Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2500
Peer-review started: September 12, 2023
First decision: September 25, 2023
Revised: October 4, 2023
Accepted: October 30, 2023
Article in press: October 30, 2023
Published online: November 27, 2023
Processing time: 76 Days and 0.7 Hours
Reducing or preventing postoperative morbidity in patients with gastric cancer (GC) is particularly important in perioperative treatment plans.
To identify risk factors for early postoperative complications of GC post-distal gastrectomy and to establish a nomogram prediction model.
This retrospective study included 131 patients with GC who underwent distal gastrectomy at the Second Hospital of Shandong University between January 2019 and February 2023. The factors influencing the development of complications after distal gastrectomy in these patients were evaluated using univariate and multivariate logistic regression analysis. Based on the results obtained, a predictive nomogram was established. The nomogram was validated using internal and external (n = 45) datasets. Its sensitivity and specificity were established by receiver operating characteristic curve analysis. Decision curve (DCA) analysis was used to determine its clinical benefit and ten-fold overfitting was used to establish its accuracy and stability.
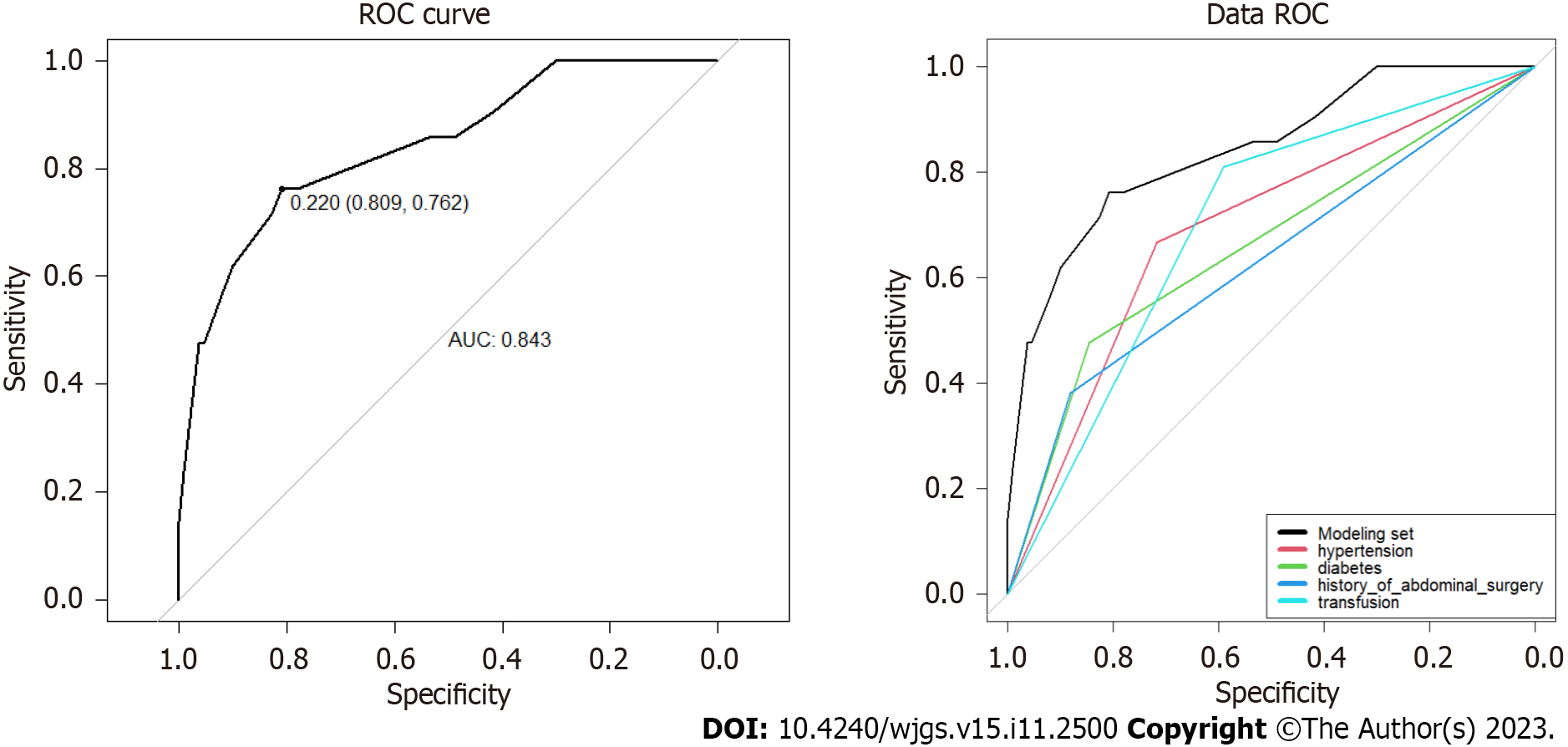
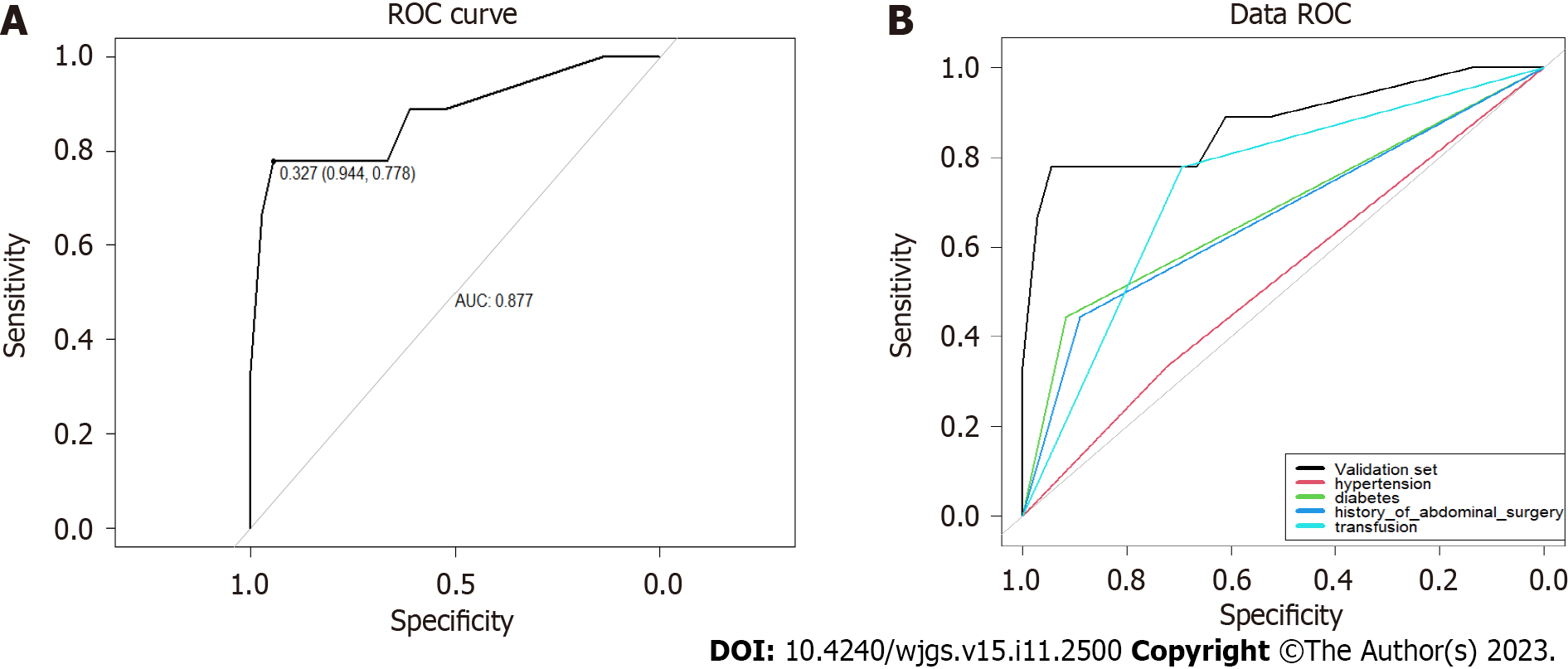
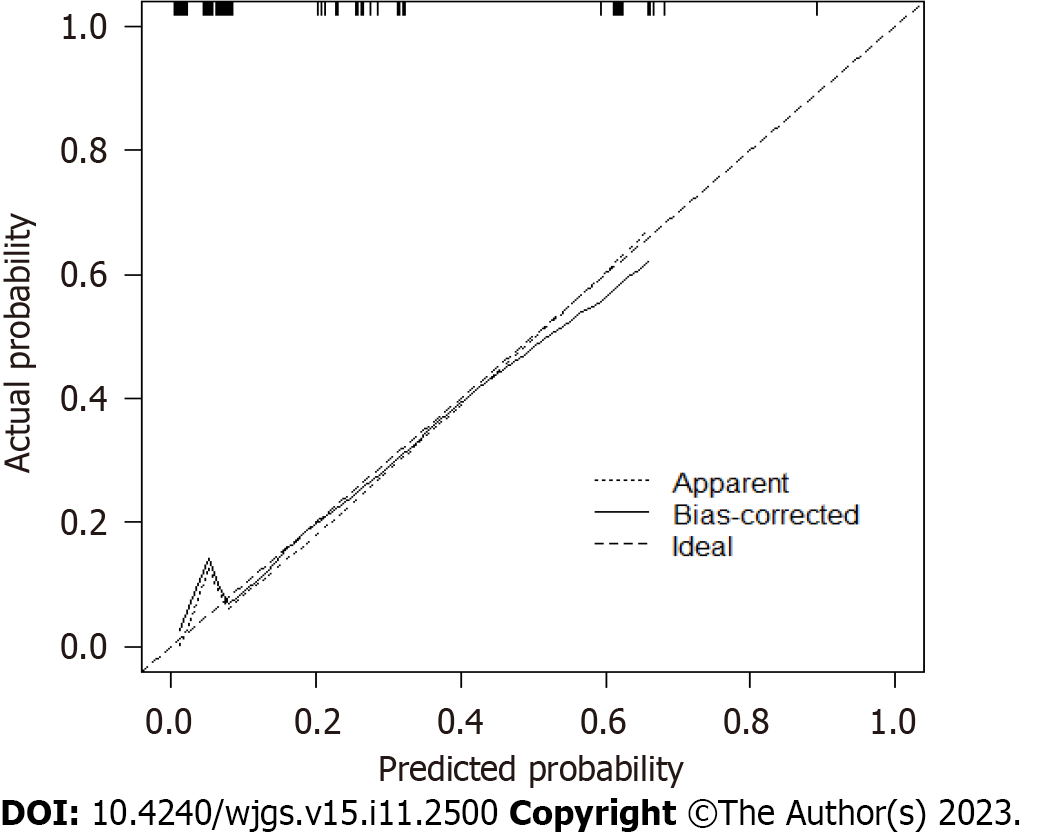
Multivariate logistic regression analysis showed that hypertension, diabetes, history of abdominal surgery, and perioperative blood transfusion were independent predictors of postoperative complications of distal gastrectomy. The modeling and validation sets showed that the area under the curve was 0.843 [95% confidence interval (CI): 0.746-0.940] and 0.877 (95%CI: 0.719-1.000), the sensitivity was 0.762 and 0.778, respectively, and the specificity was 0.809 and 0.944, respectively, indicating that the model had good sensitivity and specificity. The C-indexes of the modeling and validation datasets were 0.843 (95%CI: 0.746-0.940) and 0.877 (95%CI: 0.719-1.000), respectively. The calibration curve (Hosmer Lemeshow test: χ2 = 7.33) showed that the model had good consistency. The results of the DCA analysis indicated that this model offered good clinical benefits. The accuracy of 10-fold cross-validation was 0.878, indicating that the model had good accuracy and stability.
The nomogram prediction model based on independent risk factors related to postoperative complications of distal gastrectomy can facilitate perioperative intervention for high-risk populations and reduce the incidence of postoperative complications.
Core Tip: Using univariate and multivariate logistic regression analyses, we found that hypertension, diabetes, a history of abdominal surgery, and perioperative blood transfusion are predictors of complications after distal gastrectomy in patients with gastric cancer (GC). We then developed a novel nomogram for predicting early postoperative complications after distal gastrectomy. Using internal and external validations, we demonstrated that the model had good accuracy and stability. This model can facilitate identification of GC patients who are likely to develop complications, allowing early intervention and more appropriate management.
- Citation: Zhang B, Zhu Q, Ji ZP. Nomogram for predicting early complications after distal gastrectomy. World J Gastrointest Surg 2023; 15(11): 2500-2512
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2500.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2500
According to the Global Cancer Research Center, gastric cancer (GC) remains one of the most prevalent malignant tumors globally, contributing to the mortality due to malignant tumors[1]. In East Asia, particularly in China, GC is the third most common cancer and the second leading cause of cancer death[2]. Moreover, cancer affecting the lower one-third of the stomach is the most common type of GC[3].
At present, GC is treated comprehensively using surgery supplemented by chemotherapy, radiotherapy, immunotherapy, and targeted therapy. Radical surgical resection combined with local lymphadenectomy is currently the only curative treatment option[4]. Therefore, distal gastrectomy with D2 Lymph node dissection is recommended as a standard surgery for patients with distal GC[3]. It is suitable for clinical node positive (CN+) or T2-T4a tumors[5]. For early GC, laparoscopic distal gastrectomy was upgraded from research treatment to general practice in the 2014 version of the guidelines of the Japanese Society of Endoscopic Surgery[6]. Additionally, for advanced GC, large-scale ran
Although several studies have sought to predict postoperative complications in patients with GC, models for predicting the prognosis of patients with distal GC are lacking. Therefore, we aimed to evaluate the potential risk factors and build a nomogram model for predicting complications in individuals with distal GC.
We retrospectively collected data from all patients who underwent distal gastrectomy with D2 Lymphadenectomy between January 2019 and February 2023 at the Second Hospital of Shandong University. Eventually, we included 131 patients who underwent surgery under standard general anesthesia, followed by distal gastrectomy with D2 lymph node dissection.
The study protocol was approved by the Ethics Committee of Second Hospital of Shandong University, China. This study complied with the principles of the Declaration of Helsinki. Due to the retrospective observational nature of the study, it involved minimal risks, did not threaten the patient’s health did not require patient consent.
The inclusion criteria for this study were as follows: Distal gastrectomy with D2 lymphadenectomy, no neoadjuvant radiotherapy or chemotherapy before surgery, and complications occurring within 30-d postoperatively. The exclusion criteria were incomplete clinical data, failure to regain, distant metastasis or invasion, and conversion to open gastrectomy.
The following patient baseline data were collected at admission: Age, sex (male or female), drinking history (yes or no), smoking history (yes or no), body mass index, history of abdominal surgery (yes or no), preoperative albumin level, preoperative hemoglobin level, American Society of Anesthesiologists stage (I–VI), presence of heart, liver, kidney, lung, and brain comorbidities (yes or no), diabetes, hypertension, and coronary heart disease (yes or no), operation time, laparoscopic surgery (yes or no), robotic surgery (yes or no), R0 resection (yes or no), blood transfusion (yes or no), postoperative intraperitoneal chemotherapy (yes or no), combined organ resection (yes or no). Simultaneously, the maximum tumor diameter, tumor-related markers, histological type, gross type, tumor-node-metastasis (TNM) stage, number of lymph node resections, number of positive lymph nodes, vascular invasion (yes or no), and nerve invasion (yes or no) were also measured. During the study period, albumin, prealbumin, and hemoglobin levels were recorded preoperatively at first admission. Blood transfusion refers to blood transfusion during the period from operation to discharge.
Complications were defined as any deviation from the normal postoperative course[9]. We analyzed postoperative complications based on the Clavien-Dindo classification. Complications above level II were considered to be clinically significant. In this study, we observed early complications in patients undergoing distal gastrectomy, including abdominal bleeding, duodenal stump leakage, gastroparesis, abdominal infection, chylous leakage, pancreatic leakage, anastomotic leakage, and other related complications. The diagnosis of complications was primarily based on clinical symptoms and signs, computed tomography, endoscopy, drainage fluid, and individual laboratory examinations. The observed complications are listed in Table 1.
| Complications | Number (%) |
| Abdominal bleeding | 1 (4.8) |
| Abdominal infection | 5 (42.9) |
| Incision infection | 2 (9.5) |
| Chylous fistula | 2 (9.5) |
| Duodenal stump leakage | 4 (19.1) |
| Gastroparesis | 1 (4.8) |
| Anastomotic stomatitis | 2 (9.5) |
| Anastomotic fistula | 3 (14.3) |
| Pancreatic leakage | 1 (4.8) |
| Total | 21 |
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 26.0; IBM SPSS Inc., Armonk, NY, United States) and R software (version 4.3.0; https://www.r-project.org/). For quantitative variables with a normal distribution, values are presented as mean ± SE of the mean, while for those with a non-normal distribution, values are expressed as the median and interquartile range. Groups were compared using two independent-sample t-tests and Wilcoxon rank-sum tests (nonparametric test, Mann-Whitney U test). Categorical variables were presented as frequencies and proportions, and the chi-square test, chi-square test with correction, and Fisher’s exact test were used for comparisons between patient groups.
All variables were also categorized, and forward stepwise logistic regression models were constructed to determine involvement in postoperative morbidity. The presence or absence of postoperative complications after GC surgery were defined as the dependent variable. Univariate logistic regression analysis was used to study preoperative conditions, tumor-related factors, surgical procedures, and surgical complications after surgery. The variables that were significant (P < 0.05) in the univariate regression analysis were entered as independent variables in the multivariate logistic regression analysis, thereby establishing the regression model.
The R software (version 4.3.0) was used to construct the nomogram. The consistency between the predicted outcome probability of the model and the actual observation probability was evaluated by drawing a calibration curve represented by the consistency index (C-index). A receiver operating characteristic (ROC) curve was used to evaluate the predictive ability of the prediction model using the area under the curve (AUC). A decision curve (DCA) was drawn to evaluate the clinical benefits for patients. Finally, a ten-fold cross validation was performed to avoid overfitting and model bias. Statistical significance was set at P < 0.05.
A total of 131 patients with distal GC who underwent distal gastrectomy with D2 lymph node dissection were included in the study. Of them, 21 patients (16.03%) developed early postoperative complications. All patients were administered symptomatic treatment after a clear diagnosis, without death due to complications. The general characteristics and results of the univariate analysis of the patients are presented in Table 2. The univariate analysis between the two groups showed that prealbumin level, hypertension, diabetes, history of abdominal surgery, R0 resection, and blood transfusion were factors influencing early postoperative complications after distal gastrectomy (all P < 0.05).
| Factors | With early postoperative complications (%) | Without early postoperative complications (%) | χ2/t | P value | |
| Age (yr) | 71 (60.5-74) | 63 (5-70) | -2.14 | 0.032 | |
| Weight | 66.15 ± 11.08 | 65.85 ± 10.01 | 0.118 | 0.906 | |
| Height | 168.20 ± 6.869 | 165.52 ± 7.461 | 1.614 | 0.109 | |
| BMI | 23.312 ± 3.22 | 24.00 ± 3.11 | -0.905 | 0.367 | |
| Hemoglobin | 129 (100.5-145) | 128.5 (114.25-144.25) | -0.342 | 0.732 | |
| Albumin | 39.9 (36.35-42.75) | 41.35 (38.2-45) | -1.754 | 0.08 | |
| Prealbumin | 18.9 (14.4-22.5) | 21.45 (17.2-25.3) | -2.199 | 0.028 | |
| Alpha fetoprotein | 3.02 (2.285-4.465) | 2.495 (1.815-3.9225) | -1.383 | 0.167 | |
| Carcinoembryonic antigen | 2.065 (1.4625-2.8625) | 1.76 (1.265-3.38) | -0.511 | 0.609 | |
| CA199 | 7.3 (5-20.55) | 9.04 (5.9925-16.05) | -0.326 | 0.744 | |
| CA125 | 9.66 (6.325-19.385) | 8.685 (6.3-12.5) | -0.781 | 0.435 | |
| Number of positive lymph nodes | 2 (0-8) | 0.5 (0-6.25) | -0.643 | 0.526 | |
| Number of lymph node resections | 29 (21-40) | 28 (22-34) | -0.352 | 0.725 | |
| Tumor maximum diameter | 4.5 (3-6) | 4 (2.425-5.5) | -0.817 | 0.414 | |
| Surgical time | 250 (205-265) | 240 (210-282) | -0.141 | 0.888 | |
| Blood loss | 50 (35-100) | 50 (50-100) | -0.236 | 0.814 | |
| Organizational type | 10.664 | 0.099 | |||
| Poorly differentiated adenocarcinoma | 9 (27.3) | 24 (72.7) | |||
| Moderate to poorly differentiated adenocarcinoma | 7 (12.3) | 5 (92.3) | |||
| Moderately differentiated adenocarcinoma | 1 (7.7) | 12 (85.7) | |||
| Medium to high differentiation adenocarcinoma | 1 (12.5) | 7 (87.5) | |||
| Highly differentiated adenocarcinoma | 0 (0) | 5 (100) | |||
| Diffuse large B-cell carcinoma | 1 (100) | 0 (0) | |||
| Signet ring cell carcinoma | 2 (14.3) | 12 (87.5) | |||
| General type | 3.202 | 0.921 | |||
| Concave type | 2 (18.2) | 9 (81.8) | |||
| Shallow concave type | 1 (5.3) | 18 (94.7) | |||
| Superficial uplift type | 1 (14.3) | 6 (86.7) | |||
| Shallow flat type | 0 (0) | 1 (100) | |||
| Nodular type | 1 (33.3) | 2 (66.7) | |||
| Infiltrating ulcer type | 2 (15.4) | 11 (84.6) | |||
| Ulcerative type | 11 (19) | 47 (81) | |||
| Protuberant type | 2 (20) | 8 (80) | |||
| Diffuse infiltrative type | 1 (11.1) | 8 (88.9) | |||
| ASA | 0.428 | 0.934 | |||
| Ⅰ | 0 (0) | 1 (100) | |||
| Ⅱ | 10 (15.6) | 54 (84.4) | |||
| Ⅲ | 11 (16.9) | 54 (83.1) | |||
| Ⅳ | 0 (0) | 1 (100) | |||
| Sex | 0.21 | 0.647 | |||
| Male | 17 (16.8) | 84 (83.2) | |||
| Female | 4 (13.3) | 26 (86.7) | |||
| Transfusion | 11.342 | 0.001 | |||
| Yes | 17 (27.4) | 45 (72.6) | |||
| No | 4 (5.8) | 65 (94.2) | |||
| P stage | 3.143 | 0.37 | |||
| Ⅰ | 6 (11.5) | 46 (88.5) | |||
| Ⅱ | 5 (22.7) | 17 (77.3) | |||
| Ⅲ | 10 (19.6) | 41 (80.4) | |||
| Ⅳ | 0 (0) | 6 (100) | |||
| hypertension | 1.581 | 0.001 | |||
| Yes | 14 (31.1) | 31 (68.9) | |||
| No | 7 (8.1) | 79 (91.9) | |||
| Diabetes | 9.27 | 0.002 | |||
| Yes | 10 (37) | 17 (63) | |||
| No | 11 (10.6) | 93 (89.4) | |||
| Smoking history | 3.262 | 0.071 | |||
| Yes | 1 (3.4) | 28 (96.6) | |||
| No | 20 (19.6) | 82 (80.4) | |||
| History of drinking | 0.017 | 0.896 | |||
| Yes | 6 (15.4) | 33 (84.6) | |||
| No | 15 (16.3) | 77 (83.7) | |||
| History of abdominal surgery | 7.199 | 0.007 | |||
| Yes | 8 (38.1) | 13 (61.9) | |||
| No | 13 (11.8) | 97 (88.2) | |||
| Laparoscopy | 0.002 | 0.962 | |||
| Yes | 11 (16.2) | 57 (83.8) | |||
| No | 10 (15.9) | 53 (84.1) | |||
| Robot surgery | 0.002 | 0.962 | |||
| Yes | 10 (15.9) | 53 (84.1) | |||
| No | 11 (16.2) | 57 (83.8) | |||
| Intraperitoneal perfusion chemotherapy | 0.001 | 0.971 | |||
| Yes | 6 (16.2) | 31 (83.8) | |||
| No | 15 (16.0) | 79 (84.0) | |||
| R0 | 7.466 | 0.006 | |||
| Yes | 18 (14.3) | 108 (85.7) | |||
| No | 2 (40) | 3 (60) | |||
| Vascular invasion | 0.242 | 0.622 | |||
| Yes | 11 (14.7) | 64 (85.3) | |||
| No | 10 (17.9) | 46 (82.1) | |||
| Lymphatic invasion | 1.566 | 0.212 | |||
| Yes | 4 (10) | 36 (90) | |||
| No | 17 (18.7) | 74 (81.3) | |||
| Combined organectomy | 0.275 | 0.6 | |||
| Yes | 19 (15.6) | 103 (84.4) | |||
| No | 2 (22.2) | 7 (77.8) | |||
| Liver | 0.017 | 0.897 | |||
| Yes | 1 (14.3) | 6 (85.7) | |||
| No | 20 (16.1) | 104 (83.9) | |||
| Lung | 0.788 | 0.375 | |||
| Yes | 0 (0) | 4 (100) | |||
| No | 21 (16.5) | 106 (83.5) | |||
| Kidney | 0.788 | 0.375 | |||
| Yes | 0 (0) | 4 (100) | |||
| No | 21 (16.5) | 106 (83.5) | |||
| Heart | 2.193 | 0.144 | |||
| Yes | 5 (27.8) | 13 (72.2) | |||
| No | 16 (14.2) | 97 (85.8) | |||
| Brain | 2.329 | 0.127 | |||
| Yes | 4 (30.8) | 9 (69.2) | |||
| No | 17 (14.4) | 101 (85.6) | |||
| T stage | 0.47 | 0.493 | |||
| 1 | 6 (13) | 40 (87) | |||
| ≥ 2 | 15 (17.6) | 70 (82.4) | |||
| N stage | 0.639 | 0.888 | |||
| 0 | 9 (14.3) | 54 (85.7) | |||
| 1 | 2 (13.3) | 13 (86.7) | |||
| 2 | 3 (16.7) | 15 (83.3) | |||
| 3 | 7 (20) | 28 (80) | |||
| M stage | 1.2 | 0.273 | |||
| 0 | 21 (16.8) | 104 (83.2) | |||
| 1 | 0 (0) | 6 (100) |
Inclusion of the above significant variables in the logistic regression analysis revealed that hypertension, diabetes, a history of abdominal surgery, and blood transfusion were independent predictors of early postoperative complications after distal gastrectomy (P < 0.05) (Table 3).
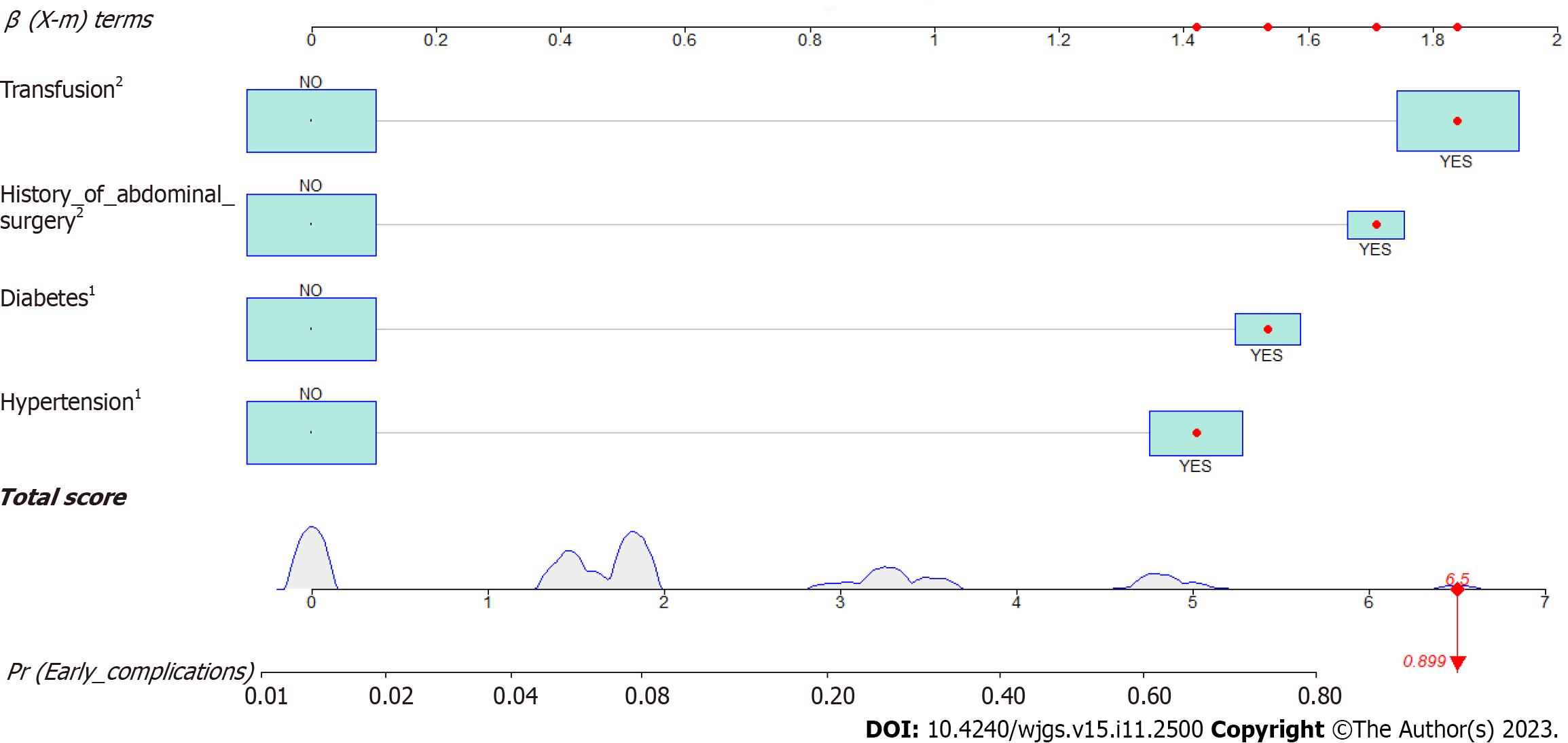
| Factors | β | S.E. | Wald value | Freedom | P value | OR value (95%CI) |
| Age (yr) | -0.017 | 0.034 | 0.263 | 1 | 0.608 | 0.983 (0.92-1.05) |
| Prealbumin | -0.035 | 0.067 | 0.275 | 1 | 0.6 | 0.966 (0.847-1.1) |
| Transfusion | 1.647 | 0.714 | 5.326 | 1 | 0.021 | 5.191 (1.282-21.02) |
| Hypertension | 1.436 | 0.602 | 5.689 | 1 | 0.017 | 4.204 (1.292-13.683) |
| Diabetes | 1.461 | 0.728 | 4.029 | 1 | 0.045 | 4.309 (1.035-17.936) |
| History of abdominal surgery | 1.75 | 0.669 | 6.853 | 1 | 0.009 | 5.757 (1.553-21.348) |
| R0 | -1.449 | 1.173 | 1.526 | 1 | 0.217 | 0.235 (0.024-2.339) |
| Constant | -1.019 | 3.136 | 0.106 | 1 | 0.745 | 0.361 |
A nomogram prediction model for early postoperative complications in patients with distal GC was constructed based on the independent risk factors identified by multivariate logistic regression analysis. As shown in Figure 1, if a patient had a history of hypertension, diabetes, and abdominal surgery before surgery and if blood transfusion was conducted during the perioperative period, the incidence of early complications after distal gastrectomy was 89.9%.
Internal validation was performed using calibration, ROC, and DCA curves, and 10-fold cross-validation. Simultaneously, data was collected from the patients at different time-points as validation sets (n = 45) for external validation.
The ROC curves for the modeling (Figure 2) and validation sets (Figure 3) yielded AUCs of 0.843 [95% confidence interval (CI): 0.746-0.940] and 0.877 (95%CI: 0.719-1.000); sensitivity of 0.762 and 0.778; and specificity of 0.809 and 0.944, respectively, indicating that the model had good sensitivity and specificity.
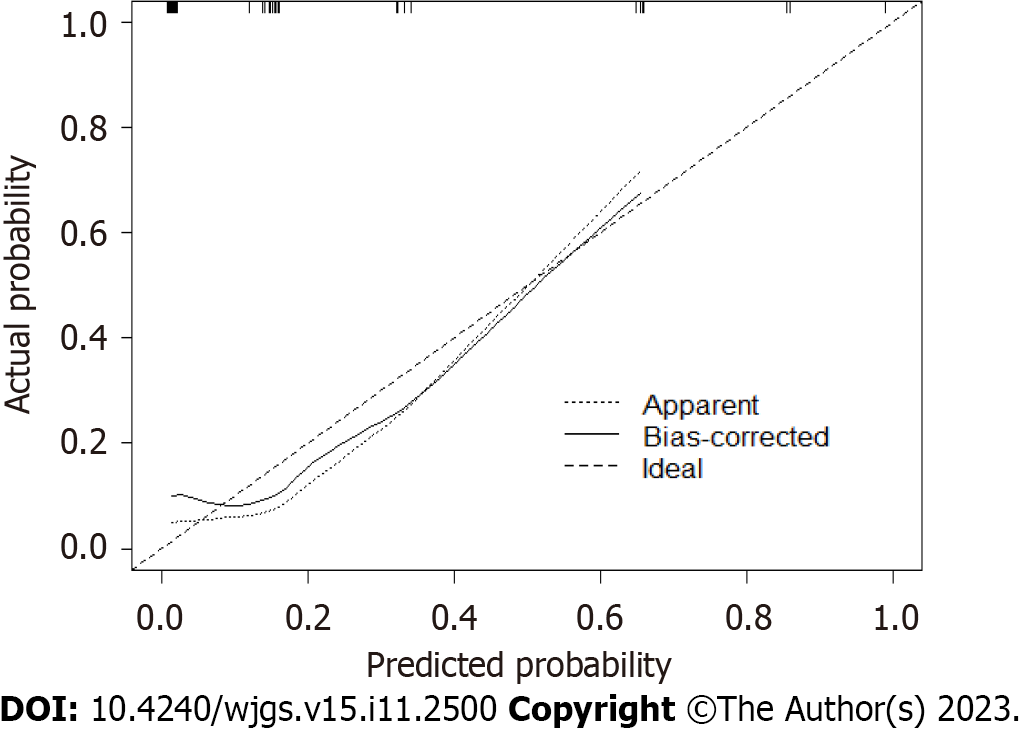
The calibration curve indicated good consistency (Hosmer-Lemeshow test: χ2 = 7.33; P = 0.501) in the evaluation of the consistency between the predicted outcome probability of the model and the actual observed outcome, represented by the C-index. The C-indexes of the modeling set (Figure 4) and validation set (Figure 5) were 0.843 (95%CI: 0.746-0.940) and 0.877 (95%CI: 0.719-1.000).
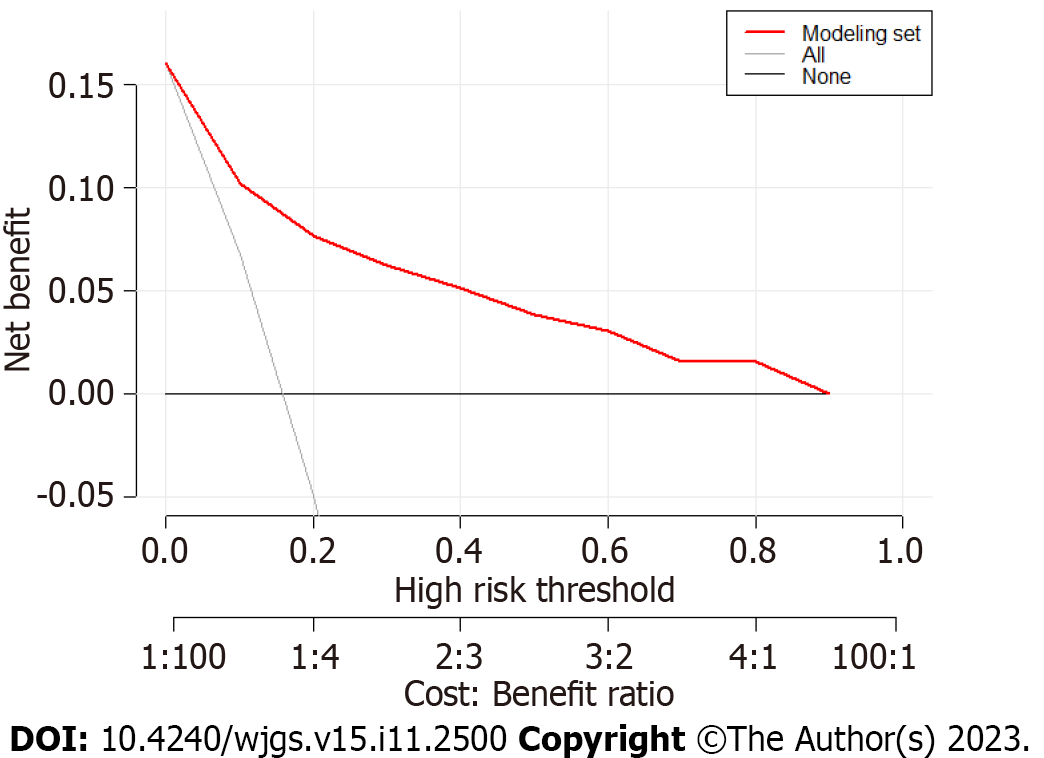
The evaluation of the degree of clinical benefit to the patients by DCA showed that the model provided good clinical benefits in the modeling set (Figure 6) and validation set (Figure 7). Ten-fold cross-validation yielded an accuracy of 0.878, indicating good accuracy and stability of the model.
This study analyzed 42 variables potentially associated with early postoperative complications in 131 patients with distal GC who underwent distal gastrectomy. The univariate and multivariate logistic regression analyses identified hypertension, diabetes, history of abdominal surgery, and perioperative blood transfusion as predictors of complications after distal gastrectomy. Using multivariate analysis, we established a novel predictive nomogram for early postoperative complications after distal gastrectomy. Internal and external validations were performed and demonstrated accuracy and stability of the model.
This study showed that hypertension is an independent risk factor for early postoperative complications after distal GC, however, the relationship between hypertension and postoperative adverse outcomes remains unclear[10]. Some studies have shown that preoperative hypertension is an important predictor of postoperative incidence rate. For example, the prediction model created by Huang et al showed that a history of hypertension in patients > 70 years old is an independent predictor of a higher surgical incidence rate[11]. However, no conclusive data are currently available to support this concept[12]. Some studies have shown that only patients with chronic hypertension whose diastolic pressure ≥ 110 mmHg have an increased risk of perioperative complications. This may be because the increased blood pressure during surgery will generally expose patients to the risk of hemodynamic instability. Hypertension is also related to an increased risk of perioperative myocardial ischemia, leading to an increased incidence of cardiovascular complications and damaged cardiac output in these patients. This, in turn, may lead to insufficient perfusion and damage to the targeted terminal organs[13-15]. Another study suggested that perioperative cardiac complications are related to intraoperative hemodynamic instability, rather than to the occurrence of hypertension during surgery. Therefore, achieving hemodynamic stability may be more important than targeting any particular intraoperative blood pressure[16]. Taken together, these findings show that effective control of blood pressure and maintaining the stability of intraoperative hemodynamics may reduce the incidence of postoperative complications, although further prospective cohort studies are required to verify this.
This study showed that diabetes is an independent risk factor for early postoperative complications in distal GC. Diabetes is a known risk factor for any postoperative complications[17]; however, the complications associated with diabetes remain controversial. Diabetes results in neutrophil dysfunction, which increases the risk of pathogen infection and reduces healing ability[18]. Additionally, it is related to tissue hypoxia and increased blood viscosity, which slows inflammatory reactions, thereby also affecting wound healing and increasing the risk of infection[18]. In addition, diabetes can also lead to lipid metabolism disorders, endothelial cell damage and dysfunction, abnormal platelet function, and vascular atherosclerosis, resulting in insufficient blood supply at the anastomoses and residual ends, thus increasing the risk of a fistula[19]. A meta-analysis revealed that the combined odds ratio of any complication for patients with as compared to patients without diabetes was 1.653 (1.487, 1.839), suggesting that diabetes is a risk factor for any postoperative complications. The two subtypes of diabetes insulin-dependent diabetes mellitus (IDDM) and non-IDDM (NIDDM)) have different incidence rates, and the risk of IDDM is higher than that of NIDDM[20]. Golinvaux et al [21] stated that compared to individuals without diabetes, individuals with IDDM had an increased risk of postoperative complications, prolonged hospital stay, postoperative adverse events, and readmission risk than those with NIDDM. In addition, complications related to IDDM were more severe than those related to NIDDM. Therefore, evaluating whether a patient has IDDM or DM (type 1 or type 2) is important during preparation for surgery.
Traditionally, a history of abdominal surgery has been considered to be a relative contraindication for laparoscopic gastrectomy, and the rate of conversion to open gastrectomy is high[22,23]. However, with the improvement of surgical instruments and accumulation of experience, postoperative surgical outcomes between patients with and without a history of abdominal surgery have not been found to be different, which contradicts the results of the present study. However, according to autopsy research reports, 75%-90% of patients who have previously undergone abdominal surgery have adhesions[24]. Beck et al[25] reported that 83% and 7% of patients who had undergone and not undergone previous abdominal surgery had intra-abdominal adhesions, which can prolong surgical time. Moreover, recent research has confirmed that surgical time is an independent risk factor for postoperative complications of GC[26]. Zhou et al[27] conducted statistical analysis on clinical data of patients undergoing GC surgery and found that longer surgery time is an independent risk factor for postoperative complications; longer the surgery time, more the stimulation and trauma to the abdominal organs, leading to an increased risk of postoperative complications. Therefore, from this perspective, a history of abdominal surgery remains a noteworthy indicator of complications.
This study showed that perioperative blood transfusion is an independent risk factor for early postoperative complications after distal gastrectomy, which may be related to an increase in the activity of regulatory T lymphocytes and the inhibition of the functions of natural killer T cells, macrophages, and monocytes, which reduces the immune function of the body. Elmi et al[28] found that patients with GC who had undergone perioperative blood transfusion had a higher risk of postoperative complications, particularly in terms of the incidence of infection. Xue et al[29] also found that perioperative blood transfusion is associated with poor prognosis in patients with gastric adenocarcinoma, particularly those with TNM III, and that patients who had received transfusions had more postoperative complications than those who had not, which is consistent with the research results of Kawakami et al[30]. Therefore, understanding the relationship between blood transfusion and postoperative complications is of great clinical significance to reduce and prevent the occurrence of complications, reduce perioperative mortality, and improve the long-term survival rate of patients.
This study had some limitations. First, this was a retrospective study, and inevitably, some unknown factors could have led to bias. Additionally, this study did not consider information regarding the postoperative survival of patients, mainly because the included patients had a shorter postoperative time; however, follow-up studies on this cohort will continue. In addition, to evaluate the performance of the model more accurately, external validation of big data from other centers is required. Nevertheless, the current results are encouraging.
In this study, preoperative and intraoperative factors were used to establish an early postoperative nomogram model. The results of this study suggest that hypertension, diabetes, a history of abdominal surgery, and perioperative blood transfusion are risk factors for early postoperative complications after distal gastrectomy. This prediction model can be used to guide the detection of early postoperative complications and has clinical reference value.
Gastric cancer (GC) remains one of the most prevalent malignant tumors globally, contributing to the mortality due to malignant tumors.
Identifying methods to reduce or prevent postoperative morbidity in patients with GC has become a key focus point.
To establish a nomogram prediction model.
We included 131 patients who underwent surgery under standard general anesthesia, followed by distal gastrectomy with D2 lymph node dissection.
The calibration curve (Hosmer Lemeshow test: χ2 = 7.33) showed that the model had good consistency. The results of the decision curve analysis indicated that this model offered good clinical benefits.
This prediction model can be used to guide the detection of early postoperative complications and has clinical reference value.
To evaluate the performance of the model more accurately, external validation of big data from other centers is required.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kalkum E, Germany; Lescinska AM, Latvia S-Editor: Qu XL L-Editor: A P-Editor: Zhao S
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64721] [Article Influence: 16180.3] [Reference Citation Analysis (177)] |
| 2. | Gong W, Zhao L, Dong Z, Dou Y, Liu Y, Ma C, Qu X. After neoadjuvant chemotherapy platelet/lymphocyte ratios negatively correlate with prognosis in gastric cancer patients. J Clin Lab Anal. 2018;32:e22364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Chen S, Chen DW, Chen XJ, Lin YJ, Xiang J, Peng JS. Postoperative complications and nutritional status between uncut Roux-en-Y anastomosis and Billroth II anastomosis after D2 distal gastrectomy: a study protocol for a multicenter randomized controlled trial. Trials. 2019;20:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Gong S, Li X, Tian H, Song S, Lu T, Jing W, Huang X, Xu Y, Wang X, Zhao K, Yang K, Guo T. Clinical efficacy and safety of robotic distal gastrectomy for gastric cancer: a systematic review and meta-analysis. Surg Endosc. 2022;36:2734-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, Shiraishi N, Kitano S. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg. 2015;39:2734-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 6. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1915] [Article Influence: 239.4] [Reference Citation Analysis (1)] |
| 7. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 609] [Article Influence: 304.5] [Reference Citation Analysis (2)] |
| 8. | Degiuli M, Sasako M, Calgaro M, Garino M, Rebecchi F, Mineccia M, Scaglione D, Andreone D, Ponti A, Calvo F; Italian Gastric Cancer Study Group. Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol. 2004;30:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518-526. [PubMed] |
| 10. | Lien SF, Bisognano JD. Perioperative hypertension: defining at-risk patients and their management. Curr Hypertens Rep. 2012;14:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Hwang SH, Park DJ, Jee YS, Kim HH, Lee HJ, Yang HK, Lee KU. Risk factors for operative complications in elderly patients during laparoscopy-assisted gastrectomy. J Am Coll Surg. 2009;208:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Ahuja K, Charap MH. Management of perioperative hypertensive urgencies with parenteral medications. J Hosp Med. 2010;5:E11-E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Varon J, Marik PE. Perioperative hypertension management. Vasc Health Risk Manag. 2008;4:615-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Hanada S, Kawakami H, Goto T, Morita S. Hypertension and anesthesia. Curr Opin Anaesthesiol. 2006;19:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Howell SJ, Sear JW, Foëx P. Hypertension, hypertensive heart disease and perioperative cardiac risk. Br J Anaesth. 2004;92:570-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Marik PE, Varon J. Perioperative hypertension: a review of current and emerging therapeutic agents. J Clin Anesth. 2009;21:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Rubel NC, Chung AS, Wong M, Lara NJ, Makovicka JL, Arvind V, Chang MS, Cho SK. 90-day Readmission in Elective Primary Lumbar Spine Surgery in the Inpatient Setting: A Nationwide Readmissions Database Sample Analysis. Spine (Phila Pa 1976). 2019;44:E857-E864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Gupta V, Winocour J, Shi H, Shack RB, Grotting JC, Higdon KK. Preoperative Risk Factors and Complication Rates in Facelift: Analysis of 11,300 Patients. Aesthet Surg J. 2016;36:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Zawada AE, Moszak M, Skrzypczak D, Grzymisławski M. Gastrointestinal complications in patients with diabetes mellitus. Adv Clin Exp Med. 2018;27:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Hou A, Cao J, Liu Y, Lou J, Li H, Ma Y, Song Y, Mi W, Liu J. Association of Diabetes Mellitus With Postoperative Complications and Mortality After Non-Cardiac Surgery: A Meta-Analysis and Systematic Review. Front Endocrinol (Lausanne). 2022;13:841256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Golinvaux NS, Varthi AG, Bohl DD, Basques BA, Grauer JN. Complication rates following elective lumbar fusion in patients with diabetes: insulin dependence makes the difference. Spine (Phila Pa 1976). 2014;39:1809-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Liao G, Wen S, Xie X, Wu Q. Laparoscopic gastrectomy for remnant gastric cancer: Risk factors associated with conversion and a systematic analysis of literature. Int J Surg. 2016;34:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Yamashita K, Miyazaki Y, Takahashi T, Masuike Y, Motoori M, Kimura Y, Kurokawa Y, Makino T, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. Safety and feasibility of laparoscopic gastrectomy for gastric cancer patients with a history of abdominal surgery. Surg Today. 2017;47:1274-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Law WL, Lee YM, Chu KW. Previous abdominal operations do not affect the outcomes of laparoscopic colorectal surgery. Surg Endosc. 2005;19:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Beck DE, Ferguson MA, Opelka FG, Fleshman JW, Gervaz P, Wexner SD. Effect of previous surgery on abdominal opening time. Dis Colon Rectum. 2000;43:1749-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Yasuda T, Sugimura K, Yamasaki M, Miyata H, Motoori M, Yano M, Shiozaki H, Mori M, Doki Y. Ten cases of gastro-tracheobronchial fistula: a serious complication after esophagectomy and reconstruction using posterior mediastinal gastric tube. Dis Esophagus. 2012;25:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Zhou J, Zhou Y, Cao S, Li S, Wang H, Niu Z, Chen D, Wang D, Lv L, Zhang J, Li Y, Jiao X, Tan X, Zhang B, Lu Y, Sun Z. Multivariate logistic regression analysis of postoperative complications and risk model establishment of gastrectomy for gastric cancer: A single-center cohort report. Scand J Gastroenterol. 2016;51:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Elmi M, Mahar A, Kagedan D, Law CH, Karanicolas PJ, Lin Y, Callum J, Coburn NG, Hallet J. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg. 2016;59:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Xue L, Chen XL, Wei-Han Z, Yang K, Chen XZ, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Impact of Perioperative Blood Transfusion on Postoperative Complications and Prognosis of Gastric Adenocarcinoma Patients with Different Preoperative Hemoglobin Value. Gastroenterol Res Pract. 2016;2016:6470857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Kawakami LE, Bonomi PB, Pereira MA, Carvalho FO, Ribeiro U Jr, Zilberstein B, Sampaio LR, Carneiro-D'Albuquerque LA, Ramos MFKP. Risk factors for blood transfusion and its prognostic implications in curative gastrectomy for gastric cancer. World J Gastrointest Surg. 2023;15:643-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |