Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2739
Peer-review started: September 28, 2023
First decision: November 1, 2023
Revised: November 9, 2023
Accepted: December 6, 2023
Article in press: December 6, 2023
Published online: December 27, 2023
Processing time: 89 Days and 17 Hours
Giant hernias present a significant challenge for digestive surgeons. The approach taken (laparoscopic vs thoracoscopic) depends largely on the preferences and skills of each surgeon, although in most cases today the laparoscopic approach is preferred.
To determine whether patients presenting inadequate laparoscopic access to the intrathoracic hernial sac obtain poorer postoperative results than those with no such problem, in order to assess the need for a thoracoscopic approach.
For the retrospective series of patients treated in our hospital for hiatal hernia (n = 112), we calculated the laparoscopic field of view and the working area accessible to surgical instruments, by means of preoperative imaging tests, to assess the likely outcome for cases inaccessible to laparoscopy.
Patients with giant hiatal hernias for whom a preoperative calculation suggested that the laparoscopic route would not access all areas of the intrathoracic sac presented higher rates of perioperative complications and recurrence during follow-up than those for whom laparoscopy was unimpeded. The difference was statistically significant. Moreover, the insertion of mesh did not improve results for the non-accessible group.
For patients with giant hiatal hernias, it is essential to conduct a preoperative evaluation of the angle of vision and the working area for surgery. When parts of the intrathoracic sac are inaccessible laparoscopically, the thoracoscopic approach should be considered.
Core Tip: In a previous study, we presented a series of mathematical formulas that can be used to assess the accessibility of large hiatal hernias to a laparoscopic approach, concluding that for some patients this technique was not viable. In the present retrospective study, we examine whether outcomes are poorer among patients whose hiatal hernias are deemed inaccessible to laparoscopy (according to the mathematical formulation applied to the preoperative imaging results).
- Citation: Pérez Lara FJ, Zubizarreta Jimenez R, Prieto-Puga Arjona T, Gutierrez Delgado P, Hernández Carmona JM, Hernández Gonzalez JM, Pitarch Martinez M. Determining the need for a thoracoscopic approach to treat a giant hiatal hernia when abdominal access is poor. World J Gastrointest Surg 2023; 15(12): 2739-2746
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2739.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2739
A hiatal hernia is the protrusion of an abdominal organ into the mediastinum through the diaphragmatic hiatus. This condition is relatively common and, given the progressive aging of the population, its prevalence is expected to increase[1]. A giant hiatal hernia is characterised by the presence of more than one third of the stomach in the chest[2]. This pathology is much less common, representing 5%-10% of all hiatal hernias[3,4]. It is associated with a wide spectrum of symptoms[4,5], typically involving chest pain, vomiting and postprandial dysphagia, due to the mechanical effect of the translocation of the stomach.
In 1919, Soresi performed the first operation to reduce a hiatal hernia and to close the diaphragmatic pillars[6] and during the past hundred years many conceptual and technical innovations have been introduced[7-10]. In 1998, the first completely laparoscopic operation based on Collis gastroplasty and Nissen fundoplication was described[11].
The basic principles for hiatal hernia repair include the complete reduction of the hernial sac and herniated structures, with sufficient dissection to optimise oesophageal mobility, followed by primary closure of the crura with nonabsorbable sutures, and then fundoplication to reduce the risk of postoperative reflux. To achieve all these goals, it is essential to properly visualise the sac at all points of intrathoracic attachment.
Recurrence during primary laparoscopic fundoplication, after hiatal repair, ranges from 1%-7%, but can reach 50% in cases of large or paraoesophageal hernias[12-15]. In this type of large hernia, we believe, as postulated in prior research[16], that it is essential to calculate working angles and angles of vision in order to determine whether the abdominal approach is feasible and safe. In the present study, we examine whether the inability to access all areas of the hernial sac might be a key factor in the recurrence of large hiatal hernias.
In this retrospective study, we evaluate all the patients operated on for hiatal hernia at our hospital from May 2006 to September 2020. The guidelines of our hospital’s ethical committee for human studies were followed at all times. The following parameters were included in the data collected: Sex, age, surgical technique, mesh placement, second intervention, type of hernia, perioperative complications, complications during follow-up, recurrence, mortality and duration of follow-up. Perioperative complications are viewed as major conditions such as pneumothorax, oesophageal perforation or splenic injury, and exclude relatively minor problems such as wound infection, ileus or mild dysphagia.
Our study hypothesis is that in large hernias where from the abdominal cavity it is not possible to access all areas of the hernia within the thoracic cavity in order to dissect the hernial sac, the risk of recurrence is much greater than when the surgeon is able to access the entire volume of the hernial sac.
The following types of case were excluded from the study: (1) When the patient was lost to follow up; (2) When no coronal imaging test was performed; and (3) When the patient had previously been operated on for a hiatal hernia and therefore presented a recurrence, not a primary hernia.
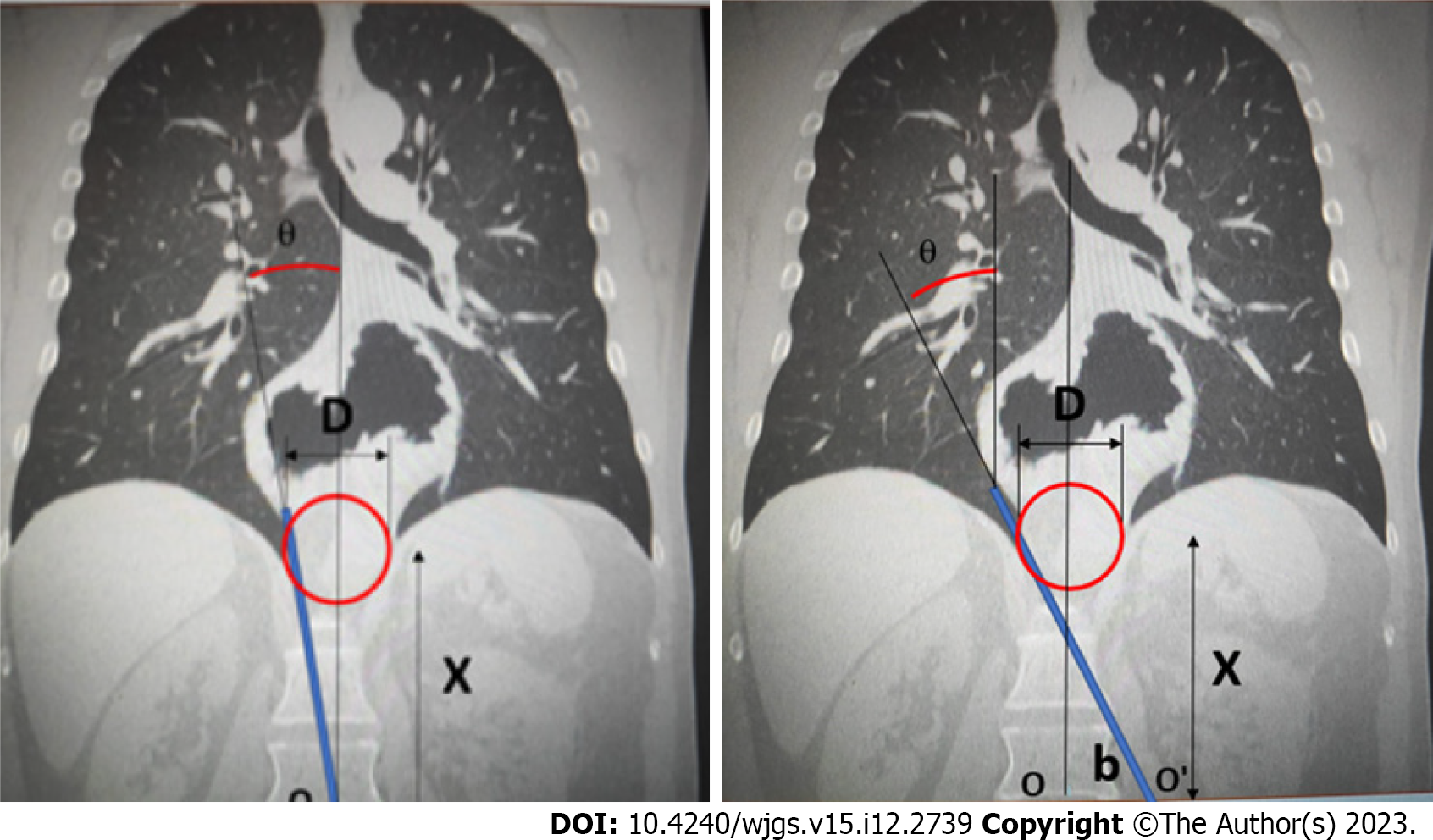
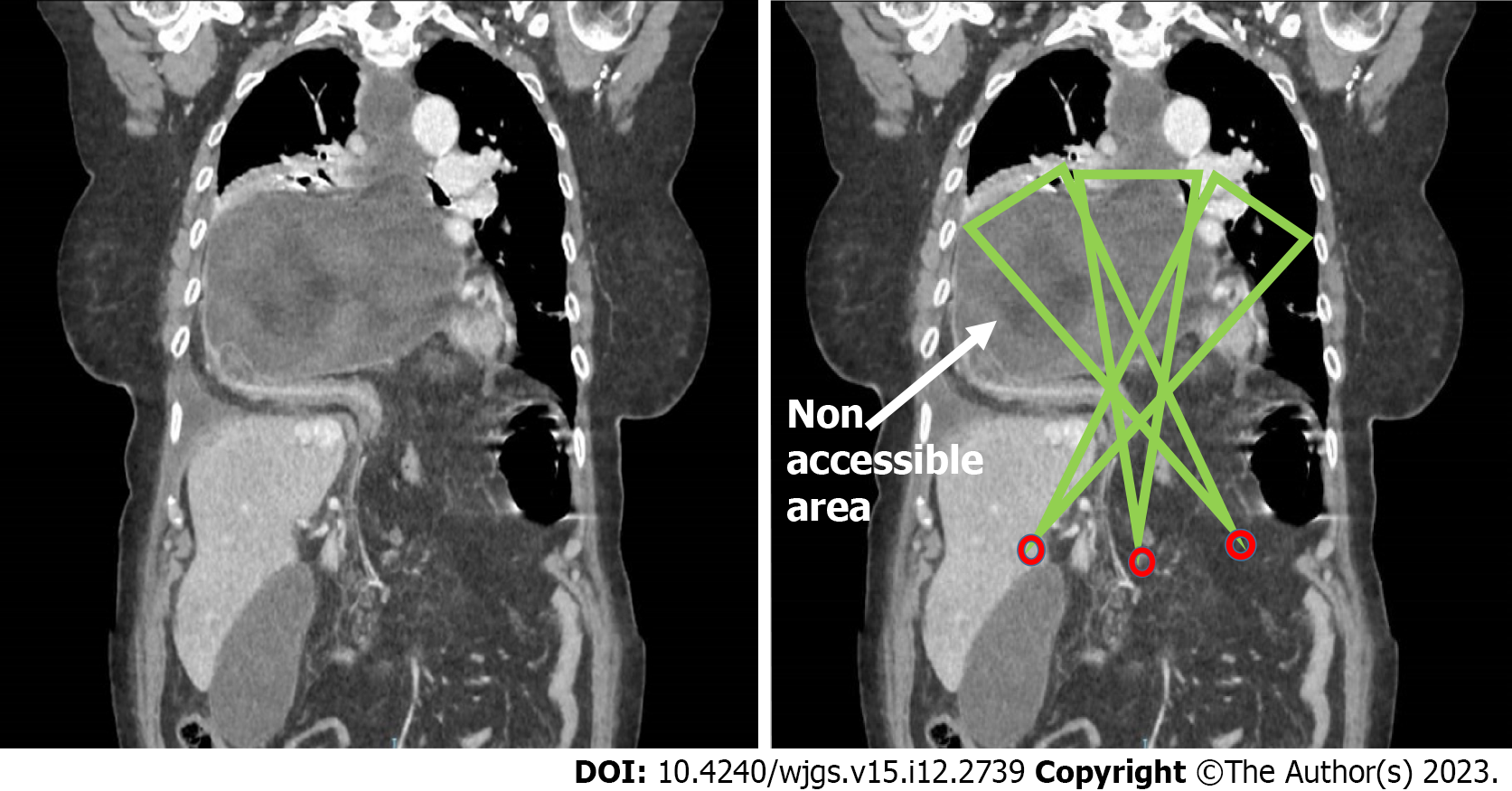
For all patients included in the study, preoperative imaging techniques were employed, in the coronal plane, by computed tomography (CT) or oesophago-gastroduodenal transit, to evaluate the angles of vision and working angles available during the intervention (angle of vision: The visual field obtained of the intrathoracic content when the laparoscope is inserted through the hernial orifice; working angle: Amplitude of instrumental access to the intrathoracic hernial content via the hiatal orifice). In this respect, the following parameters were determined: The camera angle, the diameter of the orifice and the positioning of the trocars with respect to the orifice. These data provided the basis for detailed mathematical calculations, as described previously[16], to determine the potential access, visual and/or instrumental, to the thoracic cavity (Figure 1).
The patients were classed as follows, according to the type of hernia presented: Group A, orifice < 4 cm; group B, orifice > 4 cm and accessible (i.e., enabling access both visually and with surgical instruments to all areas of the intrathoracic hernial sac); group C, orifice > 4 cm and inaccessible (not permitting visual and/or instrumental access to all areas of the intrathoracic hernial sac) (Figure 2).
The relation between each of the qualitative variables (sex, mesh placement technique, second intervention, type of hernia, perioperative complications, complications during follow-up, recurrence and mortality) and the study group (A, B and C) variable was determined by the χ2 test, under the condition that the expected values of at least 80% of the cells in the contingency table were greater than 5. If this condition was not met, Fisher’s exact test was applied.
We then determined the association between each of the quantitative variables (age and duration of follow-up) and the study group (A, B, and C variable), using the Anova test for the age variable (when the normality condition and the homogeneity of variance were fulfilled). For the non-parametric variable (the duration of follow-up), the Kruskal-Wallis test was applied.
Finally, we examined the association between recurrence and mesh placement technique, for groups B and C (logically, no mesh was employed for the cases in group A, due to the size of the hernia). For each group, Fisher’s test was applied, and the overall association was determined by the Cochran-Mantel-Haenszel method.
Table 1 summarises the results obtained, organised in contingency tables showing both absolute and relative frequencies, with each study group (A, B, and C) in its respective column. The remaining qualitative variables are listed in the rows. Our analysis shows there were no significant differences among the groups in terms of sex, mesh placement technique, second intervention or mortality. On the other hand, there were differences in terms of the type of hernia treated, the perioperative complications that occurred, complications during follow-up and recurrence. The complications and recurrences were higher in group C than in the other two groups.
| Group | |||||||||
| A | B | C | Total | ||||||
| n | % | n | % | n | % | n | % | ||
| Sex, P = 0.231 | Female | 26 | 45.6 | 26 | 61.9 | 8 | 61.5 | 60 | 53.6 |
| Male | 31 | 54.4 | 16 | 38.1 | 5 | 38.5 | 52 | 46.4 | |
| Surgery, P = 0.112 | Dor | 0 | 0 | 0 | 0 | 1 | 7.7 | 1 | 0.9 |
| Nissen | 55 | 96.5 | 41 | 97.6 | 12 | 92.3 | 108 | 96.4 | |
| Reduction | 0 | 0 | 1 | 2.4 | 0 | 0 | 1 | 0.9 | |
| Toupet | 2 | 3.5 | 0 | 0 | 0 | 0 | 2 | 1.8 | |
| Perioperative complication, P = 0.0062 | No | 56 | 98.2 | 35 | 83.3 | 10 | 76.9 | 101 | 90.2 |
| Yes | 1 | 1.8 | 7 | 16.7 | 3 | 23.1 | 11 | 9.8 | |
| Complications during follow-up, P = 0.031 | No | 36 | 63.2 | 25 | 59.5 | 3 | 23.1 | 64 | 57.1 |
| Yes | 21 | 36.8 | 17 | 40.5 | 10 | 76.9 | 48 | 42.9 | |
| Recurrence, P = 0.00052 | No | 54 | 94.7 | 35 | 83.3 | 4 | 30.8 | 93 | 83 |
| Yes | 3 | 5.3 | 7 | 16.7 | 9 | 69.2 | 19 | 17 | |
| Mortality, P = 0.131 | No | 57 | 100 | 42 | 100 | 13 | 100 | 112 | 100 |
| Yes | 57 | 100 | 42 | 100 | 13 | 100 | 112 | 100 | |
| Mesh, P = 0.00052 | No | 57 | 100 | 36 | 85.7 | 8 | 61.5 | 101 | 90.2 |
| Yes | 0 | 0 | 6 | 14.3 | 5 | 38.5 | 11 | 9.8 | |
| Paraoesophageal hernia, P = 0.0012 | No | 57 | 100 | 33 | 78.6 | 10 | 76.9 | 100 | 89.3 |
| Yes | 0 | 0 | 9 | 21.4 | 3 | 23.1 | 12 | 10.7 | |
| Secondary procedure, P = 0.282 | No | 50 | 87.7 | 40 | 95.2 | 13 | 100 | 103 | 92 |
| Yes | 7 | 12.3 | 2 | 4.8 | 0 | 0 | 9 | 8 | |
Regarding the results for the other quantitative variables: (1) Duration of follow up (Kruskal-Wallis - the mean and the interquartile range in each case) group A 120 (52, 156), group B 100 (40.5, 155.8), group C 120 (84, 145), P = 0.66; and (2) Age (Anova - means and standard deviations) group A 51.9 (13.8), group B 62.1 (13.8), group C 59.1 (14.9), P = 0.0026. By duration of follow up, there were no significant differences among the groups. However, there were statistically significant differences according to the mean age of the patients, which was lower in group A.
Finally, we analysed the association between recurrence and mesh placement technique, by groups. For group B, Fisher’s test of the association between recurrence and mesh obtained P = 0.5668, from which we conclude that in this group there was no significant association between these parameters. For group C, the corresponding value was P = 1, which also reflects independence between recurrence and mesh. The Cochran-Mantel-Haenszel test obtained the result of P = 0.4233 > 0.05. Therefore, we cannot reject the null hypothesis that the relative proportions of recurrence are independent of the mesh placement technique, within groups B and C.
Hiatal hernias have been described in 2.9%-20% of patients undergoing gastroscopies[17], but could be much more frequent, rising to perhaps 10%-50% of the general population. Four types have been distinguished: sliding (type I), paraoesophageal (type II), combined (type III) and giant paraoesophageal (type IV)[18]. A giant hiatal hernia is characterised by the presence of more than one third of the stomach within the chest[2]. It is a rare pathology, representing just 5%-10% of all hiatal hernias[3,4]. Worldwide, rising numbers of surgical interventions are being performed to treat gastrooesophageal reflux disease and/or hiatal hernias, and surgeons are encountering increasingly complicated cases, often presenting large hiatal defects.
In recent years, laparoscopic or thoracoscopic techniques involving the complete separation of the hernial sac from the thoracic cavity[19] have gradually replaced the open repair of giant hiatal hernias, to become the standard approach, resulting in lower mortality and a better quality of life, according to the GIQLI outcomes instrument[20-22]. However, since no randomised controlled trials have been performed to demonstrate which method (thoracoscopic vs laparoscopic) is superior, the choice of approach still depends largely on the preferences and skills of each surgeon, although the vast majority of surgeons currently employ the laparoscopic approach to treat hiatal hernias.
Nevertheless, the laparoscopic repair of giant hiatal hernias remains a challenging task for digestive surgeons. These defects have a high recurrence rate, ranging from 10%-42%, and can reach 50% in larger or paraoesophageal hernias[12-15]. A relevant factor in recurrences is that with the laparoscopic approach the anatomic elements may not all be clearly visible. This consideration motivated our previous study[16], in which we proposed a preoperative procedure to assess the visibility of the working area, with particular interest in determining access to the intrathoracic sac and whether the positioning of the trocars allowed us to reach the necessary intrathoracic aspects. This determination would enable us to identify the patients for whom a laparoscopic approach would not be safe and who, therefore, would require a thoracoscopic intervention. Indeed, surgeons who advocate the preferential use of transthoracic repair argue that this method provides a better visualisation of the herniated structure and thus facilitates the dissection and resection of the sac.
In the present study, we examine whether these theoretical postulates are confirmed in routine clinical activity. The results obtained show that patients whose condition is considered inaccessible via the abdominal approach (according to preoperative imaging tests) are exposed to extremely high rates of complications and recurrence (76.9% and 69.2%, respectively). Therefore, for this type of patient we propose a thoracic or combined thoracic-abdominal approach in order to access all the locations of the intrathoracic sac where adhesions to adjacent tissues must be released. Such an approach could improve the outcomes obtained by this group of patients, approximating them to those of group B patients with accessible giant hernias (among whom the rate of recurrence in our earlier study was 16.7%).
In our study, the rate of recurrence observed for patients with giant hiatal hernias (groups B and C) is close to that published in the literature (29.1%). However, we also identified the type of hernias that most strongly influences this rate in the global computation as group C, in which 69.2% of patients are subject to recurrence. Therefore, if we could bring the level of recurrence in this group in line with that presented by group B patients, by adopting the thoracic approach, and thus achieving direct visibility of all aspects of the sac, our recurrence figures would probably be 16.7% for patients with giant hiatal hernias (groups B and C) and 10.7% for the complete series (groups A + B + C). The adoption of such an approach, therefore, would significantly reduce the level of recurrence that is commonly reported for these patients.
When performing this type of intervention on large hiatal hernias, in order to safely perform the complete separation of the hernial sac, the surgical instruments employed must be able to reach all areas of the intrathoracic sac and the surgeon must have an unimpeded view of these areas.
If there is no direct vision of the area to be addressed, it will be more difficult for the surgeon to follow the steps described in point one above, thus increasing the probability of postoperative recurrence. Moreover, the dissection described in point two will be less safe, thus producing more peri- and postoperative complications. Our study confirms these consequences, showing that peri- and postoperative complications and recurrences are significantly more common in patients presenting non-accessible hernias (group C).
Despite certain advances, rates of recurrence for non-mesh large hiatal hernia repairs remain high[14]. Although the use of synthetic mesh prostheses could reduce these rates to 12%[23], their widespread adoption is limited by the fact that they can provoke potentially life-threatening complications, a risk that is often unacceptable with respect to a condition that is, in itself, benign[24-30].
In our study, the mesh improved outcomes in group B, although these findings were not statistically significant (this significance might be achieved with a larger study sample). In group C, however, the use or otherwise of mesh produced no differences, since the mesh did not address the fundamental problem considered, namely the visibility and accessibility of the intrathoracic sac.
We believe it important to note the significant period of time that has elapsed since the initial implementation of the surgical techniques discussed. Nevertheless, there has been almost no variability in the surgical technique, which in 96.5% of the patients in our study was performed by laparoscopic Nissen fundoplication, a technique that has remained the gold standard throughout this period. The only notable variation in the technique was the decision on whether to insert the mesh. We also address this question and analyse the results obtained.
Another factor that must be considered is that of the homogeneity in surgical skills when treating these patients. In our case, the patients were operated on by three surgeons, all of whom have great experience in this type of intervention and periodically refresh their technique; no patient was operated on by a surgical resident, and so we believe there is no study bias in this respect.
In conclusion, we believe that a preoperative sagittal CT imaging study should be performed for all patients with giant hiatal hernias. Among those whose condition impedes access to laparoscopic entry points, a thoracic or combined approach to the hernia should be considered. We suggest this method would reduce the risk of complication and recurrence.
The laparoscopic approach to giant hiatal hernias is often a challenge for surgeons. We propose a study to determine in which cases it would be advisable to use this method and in which circumstances thoracoscopy is to be preferred.
We consider whether it is really the case that when the hernial sac area is inaccessible during treatment of a giant hiatal hernia, the post-operatory status is always poorer.
The object of this study is to determine whether patients presenting inadequate laparoscopic access to the intrathoracic hernial sac obtain poorer postoperative results than those with no such problem, in order to assess the need for a thoracoscopic approach.
Accordingly, a retrospective study was conducted of the images of patients who underwent surgery for giant hiatal hernia, assessing the accessibility in each case and the treatment results obtained. On this basis, we compared the treatment outcomes according to accessibility.
The results were worse for patients with less accessible hiatal hernias. In these cases, it might be preferable to use thoracoscopy or to adopt a combined laparoscopic/thoracoscopic approach.
Patients whose hiatal hernias were less accessible had poorer postoperative results, a greater number of recurrences and were more likely to suffer complications.
In view of the study results obtained, we suggest a preoperative assessment should be made of all patients with a giant hiatal hernia. When the hernia is inaccessible, a thoracoscopic or combined approach should be adopted, within a prospective study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Asociacion Española de Cirujanos; Asociacion Andaluza de Cirujanos.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Ji ZL, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Davis SS Jr. Current controversies in paraesophageal hernia repair. Surg Clin North Am. 2008;88:959-978, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Morino M, Giaccone C, Pellegrino L, Rebecchi F. Laparoscopic management of giant hiatal hernia: factors influencing long-term outcome. Surg Endosc. 2006;20:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Pearson FG, Cooper JD, Ilves R, Todd TR, Jamieson WR. Massive hiatal hernia with incarceration: a report of 53 cases. Ann Thorac Surg. 1983;35:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Hill L, Kozarek R, McCallum R, Mercer CD. The Esophagus: Medical and Surgical Management 1st edition. Philadelphia: Saunders. 1988: 365. |
| 5. | Hill LD. Incarcerated paraesophageal hernia. A surgical emergency. Am J Surg. 1973;126:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 165] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Stylopoulos N, Rattner DW. The history of hiatal hernia surgery: from Bowditch to laparoscopy. Ann Surg. 2005;241:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Nissen R. [A simple operation for control of reflux esophagitis]. Schweiz Med Wochenschr. 1956;86:590-592. [PubMed] |
| 8. | Collis JL. An operation for hiatus hernia with short esophagus. J Thorac Surg. 1957;34:768-73; discussion 774. [PubMed] |
| 9. | Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg. 1967;53:33-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 393] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Maziak DE, Todd TR, Pearson FG. Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg. 1998;115:53-60; discussion 61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Johnson AB, Oddsdottir M, Hunter JG. Laparoscopic Collis gastroplasty and Nissen fundoplication. A new technique for the management of esophageal foreshortening. Surg Endosc. 1998;12:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Basso N, Rosato P, De Leo A, Genco A, Rea S, Neri T. "Tension-free" hiatoplasty, gastrophrenic anchorage, and 360 degrees fundoplication in the laparoscopic treatment of paraesophageal hernia. Surg Laparosc Endosc Percutan Tech. 1999;9:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Carlson MA, Condon RE, Ludwig KA, Schulte WJ. Management of intrathoracic stomach with polypropylene mesh prosthesis reinforced transabdominal hiatus hernia repair. J Am Coll Surg. 1998;187:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Hashemi M, Peters JH, DeMeester TR, Huprich JE, Quek M, Hagen JA, Crookes PF, Theisen J, DeMeester SR, Sillin LF, Bremner CG. Laparoscopic repair of large type III hiatal hernia: objective followup reveals high recurrence rate. J Am Coll Surg. 2000;190:553-60; discussion 560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 353] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Paul MG, DeRosa RP, Petrucci PE, Palmer ML, Danovitch SH. Laparoscopic tension-free repair of large paraesophageal hernias. Surg Endosc. 1997;11:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Lara FJP, Zubizarreta Jimenez R, Moya Donoso FJ, Hernández Gonzalez JM, Prieto-Puga Arjona T, Del Rey Moreno A, Pitarch Martinez M. Preoperative calculation of angles of vision and working area in laparoscopic surgery to treat a giant hiatal hernia. World J Gastrointest Surg. 2021;13:1638-1650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 17. | Kang JY. Systematic review: geographical and ethnic differences in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Dean C, Etienne D, Carpentier B, Gielecki J, Tubbs RS, Loukas M. Hiatal hernias. Surg Radiol Anat. 2012;34:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Lococo F, Cesario A, Meacci E, Granone P. Intrathoracic gastric perforation: a late complication of an unknown postpartum recurrent hiatal hernia. Interact Cardiovasc Thorac Surg. 2012;15:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Fullum TM, Oyetunji TA, Ortega G, Tran DD, Woods IM, Obayomi-Davies O, Pessu O, Downing SR, Cornwell EE. Open versus laparoscopic hiatal hernia repair. JSLS. 2013;17:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Hazebroek EJ, Gananadha S, Koak Y, Berry H, Leibman S, Smith GS. Laparoscopic paraesophageal hernia repair: quality of life outcomes in the elderly. Dis Esophagus. 2008;21:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Oor JE, Koetje JH, Roks DJ, Nieuwenhuijs VB, Hazebroek EJ. Laparoscopic Hiatal Hernia Repair in the Elderly Patient. World J Surg. 2016;40:1404-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Müller-Stich BP, Kenngott HG, Gondan M, Stock C, Linke GR, Fritz F, Nickel F, Diener MK, Gutt CN, Wente M, Büchler MW, Fischer L. Use of Mesh in Laparoscopic Paraesophageal Hernia Repair: A Meta-Analysis and Risk-Benefit Analysis. PLoS One. 2015;10:e0139547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Tatum RP, Shalhub S, Oelschlager BK, Pellegrini CA. Complications of PTFE mesh at the diaphragmatic hiatus. J Gastrointest Surg. 2008;12:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Huddy JR, Markar SR, Ni MZ, Morino M, Targarona EM, Zaninotto G, Hanna GB. Laparoscopic repair of hiatus hernia: Does mesh type influence outcome? A meta-analysis and European survey study. Surg Endosc. 2016;30:5209-5221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Virgilio E, Mercantini P, Cavallini M. Partial transmural gastroesophageal migration of polypropylene mesh after surgery for a recurrent hiatal hernia. Eur Rev Med Pharmacol Sci. 2016;20:3515-3516. [PubMed] |
| 27. | Bognár L, Horváth ÖP, Solt J, Jancsó G, Vereczkei A. Laparoszkópos hiatushernia-rekonstrukciót követő intraoesophagealis hálómigráció. Magy Seb. 2015;68:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Porziella V, Cesario A, Lococo F, Margaritora S, Leuzzi G, Marchese M, Petruzziello L, Costamagna G, Granone P. Complete transmural gastric migration of PTFE mesh after surgery for a recurrent hiatal hernia. Eur Rev Med Pharmacol Sci. 2012;16 Suppl 4:42-43. [PubMed] |
| 29. | Carpelan-Holmström M, Kruuna O, Salo J, Kylänpää L, Scheinin T. Late mesh migration through the stomach wall after laparoscopic refundoplication using a dual-sided PTFE/ePTFE mesh. Hernia. 2011;15:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Juhasz A, Sundaram A, Hoshino M, Lee TH, Mittal SK. Outcomes of surgical management of symptomatic large recurrent hiatus hernia. Surg Endosc. 2012;26:1501-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |