Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2596
Peer-review started: July 31, 2023
First decision: September 5, 2023
Revised: September 15, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 27, 2023
Processing time: 118 Days and 19.6 Hours
Branched chain amino acid (BCAA) supplementation has been associated with favourable outcomes in liver malignancies requiring definitive resection or liver transplantation. Currently, there are no updated systematic reviews evaluating the efficacy of perioperative BCAA supplementation in patients undergoing surgery for liver cancer.
To evaluate the efficacy of perioperative BCAA supplementation in patients undergoing surgery for liver cancer.
A systematic review of randomized control trials and observational studies was conducted on PubMed, Embase, Cochrane Library, Scopus, and Web of Science to evaluate the effect of perioperative BCAA supplementation compared to standard in-hospital diet, in liver cancer patients undergoing surgery. Clinical outcomes were extracted, and a meta-analysis was performed on relevant outcomes.
16 studies including 1389 patients were included. Perioperative BCAA administration was associated with reduced postoperative infection [risk ratio (RR) = 0.58 95% confidence intervals (CI): 0.39 to 0.84, P = 0.005] and ascites [RR = 0.57 (95%CI: 0.38 to 0.85), P = 0.005]. There was also a reduction in length of hospital stay (LOS) [weighted mean difference (WMD) = -3.03 d (95%CI: -5.49 to -0.57), P = 0.02] and increase in body weight [WMD = 1.98 kg (95%CI: 0.35 to 3.61, P = 0.02]. No significant differences were found in mortality, cancer recurrence and overall survival. No significant safety concerns were identified.
Perioperative BCAA administration is efficacious in reducing postoperative infection, ascites, LOS, and increases body weight in liver cancer patients undergoing surgical resection.
Core Tip: Liver surgery has been associated with anthropometric disturbances and systemic catabolism, which can be improved with perioperative branched chain amino acid (BCAA) supplementation. However, it remains undetermined if the reported advantages of BCAA supplementation warrant routine perioperative use. This systematic review compares sixteen studies including 1389 patients. We found that perioperative BCAA supplementation was efficacious in reducing postoperative infection, ascites, length of hospital stay and increases body weight in liver cancer patients undergoing surgical resection.
- Citation: Yap KY, Chi H, Ng S, Ng DH, Shelat VG. Effect of perioperative branched chain amino acids supplementation in liver cancer patients undergoing surgical intervention: A systematic review. World J Gastrointest Surg 2023; 15(11): 2596-2618
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2596.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2596
Liver cancer is a global health issue with an estimated incidence of over 1 million cases by 2025[1]. Secondary liver cancer is more prevalent than primary liver cancer[2-4]. Lung and colorectal primaries account for half of the cases[5]. Hepatocellular carcinoma (HCC) accounts for approximately 90 percent of all primary liver cancers[1], and is the third most common cause of cancer mortality in the world[6]. Hepatectomy and liver transplant are the predominant curative treatments for liver cancers[7,8].
Although technological innovation and adoption of prehabilitation strategies have made hepatic surgery safe, there are still substantial morbidity risks, especially in cirrhotic patients[9]. Emerging evidence suggests that levels of branched chain amino acids (BCAAs), namely valine (Val), leucine (Leu) and isoleucine (Ile), are decreased in various forms of hepatic injury[10]. As important substrates for protein synthesis and regulators of protein turnover, BCAAs are involved in the pathophysiology of HCC by affecting gene expression, apoptosis, and regeneration of hepatocytes[10]. Additionally, advanced liver diseases are usually associated with systemic catabolism and depletion of muscle mass[11].
In rat model studies, BCAAs have been reported to promote hepatocyte proliferation and suppress growth of HCC. Kim et al[12] reported that after major hepatectomy, supplementation with BCAAs helps not only to maintain a stable plasma BCAA/aromatic amino acids ratio, but also promotes liver regeneration in rats. BCAAs delay progression of carbon tetrachloride(CCl4)-induced chronic liver injury by attenuating hepatic apoptosis and stimulating the production of hepatocyte growth factors[13,14]. Miuma et al[15] reported that all three BCAAs down-regulate vascular endothelial growth factor (VEGF) expression during HCC development. Through these mechanisms, supplementation with BCAA may potentially suppress HCC development and accelerate post-surgical recovery. Furthermore, many studies have reported that preoperative malnutrition increases the risks of postoperative morbidity and mortality[16-19]. The benefits of administering BCAA to patients with HCC undergoing surgical treatment appear clear and promising.
Despite studies favouring BCAA supplementation, evidence of actual and measurable benefit is lacking. A 2012 Cochrane review showed that nutritional interventions for patients undergoing liver transplant did not offer benefits[20]. Another review demonstrated that oral BCAA supplementation improved 3-year mortality in HCC patients, but without impact on cancer recurrence[21]. In a meta-analysis on the use of supplemental BCAAs during the perioperative period in gastrointestinal cancer patients, Cogo et al[22] reported an improvement in morbidity from postoperative infection but no reduction in cancer recurrence.
Therefore, from current literature, it is unclear if the reported advantages of BCAA supplementation during surgical interventions in liver cancer warrant routine perioperative administration. Given these knowledge gaps, it is necessary to appraise the current evidence to determine if BCAA supplementation has beneficial impact on patients undergoing liver resection for various oncological indications. Thus, the aim of this systematic review and meta-analysis is to evaluate the role of perioperative BCAA supplementation in patients undergoing liver resection.
This systematic review was performed in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines[23]. This review is part of a systematic review protocol registered on PROSPERO (CRD42022341658). Search of five databases (PubMed, Embase, Cochrane Library, Scopus, and Web of Science) was conducted on July 6, 2022 for articles published since inception up to 6 July 2022. Keywords related to the terms (“BCAA” or “Branched-chain amino acid” or “Leu” or “Val” or “Ile” or “amino acid”), [(“liver” or “hepatic” or “hepatocellular”) and (“carcinoma” or “cancer” or “malignancy” or “tumour” or “neoplasm”)], (“resection” or “surgery” or “preoperative” or “perioperative” or “postoperative” or “hepatectomy” or “liver transplantation”) were used in literature search. The full search strategy is available in Supplementary Table 1.
Studies comparing outcomes of BCAA vs no supplementation in the perioperative period among liver cancer patients were considered for inclusion. Clinical trials and observational studies fulfilling the following criteria were included in the review: (1) Patients with a diagnosis of primary or secondary cancer in the liver; (2) patients underwent either hepatectomy or liver transplant; and (3) study has a control arm (placebo or normal usual diet). We excluded studies with patients undergoing liver surgery for other indications, or undergoing treatment procedures for liver cancer, such as radiofrequency ablation or transarterial chemoembolisation, without surgical intervention. All other studies were included, and details of source databases used in each included study were collected. The details of inclusion and exclusion criteria of this review according to the Population, Intervention, Comparison, Outcomes and Study framework are documented in Supplementary Table 2.
Three reviewers (Yap KY, Chi H, Ng S) independently performed the literature search and data extraction and all disagreements were resolved by mutual consensus. Data extracted include information on patient demographics (number of patients, age, sex, comorbid liver disease), cancer type and histopathology (HCC or metastatic or other cancers, tumour size and number, stage of cancer), surgical details (extent of hepatectomy, type of liver transplant) and mean duration of follow-up.
Risk of bias and quality of studies were assessed. For randomised control trials (RCTs), quality control was performed by two co-authors (Yap KY and Chi H) using the Cochrane Risk of Bias tool 2[24], which assesses five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. For observational studies, quality control was performed using the ROBINS-1 tool[25], which assesses seven domains in total: for pre-intervention (confounding and participation selection), during intervention (classification of intervention) and post-intervention (deviations from intended interventions, missing data, measurement of outcomes and selection of reported results) stages.
Primary outcomes of interest in this review were perioperative and oncological outcomes. Perioperative outcomes include postoperative morbidity, mortality, and length of stay (LOS). Oncological outcomes include recurrence and overall survival (OS). Secondary outcomes were changes in serum albumin, anthropometrics, and overall quality of life (QOL) in patients.
Postoperative infections were defined as any infectious complication arising in the postoperative period, including surgical site infections, septic complications, urinary tract infections, chest infections, liver abscesses and infected ascites. Ascites was defined as either new onset postoperative ascites or refractory ascites requiring diuretic agent for control. All-cause mortality was derived from OS in most studies, with a minimal follow-up time of 3 years (Supplementary Table 3). Recurrence was defined as reappearance of tumour with typical findings on imaging modalities. Changes in serum albumin were determined by comparing preoperative to postoperative measurements reported at 6- and 12-mo intervals. Anthropometrics reported by studies include body weight change, triceps skin-fold thickness and mid-arm circumference, but only body weight changes were included in this analysis.
Review Manager version 5.4 was used to pool and analyse results with reference to approaches from the Cochrane Handbook[26]. In studies without SD, P-values or confidence intervals (CI) were converted to SD. For studies without SD, P-values, and CI, we used the square-root of weighted mean variance of all other studies to estimate the SD[27]. Pre-intervention baseline imbalances were corrected using the simple analysis of change scores method for panel data and longitudinal outcomes. In studies reporting the outcome in different scales, a simple unit conversion was performed. Inverse variance was used to derive the pooled outcomes. The random-effects model was used in accounting for between-study variance. I2 and τ2 statistics were used to present between-study heterogeneity: Low heterogeneity (I2 < 30%), moderate heterogeneity (I2 30%-60%), and substantial heterogeneity (I2 > 60%). Two-sided P value of < 0.05 was regarded as significant[26,28,29]. The statistical methods of this study were reviewed by Vishal G Shelat from National University of Singapore.
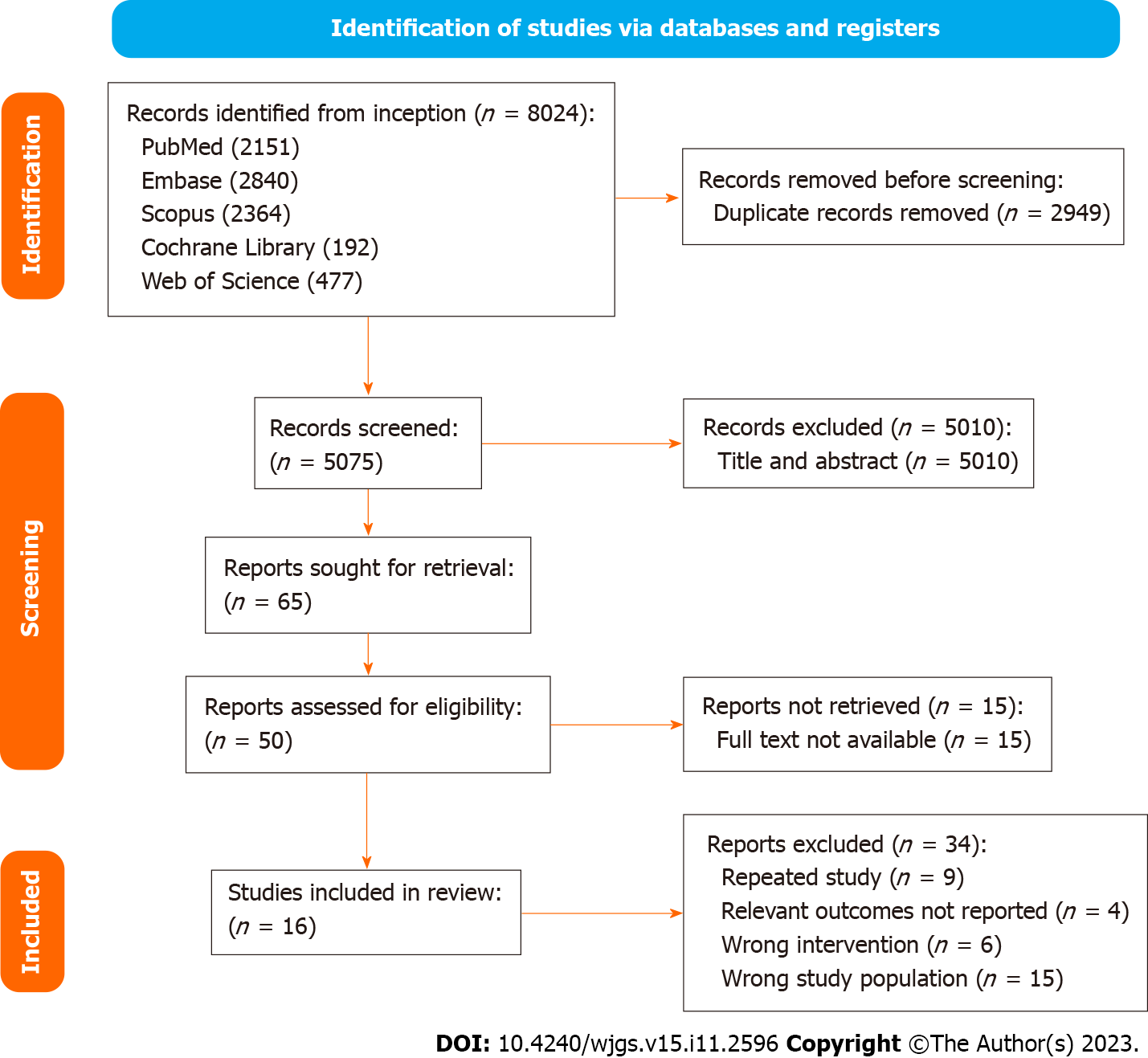
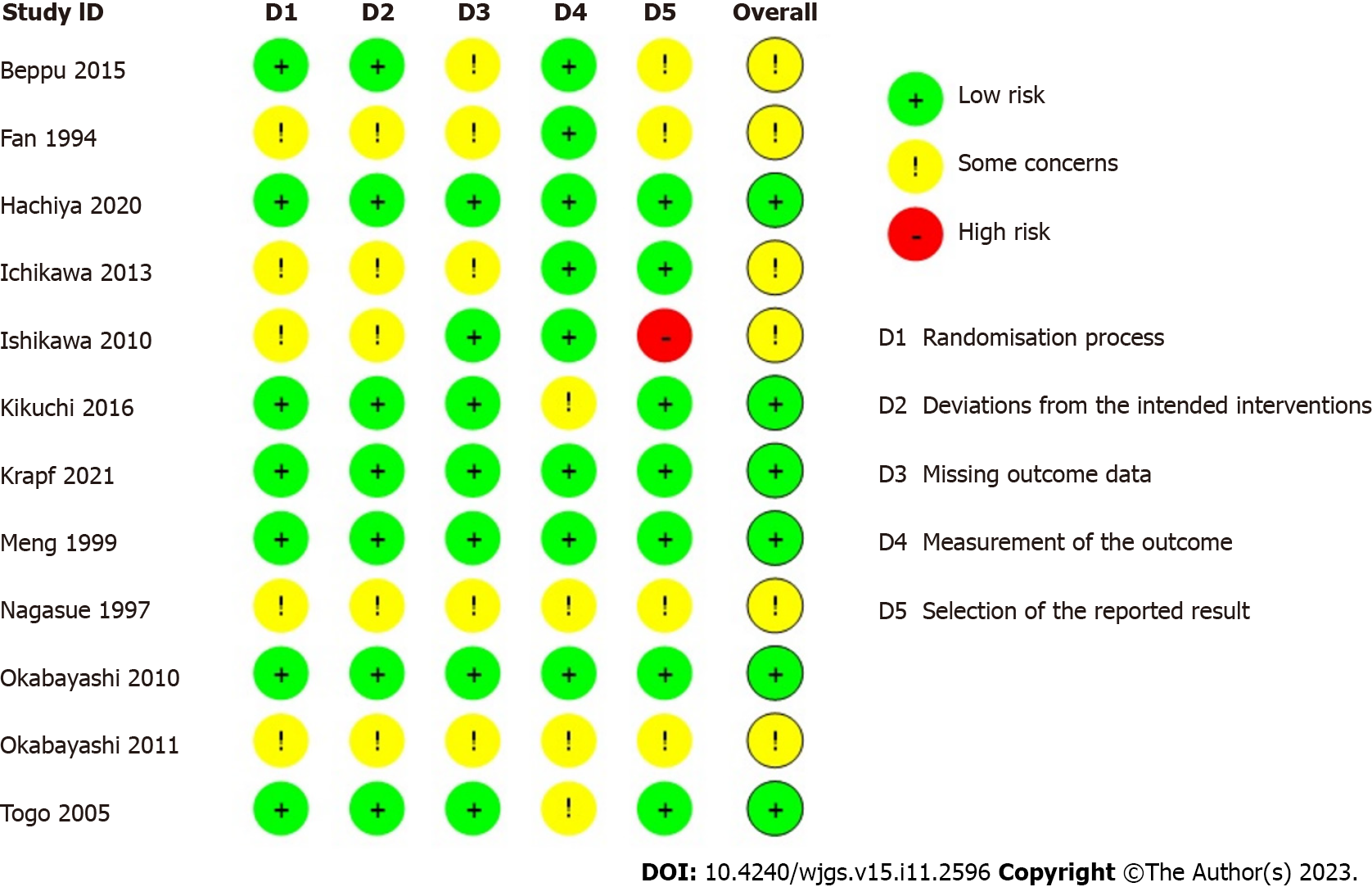
A systematic search identified 8024 studies, of which 2949 duplicate studies were excluded. Subsequent screening of title and abstract performed independently by authors (Yap KY, Chi H, Ng S) identified 50 studies for full-text evaluation. Finally, 12 prospective RCTs and 4 non-randomised studies (1 non-randomised trial and 3 observational studies) were included. A detailed PRISMA diagram is shown in Figure 1. The studies included were assessed for risk of bias, with a summary of the assessment shown in Figure 2 and Table 1 for trials and non-interventional studies, respectively. The PRISMA checklist is appended in Supplementary Figure 1.
| Ref. | Confounding factor bias | Selection bias | Bias in classification of interventions | Bias due to deviations from intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Ardito et al[45] | Low | Low | Low | Low | Low | Low | Low | Low |
| Kobayashi et al[36] | Low | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Okabayashi et al[33] | Low | Low | Low | Low | Low | Low | Low | Low |
| Shirabe et al[37] | Low | Low | Low | Low | Low | Low | Low | Low |
The sixteen studies comprised a total cohort of 1389 patients. 645 patients were randomized into the intervention group, consisting of various perioperative regimens of BCAA supplementation, and 744 patients into the control group. The total sample mean age is 60.5 years, intervention mean age is 62.3 years, control mean age is 58.9 years, and the total sample male is 78.7%. Table 2 summarises the baseline data of included studies. The subsequent tables outline surgical details (Table 3) and the presence and severity of comorbid liver diseases (Table 4).
| Ref. | Country/year | Study design | Sample size | Gender (M/F) | Male (%) | Age (yr) | Pathology | |||||||
| Intervention | Control | Total | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Beppu et al[38] | Japan, 2015 | RCT | 13 | 15 | 28 | 9/4 | 10/5 | 69.2 | 66.7 | 64.7 (30.0) | 68.4 (18.0) | Metastasis | 1 | 2 |
| HCC | 11 | 10 | ||||||||||||
| Cholangiocarcinoma | 1 | 3 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Fan et al[43] | Hong Kong, 1994 | RCT | 64 | 60 | 124 | 56/9 | 53/7 | 87.5 | 88.3 | 51 (33.4) | 53 (35) | Metastasis | 0 | 0 |
| HCC | 64 | 60 | ||||||||||||
| Cholangiocarcinoma | 0 | 0 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Hachiya et al[39] | Japan, 2020 | RCT | 74 | 80 | 154 | 59/15 | 66/14 | 79.7 | 82.5 | NR | NR | Metastasis | 0 | 0 |
| HCC | 74 | 80 | ||||||||||||
| Cholangiocarcinoma | 0 | 0 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Ichikawa et al[40] | Japan, 2013 | RCT | 26 | 30 | 56 | 20/6 | 18/12 | 76.9 | 60 | 64.5 (11.4) | 64.7 (9.8) | Metastasis | 0 | 0 |
| HCC | 26 | 30 | ||||||||||||
| Cholangiocarcinoma | 0 | 0 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Ishikawa et al[30] | Japan, 2010 | RCT | 10 | 10 | 20 | NR | NR | NR | NR | NR | NR | Metastasis | 2 | 1 |
| HCC | 7 | 7 | ||||||||||||
| Cholangiocarcinoma | 1 | 2 | ||||||||||||
| Others1 | 1 | 3 | ||||||||||||
| Kikuchi et al[41] | Japan, 2016 | RCT | 39 | 38 | 77 | 31/8 | 29/9 | 79.5 | 76.3 | 69.4 (7.5) | 71.9 (7.4) | Metastasis | 0 | 0 |
| HCC | 39 | 38 | ||||||||||||
| Cholangiocarcinoma | 0 | 0 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Krapf et al[44] | Austria, 2021 | RCT | 12 | 9 | 21 | NR | NR | NR | NR | NR | NR | Metastasis | NR | NR |
| HCC | NR | NR | ||||||||||||
| Cholangiocarcinoma | NR | NR | ||||||||||||
| Others | NR | NR | ||||||||||||
| Meng et al[31] | Hong Kong, 1999 | RCT | 21 | 23 | 44 | 19/2 | 18/5 | 90.5 | 78.3 | 51.5 (10.8) | 53.3 (12.8) | Metastasis | 0 | 0 |
| HCC | 21 | 23 | ||||||||||||
| Cholangiocarcinoma | 0 | 0 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Nagasue et al[32] | Japan, 1997 | RCT | 67 | 65 | 132 | 54/13 | 55/10 | 80.6 | 84.6 | NR | NR | Metastasis | NR | NR |
| HCC | NR | NR | ||||||||||||
| Cholangiocarcinoma | NR | NR | ||||||||||||
| Others | NR | NR | ||||||||||||
| Okabayashi et al[33] | Japan, 2010 | RCT | 13 | 13 | 26 | 9/4 | 8/5 | 69.2 | 61.5 | 68.2 (11.0) | 63.5 (5.7) | Metastasis | 0 | 0 |
| HCC | 8 | 7 | ||||||||||||
| Adenocarcinoma | 5 | 6 | ||||||||||||
| Cholangiocarcinoma | 0 | 0 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Okabayashi et al[34] | Japan, 2011 | RCT | 40 | 36 | 76 | 29/11 | 24/12 | 72.5 | 66.7 | 68.7 (7.6) | 65.1 (11.3) | Metastasis | 0 | 0 |
| HCC | 32 | 26 | ||||||||||||
| Cholangiocarcinoma | 8 | 10 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Togo et al[42] | Japan, 2005 | RCT | 21 | 22 | 43 | 17/5 | 17/6 | 81 | 77.3 | 66.5 (4.5) | 64.3 (9.1) | Metastasis | NR | NR |
| HCC | NR | NR | ||||||||||||
| Cholangiocarcinoma | NR | NR | ||||||||||||
| Others | NR | NR | ||||||||||||
| Ardito et al[45] | Japan, 2020 | Retrospective | 107 | 205 | 312 | NR | NR | NA | NA | NR | NR | Metastasis | 83 | 163 |
| Cohort | HCC | 18 | 36 | |||||||||||
| Cholangiocarcinoma | 6 | 6 | ||||||||||||
| Others2 | 6 | 48 | ||||||||||||
| Okabayashi et al[35] | Japan, 2008 | Retrospective | 40 | 72 | 112 | 29/11 | 55/17 | 72.5 | 76.4 | 65.7 (8.6) | 68.3 (8.1) | Metastasis | 0 | 0 |
| Cohort | HCC | 40 | 72 | |||||||||||
| Cholangiocarcinoma | 0 | 0 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Shirabe et al[37] | Japan, 2011 | Retrospective | 72 | 56 | 128 | NR | NR | NR | NR | NR | NR | Metastasis | NR | NR |
| Cohort | HCC | 72 | 56 | |||||||||||
| Cholangiocarcinoma | NR | NR | ||||||||||||
| Others | NR | NR | ||||||||||||
| Kobayashi et al[36] | Japan, 2019 | Non- randomised trial | 26 | 10 | 36 | 21/5 | 5/5 | 80.8 | 50 | 69.2 (29.0) | 64.8 (26.7) | Metastasis | 4 | 2 |
| HCC | 22 | 8 | ||||||||||||
| Cholangiocarcinoma | 0 | 0 | ||||||||||||
| Others | 0 | 0 | ||||||||||||
| Ref. | Surgical method | Intervention | Control | Blood loss/mL1 | ||
| Intervention | Control | |||||
| Ardito et al[45], 2020 | Hepatectomy | Minimally invasive liver surgery | 33 | 74 | NR | NR |
| Major hepatic resection | 16 | 62 | ||||
| Multiple resections | 43 | 44 | ||||
| Total | 92 | 180 | ||||
| Beppu et al[38], 2015 | Hepatectomy | Right hemihepatectomy | 8 | 7 | NR | NR |
| Left hemihepatectomy | 0 | 1 | ||||
| Sectionectomy | 1 | 3 | ||||
| Left hemihepatectomy + sectionectomy | 0 | 1 | ||||
| Total | 9 | 12 | ||||
| Fan et al[43], 1994 | Hepatectomy | Major hepatic resection | 47 | 42 | 2600 (300-20000) | 1900 (400-10500) |
| Minor hepatic resection | 17 | 18 | ||||
| Total | 64 | 60 | ||||
| Hachiya et al[39], 2020 | Hepatectomy | Non-anatomical resections | 19 | 25 | 348 (5-3400) | 359 (5-3741) |
| Anatomical resections | 55 | 55 | ||||
| Total | 74 | 80 | ||||
| Ichikawa et al[40], 2013 | Hepatectomy | Major hepatic resection | 10 | 14 | 716 +/- 704 | 492 +/- 329 |
| Limited resection of the liver | 16 | 16 | ||||
| Total | 26 | 30 | ||||
| Ishikawa et al[30], 2010 | Hepatectomy | Major hepatic resection | 7 | 5 | 802.7 +/- 350.6 | 838.8 +/- 597.6 |
| Minor hepatic resection | 6 | 8 | ||||
| Total | 13 | 13 | ||||
| Kikuchi et al[41], 2016 | Hepatectomy | Partial hepatectomy | 13 | 12 | 665.7 +/- 528.9 | 578.5 +/- 492.1 |
| Segmentectomy | 0 | 6 | ||||
| Bisgmentectomy/sectionectomy | 13 | 10 | ||||
| Bisectionectomy or more | 13 | 10 | ||||
| Total | 39 | 38 | ||||
| Kobayashi et al[36], 2019 | Hepatectomy | Major hepatic resection | 4 | 0 | 454 (140-5103) | 365 (35-3650) |
| Minor hepatic resection | 22 | 10 | ||||
| Total | 26 | 10 | ||||
| Krapf et al[44], 2021 | Liver transplant- Hepatectomy | Major hepatic resection | 24 | 12 | NR | NR |
| Hepatic resection | 9 | 12 | ||||
| Total | 33 | 24 | ||||
| Meng et al[31], 1999 | Hepatectomy | Major hepatic resection | 13 | 18 | NR | NR |
| Minor hepatic resection | 8 | 5 | ||||
| Total | 21 | 23 | ||||
| Nagasue et al[32], 1997 | Hepatectomy | Major hepatic resection | 19 | 26 | NR | NR |
| Minor hepatic resection | 48 | 39 | ||||
| Total | 67 | 65 | ||||
| Okabayashi et al[35], 2008 | Hepatectomy | Major hepatic resection | 10 | 19 | 516 +/- 354 | 821 +/- 552 |
| Minor hepatic resection | 30 | 53 | ||||
| Total | 40 | 72 | ||||
| Okabayashi et al[33], 2010 | Hepatectomy | Major hepatic resection | 5 | 7 | 1252 +/- 1205 | 669 +/- 575 |
| Minor hepatic resection | 8 | 6 | ||||
| Total | 13 | 13 | ||||
| Okabayashi et al[34], 2011 | Hepatectomy | Hemihepatectomy | 4 | 4 | 945 +/- 827 | 676 +/- 695 |
| Segmentectomy | 12 | 10 | ||||
| Limited hepatic resection | 24 | 22 | ||||
| Total | 40 | 36 | ||||
| Shirabe et al[37], 2011 | Liver transplant | Total | 129 | 107 | 7388 +/- 9031 | 6448 +/- 6547 |
| Togo et al[42], 2005 | Hepatectomy | Partial hepatectomy | 8 | 8 | 1163 +/- 853 | 1209 +/- 872 |
| Segmentectomy | 7 | 8 | ||||
| Sectionectomy | 4 | 3 | ||||
| Hemihepatectomy | 2 | 3 | ||||
| Total | 21 | 22 | ||||
| Ref. | Hepatitis | Cirrhosis | Child-Pugh Score | ||||
| Intervention | Control | Intervention | Control | Intervention | Control | ||
| Ardito et al[45], 2020 | NR | NR | NR | NR | A | NR | NR |
| B | NR | NR | |||||
| C | NR | NR | |||||
| Beppu et al[38], 20151 | 9 | 8 | NR | NR | A | NR | NR |
| B | NR | NR | |||||
| C | NR | NR | |||||
| Fan et al[43], 1994 | 18 | 12 | 39 | 33 | A | NR | NR |
| B | NR | NR | |||||
| C | NR | NR | |||||
| Hachiya et al[39], 2020 | 74 | 80 | NR | NR | A | 61 | 64 |
| B | 13 | 16 | |||||
| C | 0 | 0 | |||||
| Ichikawa et al[40], 2013 | 10 | 7 | 16 | 23 | A | 21 | 25 |
| B | 5 | 5 | |||||
| C | 0 | 0 | |||||
| Ishikawa et al[30], 20101 | 4 | 2 | 5 | 5 | A | 10 | 12 |
| B | 1 | 1 | |||||
| C | 0 | 0 | |||||
| Kikuchi et al[41], 2016 | 39 | 38 | NR | NR | A | 39 | 38 |
| B | 0 | 0 | |||||
| C | 0 | 0 | |||||
| Kobayashi et al[36], 20191 | 20 | 6 | 12 | 8 | A | NR | NR |
| B | NR | NR | |||||
| C | NR | NR | |||||
| Krapf et al[44], 2021 | NR | NR | NR | NR | A | NR | NR |
| B | NR | NR | |||||
| C | NR | NR | |||||
| Meng et al[31], 1999 | 18 | 19 | 15 | 15 | A | 17 | 20 |
| B | 4 | 3 | |||||
| C | 0 | 0 | |||||
| Nagasue et al[32], 1997 | 21 | 10 | 46 | 53 | A | 53 | 50 |
| B | 13 | 14 | |||||
| C | 1 | 1 | |||||
| Okabayashi et al[35], 2008 | 33 | 54 | NR | NR | A | 33 | 62 |
| B | 7 | 10 | |||||
| C | 0 | 0 | |||||
| Okabayashi et al[33], 2010 | 8 | 7 | NR | NR | A | 10 | 11 |
| B | 3 | 2 | |||||
| C | 0 | 0 | |||||
| Okabayashi et al[34], 20111 | 22 | 16 | NR | NR | A | 28 | 25 |
| B | 12 | 10 | |||||
| C | 0 | 0 | |||||
| Shirabe et al[37], 2011 | NR | NR | 26 | 18 | A | 3 | 18 |
| B | 36 | 39 | |||||
| C | 90 | 50 | |||||
| Togo et al[42], 2005 | 21 | 22 | 21 | 22 | A | 15 | 17 |
| B | 7 | 5 | |||||
| C | 0 | 0 | |||||
The BCAA supplementation used were mainly Aminoleban EN (Ajinomoto Pharma, Tokyo, Japan)[30-37] and Livact (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan)[37-42]. The compositions of Aminoleban EN and Livact are compared in Supplementary Table 4. Three studies used generic BCAA supplements[43-45]. The BCAA supplements were administered perioperatively in varying dose and duration, while patients in the control groups did not receive BCAA supplements for the same specified duration. Table 5 summarises the intervention and control protocol of all included studies. Patients were not blinded due to the lack of suitable placebos that share similar taste to the BCAA supplements.
| Ref. | Intervention type | Intervention protocol1 | Control protocol | Follow up period2 | ||||||||
| Pre-operative regime | Total BCAA received (g)/d | Duration | Post-operative regime | Total BCAA received (g)/d | Duration | Intervention | Control | |||||
| Mean | Range | Mean | Range | |||||||||
| Ardito et al[45], 2020 | Preoperative, postoperative | BCAA 500 mg 2 tablets TDS with personalised diet (ERAS) | 3 | 2/52 | BCAA 500 mg 2 tablets TDS (ERAS) | 3 | 1/12 | Normal usual diet (ERAS) | NR | NR | NR | NR |
| Beppu et al[38], 2015 | Preoperative, postoperative | Livact 4.15 g BD | 8 | 6/12 | NIL | NIL | NIL | Normal usual diet | NR | NR | NR | NR |
| Fan et al[43], 1994 | Preoperative, postoperative | BCAA 1.5 g/kg | Weight dependent | 1/52 | BCAA 1.5 g/kg | Weight dependent | 1/52 | Normal usual diet | NR | NR | NR | NR |
| Hachiya et al[39], 2020 | Postoperative | NIL | NIL | NIL | Livact 4 g TDS | 12 | 4 yr | Normal usual diet | 21.8 | 1.2-48 | NR | NR |
| Ichikawa et al[40], 2013 | Preoperative, postoperative | Livact 4.74 g TDS | 12 | 2/52 | Livact 4.74 g TDS | 12 | ≥ 6/12 | Normal usual diet | 39.5 mo | 7-48 mo | 36.0 mo | 6-50 mo |
| Ishikawa et al[30], 2010 | Preoperative, postoperative | Aminoleban EN 50 g BD | 11.123 | 2/52 | Aminoleban EN 50 g BD | 11.123 | 1/52 | Normal usual diet | NR | NR | NR | NR |
| Kikuchi et al[41], 2016 | Preoperative, postoperative | Livact 4.74 g TDS | 12 | 1/12 | Livact 4.74 g TDS | 12 | 1 yr | Normal usual diet (35-40 kcal/kg/d) + 4.74 g Livact TDS × 1 yr post-operatively | NR | NR | NR | NR |
| Kobayashi et al[36], 2019 | Preoperative, postoperative | Aminoleban EN 50 g ON | 5.5615 | 2/52 | Aminoleban EN ON | 5.5615 | 12/52 | Normal usual diet | NR | NR | NR | NR |
| Krapf et al[44], 2021 | Postoperative | NIL | NIL | NIL | High BCAA diet | NA | 2/52 | Normal usual diet (standard isocaloric meal plan) | NR | NR | NR | NR |
| Meng et al[31], 1999 | Postoperative | NIL | NIL | NIL | Aminoleban EN TDS with 40 g protein/d + 6300 kJ/d | NA | 12/52 | Normal usual diet (80 g protein/d + 6300 kJ/d) | 511.6d | 6-982 d | 512.7d | 48-983 d |
| Nagasue et al[32], 1997 | Postoperative | NIL | NIL | 2/52 | Aminoleban EN 50 g BD | 11.123 | ≥ 1 yr | Normal usual diet | 35.8 mo (17.9) | NR | 36.0 mo (17.7) | NR |
| Okabayashi et al[35], 2008 | Preoperative | Aminoleban EN 50 g BD | 11.123 | 2/52 | NIL | NIL | NIL | Normal usual diet | 16.3 mo | 2-47 mo | 23.3 mo | 2-84 mo |
| Okabayashi et al[33], 2010 | Preoperative | Aminoleban EN 50 g BD | 11.123 | 2/52 | NIL | NIL | NIL | Normal usual diet | NR | NR | NR | NR |
| Okabayashi et al[34], 2011 | Preoperative, postoperative | Aminoleban EN 50 g BD | 11.123 | 2/52 | Aminoleban EN 50 g BD | 11.123 | ≥ 6/12 | Normal usual diet | NR | NR | NR | NR |
| Shirabe et al[37], 2011 | Preoperative | Regime 1: Livact 3 packets OD Regime 2: Aminoleban EN 50 g 1 to 3 packets | Regime 1: 12Regime 2: 5.5615-16.6845 | > 1/12 | NIL | NIL | NIL | Normal usual diet | NR | NR | NR | NR |
| Togo et al[42], 2005 | Post-operative | NIL | NIL | NIL | Livact 4.74 g TDS | 12 | 1 yr | Normal usual diet | NR | NR | NR | NR |
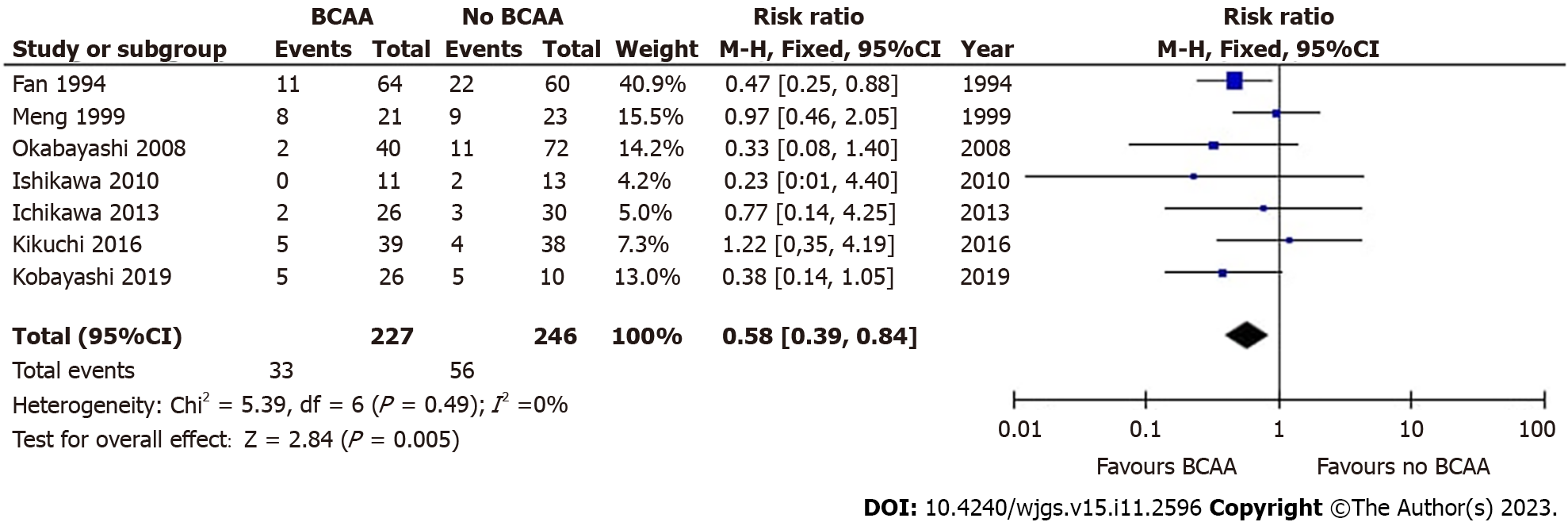
Seven out of sixteen studies[30,31,35,36,40,41,43] including 473 patients (227 in the BCAA group and 246 in the control group) reported data on postoperative infections. There was low statistical heterogeneity between studies (I2 = 0%). Postoperative infections were found to be significantly lower in the BCAA group [risk ratio (RR) = 0.58 (95%CI: 0.39 to 0.84), P = 0.005] (Figure 3).
In a study involving BCAA supplementation in liver transplant patients, Shirabe et al[37] discovered that normal usual diet without BCAA supplementation significantly increased the risk of postoperative bacteraemia [OR = 4.32 (95%CI: 1.137 to 16.483), P = 0.031]. Although more than half of the study population had liver cancer, the study was not included in the meta-analysis as it was unclear if the effect of BCAA supplementation was generalisable to liver cancer patients.
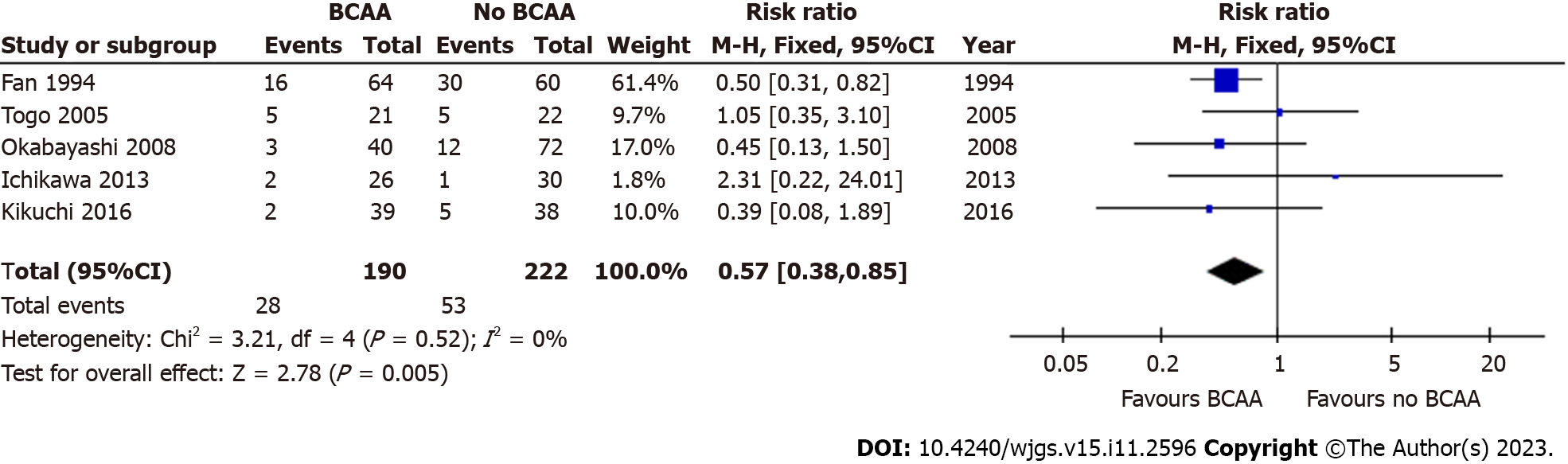
Five out of sixteen studies[35,40-43] including 412 patients (190 in the BCAA group and 222 in the control group) reported data on postoperative ascites. There was low statistical heterogeneity between studies (I2 = 0%). Postoperative ascites was found to be significantly lower in the BCAA group [RR = 0.57 (95%CI: 0.38 to 0.85), P = 0.005] (Figure 4). Additionally, Kikuchi et al[41] reported that the incidence of refractory ascites and/or pleural effusion in the BCAA group was significantly lower than in the non-BCAA group (P = 0.047).
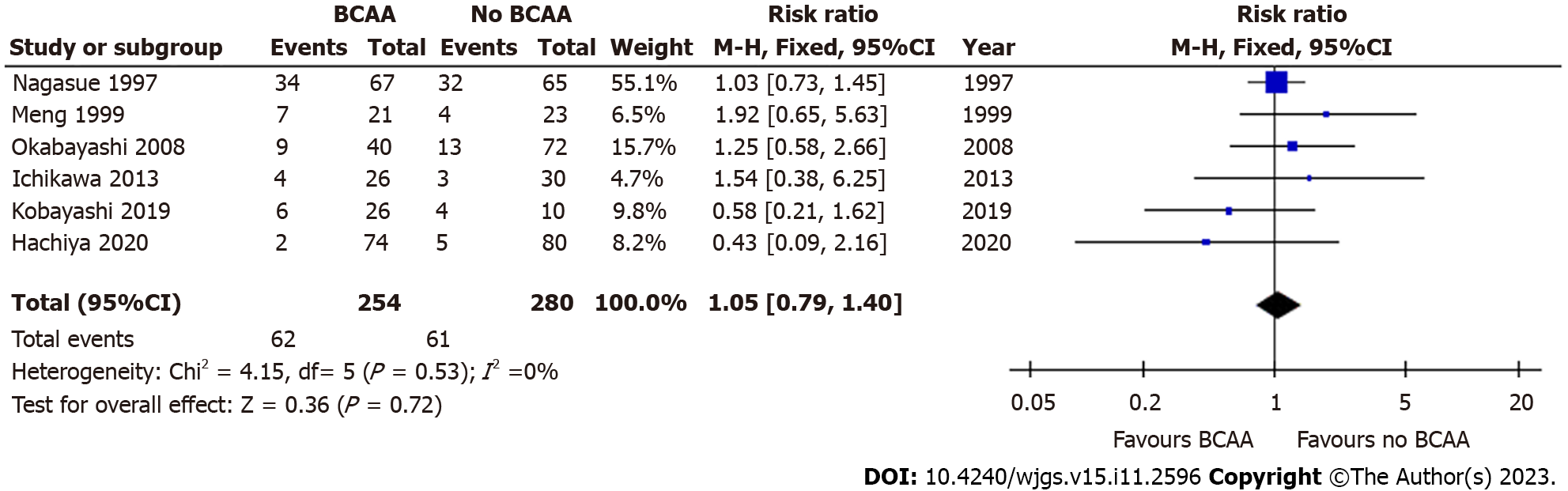
Six out of sixteen studies[31,32,35,36,39,40] including 534 patients (254 in the BCAA group and 280 in the control group) reported data on mortality due to all causes (> 3 years follow-up). Follow-up periods were between 3-4 years for most included studies, and 3.5-6 years for one study. There was low statistical heterogeneity between included studies (I2 = 14%). There was no evidence of significant difference between the BCAA and control groups for all-cause mortality [RR = 1.05 (95%CI: 0.79 to 1.40), P = 0.72] (Figure 5). A separate analysis did not find any significant difference in 90-day mortality between the BCAA and control group [RR = 1.69 (95%CI: 0.23 to 12.24), P = 0.60] (Supplementary Figure 2).
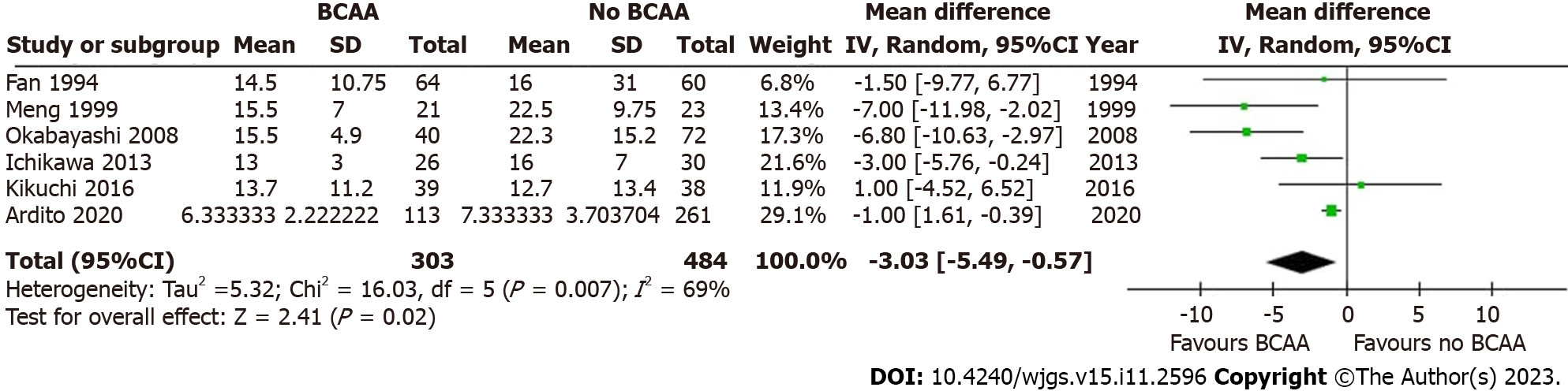
Six out of sixteen studies[31,35,40,41,43,45] including 787 patients (303 in the BCAA group and 484 in the control group) reported LOS data. There was considerable statistical heterogeneity between studies (I2 = 69%), and a random effects model was employed. LOS was reduced by 3.03 d in the BCAA group compared to controls [weighted mean difference (WMD) = -3.03 d (95%CI: -5.49 to -0.57), P = 0.02] (Figure 6).
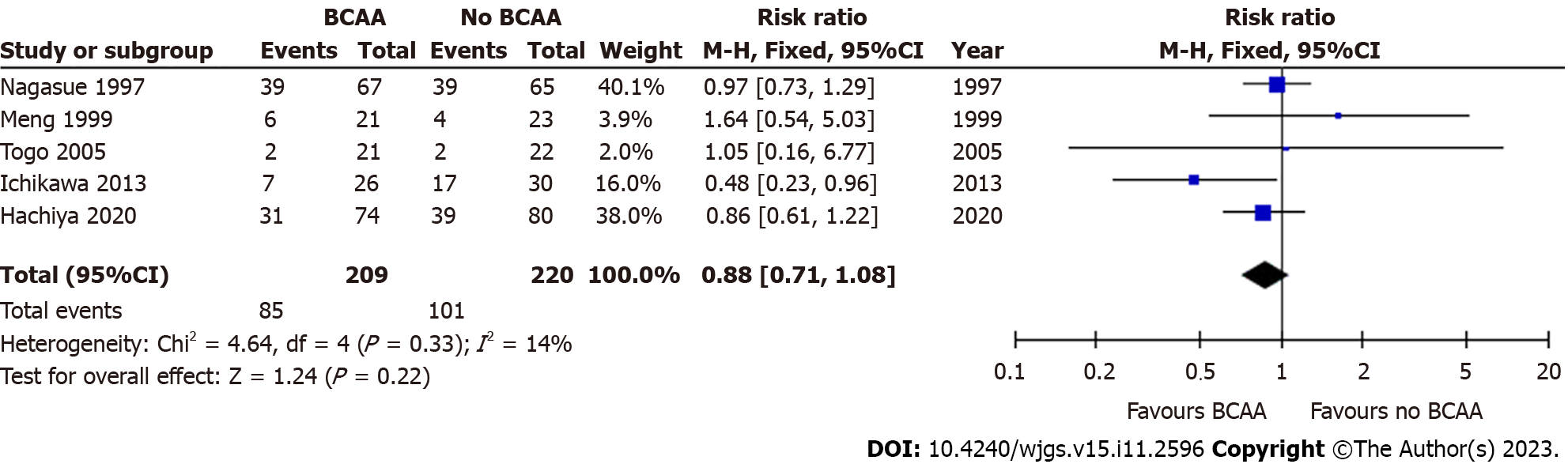
Five out of sixteen studies [31,32,39,40,42] including 429 patients (209 in the BCAA group and 220 in the control group) reported data on cancer recurrence. Median follow-up period varied from 12 to 30 mo. One author reported recurrence but was not included in analysis as recurrence was not an end point in the original study[34]. A subgroup analysis in Hachiya et al[39] of patients under 72 years of age with haemoglobin A1C levels below 6.4% revealed that recurrence-free survival was higher in the BCAA group compared to the control group (P = 0.015). There was low statistical heterogeneity between included studies (I2 = 0%). There was no statistically significant difference between the BCAA and control groups for recurrence [RR = 0.88 (95%CI: 0.71 to 1.08), P = 0.22] (Figure 7).
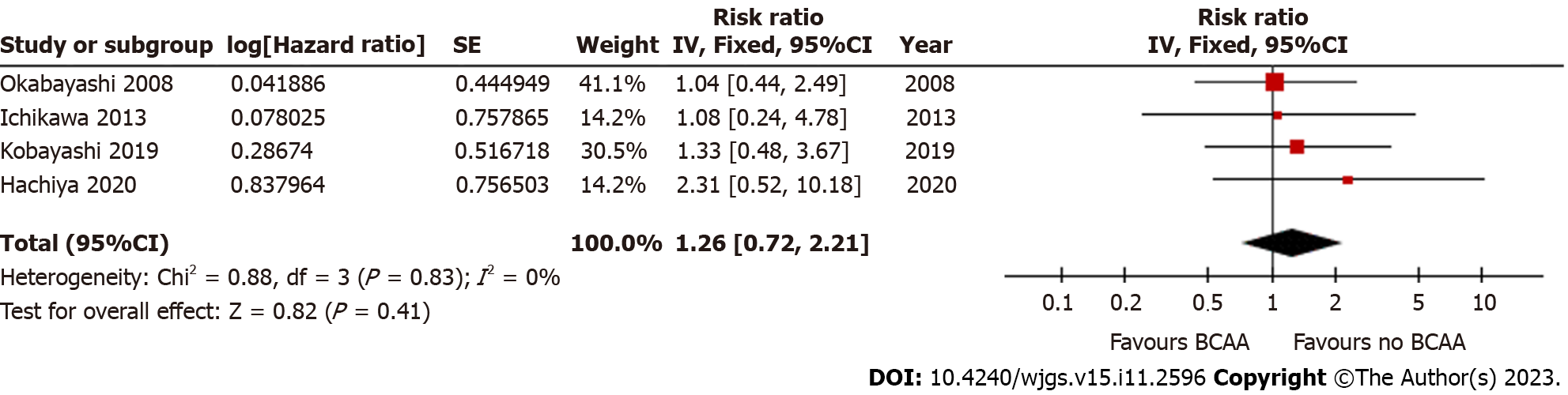
Four out of sixteen studies[35,36,39,40] reported OS data. There was low statistical heterogeneity (I2 = 0%). OS did not differ significantly between the BCAA and control groups [hazard ratio = 1.26 (95%CI: 0.72 to 2.21), P = 0.41] (Figure 8).
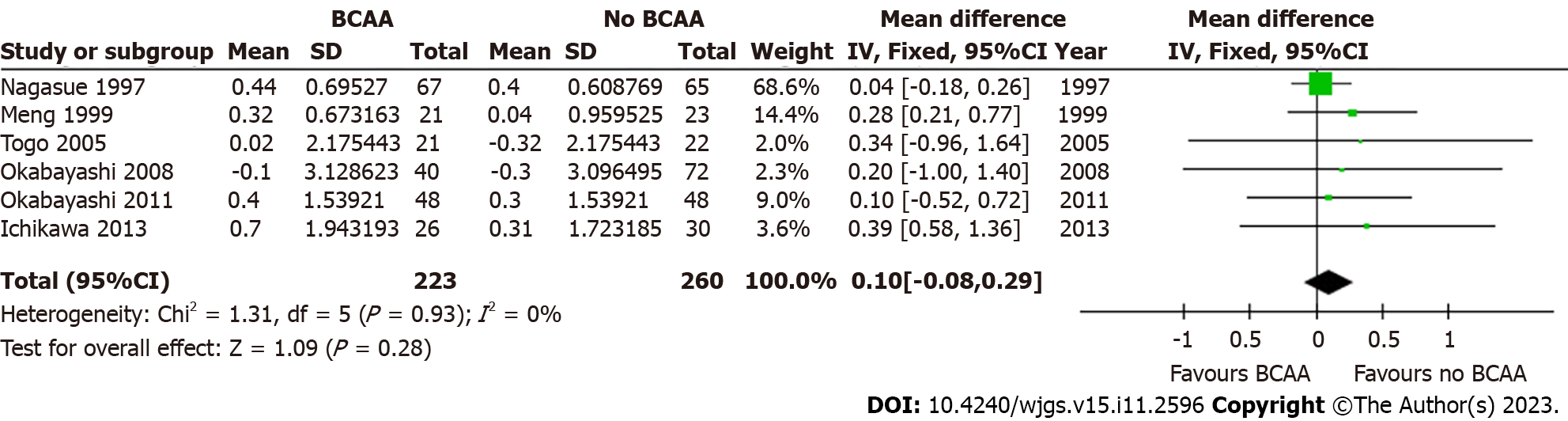
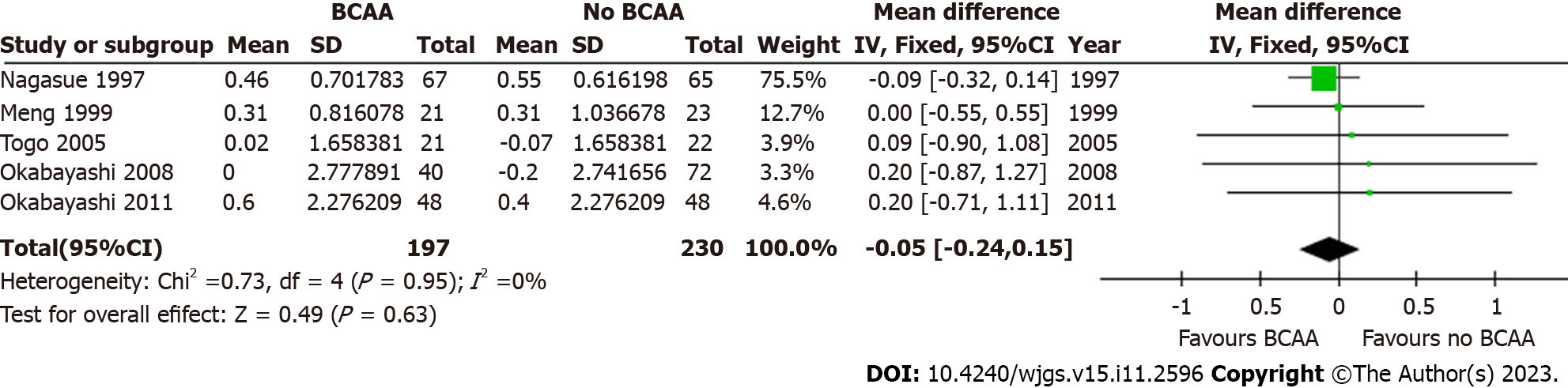
Five out of sixteen studies[31,32,34,35,42] including 427 patients (197 in the BCAA group and 230 in the control group) reported data on change in serum albumin. There was low statistical heterogeneity (I2 = 0%). While individual studies reported faster albumin increase in the intervention group compared to the control group[22], change in serum albumin was not significant at 6 mo [WMD = 0.10 (95%CI: -0.08 to 0.29), P = 0.28] and 12 mo [WMD = -0.05 (95%CI: -0.24 to 0.15), P = 0.63] (Figures 9 and 10).
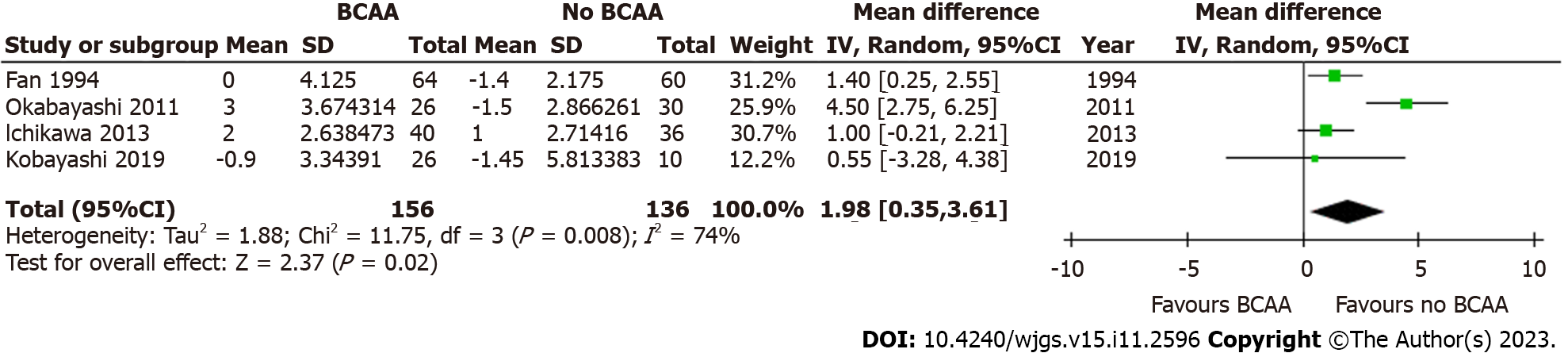
Four out of sixteen studies[34,36,40,43] including 292 patients (156 in the BCAA group and 136 in the control group) reported data on postoperative body weight change. There was considerable heterogeneity between studies (I2 = 74%), and a random effects model was employed. Postoperative body weight increased significantly by 1.98 kg in the BCAA group [WMD = 1.98 kg (95%CI: 0.35 to 3.61), P = 0.02] (Figure 11).
Three out of sixteen studies[32,34,44] reported data on QOL measures. Krapf et al[44] used the European Organisation for Research and Treatment of Cancer QOL Questionnaire Core 30 (EORTC QLQ-C30) to look at physical, psychological, and social functions across 30 questions, and found that there was no significant difference between study groups. However, the study authors noted that patients in the BCAA group had a greater amount of food intake and significantly better subjective rating of the meals.
Okabayashi et al[34] using the short-form 36 (SF-36) health sheet reported significant improvement in all 8 parameters (physical functioning, role physical, bodily pain, general health perceptions, vitality, social functioning, role emotional and mental health) in the BCAA group at the 12-month follow-up while the control group shown no significant differences in postoperative QOL.
Nagasue et al[32] used the Kanovsky scale to evaluate performance status and reported that the percentage change from baseline to the 12 mo follow-up was significantly higher in the BCAA group.
It is worth nothing that two of the sixteen studies included in this review described unique outcomes of BCAA supplementation. Beppu et al[38] found that patients undergoing portal vein embolisation (PVE) and subsequent major hepatectomy had significantly greater functional liver regeneration after PVE (P = 0.079) and better postoperative outcomes in the BCAA group compared to controls. A 2010 study by Okabayashi et al[33] concluded that BCAA supplementation resulted in significantly reduced immediate postoperative insulin resistance (P = 0.039), improved blood glucose levels and decreased need for insulin therapy in liver cancer patients.
This study shows perioperative BCAAs supplementation increases body weight, reduces infection, LOS and ascites in cancer patients undergoing liver surgery.
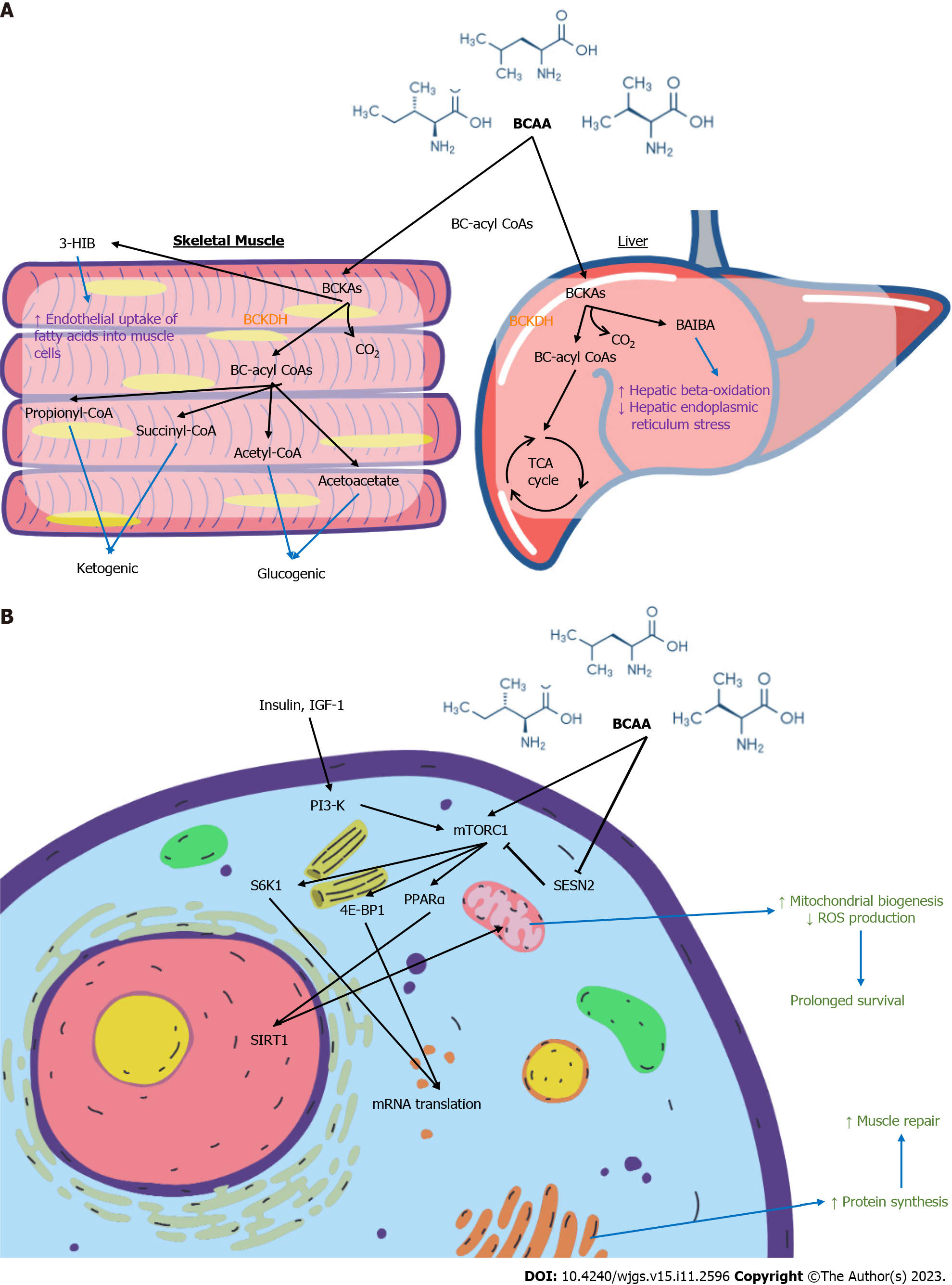
BCAAs are essential amino acids and contribute to protein synthesis, act as precursors of the tricarboxylic acid cycle intermediates and are involved in key signalling pathways[46-48]. Figure 12A summarises the molecular effects of BCAA on skeletal muscle and liver. Figure 12B summarises the cellular signalling pathways involving BCAA with downstream effects. Leu, the most abundant amino acid, plays a major role in protein synthesis and cellular growth. It promotes mammalian target of rapamycin (mTOR) pathway activation by binding to Sestrin2, preventing the latter from inhibiting mTOR complex 1 activity[49]. This contributes to downstream phosphorylation of ribosomal proteins and upregulates mRNA translation[50]. Val catabolites also serve signalling functions, particularly 3-hydroxyisobutyrate (3-HIB) and beta-amino-isobutyric acid (BAIBA). 3-HIB promotes the endothelial uptake of fatty acids into muscle, acting as an intermediary between protein and lipid metabolism[51]. BAIBA promotes hepatic beta-oxidation and reduce hepatic endoplasmic reticulum stress[52]. Ile is less well-studied. Some authors have postulated its immune modulating role in inducing the expression of host defence peptides and improving innate immunity by maintaining skin mucus barrier[53,54].
Over 85% of liver cancer patients are cachectic[55], resulting from malignancy and protein energy malnutrition on a background of cirrhosis[56]. Liver surgeries are often associated with ischemia-reperfusion periods. Poor nutritional status further exacerbates ischemic injury by accelerating glycolysis and rapidly depleting adenosine triphosphate, leading to irreversible cellular necrosis[57-59]. BCAA supplementation is well-established in liver disease. The European Society for Clinical Nutrition and Metabolism[60] recommend long-term oral BCAA supplements (0.25 g × kg-1 × d-1) in patients with advanced cirrhosis to improve event-free survival or QOL. The American Association for the Study of Liver[61] recommend BCAA administration as an alternative or additional agent to treat hepatic encephalopathy in patients who are unresponsive to conventional therapy. However, the evidence of benefit for BCAAs in liver cancer patients managed by surgical approaches is less clear.
Despite many studies reporting a strong association between malnutrition and poor surgical outcomes, oncological patients often lack opportune time to delay treatment for nutritional optimisation. In a study of nutrition and HCC, Huang et al[62] noted that patients assessed by dietitians to be malnourished had a significantly higher rate of major complications[63]. With an increasing focus on optimisation of postoperative surgical recovery, enhanced recovery after surgery protocols and prehabilitation initiatives have also emphasised the importance of perioperative nutrition[64].
This review investigated perioperative and oncological outcomes of BCAA supplementation in patients undergoing surgery for liver cancers unlike the previous review that included diverse oncological diagnoses[22]. We demonstrated that BCAA supplementation significantly reduced postoperative complications, with over 40% relative risk reduction of postoperative infection and ascites. The BCAA group also had slightly higher body weight and performed better on both perceived and actual QOL metrics, suggesting that BCAA intake can optimise recovery and function after surgery.
BCAA supplementation had no impact on cancer recurrence and OS. This is consistent with the conclusions of recent meta-analyses[22,65]. The relationship between BCAA and liver cancer at the molecular level has been explored[66-68]. BCAAs suppress the development of liver cancers in rodent models[69,70], presumably improving insulin resistance in obesity or diabetes mellitus. Insulin resistance is involved in the pathogenesis of HCC, as insulin has oncogenic properties on HCC cells, stimulating cell growth and inducing anti-apoptotic activity[71,72]. Another study postulated suppression of VEGF expression in tumour cells as an alternative mechanism[73]. The catabolism of BCAAs has also been extensively implicated in carcinogenesis[67] through various molecular pathways, including accumulation of branched-chain α-ketoacids and activation of the mTORC1 pathway[74,75]. Ericksen et al[76] validated oncogenic pathways and linked high dietary BCAA intake to tumour burden and mortality specifically in HCC patients.
Given the apparent contradictory effects of BCAA on liver cancer development and prognosis, it may be challenging to interpret our findings. BCAAs may potentially improve liver function and body weight postoperatively but also contribute to tumour recurrence via the above-mentioned pathways. The beneficial oncological effects of BCAA supplementation remain inconclusive.
While oncological outcomes do not support routine use of BCAA supplementation in the perioperative period, our review found that the risk of postoperative infection and ascites were significantly lowered in BCAA groups.
Postoperative infection is a frequent complication of hepatic resection, with reported rates of up to 25%[77,78]. It can be associated with significant morbidity in the absence of early recognition and treatment[79]. After major liver surgery, impairment of innate immune function increases host susceptibility to infection[80]. Furthermore, surgery in HCC patients results in higher risk of infectious sequelae, presumably due to underlying cirrhosis and chronic liver dysfunction, bile leak with risk of abdominal sepsis, and postoperative pneumonia due to upper abdominal incision[81]. Another major risk factor for infection is malnutrition[82-85]. BCAA supplementation directly improves patients’ preoperative nutritional status[86]. BCAAs also play an essential role in immune cell function relating to protein synthesis[87], and in immune regulation in patients with advanced cirrhosis[88-90].
Postoperative ascites is reported in 5% to 56% patients undergoing hepatectomy[91], and is associated with liver failure[92,93]. Chan et al[94] described higher 1-year mortality and lower recurrence-free survival rates attributed to postoperative ascites.
BCAAs increase the synthesis and secretion of albumin by hepatocytes[95], and improve impaired metabolic turnover of albumin in cirrhotic patients[96]. Fukushima et al[97] reported that BCAAs effectively improved the oxidisation/reduction imbalance of albumin in cirrhosis. In our study, BCAA supplementation did not significantly increase postoperative serum albumin levels. The underlying mechanisms behind the demonstrated efficacy of BCAA supplementation in reducing postoperative ascites remain uncertain.
2 out of 3 included studies[34,44] demonstrated significant improvement in QOL with BCAAs, but differing methodologies and domains for assessment were employed. Krapf et al[44] noted a significantly higher subjective rating of high BCAA content diet compared to normal usual diet, despite similar preparation methods and staff. If such an outcome is indeed replicated in future QOL studies, it may represent a potential benefit of BCAA supplementation in improving oral intake and avoiding malnutrition in post-surgical patients. These results are consistent with QOL improvements seen in RCTs on BCAA supplementation in cirrhosis[98,99].
Overall, more robust, large-scale clinical studies of surgical patients with liver cancer would need to be conducted with standardisation of total calorie and protein intake in intervention and control groups to conclude on the risk-benefit calculation of BCAA supplementation. Future studies should report BCAA to total protein intake ratio, protein to calorie ratio and type of BCAA to understand cause effect relation on perioperative outcomes of BCAA supplementation.
We reviewed side effects reported from routine BCAA ingestion in the included studies. Most studies did not report side effects with the exception that one RCT[43] reported 2 events (3%) relating to intravenous administration, while another[32] reported that 3 patients were unable to continue with BCAA administration due to adverse reactions. Overall, BCAAs have an extremely low incidence of side effects in the included studies.
The strengths of this study include data pooling from newer RCTs and multiple real-world observational studies. While RCTs are considered the gold standard for ascertaining the efficacy and safety of a treatment, their methodologies may limit generalizability. The inclusion of observational studies provides more generalizable results applicable in the real-world situation. Since both types of study design have their strengths and limitations, they provide insight into the efficacy of BCAA supplementation.
Though many Japanese studies were included, exclusion of non-English articles may have led to language biases as a potential confounding factor in our conclusions. The included studies had varying doses of BCAA supplementation and a lack of data on composition of normal usual diet in the participating institutions. Most of the patients in this review had Child-Pugh score A cirrhosis and thus might not be malnourished. Further, there is no population data to suggest that HCC patients have deficiency of BCAA. A lack of long-term follow-up data in the included studies makes it difficult to study the impact of BCAA supplementation on oncological outcomes.
Perioperative BCAA administration increases body weight and reduces postoperative infection, ascites, and LOS in liver cancer patients undergoing surgery.
Branched chain amino acids (BCAA) show promising results in improving surgical outcomes in liver cancer patients and potential for routine use.
Current studies on BCAA supplementation show varying results but with no clear conclusion and no updated reviews on the matter.
To provide the most updated review on whether BCAA supplementation provides measurable benefits in liver cancer patients for surgical intervention.
Current trials and studies on BCAA supplementation in liver cancer patients undergoing surgery were appraised by three independent authors. Studies were identified and data extracted for meta-analysis of the relevant outcomes.
Perioperative BCAA supplementation reduced postoperative infections, length of stay and increased body weight in the studied patient groups but did not improve mortality, oncological recurrence, and long-term survival.
This review has shown that BCAA supplementation improves postoperative outcomes with no significant side effects. However, benefits on oncological outcomes remain inconclusive.
This review highlights the possible routine use of BCAA for liver cancer patients for surgical intervention. Further clinical research can be directed at assessing optimal BCAA supplementation regime for such patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Singapore Medical Association, 12617I.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Goja S, India S-Editor: Qu XL L-Editor: A P-Editor: Wu RR
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3826] [Article Influence: 956.5] [Reference Citation Analysis (3)] |
| 2. | Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol. 2006;23:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Ahmed I, Lobo DN. Malignant tumours of the liver. Surgery (Oxford). 2009;27:30-37. [DOI] [Full Text] |
| 4. | Kasper HU, Drebber U, Dries V, Dienes HP. [Liver metastases: incidence and histogenesis]. Z Gastroenterol. 2005;43:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Wang ZG, He ZY, Chen YY, Gao H, Du XL. Incidence and survival outcomes of secondary liver cancer: a Surveillance Epidemiology and End Results database analysis. Transl Cancer Res. 2021;10:1273-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Sheriff S, Madhavan S, Lei GY, Chan YH, Junnarkar SP, Huey CW, Low JK, Shelat VG. Predictors of mortality within the first year post-hepatectomy for hepatocellular carcinoma. J Egypt Natl Canc Inst. 2022;34:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Lim MS, Goh GB, Chang JP, Low JK, Shelat VG, Huey TC, Dan YY, Kow A, Shridhar I, Tan PS, Junnarkar SP, Tan CK. A study of 3013 cases of hepatocellular carcinoma: Etiology and therapy before and during the current decade. JGH Open. 2021;5:1015-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Tsim NC, Frampton AE, Habib NA, Jiao LR. Surgical treatment for liver cancer. World J Gastroenterol. 2010;16:927-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Lei GY, Shen L, Junnarkar SP, Huey CT, Low J, Shelat VG. Predictors of 90-Day Mortality following Hepatic Resection for Hepatocellular Carcinoma. Visc Med. 2021;37:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. Transl Gastroenterol Hepatol. 2018;3:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Choudry HA, Pan M, Karinch AM, Souba WW. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J Nutr. 2006;136:314S-318S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Kim SJ, Kim DG, Lee MD. Effects of branched-chain amino acid infusions on liver regeneration and plasma amino acid patterns in partially hepatectomized rats. Hepatogastroenterology. 2011;58:1280-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Kuwahata M, Kubota H, Kanouchi H, Ito S, Ogawa A, Kobayashi Y, Kido Y. Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr Res. 2012;32:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Tomiya T, Omata M, Fujiwara K. Significance of branched chain amino acids as possible stimulators of hepatocyte growth factor. Biochem Biophys Res Commun. 2004;313:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Miuma S, Ichikawa T, Arima K, Takeshita S, Muraoka T, Matsuzaki T, Ootani M, Shibata H, Akiyama M, Ozawa E, Miyaaki H, Taura N, Takeshima F, Nakao K. Branched-chain amino acid deficiency stabilizes insulin-induced vascular endothelial growth factor mRNA in hepatocellular carcinoma cells. J Cell Biochem. 2012;113:3113-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Zheng HL, Lu J, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu R, Huang CM, Zheng CH. Effects of Preoperative Malnutrition on Short- and Long-Term Outcomes of Patients with Gastric Cancer: Can We Do Better? Ann Surg Oncol. 2017;24:3376-3385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Chan KS, Chia CLK, Ng FKL, Seow WHJ, Leong DY, Shelat VG. Impaired Handgrip Strength Does Not Predict Postoperative Morbidity in Major Hepatobiliary Surgery. J Surg Res. 2020;256:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lee B, Han HS, Yoon YS, Cho JY, Lee JS. Impact of preoperative malnutrition, based on albumin level and body mass index, on operative outcomes in patients with pancreatic head cancer. J Hepatobiliary Pancreat Sci. 2021;28:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 19. | Leide da Silva Nunes F, Calado Ferreira Pinheiro Gadelha P, Damasceno de Souza Costa M, Carolina Ribeiro de Amorim AC, Bezerra da Silva Mda G. Nutritional status and its impact on time and relocation in postoperative complications of abdominal patients undergoing surgery. Nutr Hosp. 2014;30:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Langer G, Großmann K, Fleischer S, Berg A, Grothues D, Wienke A, Behrens J, Fink A. Nutritional interventions for liver-transplanted patients. Cochrane Database Syst Rev. 2012;CD007605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Chen L, Chen Y, Wang X, Li H, Zhang H, Gong J, Shen S, Yin W, Hu H. Efficacy and safety of oral branched-chain amino acid supplementation in patients undergoing interventions for hepatocellular carcinoma: a meta-analysis. Nutr J. 2015;14:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Cogo E, Elsayed M, Liang V, Cooley K, Guerin C, Psihogios A, Papadogianis P. Are Supplemental Branched-Chain Amino Acids Beneficial During the Oncological Peri-Operative Period: A Systematic Review and Meta-Analysis. Integr Cancer Ther. 2021;20:1534735421997551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39563] [Article Influence: 9890.8] [Reference Citation Analysis (2)] |
| 24. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 14983] [Article Influence: 2497.2] [Reference Citation Analysis (0)] |
| 25. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10723] [Article Influence: 1191.4] [Reference Citation Analysis (2)] |
| 26. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Aug 2023. [cited August 2023]. Available from: https://training.cochrane.org/handbook. |
| 27. | Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1155] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 28. | Walter SD, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol. 2007;60:849-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Council for International Organizations of Medical Sciences (CIOMS). Evidence Synthesis and Meta-Analysis for Drug Safety: Report of CIOMS Working Group X. 2016;. [DOI] [Full Text] |
| 30. | Ishikawa Y, Yoshida H, Mamada Y, Taniai N, Matsumoto S, Bando K, Mizuguchi Y, Kakinuma D, Kanda T, Tajiri T. Prospective randomized controlled study of short-term perioperative oral nutrition with branched chain amino acids in patients undergoing liver surgery. Hepatogastroenterology. 2010;57:583-590. [PubMed] |
| 31. | Meng WC, Leung KL, Ho RL, Leung TW, Lau WY. Prospective randomized control study on the effect of branched-chain amino acids in patients with liver resection for hepatocellular carcinoma. Aust N Z J Surg. 1999;69:811-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Nagasue N, Yu-Chung C, Fukuda T, Hamazoe R, Handa Y, Hayashi T, Horisawa H, Kanamori H, Kawaguchi H, Kishi K, Kishimoto H, Kohno H, Kubota H, Kudo H, Kuratsuka H, Mishima I, Miyazaki Y, Nishimura O, Okita K, Takeuchi T, Taniguchi H, Yoshioka H, Yukaya H, Yumura M, Watanabe S. Long-term oral administration of branched chain amino acids after curative resection of hepatocellular carcinoma: a prospective randomized trial. The San-in Group of Liver Surgery. Br J Surg. 1997;84:1525-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Namikawa T, Maeda H, Yatabe T, Hanazaki K. Preoperative oral supplementation with carbohydrate and branched-chain amino acid-enriched nutrient improves insulin resistance in patients undergoing a hepatectomy: a randomized clinical trial using an artificial pancreas. Amino Acids. 2010;38:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Okabayashi T, Iyoki M, Sugimoto T, Kobayashi M, Hanazaki K. Oral supplementation with carbohydrate- and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids. 2011;40:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Okabayashi T, Nishimori I, Sugimoto T, Maeda H, Dabanaka K, Onishi S, Kobayashi M, Hanazaki K. Effects of branched-chain amino acids-enriched nutrient support for patients undergoing liver resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1869-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Kobayashi K, Kaneko J, Yamaguchi T, Kawaguchi Y, Arita J, Akamatsu N, Ishizawa T, Sekine R, Ijichi H, Kubota N, Fukatsu K, Kokudo N, Hasegawa K. Late-Evening Carbohydrate and Branched-Chain Amino Acid Snacks Improve the Nutritional Status of Patients Undergoing Hepatectomy Based on Bioelectrical Impedance Analysis of Body Composition. Gastrointest Tumors. 2019;6:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Shirabe K, Yoshimatsu M, Motomura T, Takeishi K, Toshima T, Muto J, Matono R, Taketomi A, Uchiyama H, Maehara Y. Beneficial effects of supplementation with branched-chain amino acids on postoperative bacteremia in living donor liver transplant recipients. Liver Transpl. 2011;17:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Beppu T, Nitta H, Hayashi H, Imai K, Okabe H, Nakagawa S, Hashimoto D, Chikamoto A, Ishiko T, Yoshida M, Yamashita Y, Baba H. Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: a randomized controlled trial. J Gastroenterol. 2015;50:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Hachiya H, Aoki T, Iso Y, Shimizu T, Tago K, Park KH, Sakuraoka Y, Shiraki T, Mori S, Kubota K. Effects of branched-chain amino acids on postoperative tumor recurrence in patients undergoing curative resection for hepatocellular carcinoma: A randomized clinical trial. J Hepatobiliary Pancreat Sci. 2020;27:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Ichikawa K, Okabayashi T, Maeda H, Namikawa T, Iiyama T, Sugimoto T, Kobayashi M, Mimura T, Hanazaki K. Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today. 2013;43:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Kikuchi Y, Hiroshima Y, Matsuo K, Kawaguchi D, Murakami T, Yabushita Y, Endo I, Taguri M, Koda K, Tanaka K. A Randomized Clinical Trial of Preoperative Administration of Branched-Chain Amino Acids to Prevent Postoperative Ascites in Patients with Liver Resection for Hepatocellular Carcinoma. Ann Surg Oncol. 2016;23:3727-3735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Togo S, Tanaka K, Morioka D, Sugita M, Ueda M, Miura Y, Kubota T, Nagano Y, Matsuo K, Endo I, Sekido H, Shimada H. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition. 2005;21:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 261] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Krapf J, Schuhbeck A, Wendel T, Fritz J, Scholl-Bürgi S, Bösmüller C, Oberhuber R, Margreiter C, Maglione M, Stättner S, Messner F, Berchtold V, Braunwarth E, Primavesi F, Cardini B, Resch T, Karall D, Öfner D, Margreiter R, Schneeberger S. Assessment of the Clinical Impact of a Liver-Specific, BCAA-Enriched Diet in Major Liver Surgery. Transplant Proc. 2021;53:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Ardito F, Lai Q, Rinninella E, Mimmo A, Vellone M, Panettieri E, Adducci E, Cintoni M, Mele MC, Gasbarrini A, Giuliante F. The impact of personalized nutritional support on postoperative outcome within the enhanced recovery after surgery (ERAS) program for liver resections: results from the NutriCatt protocol. Updates Surg. 2020;72:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Mattick JSA, Kamisoglu K, Ierapetritou MG, Androulakis IP, Berthiaume F. Branched-chain amino acid supplementation: impact on signaling and relevance to critical illness. Wiley Interdiscip Rev Syst Biol Med. 2013;5:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Matthews DE. Observations of branched-chain amino acid administration in humans. J Nutr. 2005;135:1580S-1584S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 756] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 49. | Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 925] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 50. | Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S-231S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 396] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 51. | Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL, Arany Z. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 52. | Shi CX, Zhao MX, Shu XD, Xiong XQ, Wang JJ, Gao XY, Chen Q, Li YH, Kang YM, Zhu GQ. β-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci Rep. 2016;6:21924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 53. | Yin L, Zhao Y, Zhou XQ, Yang C, Feng L, Liu Y, Jiang WD, Wu P, Zhou J, Zhao J, Jiang J. Effect of dietary isoleucine on skin mucus barrier and epithelial physical barrier functions of hybrid bagrid catfish Pelteobagrus vachelli × Leiocassis longirostris. Fish Physiol Biochem. 2020;46:1759-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Gu C, Mao X, Chen D, Yu B, Yang Q. Isoleucine Plays an Important Role for Maintaining Immune Function. Curr Protein Pept Sci. 2019;20:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 55. | Wie GA, Cho YA, Kim SY, Kim SM, Bae JM, Joung H. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition. 2010;26:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Stickel F, Inderbitzin D, Candinas D. Role of nutrition in liver transplantation for end-stage chronic liver disease. Nutr Rev. 2008;66:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Caraceni P, Nardo B, Domenicali M, Turi P, Vici M, Simoncini M, De Maria N, Trevisani F, Van Thiel DH, Derenzini M, Cavallari A, Bernardi M. Ischemia-reperfusion injury in rat fatty liver: role of nutritional status. Hepatology. 1999;29:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Gasbarrini A, Borle AB, Farghali H, Caraceni P, Van Thiel D. Fasting enhances the effects of anoxia on ATP, Cai2+ and cell injury in isolated rat hepatocytes. Biochim Biophys Acta. 1993;1178:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Cornide-Petronio ME, Álvarez-Mercado AI, Jiménez-Castro MB, Peralta C. Current Knowledge about the Effect of Nutritional Status, Supplemented Nutrition Diet, and Gut Microbiota on Hepatic Ischemia-Reperfusion and Regeneration in Liver Surgery. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 60. | Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Plauth M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr. 2020;39:3533-3562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 61. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1405] [Article Influence: 127.7] [Reference Citation Analysis (1)] |
| 62. | Huang TH, Hsieh CC, Kuo LM, Chang CC, Chen CH, Chi CC, Liu CH. Malnutrition associated with an increased risk of postoperative complications following hepatectomy in patients with hepatocellular carcinoma. HPB (Oxford). 2019;21:1150-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 63. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8561] [Article Influence: 535.1] [Reference Citation Analysis (0)] |
| 64. | Wang B, Shelat VG, Chow JJL, Huey TCW, Low JK, Woon WWL, Junnarkar SP. Prehabilitation Program Improves Outcomes of Patients Undergoing Elective Liver Resection. J Surg Res. 2020;251:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 65. | McKay BP, Larder AL, Lam V. Pre-Operative vs. Peri-Operative Nutrition Supplementation in Hepatic Resection for Cancer: A Systematic Review. Nutr Cancer. 2019;71:179-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Wang J, Wang W, Zhu F, Duan Q. The role of branched chain amino acids metabolic disorders in tumorigenesis and progression. Biomed Pharmacother. 2022;153:113390. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 67. | Ananieva EA, Wilkinson AC. Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr Metab Care. 2018;21:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 68. | Lo EKK, Felicianna, Xu JH, Zhan Q, Zeng Z, El-Nezami H. The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 69. | Iwasa J, Shimizu M, Shiraki M, Shirakami Y, Sakai H, Terakura Y, Takai K, Tsurumi H, Tanaka T, Moriwaki H. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci. 2010;101:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Cha JH, Bae SH, Kim HL, Park NR, Choi ES, Jung ES, Choi JY, Yoon SK. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 2013;8:e77899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Kang S, Song J, Kang H, Kim S, Lee Y, Park D. Insulin can block apoptosis by decreasing oxidative stress via phosphatidylinositol 3-kinase- and extracellular signal-regulated protein kinase-dependent signaling pathways in HepG2 cells. Eur J Endocrinol. 2003;148:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Hagiwara A, Nishiyama M, Ishizaki S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J Cell Physiol. 2012;227:2097-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Yoshiji H, Noguchi R, Kitade M, Kaji K, Ikenaka Y, Namisaki T, Yoshii J, Yanase K, Yamazaki M, Tsujimoto T, Akahane T, Kawaratani H, Uemura M, Fukui H. Branched-chain amino acids suppress insulin-resistance-based hepatocarcinogenesis in obese diabetic rats. J Gastroenterol. 2009;44:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Tian Q, Yuan P, Quan C, Li M, Xiao J, Zhang L, Lu H, Ma T, Zou L, Wang F, Xue P, Ni X, Wang W, Liu L, Wang Z, Zhu F, Duan Q. Phosphorylation of BCKDK of BCAA catabolism at Y246 by Src promotes metastasis of colorectal cancer. Oncogene. 2020;39:3980-3996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 75. | Chi R, Yao C, Chen S, Liu Y, He Y, Zhang J, Ellies LG, Wu X, Zhao Q, Zhou C, Wang Y, Sun H. Elevated BCAA Suppresses the Development and Metastasis of Breast Cancer. Front Oncol. 2022;12:887257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 76. | Ericksen RE, Lim SL, McDonnell E, Shuen WH, Vadiveloo M, White PJ, Ding Z, Kwok R, Lee P, Radda GK, Toh HC, Hirschey MD, Han W. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab. 2019;29:1151-1165.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 77. | Lai HF, Chau IY, Lei HJ, Chou SC, Hsia CY, Kao YC, Chau GY. Postoperative fever after liver resection: Incidence, risk factors, and characteristics associated with febrile infectious complication. PLoS One. 2022;17:e0262113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 78. | Moreno Elola-Olaso A, Davenport DL, Hundley JC, Daily MF, Gedaly R. Predictors of surgical site infection after liver resection: a multicentre analysis using National Surgical Quality Improvement Program data. HPB (Oxford). 2012;14:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 79. | D'Amico D, Cillo U. Impact of severe infections on the outcome of major liver surgery: a pathophysiologic and clinical analysis. J Chemother. 1999;11:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 81. | Uchiyama K, Ueno M, Ozawa S, Kiriyama S, Kawai M, Hirono S, Tani M, Yamaue H. Risk factors for postoperative infectious complications after hepatectomy. J Hepatobiliary Pancreat Sci. 2011;18:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Figueiredo F, Dickson ER, Pasha T, Kasparova P, Therneau T, Malinchoc M, DiCecco S, Francisco-Ziller N, Charlton M. Impact of nutritional status on outcomes after liver transplantation. Transplantation. 2000;70:1347-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, Corradini SG, Siciliano M, Farcomeni A, Attili AF, Berloco P, Rossi M. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 84. | Harrison J, McKiernan J, Neuberger JM. A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transpl Int. 1997;10:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Kaido T, Mori A, Oike F, Mizumoto M, Ogura Y, Hata K, Yoshizawa A, Iida T, Uemoto S. Impact of pretransplant nutritional status in patients undergoing liver transplantation. Hepatogastroenterology. 2010;57:1489-1492. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 86. | Masuda T, Shirabe K, Yoshiya S, Matono R, Morita K, Hashimoto N, Ikegami T, Yoshizumi T, Baba H, Maehara Y. Nutrition support and infections associated with hepatic resection and liver transplantation in patients with chronic liver disease. JPEN J Parenter Enteral Nutr. 2013;37:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Calder PC. Branched-chain amino acids and immunity. J Nutr. 2006;136:288S-293S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 88. | Nakamura I, Ochiai K, Imawari M. Phagocytic function of neutrophils of patients with decompensated liver cirrhosis is restored by oral supplementation of branched-chain amino acids. Hepatol Res. 2004;29:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Nakamura I, Ochiai K, Imai Y, Moriyasu F, Imawari M. Restoration of innate host defense responses by oral supplementation of branched-chain amino acids in decompensated cirrhotic patients. Hepatol Res. 2007;37:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Tsukishiro T, Shimizu Y, Higuchi K, Watanabe A. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J Gastroenterol Hepatol. 2000;15:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Ishizawa T, Hasegawa K, Kokudo N, Sano K, Imamura H, Beck Y, Sugawara Y, Makuuchi M. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 92. | Fernández-Esparrach G, Sánchez-Fueyo A, Ginès P, Uriz J, Quintó L, Ventura PJ, Cárdenas A, Guevara M, Sort P, Jiménez W, Bataller R, Arroyo V, Rodés J. A prognostic model for predicting survival in cirrhosis with ascites. J Hepatol. 2001;34:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 93. | Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, Fisher RA, Mihas AA. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 94. | Chan KM, Lee CF, Wu TJ, Chou HS, Yu MC, Lee WC, Chen MF. Adverse outcomes in patients with postoperative ascites after liver resection for hepatocellular carcinoma. World J Surg. 2012;36:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Okuno M, Moriwaki H, Kato M, Muto Y, Kojima S. Changes in the ratio of branched-chain to aromatic amino acids affect the secretion of albumin in cultured rat hepatocytes. Biochem Biophys Res Commun. 1995;214:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 97. | Fukushima H, Miwa Y, Shiraki M, Gomi I, Toda K, Kuriyama S, Nakamura H, Wakahara T, Era S, Moriwaki H. Oral branched-chain amino acid supplementation improves the oxidized/reduced albumin ratio in patients with liver cirrhosis. Hepatol Res. 2007;37:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, Kumada H; Long-Term Survival Study Group. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 99. | Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R; Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |