Published online Dec 27, 2023. doi: 10.4240/wjgs.v15.i12.2727
Peer-review started: September 11, 2023
First decision: October 9, 2023
Revised: October 18, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: December 27, 2023
Processing time: 106 Days and 22.5 Hours
Clinical factors predicting graft survival (GS) after ABO-incompatible (ABOi) liver transplantation (LT), and differences between recipients with and without hepatocellular carcinoma (HCC) are unclear.
To analyze the impact of serial serum tacrolimus trough concentration in recipients with or without HCC) in ABOi living-donor liver transplantation (LDLT).
We analyzed a historical cohort of 89 recipients who underwent ABOi LDLT, including 47 patients with HCC.
The 1-, 3-, 5-, and 10-year GS rates were 85.9%, 73.3%, 71.4%, and 71.4%, respectively, and there were no significant differences between HCC and non-HCC recipients. In multivariate Cox-regression analyses, tacrolimus trough concentrations below 5.4 ng/mL at 24 wk post-LT, in addition to the antibody-mediated rejection (AMR) were associated with poor-graft outcomes. In HCC patients, AMR [hazard ratio (HR) = 63.20, P < 0.01] and HCC recurrence (HR = 20.72, P = 0.01) were significantly associated with poor graft outcomes. HCCs outside Milan criteria, and tacrolimus concentrations at 4 wk post-LT > 7.3 ng/mL were significant predictive factors for HCC recurrence. After propensity score matching, patients with high tacrolimus concentrations at 4 wk had significantly poor recurrence-free survival.
Elevated tacrolimus levels at 4 wk after ABOi LDLT have been found to correlate with HCC recurrence. Therefore, careful monitoring and control of tacrolimus levels are imperative in ABOi LT recipients with HCC.
Core Tip: Maintenance immunosuppression with calcineurin inhibitors (CNIs) such as tacrolimus or cyclosporine is the current standard regimen for liver transplantation (LT) patients, and this might be important in the preventing antibody-mediated rejection in ABO-incompatible (ABOi) LT. However, increased exposure to CNIs, especially in the first month after LT, is reported to be a risk factor for HCC recurrence. Nevertheless, the appropriate adjustment of immunosuppressant doses in ABOi LT recipients with and without HCC has not yet been determined. In this study, greater early exposure to tacrolimus was associated with HCC recurrence, whereas lesser exposures at later time points was associated with poorer long-term graft outcomes, suggesting that adjustment of serum tacrolimus concentration according to the presence of HCC may be required to improve graft outcomes.
- Citation: Han JW, Choi JY, Jung ES, Kim JH, Cho HS, Yoo JS, Sung PS, Jang JW, Yoon SK, Choi HJ, You YK. Association between the early high level of serum tacrolimus and recurrence of hepatocellular carcinoma in ABO-incompatible liver transplantation. World J Gastrointest Surg 2023; 15(12): 2727-2738
- URL: https://www.wjgnet.com/1948-9366/full/v15/i12/2727.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i12.2727
Early attempts to perform ABO-incompatible (ABOi) liver transplantation (LT) under conventional immunosuppression showed that ABO incompatibility is a predictor of poor graft survival (GS)[1,2]. Reduced GS may be associated with antibody-mediated rejection (AMR), resulting in vascular or biliary complications, and hepatic necrosis; these effects may be due to the expression of ABO blood group antigens on vascular and biliary epithelia[3]. A recent report from a Korean nationwide study also showed that ABOi transplantation was an independent risk factor for graft rejection[4]. Nevertheless, the use of ABOi LT allows the expansion of the donor pool and currently comprises approximately 20% of living-donor LT (LDLT) surgeries[5].
Various desensitization protocols have been utilized, but the introduction of rituximab with ABOi LT produced comparable graft outcomes to ABO-compatible (ABOc) LT[6-8]. Kim et al[6] showed that two-year survival rates were 85% and 83% in ABOi and ABOc LT groups, respectively, and were not statistically different. In addition, another study reported that five-year GS was not different between the ABOi and ABOc LT groups (89.9% vs 91.2%), although AMR and biliary complications occurred more frequently in the ABOi group[8]. In LT recipients with hepatocellular carcinoma (HCC), tumor recurrence and overall survival (OS) were also not different between the ABOi and ABOc LT groups[9]. However, these studies could not clarify the risk factors affecting graft outcome, especially in the subgroup of ABOi LT recipients.
Maintenance immunosuppression with calcineurin inhibitors (CNIs) such as tacrolimus or cyclosporine is the current standard regimen for LT patients[10], although the optimal protocol has not yet been established. Increased exposure to CNIs, especially in the first month after LT, is reported to be a risk factor for HCC recurrence[11]. Recurrence of HCC during maintenance CNI therapy following LT tends to be associated with poor survival[12]. On the other hand, main
In the present study, we investigated the long-term graft outcomes of ABOi LDLT and the associated risk factors. In addition, we performed subgroup analyses of GS and associated risk factors according to the presence or absence of HCC. Of note, we analyzed the impact of serial serum tacrolimus trough concentration in recipients with or without HCC.
A historical cohort of 89 recipients who received ABOi LDLT from October 2010 to March 2023 at Seoul St. Mary’s Hospital (Seoul, Republic of Korea) was analyzed retrospectively. Clinical, laboratory, radiological, and historical findings were collected for each patient. The presence of HCC was initially diagnosed based on pre-transplant imaging studies, including computed tomography (CT) or magnetic resonance imaging (MRI), and was confirmed in the explanted liver. Anti-A or anti-B Isoagglutinin (IA) titer and the percentage of CD19/CD20+ lymphocytes were measured as previously described[8]. Human leukocyte antigen (HLA) typing, panel reactive antibody (PRA) testing, and T-cell/B-cell crossmatching were performed preoperatively, as previously described[14]. The trough level of tacrolimus was measured immediately before the administration of the next dose of the medication. The tacrolimus level was measured serially daily up until the first week post-transplant, three times a week until 4 wk post-transplant, and following discharge, it was carried out at each outpatient visit, typically at intervals ranging from a minimum of 2 wk to about 12 wk depending on the patients’ status. The median observation duration was 30.1 mo, and the study patients were observed until re-transplantation, death, or the last visit to the clinic. Biliary stricture and vascular complications following LT were defined using radiologic examinations including CT or MRI scans, as previously described[15]. Acute cellular rejection (ACR) was diagnosed clinically or in liver biopsy specimens[16]. In patients with HCC, CT scans were performed every three months until 12 mo after LT, followed by examinations every six months during the next two years and every 12 mo thereafter. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (approval number: KC22RISI0921) and was performed according to the Declaration of Helsinki.
A single dose of rituximab (300 or 375 mg/m2) was administered 14 d before the transplantation. From 7 d before the transplantation, plasma exchanges were done, and the target IA titer was 1:16. Basiliximab was injected on the day of and 4 d after LT. Immunosuppression was performed as previously described[17]. Tacrolimus level was tried to be maintained at 7–10 ng/mL for the 1st month after LT and at 5–7 ng/mL following that, unless they were tapered or stopped due to the use of mTOR inhibitors. Prednisolone was tapered out 1 mo after LT, and MMF was stopped 6 mo after LT. There was no difference in the protocol of induction or maintenance for HCC/non-HCC patients. mTOR inhibitor was not used routinely in our cohort. When high IA titer peak was highlighted in the follow-up after LT, several sessions of plasmapheresis were done as previously described[18].
AMR was diagnosed as previously reported[8]. Briefly, AMR was diagnosed as a greater than two-fold elevation of aminotransferase or total bilirubin compared with baseline level, together with an IA titer ≥ 1:64. Radiologic examinations including ultrasonography, CT, or MRI scans were used to exclude other causes of liver function abnormalities. The presence of ABOi-associated biliary strictures was defined as diffuse intrahepatic strictures on radiologic examinations, without vascular complications including hepatic artery thrombosis. Liver biopsy and C4d staining were used to confirm the occurrence of AMR. If AMR was diagnosed clinically or pathologically, plasmapheresis was used to reduce the IA titer to less than 1:32.
We used propensity-score matching (PSM) to adjust for differences in baseline characteristics between the high (n = 13) and low (n = 34) groups according to the tacrolimus trough concentrations at 4 wk. Variables such as gender, age, tumor markers, occurrence of ACR or AMR, biliary complications, hepatic artery thrombosis, use of mTOR inhibitor, outside the Milan criteria according to radiologic or pathologic criteria, tumor size, and tumor numbers were included. One-to-one nearest-neighbor matching within a caliper size of 0.20 was used. PSM analyses resulted in the selection of 12 patients in each group.
The median (interquartile) was used to present continuous variables, and the number (%) was used to present categorical variables. Continuous variables were compared using Mann-Whitney U tests, and categorical variables were compared using Chi-square tests. Logistic regression analyses were used to identify the risk factors for graft loss. Cox regression analyses were used to identify the time-dependent factors associated with GS. Factors with a P value < 0.01 in the univariate analyses were included in the multivariate analyses. The odds ratio and confidence interval (CI), as well as the hazard ratio (HR) and CI, were obtained from logistic and Cox regression analysis, respectively. Kaplan-Meier curves were used to assess GS in each risk group. Statistical significance was noted when P < 0.05. The optimal tacrolimus cutoff concentration was determined using the area under the receiver operating characteristic curves and Youden’s index. All analyses were performed using R statistical software version 4.0.3 (R Foundation, Vienna, Austria) or GraphPad Prism version 8 (GraphPad Software, San Diego, CA).
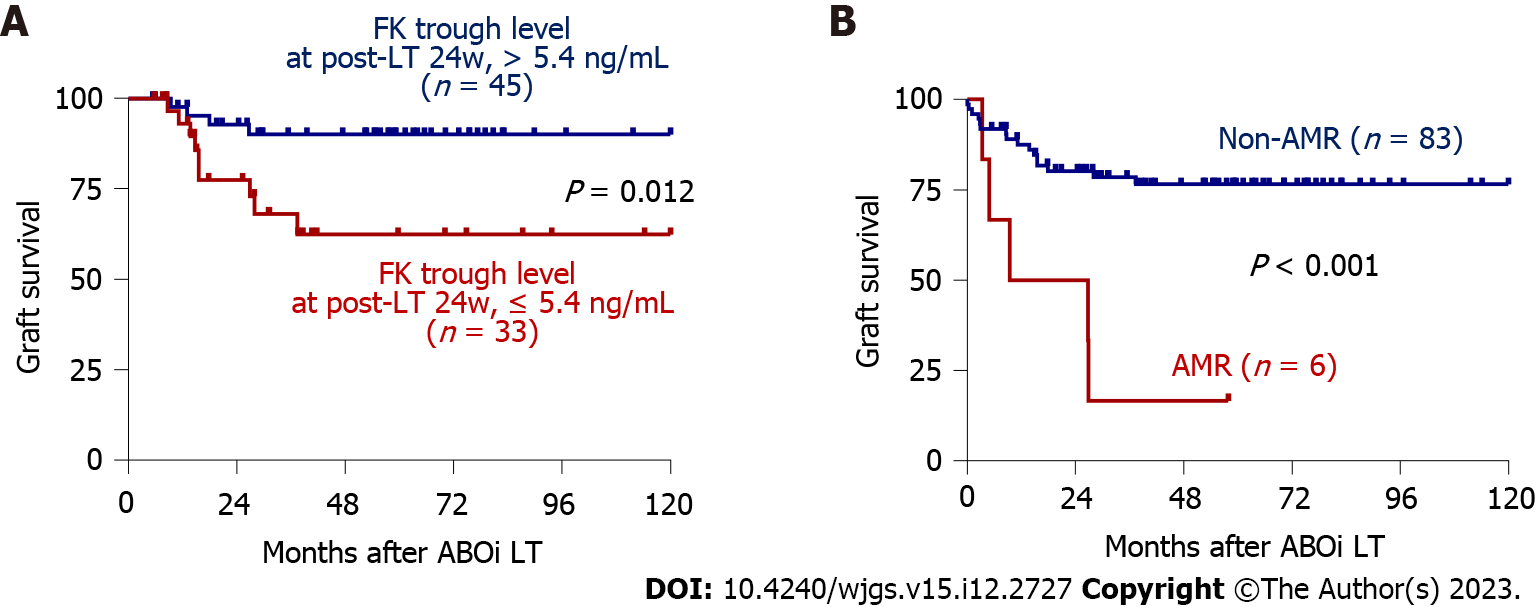
Imaging and histologic studies indicated HCC was present in 52.8% (47/89) of study patients. The one-, three-, five-, and ten-year GS rates were 85.9%, 73.3%, 71.4%, and 71.4%, respectively (Supplementary Figure 1A), and did not differ in the HCC (n = 47) and non-HCC (n = 42) groups. Graft and overall survivals were not different between non-HCC and HCC groups (Supplementary Figure 1B and C). Trough concentrations of tacrolimus at each time point were not different in the HCC and non-HCC groups (Supplementary Figure 2A). To compare the clinical characteristics according to GS, we divided the study subjects into the one-year graft loss (n = 10) and one-year GS (n = 79) groups (Supplementary Table 1). There were no significant differences about sex, recipient and donor ages, etiology of liver disease, or percentage of patients with HCC between the two groups. There were no differences in the results of preoperative laboratory and liver function tests. Positivity of crossmatching was reported in a greater percentage of the one-year GS group than in the graft loss group (72/79, 91.1% vs 5/10, 50.0%, respectively; P = 0.002), but there was no difference in HLA-mismatching between the two groups. There were no differences in the percentages of CD19 or CD20+ lymphocytes, presence of ACR, biliary/vascular complications, dose of rituximab, and concurrent usage of mTOR inhibitors between the two groups. In addition, there was no significant differences in the pre- and post-operative IA titers, between graft loss and survival groups (Supplementary Figure 3). Concerning serial measurements of tacrolimus trough concentrations, higher tacrolimus concentrations were reported in the graft loss group at 4 wk post-LT than in the GS group (median 8.7 vs 5.9 ng/mL, respectively; P = 0.012). When we divided study subjects into five-year graft loss (n = 20) and GS (n = 69) groups, AMR differed between the two groups (1/69, 1.4% in the GS group vs 5/20, 25.0% in the graft loss group, P = 0.001), and concurrent usage of mTOR inhibitors also differed between the two groups (10/69, 14.5% in the GS group vs 8/20, 40.0% in the graft loss group, P = 0.029).
To investigate the factors associated with GS in the total patient group, univariate and multivariate Cox regression analyses were performed. Tacrolimus trough concentrations at 16 and 24 wk post-LT and AMR were significant factors in the univariate analysis, and tacrolimus trough concentration at 24 wk post-LT (HR = 0.75, P = 0.048) and AMR (HR = 5.41, P = 0.013) remained significant factors in the multivariate analysis (Table 1). The optimal cutoff for 24-week tacrolimus concentration was 5.4 ng/mL (Supplementary Figure 4A), and GS differed significantly in patients with concentrations > 5.4 ng/mL (n = 45) and those with ≤ 5.4 ng/mL (n = 33) (P = 0.012, Figure 1A) and in patients with AMR (n = 6) and without AMR (n = 83) (P < 0.001, Figure 1B). We further analyzed risk factors for AMR using univariate and multivariate analyses, and post-LT peak IA titer was a risk factor identified in the multivariate analysis (P = 0.022, Supple
| Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Tacrolimus level, post-LT 16 wk | 0.82 (0.68-0.99) | 0.040 | NS | |
| Tacrolimus level, post-LT 24 wk | 0.71 (0.52-0.96) | 0.029 | 0.75 (0.56-0.99) | 0.048 |
| AMR | 6.01 (2.17-16.68) | 0.001 | 5.41 (1.44-20.40) | 0.013 |
Supplementary Table 4 presents the clinical characteristics of the HCC (n = 47) and non-HCC (n = 42) groups. Males were more frequent in the HCC group than in the non-HCC group (40/47, 85.1% vs 24/42, 57.1%, P = 0.007), and the patients in the HCC group were also older (median 57 vs 52 years, P = 0.009). Hepatitis B virus was the most common etiology in the HCC group (37/47, 78.7%), whereas alcohol was most common in the non-HCC group (19/42, 45.2%). Liver functions, as assessed using the Child-Pugh score and model of end-stage liver disease, were better in the HCC subgroup than in the non-HCC subgroup. Graft loss and re-transplantation did not differ between the two groups. In the HCC subgroup, 38.3% (18/47, 38.3%) of patients were classified as beyond the Milan criteria before and after LT, respectively, and recurrence was reported in 27.7% (13/47). Vascular invasion and alpha-fetoprotein (AFP) levels before downstaging treatment and LT were significantly worse in the recurrence group (Supplementary Table 5). When we investigated the factors associated with GS in each subgroup (Table 2), we found tacrolimus trough concentration at 24 wk post-LT (HR = 0.46, P = 0.026) was a significant factor in the multivariate analysis in the non-HCC group. On the other hand, in the HCC subgroup, AMR (HR = 63.20, P < 0.001) and HCC recurrence (HR = 20.72, P = 0.005) were significant factors in the multivariate analysis.
| Non-HCC | HCC | |||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| B cell crossmatching, positive | 0.19 (0.05-0.73) | 0.016 | NS | |||||
| Tacrolimus level, post-LT 24 week | 0.42 (0.23-0.88) | 0.019 | 0.46 (0.23-0.91) | 0.026 | ||||
| AMR | 3.39 (0.70-16.36) | 0.099 | NS | 11.33 (2.88-44.57) | 0.001 | 63.20 (6.49-614.78) | < 0.001 | |
| HCC recurrence | 5.31 (1.48-19.02) | 0.010 | 20.72 (2.48-173.32) | 0.005 | ||||
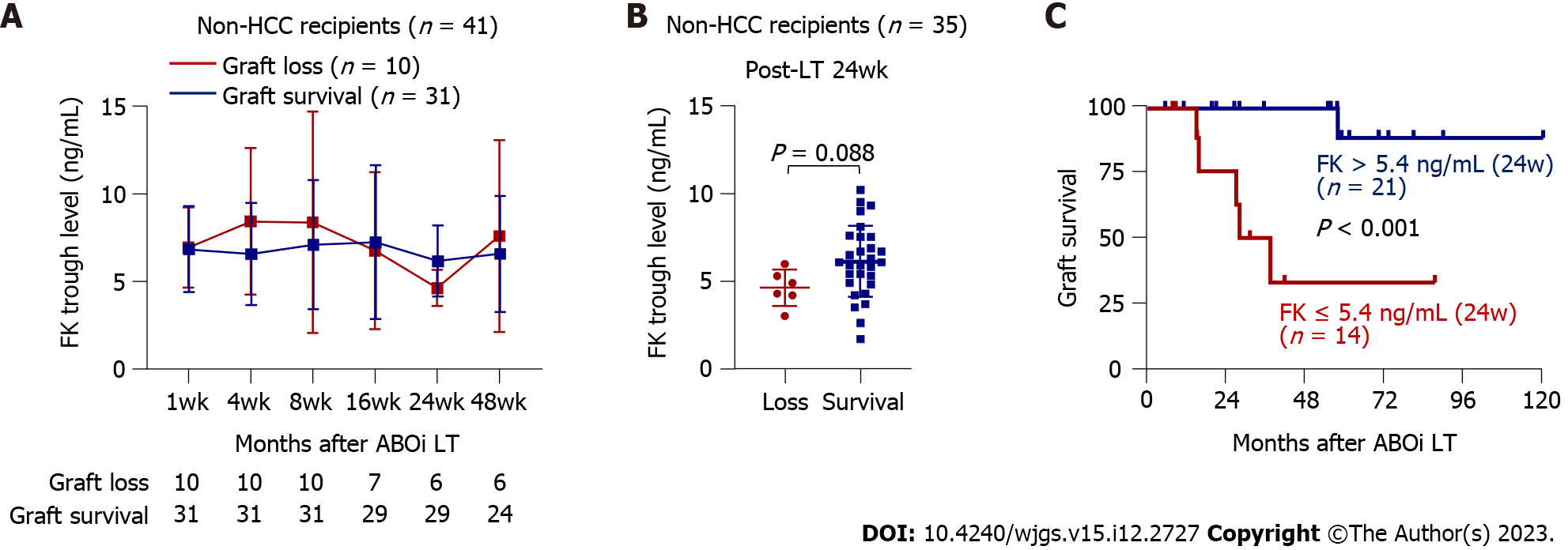
We next compared the tacrolimus trough concentrations at each time point in patients with graft loss (n = 10) and GS (n = 31) (Figure 2A). The tacrolimus concentration at 24 wk post-LT tended to be greater in the GS group than in the graft loss group (mean 6.1 vs 4.6 ng/mL, P = 0.088) (Figure 2A and B). In addition, when we categorized these patients into those with tacrolimus trough concentration > 5.4 ng/mL (n = 21) and ≤ 5.4 ng/mL (n = 14), the former subgroup had better GS (P < 0.001) (Figure 2C), which was consistent with the analyses of the total group. Furthermore, we investigated whether cumulative tacrolimus level from 20 to 28 wk after LT is associated with GS in non-HCC recipients. As a result, lower cumulative tacrolimus level from week 20 to 28 ≤ 296.8 ng/mL was associated with poor GS (Supplementary Figure 5A). We further obtained the tacrolimus level at 20-24 wk and 24-28 wk after LT and calculated the mean tacrolimus level to identify its association with GS in non-HCC patients. As a result, the mean level of tacrolimus ≤ 5.0 ng/mL from week 20 to 28 was associated with poor GS in non-HCC patients (Supplementary Figure 5B).
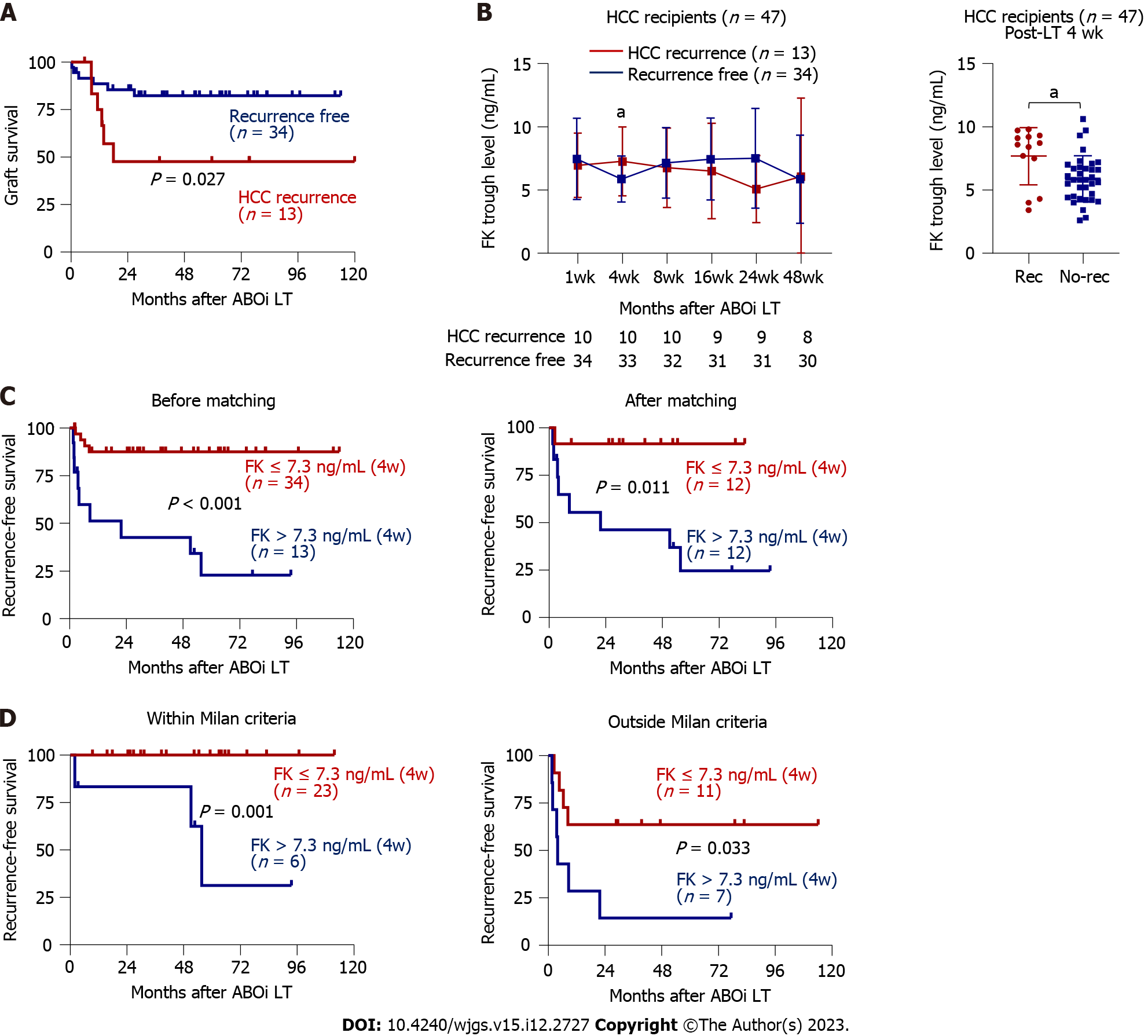
Figure 3A shows the significant difference in GS between recurrence and non-recurrence groups. Because HCC recurrence was an independent predictor of GS, we performed univariate and multivariate Cox regression analyses as shown in Table 3 for identifying the factors associated with the HCC recurrence. As a result, serum AFP concentrations greater than 200 ng/mL (the cutoff used in the previous reports[19,20]), tacrolimus concentration at 4 wk post-LT, and pre- or post-operative outside Milan criteria were risk factors in the univariate analysis. Furthermore, tacrolimus concentration at 4 wk post-LT (HR = 1.37, P = 0.024) and pre-operative radiologic outside Milan criteria (HR = 8.61, P = 0.004) remained significant factors in the multivariate analysis. Among the serial tacrolimus measurements, that obtained at 4 wk post-LT was the only one with significantly different tacrolimus concentrations in the recurrence (n = 13) and non-recurrence (n = 34) groups (Figure 3B). Recipients with HCC recurrence tended to have lower tacrolimus concentrations 24 wk post-LT (Figure 3B), although it was not statistically significant, which might be due to the early recurrence and use of everolimus with minimized tacrolimus doses.
| Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| AFP > 200 | 4.07 (1.32-12.53) | 0.014 | NS | |
| B-cell crossmatching, positive | 0.29 (0.08-1.08) | 0.065 | NS | |
| HLA-C mismatching | 0.36 (0.12-1.11) | 0.066 | NS | |
| Tacrolimus level, post-LT 4 wk | 1.28 (1.00-1.65) | 0.046 | 1.37 (1.04-1.80) | 0.024 |
| Over Milan – preoperative radiologic | 13.11 (3.54-48.55) | < 0.001 | 8.61 (1.97-37.70) | 0.004 |
| Over Milan – pathologic | 6.69 (2.05-21.83) | 0.002 | NS | |
When we analyzed the difference in the HCC recurrence between ACR (n = 7) and non-ACR (n = 40), as well as total rejection (ACR + AMR, n = 9) and non-rejection (n = 38), we found no differences between those ACR and non-ACR, and rejection and non-rejection groups (Supplementary Figure 6A). Finally, we divided patients into before (n = 12) and after (n = 35) the era of everolimus, and there was no difference in recurrence between two groups (Supplementary Figure 6B).
The recurrence group had greater tacrolimus trough concentrations at 4 wk post-LT than the non-recurrence group (8.6 vs 5.8 ng/mL, P = 0.011, Figure 3B), and the optimal cutoff point was 7.3 ng/mL (Supplementary Figure 4B). There were significant differences between patients with tacrolimus trough concentration > 7.3 ng/mL (n = 13) and ≤ 7.3 ng/mL (n = 34) (P < 0.001, Figure 3C): The one-, three-, five-, and ten-year recurrence-free survival (RFS) rates were 51.3%, 42.7%, 34.2%, and 22.8% in the > 7.3 group, respectively, and 87.5%, 87.5%, 87.5%, and 87.5% in the ≤ 7.3 group, which were not different with HCC patients with ABOc LT (Supplementary Figure 7A). PSM was successfully performed (Table 4), and significant difference in HCC recurrence according to the pre-defined cutoff was sustained after PSM (P = 0.011, Figure 3C). Furthermore, when we calculated the cumulative level of tacrolimus, higher cumulative tacrolimus level from LT to week 4 > 200.2 ng/mL was also associated with a higher future risk of HCC recurrence, which is in line with the previous report[21] (Supplementary Figure 7B). In addition, we analyzed whether the mean tacrolimus trough level within 4 wk after LT, which was obtained from week 1/2/3/4 timepoints, is associated with the HCC recurrence, and a higher mean level of tacrolimus from LT to week 4 > 8.0 ng/mL was associated with a higher risk of HCC recurrence (Supplementary Figure 7C). We further analyzed whether there is any difference in the HCC recurrence between patients with serum tacrolimus levels of > 10 ng/mL and ≤ 10 ng/mL at 1 or 2 wk after LT using the cut-off which has been suggested as high level in the previous report[21] (Supplementary Figure 7D), but, there was no significant difference in HCC recurrence using a cut-off tacrolimus level of 10 ng/mL at week 1 or 2. When we performed subgroup analyses according to the tumors within Milan criteria (n = 29), and outside Milan criteria (n = 18), the patients with tacrolimus trough concentration > 7.3 ng/mL at 4 wk post-LT also had poorer RFS (Figure 3D). When we performed subgroup analyses according to the vascular invasion and AFP levels, high tacrolimus at 4 wk was also associated with poor RFS (Supplementary Figure 7E and F).
| Before matching | After matching | |||||
| Low FK 4wk, n = 34 | High FK 4wk, n = 13 | P value | Low FK 4wk, n = 12 | High FK 4wk, n = 12 | P value | |
| Male gender | 31 (91.2) | 9 (69.2) | 0.152 | 10 (83.3) | 8 (66.7) | 0.637 |
| Age (yr) | 57.0 (54.0; 62.0) | 54.0 (46.0; 59.0) | 0.103 | 54.0 (52.5; 58.5) | 54.0 (46.0; 62.0) | 0.862 |
| AFP (ng/mL) | 9.6 (3.2; 31.0) | 17.5 (12.0; 28.8) | 0.216 | 15.3 (3.5; 40.0) | 16.5 (10.2; 263.8) | 0.713 |
| PIVKA (mAU/mL) | 20.5 (12.0; 119.0) | 16.0 (13.0; 26.0) | 0.625 | 21.5 (14.5; 87.5) | 15.0 (12.5; 62.5) | 0.506 |
| MELD | 9.0 (7.0; 14.0) | 8.0 (7.0; 10.0) | 0.234 | 7.5 (4.5;12.2) | 7.0 (4.5; 8.0) | 0.62 |
| ACR | 4 (11.8) | 3 (23.1) | 0.606 | 3 (25.0) | 2 (16.7) | 1 |
| AMR | 2 (5.9) | 1 (7.7) | 1 | 2 (16.7) | 1 (8.3) | 1 |
| Biliary complication | 14 (41.2) | 5 (38.5) | 1 | 6 (50.0) | 5 (41.7) | 1 |
| Hepatic artery thrombosis | 1 (2.9) | 1 (7.7) | 1 | 1 (8.3) | 1 (8.3) | 1 |
| mTOR inhibitor | 8 (23.5) | 5 (38.5) | 0.51 | 3 (27.3) | 3 (27.3) | 1 |
| Radiologic over Milan | 8 (23.5) | 7 (53.8) | 0.1 | 2 (16.7) | 6 (50.0) | 0.194 |
| Pathologic over Milan | 10 (29.4) | 5 (38.5) | 0.806 | 3 (25.0) | 4 (33.3) | 1 |
| Downstaging treatment | 14 (41.2) | 5 (38.5) | 1 | 4 (33.3) | 4 (33.3) | 1 |
| Tumor largest size, cm | 1.5 (0.0; 3.2) | 3.0 (1.6; 4.5) | 0.174 | 1.8 (0.0; 2.3) | 2.5 (0.8; 4.0) | 0.331 |
| Tumor number | 1.0 (0.0; 2.0) | 1.0 (1.0; 2.0) | 0.303 | 1.0 (0.0; 1.5) | 1.0 (0.5; 2.5) | 0.336 |
This study evaluated the short- and long-term outcomes of ABOi LT and compared the GS between HCC and non-HCC recipients. The results indicated that the one- and five-year GS rates were comparable between the groups, and that AMR was a common risk factor predicting poor GS. These results suggest that intensive monitoring and treatment of AMR is the most important strategy for improving the long-term outcomes associated with ABOi LT. To our knowledge, this is the first to include serial measurements of trough tacrolimus concentrations in the analyses combined with an evaluation of any associated clinical outcomes in the patient subgroups. Greater exposure to tacrolimus 4 wk after ABOi LT was associated with HCC recurrence, whereas lower exposure at later time points was associated with poorer long-term graft outcomes.
The long-term outcomes of ABOi LT have been reported in several studies. Data from a long-term Japanese registry showed a 5-year OS rate of 74.0%[22]. Korean studies have reported 5-year survival rates of 74%–86%, reporting no differences between the HCC and non-HCC groups[6,8,9,23,24], which is consistent with our findings. In contrast to previous studies, we analyzed the risk factors for poor survival including AMR and HCC recurrence in ABOi LT. In particular, previous studies did not identify the risk factors associated with AMR, therefore we investigated the potential factors in detail including serial tacrolimus concentrations, PRA, T-cell or B-cell cross-matching, and HLA typing, although we did not identify any related factors. As a result, we showed that tacrolimus concentrations were differentially associated with graft outcomes in HCC and non-HCC patients.
The key finding of this study is that early high exposure to tacrolimus can be associated with poor outcomes in HCC patients who undergo ABOi LT, as has been shown in cases of ABOc LT. The trough concentration of tacrolimus in the maintenance phase can be adjusted according to whether or not the LT recipients have HCC. Regardless of ABO compatibility, the recommended tacrolimus concentration is 5–10 ng/mL during the first 3 mo after LT and 5–8 ng/mL for the next 3–12 mo, followed by a further reduction to approximately 3–6 ng/mL if tacrolimus is not combined with other immunosuppressants such as MMF or everolimus[25]. Another guideline recommends that the tacrolimus concentration should be set at 6–10 ng/mL during the first 3 mo and subsequently reduced and maintained below 5 ng/mL for 12 mo after LT[26]. However, CNIs can promote tumor growth owing to the overexpression of transforming growth factor-beta1[27] and therefore increase tumor recurrence in a rat model of HCC[28]. A previous clinical study confirmed that early exposure to high concentrations of CNIs within the first month after LT was significantly associated with HCC recurrence, with a cutoff value of 10 ng/mL[11]. Another study showed that tacrolimus levels > 8 ng/mL 20 d after ABOc LT were associated with HCC recurrence[29]. Our cutoff level for tacrolimus was 7.3 ng/mL at 4 wk, although this level requires further validation. A recent report also showed that early cumulative high tacrolimus exposure, and not just one level at a single timepoint, was associated with HCC recurrence[21], We also confirmed this finding in the present study. However, the occurrence of AMR was not related to serum tacrolimus levels, emphasizing that minimizing CNIs is also feasible with ABOi LT, particularly in HCC patients.
The HCC recurrence rate following LT is reportedly 8%–20%[30], whereas we identified a recurrence rate of 27.7%. This finding might have been associated with the inclusion of more patients who did not meet the Milan criteria compared to the number of such patients recruited in previous studies. Preoperative recipient and tumor factors, such as the size and number of tumors, AFP levels, and vascular invasion[30], are critically associated with recurrence. One study including a considerable number of ABOi LT patients revealed that pre-LT HCC treatment, higher tumor marker levels, and lymphovascular invasion were related to HCC recurrence[9]. Our study showed that outside Milan, vascular invasion and high AFP levels are related to HCC recurrence in ABOi LT. We then adjusted for these conventional factors and subsequently showed that a high tacrolimus level at week 4 remained a significant factor for HCC recurrence, further underscoring the importance of minimizing CNIs. To achieve this goal, strategies other than rituximab and CNIs, including intravenous immunoglobulins associated with plasmapheresis or the addition of everolimus, together with the close monitoring of IA titers, requires further validation.
Tacrolimus may play an important role in inhibiting B-lymphocytes[31], but no consensus exists on the optimum trough concentration of tacrolimus in ABOi LT recipients. Due to concerns about AMR, many clinicians maintain a higher level of tacrolimus in the early phase of ABOi LT. The mechanism of suppression of B cell activation by CNIs has not been clearly elucidated; however, one study reported that tacrolimus can suppress lymph node or circulating follicular helper T cells, differentiation of plasmablasts, and antibody production. This suggests that inadequate tacrolimus concentrations may be associated with acute or chronic AMR in solid organ transplantation, in addition to ACR[23]. Although data from ABOi LT patients are lacking, a lower CNI concentration is associated with an increased risk of donor-specific antibody formation after kidney transplantation (KT)[32,33]. In line with this finding, low tacrolimus levels at 24 wk as well as cumulative levels between 20 and 28 wk were associated with poor long-term outcomes in non-HCC patients in our study. In particular, one non-HCC LT recipient with a low tacrolimus level at 24 wk post-LT died from late AMR, and two recipients lost grafts due to ACR-induced hepatic failure. However, one study reported no difference in the incidence of donor-specific antibody (DSA) when tacrolimus concentrations were controlled within a narrow range of 4–6 ng/mL in KT. This strategy can also be applied to ABOi LT, particularly in HCC patients[34]. Our findings suggest that considering both DSA and HCC recurrence, maintaining a narrow range of tacrolimus concentrations, such as between 5.4 and 7.3 ng/mL, may be helpful in ABOi LT. However, the optimal cutoff must be validated in larger prospective studies.
Our study had some limitations. First, this was a single-center, small-sized, retrospective study. Second, we did not evaluate the impact of antitumor treatment including transarterial chemoembolization or systemic chemotherapies on HCC recurrence as previously reviewed, because this study did not focus on the HCC treatment strategy following post-LT recurrence. Third, the long-term adverse effects of tacrolimus including renal dysfunction, which we had previously analyzed[17] were not considered risk factors for long-term survival. Larger multicenter studies that consider the heterogeneity and dynamic changes in the risk factors of LT recipients are warranted to develop a predictive model for ABOi LT recipients.
To our knowledge, this is the first study to demonstrate that early exposure to high tacrolimus concentrations may be associated with HCC recurrence and poor GS in ABOi LDLT recipients with HCC, whereas low tacrolimus concentrations at 24 wk post-LT were associated with poor GS in the non-HCC group. AMR was a common risk factor in both subgroups, emphasizing the need for close monitoring of IA titers after ABOi LT. Future studies are required to validate our results and establish a proper strategy for immunosuppressive therapy in ABOi LT.
ABO-incompatible (ABOi) liver transplantation (LT) has posed significant challenges due to its impact on graft survival and the occurrence of complications like antibody-mediated rejection (AMR). The outcomes of ABOi living-donor LT (LDLT) have shown considerable variability in previous studies. This research aimed to investigate the long-term outcomes of ABOi LDLT and associated risk factors.
Understanding the factors that influence graft survival (GS) and complications in ABOi LDLT recipients is essential for improving the clinical outcomes. Identifying the impact of tacrolimus concentration on graft outcomes, especially in patients with or without hepatocellular carcinoma (HCC), can provide valuable insights for optimizing immunosuppressive therapy.
The primary objectives of this study were to assess the long-term graft outcomes in ABOi LDLT and analyze the associated risk factors. Subgroup analyses were performed to evaluate GS and risk factors based on the presence or absence of HCC. Additionally, the study aimed to investigate the influence of serial serum tacrolimus trough concentration on graft outcomes in both HCC and non-HCC recipients.
A retrospective analysis was conducted on a cohort of 89 ABOi LDLT recipients. Various clinical, laboratory, and radiological data were collected for each patient. Desensitization and immunosuppression protocols were followed, including the use of rituximab and tacrolimus. The impact of tacrolimus trough concentration on GS and HCC recurrence was analyzed using statistical methods.
The study found that AMR was a common risk factor affecting GS in both HCC and non-HCC ABOi LDLT recipients. Early exposure to high tacrolimus concentrations was associated with HCC recurrence, while lower concentrations at later time points were linked to poorer long-term graft outcomes in non-HCC patients. Maintaining a narrow range of tacrolimus concentrations between 5.4 and 7.3 ng/mL may be beneficial.
This research highlighted the importance of monitoring and managing the calcineurin inhibitor (CNI) concentrations in ABOi LDLT recipients. The study also suggested that tailoring tacrolimus concentration based on the presence of HCC can optimize graft outcomes. Minimizing CNIs, especially in HCC patients, may reduce the risk of HCC recurrence.
Future studies should validate the optimal tacrolimus concentration cutoffs and explore additional strategies to improve the outcomes of ABOi LDLT recipients. Multicenter studies considering various risk factors are warranted to develop predictive models for this patient population.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lu GR, China; Ullah K, Pakistan S-Editor: Lin C L-Editor: A P-Editor: Wu RR
| 1. | Farges O, Kalil AN, Samuel D, Saliba F, Arulnaden JL, Debat P, Bismuth A, Castaing D, Bismuth H. The use of ABO-incompatible grafts in liver transplantation: a life-saving procedure in highly selected patients. Transplantation. 1995;59:1124-1133. [PubMed] |
| 2. | Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990;336:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 223] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Oh J, Kim JM. Immunologic strategies and outcomes in ABO-incompatible living donor liver transplantation. Clin Mol Hepatol. 2020;26:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Kim JM, Kim DG, Kim J, Lee K, Lee KW, Ryu JH, Kim BW, Choi DL, You YK, Kim DS, Nah YW, Kang KJ, Cho JY, Hong G, Yu HC, Moon JI, Choi D, Hwang S, Kim MS. Outcomes after liver transplantation in Korea: Incidence and risk factors from Korean transplantation registry. Clin Mol Hepatol. 2021;27:451-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Egawa H, Ohdan H, Saito K. Current Status of ABO-incompatible Liver Transplantation. Transplantation. 2023;107:313-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Kim JM, Kwon CH, Joh JW, Han SB, Sinn DH, Choi GS, Kang ES, Lee JH, Kim GS, Lee SK. Case-matched comparison of ABO-incompatible and ABO-compatible living donor liver transplantation. Br J Surg. 2016;103:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Kim JM, Kwon CH, Joh JW, Kang ES, Park JB, Lee JH, Kim SJ, Paik SW, Lee SK, Kim DW. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol. 2013;59:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Kim WJ, Sin MH, Yoon YI, Kang WH, Kim SH, Tak EY. ABO-Incompatible Adult Living Donor Liver Transplantation Under the Desensitization Protocol With Rituximab. Am J Transplant. 2016;16:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Yoon YI, Song GW, Lee SG, Hwang S, Kim KH, Kim SH, Kang WH, Cho HD, Jwa EK, Kwon JH, Tak EY, Kirchner VA. Outcome of ABO-incompatible adult living-donor liver transplantation for patients with hepatocellular carcinoma. J Hepatol. 2018;68:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Kang I, Lee JG, Choi SH, Kim HJ, Han DH, Choi GH, Kim MS, Choi JS, Kim SI, Joo DJ. Impact of everolimus on survival after liver transplantation for hepatocellular carcinoma. Clin Mol Hepatol. 2021;27:589-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O'Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D, Thorburn D, Briceño J, De la Mata M, Burroughs AK. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Kim JM. Can hepatocellular carcinoma recurrence be prevented after liver transplantation? Clin Mol Hepatol. 2021;27:562-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Lee SD, Kim SH, Kong SY, Kim YK, Lee SA, Park SJ. ABO-incompatible living donor liver transplantation without graft local infusion and splenectomy. HPB (Oxford). 2014;16:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Chung BH, Yun JT, Ha SE, Kim JI, Moon IS, Choi BS, Park CW, Kim YS, Yang CW. Combined use of rituximab and plasmapheresis pre-transplant increases post-transplant infections in renal transplant recipients with basiliximab induction therapy. Transpl Infect Dis. 2013;15:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Han JW, Choi JY, Lee SK, Sung PS, Jang JW, Yoon SK, Choi YH, Lee IS, Oh JS, Chun HJ, Choi HJ, You YK. Long-term Clinical Outcomes and Predictive Factors for Living-donor Liver Transplant Recipients With Biliary Strictures. Transplantation. 2022;106:1990-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 16. | Han JW, Joo DJ, Kim JH, Rha MS, Koh JY, Park HJ, Lee JG, Kim MS, Kim SI, Shin EC, Park JY, Park SH. Early reduction of regulatory T cells is associated with acute rejection in liver transplantation under tacrolimus-based immunosuppression with basiliximab induction. Am J Transplant. 2020;20:2058-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Sung PS, Han JW, Seo C, Ahn J, Lee SK, Nam HC, Choi HJ, You YK, Jang JW, Choi JY, Yoon SK. Real-Life Experience of mTOR Inhibitors in Liver Transplant Recipients in a Region Where Living Donation Is Predominant. Front Pharmacol. 2021;12:685176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Lee SH, Choi HJ, You YK, Kim DG, Na GH. ABO incompatible living donor liver transplantation: a single center experience. The Journal of the Korean Society for Transplantation. 2018;32:84-91. |
| 19. | Suh KS, Lee HW. Liver transplantation for advanced hepatocellular carcinoma: how far can we go? Hepat Oncol. 2015;2:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Hong G, Suh KS, Suh SW, Yoo T, Kim H, Park MS, Choi Y, Paeng JC, Yi NJ, Lee KW. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol. 2016;64:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Rodríguez-Perálvarez M, Colmenero J, González A, Gastaca M, Curell A, Caballero-Marcos A, Sánchez-Martínez A, Di Maira T, Herrero JI, Almohalla C, Lorente S, Cuadrado-Lavín A, Pascual S, López-Garrido MÁ, González-Grande R, Gómez-Orellana A, Alejandre R, Zamora-Olaya J, Bernal-Bellido C; Chronic immunosuppression, cancer Spanish consortium. Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation. Am J Transplant. 2022;22:1671-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 22. | Umeshita K, Eguchi S, Egawa H, Haga H, Kasahara M, Kokudo N, Sakisaka S, Takada Y, Tanaka E, Eguchi H, Uemoto S, Ohdan H. Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society. Hepatol Res. 2019;49:964-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Wallin EF, Hill DL, Linterman MA, Wood KJ. The Calcineurin Inhibitor Tacrolimus Specifically Suppresses Human T Follicular Helper Cells. Front Immunol. 2018;9:1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Kim SH, Lee EC, Na BG, Park SJ. Impact of ABO-incompatibility on hepatocellular carcinoma recurrence after living donor liver transplantation. Eur J Surg Oncol. 2019;45:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Cillo U, De Carlis L, Del Gaudio M, De Simone P, Fagiuoli S, Lupo F, Tisone G, Volpes R. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: consensus recommendations from an Italian Working Group. Hepatol Int. 2020;14:930-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Martins-Filho SN, Alves VAF, Wakamatsu A, Maeda M, Craig AJ, Assato AK, Villacorta-Martin C, D'Avola D, Labgaa I, Carrilho FJ, Thung SN, Villanueva A. A phenotypical map of disseminated hepatocellular carcinoma suggests clonal constraints in metastatic sites. Histopathology. 2019;74:718-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, Suthanthiran M. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003;76:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Ogawa T, Tashiro H, Miyata Y, Ushitora Y, Fudaba Y, Kobayashi T, Arihiro K, Okajima M, Asahara T. Rho-associated kinase inhibitor reduces tumor recurrence after liver transplantation in a rat hepatoma model. Am J Transplant. 2007;7:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Abrahamsson J, Sternby Eilard M, Rizell M, Bennett W, Åberg F. Reduced calcineurin inhibitor exposure with antibody induction and recurrent hepatocellular carcinoma after liver transplantation. Scand J Gastroenterol. 2022;57:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 31. | Zhou W, Ohdan H, Asahara T. Calcineurin inhibitors block B-1 cell differentiation: the relevance to immunosuppressive treatment in ABO-incompatible transplantation. Transplant Proc. 2005;37:1808-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1243] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 33. | Opelz G, Döhler B. Effect on kidney graft survival of reducing or discontinuing maintenance immunosuppression after the first year posttransplant. Transplantation. 2008;86:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Unagami K, Ishida H, Furusawa M, Kitajima K, Hirai T, Kakuta Y, Toki D, Shimizu T, Omoto K, Okumi M, Nitta K, Tanabe K. Influence of a low-dose tacrolimus protocol on the appearance of de novo donor-specific antibodies during 7 years of follow-up after renal transplantation. Nephrol Dial Transplant. 2021;36:1120-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |