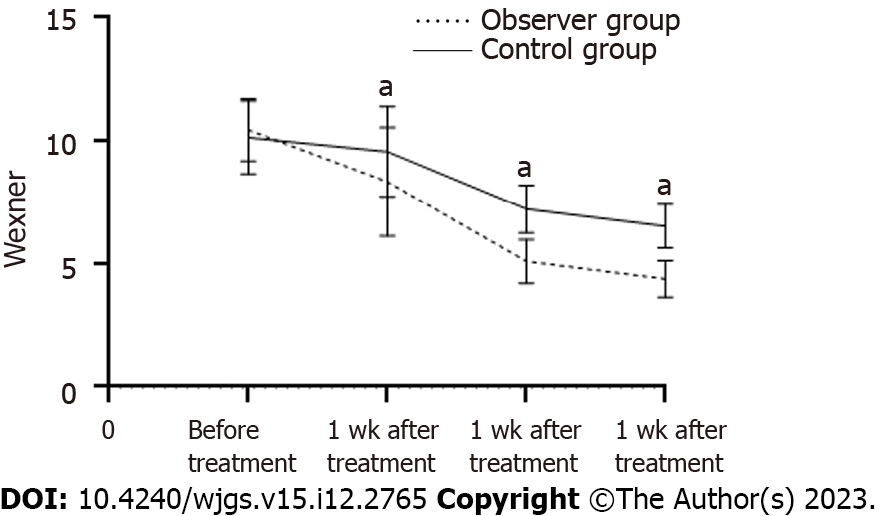
The Wexner Anal Incontinence Scale score showed no considerable variance between the two groups before treatment (P > 0.05). Following treatment, there was an average reduction in scores for both groups (P < 0.05). The scores of the experimental group were substantially lower than those of the control group after one, two, and four weeks of treatment (P < 0.05, Table 3, Figure 2).
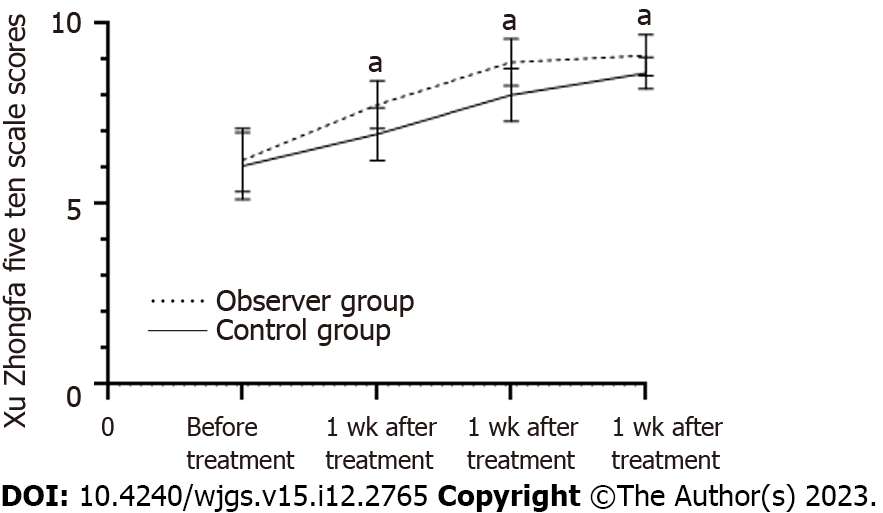
Before therapy, there was no statistically significant variance between the two groups in the Xu Zhongfa 5-item, 10-point scale scores (P > 0.05). The scores in both groups improved following treatment (P < 0.05), and the experimental group’s scores considerably outperformed the control group’s scores after one week, two weeks, and four weeks of therapy (P < 0.05, Table 4, Figure 3).
Before the treatment, there were no discernible differences between the observational and control groups’ measurements of anal pressure (P > 0.05). Following treatment, the rectal defecation threshold, anal resting pressure, and maximum tolerance volume of the two groups were increased, and the indicators in the observational group were considerably higher than those in the control group (P < 0.05, Table 5).
| Group | n | Anal resting pressure | Rectal defecation threshold | Maximum tolerance volume | |||
| Before treatment | After 4 wk of treatment | Before treatment | After 4 wk of treatment | Before treatment | After 4 wk of treatment | ||
| Observation al group | 25 | 49.51 ± 3.53 | 59.73 ± 2.12a | 85.32 ± 3.34 | 115.54 ± 5.62a | 122.35 ± 9.84 | 134.12 ± 7.82a |
| Control group | 25 | 49.34 ± 3.62 | 54.41 ± 2.86a | 85.09 ± 3.62 | 107.31 ± 6.49a | 120.18 ± 10.45 | 128.36 ± 11.41a |
| T value | 0.168 | 7.472 | 0.233 | 4.793 | 0.756 | 2.082 | |
| P value | 0.867 | 0.000 | 0.816 | 0.000 | 0.453 | 0.043 | |
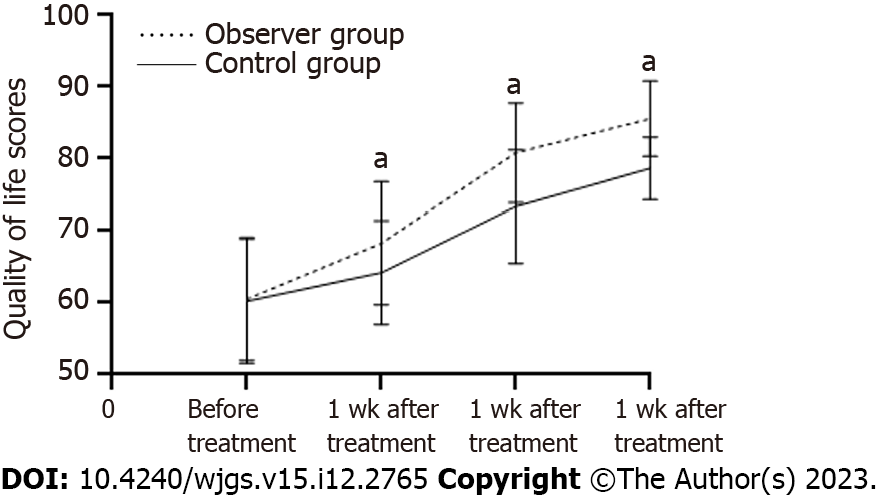
Before therapy, the two groups showed no variance in the QoL score (P > 0.05). The results showed that both groups had improved scores after receiving treatment. Additionally, the scores of the observation group were substantially more significant than those of the control group following 1, 2, and 4 wk of administration (P < 0.05, Table 6, Figure 4).
The occurrence of side effects within the two groups did not show a significant variance (P > 0.05), as shown in Table 7.
| Group | n | Local bleeding (cases) | Dizziness (cases) | Fever (cases) | Total incidence (%) |
| Observation group | 25 | 1 | 1 | 0 | 8.00 |
| Control group | 25 | 0 | 1 | 0 | 4.00 |
| χ2 | 0.355 | ||||
| P value | 0.552 |
Based on incomplete statistics[9], 3/4 of patients with rectal cancer have low-middle rectal cancer. With the continuous development of surgical technology, many patients with medium-low rectal cancer receive low or ultralow anal preservation surgery. Anal preservation surgery can help patients avoid permanent ostomy surgery, but the occurrence of postoperative LARS will seriously affect the quality of a patient’s life[10]. The specific pathogenesis of LARS after rectal cancer surgery remains unclear. Most investigations have found that the incidence of LARS may be correlated with factors such as increased neorectum motility, anal sphincter dysfunction, and nerve damage. No specific treatment methods have been confirmed in clinical treatment, and symptomatic treatment measures such as biofeedback and pelvic rehabilitation therapy are mostly adopted. Among them, defecation training therapy mainly adopts anal lifting, anal retraction, and defecation reflex training to promote intestinal peristalsis and then reduce the threshold of rectal distention sensation to achieve a therapeutic effect[11].
In traditional Chinese medicine, there is no disease called “postoperative LARS of rectal cancer”; however, this condition is included in the categories of “constipation” and “diarrhea” according to its clinical symptoms and signs, and the defecation function of patients is closely associated with the anus. Previous studies have pointed out[12] that intraoperative sympathetic and parasympathetic nerve injury is an essential factor causing postoperative LARS in patients with rectal cancer. Electroacupuncture therapy is a kind of external treatment in traditional Chinese medicine that produces a continuous stimulus on the human body surface, which helps to repair the nerve injury of patients and further relieves the symptoms of defecation disorder[13]. Baliao points selected by electroacupuncture in this study belong to the Taiyang Bladder Meridian of Foot, which affects diseases of the urinary, anal and intestinal systems. From the perspective of anatomical structure, there are abundant sacral nerves near Baliao points, and the sacral nerves innervate the muscles around the anus. Some studies have found[14] that treating patients undergoing mixed hemorrhoidal surgery by acupuncture at Baliao points can improve the clinical efficacy, relieve anorectal pressure, reduce postoperative anal distension pain, and promote postoperative recovery.
In this study, electroacupuncture was applied with defecation training to treat postoperative LARS patients with rectal cancer. The observation group showed a significant improvement in the LARSS grade, a lower LARSS score, and a lower Wexner anal incontinence scale score compared to the control group. The Xu Zhongfa 5-item 10-point scale score was higher in the experimental group. The present findings indicate that electroacupuncture is an effective treatment for LARS after rectal cancer surgery and can improve the clinical symptoms experienced by patients. Following treatment, the observation group’s anal resting pressure, anal canal systolic pressure, and maximum tolerated volume were substantially higher than those of the control group. The EORTC QLQ-C30 score was significantly different from that in the control group, indicating that adding electroacupuncture was more beneficial to the standard of the patient’s life and improved the anal pressure indicators. The reason may be that electroacupuncture therapy can produce continuous and effective stimulation to patients’ Baihui, Yintang, Tianshu, and Qihai acupoints, thus facilitating patients’ intraoperative nerve injury, enhancing autonomic nerve innervation, and relieving the symptoms of anal sphincter dysfunction[15]. Combined with defecation training, electroacupuncture therapy can further improve patients’ intestinal function and alleviate their clinical symptoms. Additionally, there was no statistically significant difference in the occurrence of negative side effects between the two groups during treatment, demonstrating that the use of hot compress therapy and electroacupuncture would not increase negative side effects and confirming a high degree of safety.
In summary, electroacupuncture effectively alleviated patients’ clinical symptoms, improved the level of anal pressure indicators and QoL, showed promising clinical efficacy and good safety in treating postoperative LARS for rectal cancer and is worthy of promotion in clinical practice. There are some deficiencies in this study. If the sample size is too small, it is a retrospective study. In the future, the sample size will be expanded and multi-center prospective trials will be carried out to further verify the research conclusions.
In the future, the effect of electroacupuncture on the prevention and treatment of other postoperative complications in patients with rectal cancer will be further studied, and more laboratory indicators will be added to explore the mechanism of electroacupuncture.
The results of this study show that electroacupuncture is effective in treating low anterior resection syndrome (LARS) after rectal cancer surgery. It can effectively improve the clinical symptoms and anal pressure indicators of patients, improve the quality of life (QoL), and help to provide a convenient and effective treatment for clinical prevention and treatment of LARS after rectal cancer surgery.
The results of this study showed that compared with the simple western medicine treatment, the combination of electroacupuncture therapy on the basis of conventional western medicine treatment was helpful to improve the LARS Scale (LARSS) score classification and anal pressure index, reduce the LARSS score, Wexner anal incontinence scale score, improve the Xu Zhongfa five ten scale score and QoL, with less adverse reactions, but the impact on postoperative laboratory indicators of rectal cancer patients still needs further study.
This study mainly adopts a retrospective cohort study. The observation indexes mainly analyze the changes of Wexner anal incontinence scale score, Xu Zhongfa five-tenth scale score and European Organization of Research and Treatment of Cancer QoL C30 score at different time points, which is helpful to dynamically observe the application value of electroacupuncture therapy.
This study mainly verifies the effect of electroacupuncture therapy on preventing and reducing the severity of LARS, which is helpful to improve the QoL of patients and promote their postoperative recovery. It is helpful to further promote electroacupuncture therapy and prevent the occurrence of LARS in patients with rectal cancer.
This study mainly discusses the effect of electroacupuncture therapy on prevention and treatment, reduction of the severity of LARS and postoperative QoL of patients, so as to provide reference for clinical prevention and treatment.
Some patients with rectal cancer are prone to LARS after receiving sphincter-preserving surgery, with a high incidence and a greater impact on the prognosis of patients. Electroacupuncture therapy is a continuous external treatment of traditional Chinese medicine through the human body surface, which helps to improve the patient’s nerve injury and further alleviate the symptoms of defecation disorders. This study mainly analyzes the application value of electroacupuncture therapy in the prevention and treatment of postoperative LARS in patients with rectal cancer, in order to provide a basis for clinical prevention and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ruengwongroj P, Thailand; Torres-Castillo S, Mexico S-Editor: Qu XL L-Editor: A P-Editor: Yu HG
| 1. | Nguyen TH, Chokshi RV. Low Anterior Resection Syndrome. Curr Gastroenterol Rep. 2020;22:48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Bulfone G, Del Negro F, Del Medico E, Cadorin L, Bressan V, Stevanin S. Rehabilitation strategies for low anterior resection syndrome. A systematic review. Ann Ist Super Sanita. 2020;56:38-47. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Ye L, Huang M, Huang Y, Yu K, Wang X. Risk factors of postoperative low anterior resection syndrome for colorectal cancer: A meta-analysis. Asian J Surg. 2022;45:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Christensen P, Im Baeten C, Espín-Basany E, Martellucci J, Nugent KP, Zerbib F, Pellino G, Rosen H; MANUEL Project Working Group. Management guidelines for low anterior resection syndrome - the MANUEL project. Colorectal Dis. 2021;23:461-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 5. | Keane C, Fearnhead NS, Bordeianou L, Christensen P, Espin Basany E, Laurberg S, Mellgren A, Messick C, Orangio GR, Verjee A, Wing K, Bissett I; LARS International Collaborative Group. International consensus definition of low anterior resection syndrome. Colorectal Dis. 2020;22:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 747] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 7. | Alavi K, Chan S, Wise P, Kaiser AM, Sudan R, Bordeianou L. Fecal Incontinence: Etiology, Diagnosis, and Management. J Gastrointest Surg. 2015;19:1910-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Arraras JI, Suárez J, Arias de la Vega F, Vera R, Asín G, Arrazubi V, Rico M, Teijeira L, Azparren J. The EORTC Quality of Life questionnaire for patients with colorectal cancer: EORTC QLQ-CR29 validation study for Spanish patients. Clin Transl Oncol. 2011;13:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 9. | Sandberg S, Asplund D, Bisgaard T, Bock D, González E, Karlsson L, Matthiessen P, Ohlsson B, Park J, Rosenberg J, Skullman S, Sörensson M, Angenete E. Low anterior resection syndrome in a Scandinavian population of patients with rectal cancer: a longitudinal follow-up within the QoLiRECT study. Colorectal Dis. 2020;22:1367-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Dulskas A, Smolskas E, Kildusiene I, Samalavicius NE. Treatment possibilities for low anterior resection syndrome: a review of the literature. Int J Colorectal Dis. 2018;33:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Özin Y, Öztürk Ö, Tenlik I, Yüksel S, Bacaksız F, Arı D, Ramadan SU, Yalınkılıç ZM. Efficacy of combination of biofeedback therapy and pelvic floor muscle training in dyssynergic defecation. Acta Gastroenterol Belg. 2021;84:577-583. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Sun R, Dai Z, Zhang Y, Lu J, Xiao Y. The incidence and risk factors of low anterior resection syndrome (LARS) after sphincter-preserving surgery of rectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2021;29:7249-7258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Bai H, Gu RJ, Chen LY, Qian Y, Yu ML, Xu SL, Xia XF, Liu YC, Zhang HR, Gu YH, Lu SF. Electroacupuncture interventions alleviates myocardial ischemia reperfusion injury through regulating gut microbiota in rats. Microvasc Res. 2021;138:104235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Ye S, Zhou J, Guo X, Jiang X. Three Acupuncture Methods for Postoperative Pain in Mixed Hemorrhoids: A Systematic Review and Network Meta-Analysis. Comput Math Methods Med. 2022;2022:5627550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Wang L, An J, Song S, Mei M, Li W, Ding F, Liu S. Electroacupuncture preserves intestinal barrier integrity through modulating the gut microbiota in DSS-induced chronic colitis. Life Sci. 2020;261:118473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |