Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2490
Peer-review started: August 13, 2023
First decision: September 20, 2023
Revised: October 3, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 27, 2023
Processing time: 105 Days and 20.3 Hours
Portal hypertension (PHT) in patients with alcoholic cirrhosis causes a range of clinical symptoms, including gastroesophageal varices and ascites. The hepatic venous pressure gradient (HVPG), which is easier to measure, has replaced the portal venous pressure gradient (PPG) as the gold standard for diagnosing PHT in clinical practice. Therefore, attention should be paid to the correlation between HVPG and PPG.
To explore the correlation between HVPG and PPG in patients with alcoholic cirrhosis and PHT.
Between January 2017 and June 2020, 134 patients with alcoholic cirrhosis and PHT who met the inclusion criteria underwent various pressure measurements during transjugular intrahepatic portosystemic shunt procedures. Correlations were assessed using Pearson’s correlation coefficient to estimate the correlation coefficient (r) and determination coefficient (R2). Bland-Altman plots were constructed to further analyze the agreement between the measurements. Disagreements were analyzed using paired t tests, and P values < 0.05 were considered statistically significant.
In this study, the correlation coefficient (r) and determination coefficient (R2) between HVPG and PPG were 0.201 and 0.040, respectively (P = 0.020). In the 108 patients with no collateral branch, the average wedged hepatic venous pressure was lower than the average portal venous pressure (30.65 ± 8.17 vs. 33.25 ± 6.60 mmHg, P = 0.002). Hepatic collaterals were identified in 26 cases with balloon occlusion hepatic venography (19.4%), while the average PPG was significantly higher than the average HVPG (25.94 ± 7.42 mmHg vs 9.86 ± 7.44 mmHg; P < 0.001). The differences between HVPG and PPG < 5 mmHg in the collateral vs no collateral branch groups were three cases (11.54%) and 44 cases (40.74%), respectively.
In most patients, HVPG cannot accurately represent PPG. The formation of hepatic collaterals is a vital reason for the strong underestimation of HVPG.
Core Tip: Portal hypertension (PHT) in alcoholic cirrhosis causes a range of clinical symptoms, including gastroesophageal varices and ascites. Because it is easier to measure, the hepatic venous pressure gradient (HVPG) has replaced the portal venous pressure gradient (PPG) as the gold standard for diagnosing PHT in clinical practice. However, our study showed a poor correlation between HVPG and PPG in patients with PHT and alcoholic cirrhosis. The underestimation of HVPG may be related to the formation of hepatic collaterals.
- Citation: Zhang D, Wang T, Yue ZD, Wang L, Fan ZH, Wu YF, Liu FQ. Hepatic venous pressure gradient: Inaccurately estimates portal venous pressure gradient in alcoholic cirrhosis and portal hypertension. World J Gastrointest Surg 2023; 15(11): 2490-2499
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2490.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2490
Alcoholic cirrhosis is a vital factor in portal hypertension (PHT), with an increased prevalence in recent years[1]. The blood alcohol concentration in those with extended periods of heavy drinking tends to exceed the recommended consumption limits by far. With intrahepatic vasoconstriction, the blood flow is decreased, and hemodynamic disorder occurs, which can trigger hepatic microvascular disturbances and hypoxemia[2]. Hepatocellular necrosis can occur, leading to fibrosis. This gradual development causes the segmentation and destruction of the normal structure of the hepatic lobules, with the occurrence of pseudolobules and nodular regeneration of hepatocytes, development of alcoholic cirrhosis[3], and progression to PHT. The initial stage has no characteristic symptoms, while a series of clinical patterns may occur during decompensation in patients with alcoholic cirrhosis, including gastroesophageal varices, gastro
Few studies have deeply explored the relationship between HVPG and PPG in patients with alcoholic cirrhosis and PHT. Therefore, whether HVPG can represent PPG remains controversial.
The present study aimed to examine the correlation between HVPG and PPG in patients with alcoholic cirrhosis and complications of PHT as well as determine whether HVPG can represent PPG.
We performed a retrospective study of patients with alcoholic cirrhosis and PHT complications who underwent transjugular intrahepatic portosystemic shunt (TIPS) placement and were admitted to our hospital between June 2017 and June 2020. Approval for the study was obtained from the ethics committee of our institution, and all patients provided informed consent to undergo TIPS creation and pressure measurements. The following inclusion criteria were applied: (1) Indication for TIPS; (2) age of 18-75 years; (3) elective TIPS surgery; and (4) normal hepatic veins and IVC. The following exclusion criteria were applied: (1) Portal vein tumor thrombus; (2) arteriovenous fistula; (3) portal vein thrombosis affecting blood flow (e.g., generally occurring over one-third of the main portal vein); (4) administration of drugs affecting PVP within 1 wk; and (5) intraoperative factors affecting the accuracy of manometry, such as gallbladder cardiac reflex and incomplete balloon closure.
A total of 134 patients with alcoholic cirrhosis and PHT complications undergoing TIPS were included in this study. Various venous pressures were measured during the TIPS procedure, and HVPG and PPG were calculated. Patient characteristics are summarized in Table 1. Patients were aged from 18–75 years old (average, 55.02 ± 10.65 years) and included 119 men (88.8%) and 15 women (11.2%). Of the 134 patients, 77 cases were complicated by gastrointestinal bleeding (57.5%), 46 had refractory ascites (34.3%), 6 had gastrointestinal bleeding associated with refractory ascites (4.5%), and 5 had other conditions (e.g., shunt restenosis and abdominal pain). According to the Child-Pugh classification, 52 (38.8%) patients had class A, 68 (50.8%) had class B, and 14 (10.4%) had class C liver disease. Fifteen patients with alcoholic cirrhosis and PHT also had hepatocellular carcinoma.
| Collateral branch (n = 26) | No collateral branch (n = 108) | P value | |
| Sex (male/female) | 24/2 (92.3/7.7%) | 95/13 (88.0/12.0%) | 0.776 |
| Age (yr)1 | 52.3 ± 10.7 | 55.7 ± 10.6 | 0.143 |
| Indications (n) | 0.865 | ||
| Gastrointestinal bleeding | 17 | 60 | |
| Refractory ascites | 7 | 39 | |
| Both | 1 | 5 | |
| Other conditions | 1 | 4 | |
| Child-Puph score1 | 7.2 ± 1.7 | 7.3 ± 1.7 | 0.878 |
| Child-Pugh class | 0.644 | ||
| A | 8 (30.8%) | 44 (40.7%) | |
| B | 15 (57.7%) | 53 (49.1%) | |
| C | 3 (11.5%) | 11 (10.2%) | |
| Hepatocellular carcinoma | 3 (11.5%) | 12 (11.1%) | > 0.999 |
Preoperatively, all patients underwent various examinations, including a complete blood count, biochemical tests; quantitation of liver function; analysis of the indocyanine green retention rate at 15 min, blood ammonia level, blood type, coagulation status, and tumor markers; electrocardiogram; portal vein ultrasound; and enhanced abdominal CT and/or magnetic resonance examination. The coagulation status, platelet count, and bilirubin, albumin, and hemoglobin levels were adjusted to adapt to interventional surgery. The effects and risks of the surgery were explained to the patients and their families, and informed consent was obtained. The ethics committee of the hospital approved the protocol [2018(01)], and all patients provided written informed consent to participate in the study.
The method for measuring the hepatic pressure has been described previously[8]. Briefly, after routine disinfection and towel placement, the right internal jugular vein was punctured under local anesthesia and intubation was performed. The Rösch-Uchida Transjugular Liver Access Set (RUPS-100, specialized for TIPS; Cook Medical, United States) was placed in the right atrium and IVC, and the pressure was measured. A Fogarty balloon catheter (Edwards Lifesciences, United States) was inserted through the 10-French outer sheath. Under the guidance of the guidewire, the catheter was passed through the superior vena cava, right atrium, and IVC and was then advanced into the hepatic vein. The balloon-tipped catheter was placed 3–5 cm peripheral to the junction of the hepatic vein and the IVC. WHVP and FHVP measurements were obtained before and after occlusion of the hepatic vein using a balloon inflated with 5 mL of contrast medium. All measurements were performed three times. Pressure values were recorded when they were stable, and the mean WHVP and FHVP values were taken; HVPG was subsequently calculated. After measurements, the balloon was blocked to obtain occluded hepatic venography (15 mL of contrast agent, 5 mL/s, pressure 200–300 psi), and WHVP and FHVP were remeasured. After observing the balloon catheter occlusion after balloon expansion, the balloon catheter position was adjusted for retesting and angiography once occlusion occurred. The hepatic parenchyma and portal vein were punctured through the IVC or hepatic vein. After a successful puncture, a pigtail catheter was inserted into the splenic or superior mesenteric vein for venography. Before shunting, the pressure of the main trunk of the portal vein was measured three times for an average value, and PPG was calculated. Subsequently, the liver tissue from the preshunt channel was obtained, and the shunt channel was established. The pressure of the main trunk of the portal vein was remeasured three times for an average value, and PPG was calculated. An indwelling catheter was placed in the portal vein for at least 24 h postoperatively, and PVP was measured three times daily. IVCP and right atrial pressure measurements were repeated three times during extubation to obtain average values.
Statistical analyses were performed using SPSS (version 25.0; IBM SPSS Statistics, NC, United States) and GraphPad Prism 8 (GraphPad, Inc., La Jolla, CA, United States). The results are expressed as mean ± SD. Correlations were assessed using Pearson’s correlation coefficient to estimate the correlation coefficient (r) and the determination coefficient (R2). Bland-Altman plots were constructed to further analyze the agreement between measurements. Differences between PPG and HVPG, WHVP and PVP, and FHVP and IVCP were analyzed using paired t-test. Statistical significance was set at P values < 0.05.
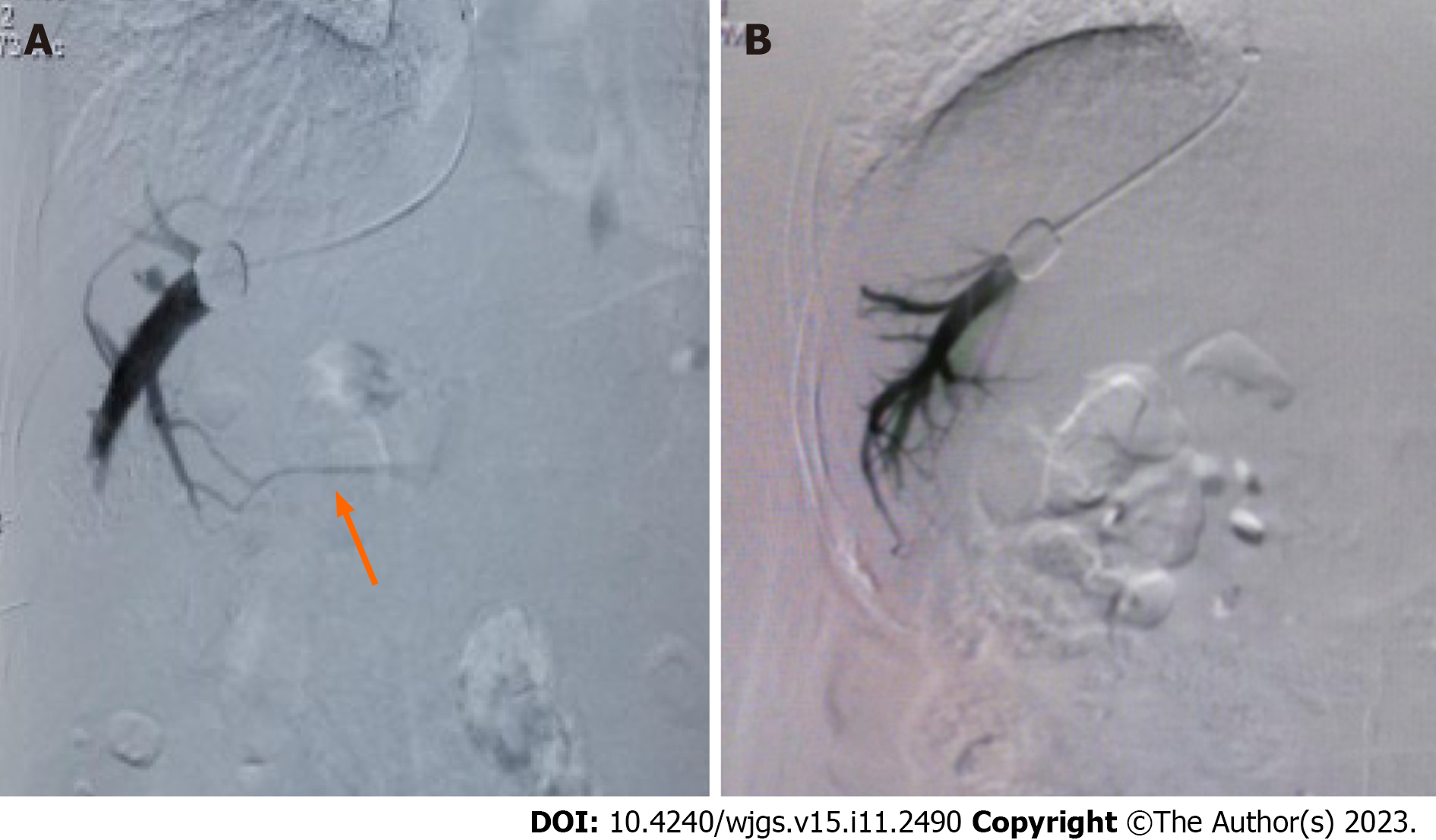
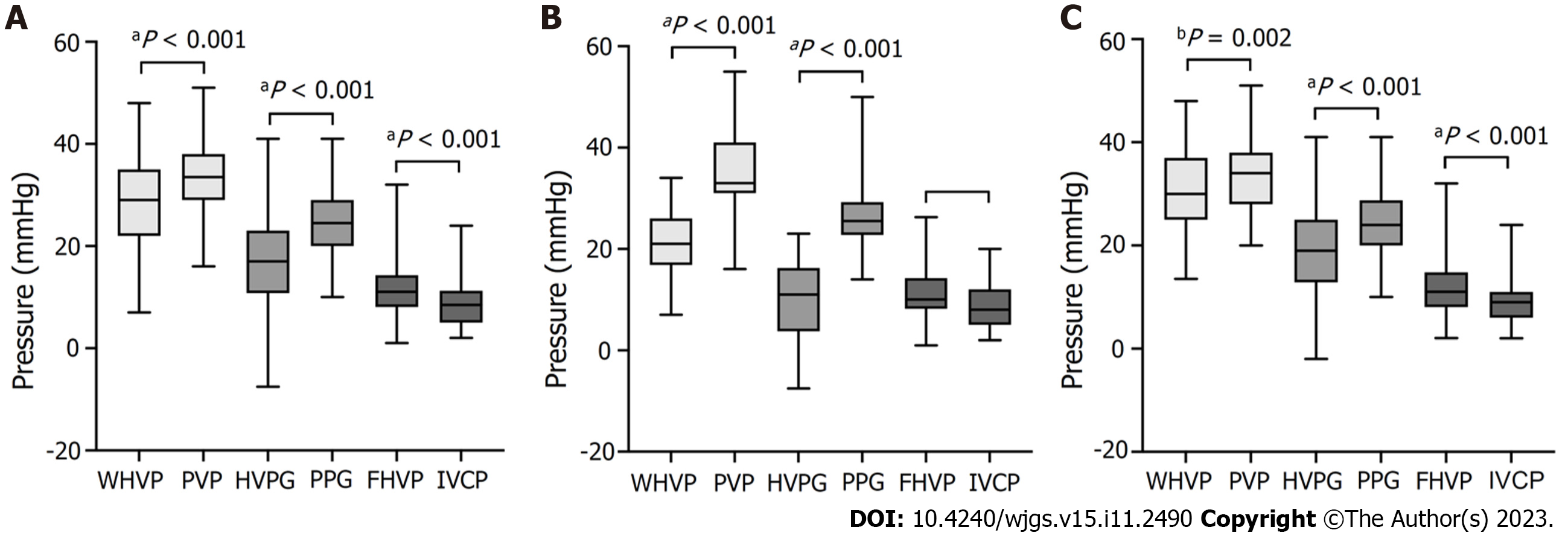
Among the 134 patients with alcoholic cirrhosis with PHT complications, intraoperative venograms showed portal vein collateral formation in 26 (19.40%) patients and no portal vein collateralization in 108 (80.60%) patients (Figure 1). As shown in Figure 2, WHVP and HVPG were significantly lower than PVP and HVPG, respectively. The average FHVP was higher than the average IVCP.
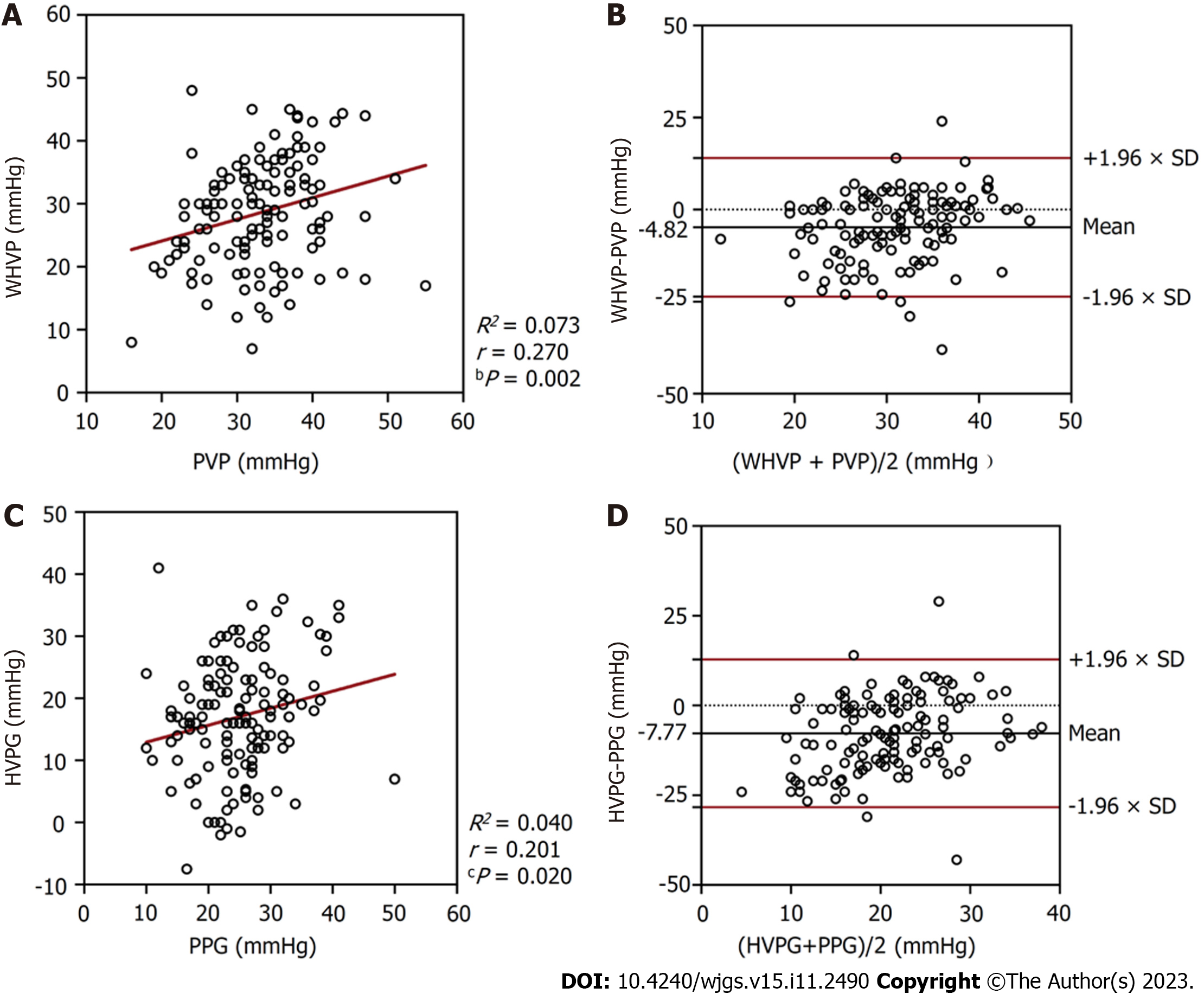
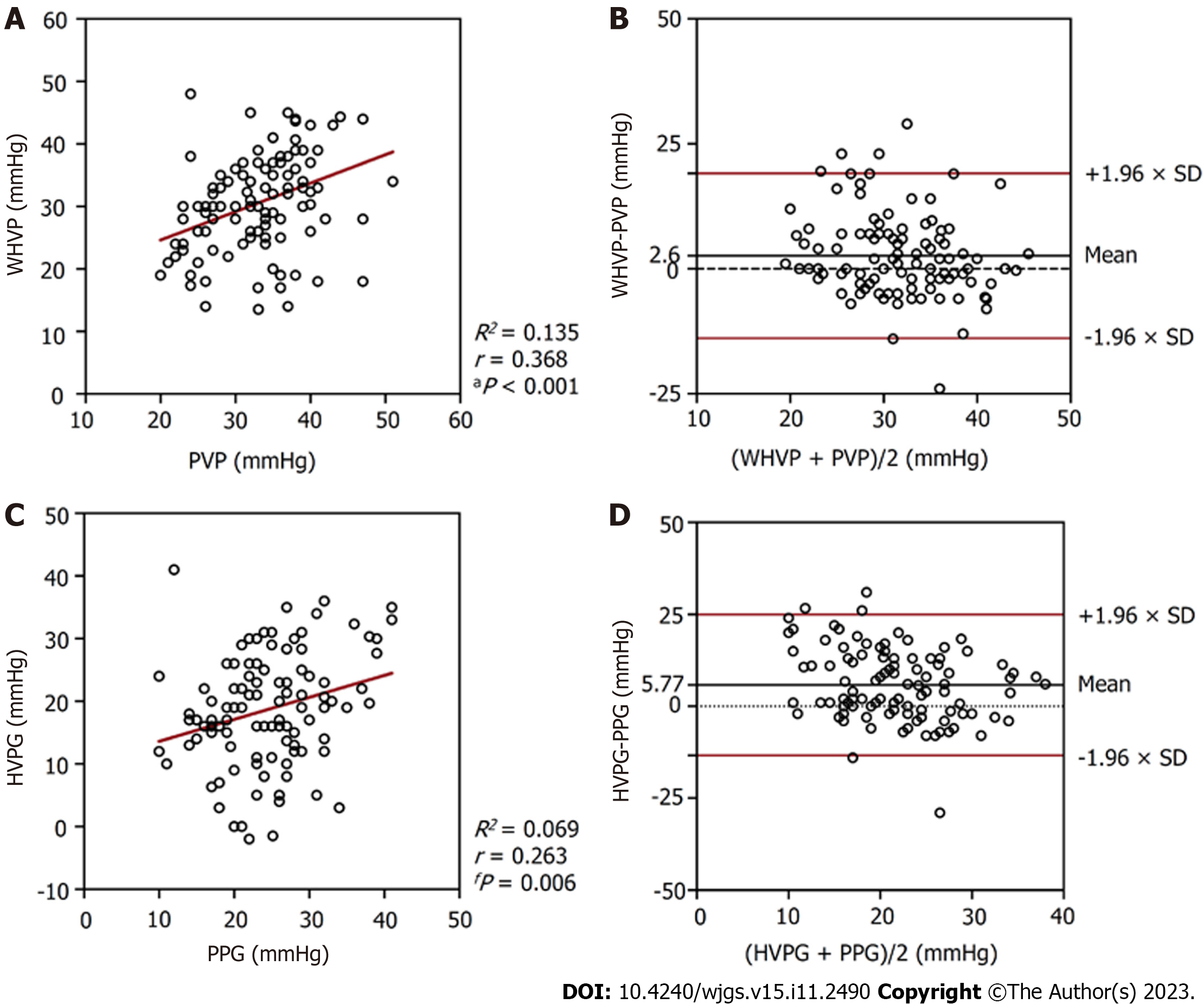
In the 134 patients with alcoholic cirrhosis with PHT complications, the average WHVP was lower than the average PVP (28.70 ± 8.78 mmHg vs 33.58 ± 6.91 mmHg; P < 0.001) (Table 2). The r and R2 values between WHVP and PVP were 0.270 and 0.073, respectively (P = 0.002), and the average difference between them was -4.82 ± 9.60 mmHg [95% limits of agreement (LoA) -23.64 to 13.99] (Table 3 and Figure 3). The average HVPG was lower than the average PPG (16.96 ± 9.41 mmHg vs 24.73 ± 6.92 mmHg; P < 0.001) (Table 2). The r and R2 values between the HVPG and PPG were 0.201 and 0.040, respectively (P = 0.020), and the average difference between them was -7.77 ± 10.50 mmHg (95% LoA -28.35 to 12.80) (Table 3 and Figure 3).
| WHVP (mmHg) | PVP (mmHg) | P value | 95%CI | HVPG (mmHg) | PPG (mmHg) | P value | 95%CI | |
| With collateral branches (n = 26) | 20.89 ± 6.69 | 34.96 ± 8.08 | < 0.001 | 10.52-17.63 | 9.86 ± 7.44 | 25.94 ± 7.42 | < 0.001 | 12.30-19.86 |
| No collateral branch (n = 108) | 30.65 ± 8.17 | 33.25 ± 6.60 | 0.002 | 0.99-4.20 | 18.67 ± 9.05 | 24.44 ± 6.79 | < 0.001 | 3.91-7.64 |
| Total (n = 134) | 28.70 ± 8.78 | 33.58 ± 6.91 | < 0.001 | 3.18-6.46 | 16.96 ± 9.41 | 24.73 ± 6.92 | < 0.001 | 5.98-9.57 |
| WHVP and PVP | HVPG and PPG | |||||||
| r | R2 | P value | 95% LoA (mmHg) | r | R2 | P value | 95% LoA (mmHg) | |
| With collateral branches (n = 26) | 0.303 | 0.092 | 0.133 | -3.16-31.31 | 0.208 | 0.043 | 0.309 | -2.25-34.40 |
| No collateral branch (n = 108) | 0.368 | 0.135 | < 0.001 | -13.88-19.07 | 0.263 | 0.069 | 0.006 | -13.41-24.95 |
| Total (n = 134) | 0.270 | 0.073 | 0.002 | -23.64-13.99 | 0.201 | 0.040 | 0.020 | -28.35-12.80 |
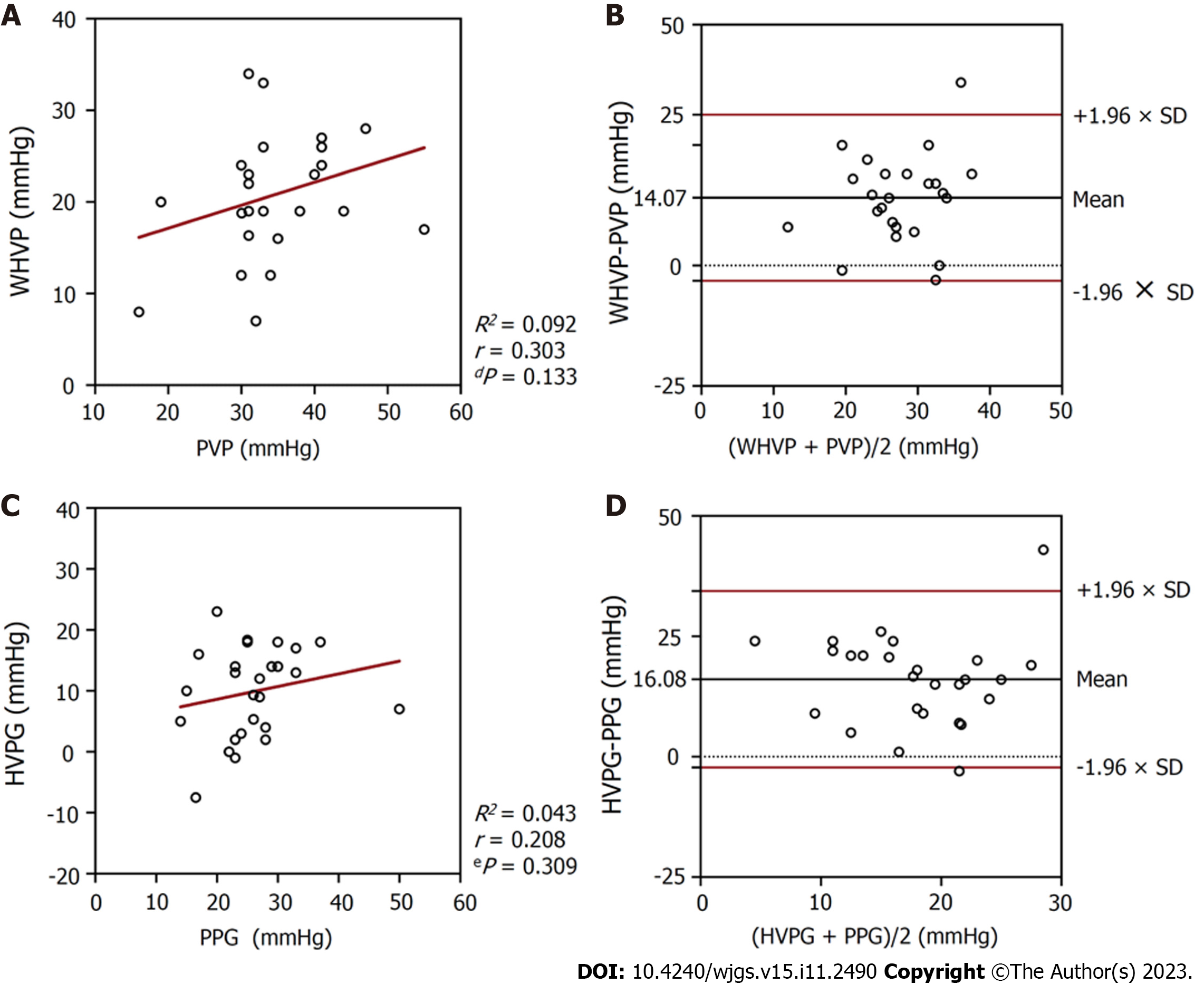
In the 26 patients with collateral branches, the average WHVP was lower than the average PVP (20.89 ± 6.69 mmHg vs 34.96 ± 8.08 mmHg; P < 0.001) (Table 2). The r and R2 values between WHVP and PVP were 0.303 and 0.092, respectively (P = 0.133), and the average difference between them was 14.07 ± 8.79 mmHg (95% LoA -3.16 to 31.31) (Table 3 and Figure 4). The average HVPG was lower than the average PPG (9.86 ± 7.44 mmHg vs 25.94 ± 7.42 mmHg; P < 0.001) (Table 2). The r and R2 values between the HVPG and PPG were 0.208 and 0.043, respectively (P = 0.309), and the average difference between them was 16.08 ± 9.35 mmHg (95% LoA -2.25 to 34.40) (Table 3 and Figure 4).
In the 108 patients with no collateral branches, the average WHVP was lower than the average PVP (30.65 ± 8.17 mmHg vs 33.25 ± 6.60 mmHg; P = 0.002) (Table 2). The r and R2 values between WHVP and PVP were 0.368 and 0.135, respectively (P < 0.001), and the average difference between them was 2.60 ± 8.41 mmHg (95% LoA -13.88 to 19.07) (Table 3 and Figure 5). The average HVPG was lower than the average PPG (18.67 ± 9.05 mmHg vs 24.44 ± 6.79 mmHg; P < 0.001) (Table 2). The r and R2 values between the HVPG and PPG were 0.263 and 0.069, respectively (P = 0.006), and the average difference between them was 5.77 ± 9.79 mmHg (95% LoA -13.41 to 24.95 mmHg (mean) (Table 3 and Figure 5).
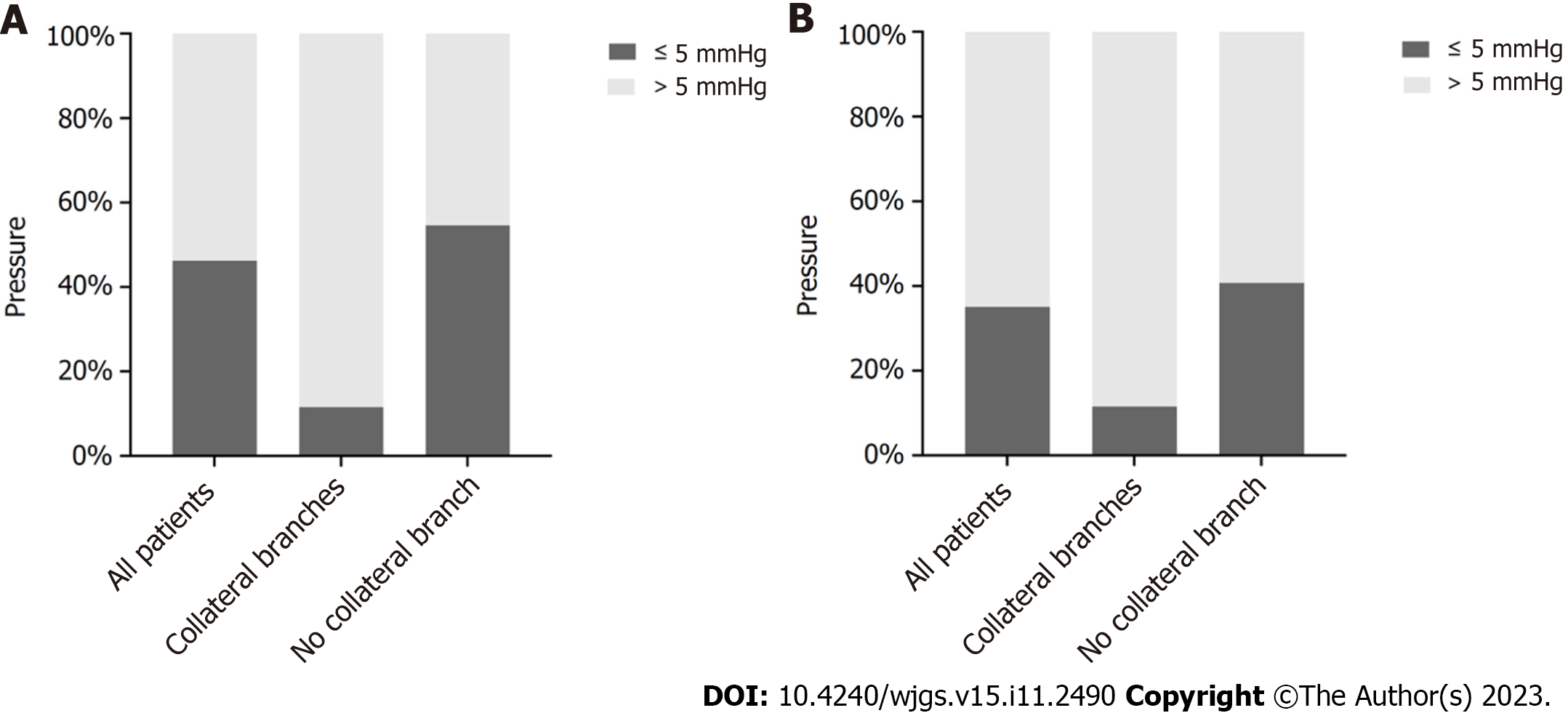
As shown in Figure 6, 3 (11.54%) patients in the collateral branches group and 59 (54.63%) patients in the no collateral branches groups had differences between WHVP and PVP of less than 5 mmHg. In addition, 3 (11.54%) patients in the collateral branches group and 44 (40.74%) patients in the no collateral branches groups had differences between HVPG and PPG of less than 5 mmHg.
Alcoholic cirrhosis associated with PHT is a common liver disease. Based on the pathological changes in the liver, significant changes in liver hemodynamics occur, and vascular resistance is increased, causing gradually elevated PVP. Clinically, the symptoms gradually become more conspicuous and their severity is directly associated with the degree of PVP[9]. In clinical practice, the degree of PHT in patients with liver cirrhosis is mainly evaluated based on clinical symptoms, signs, imaging examinations, and gastroscopy; however, these evaluation methods typically have a low sensitivity. Accurate assessments require direct PVP measurements[10]. Comparing the risks and benefits of the assessment, the technique is too complicated, and the trauma is too severe. In recent years, many researchers have measured the hepatic vein pressure instead of directly measuring PVP as the gold standard to reflect PVP in clinical applications[11,12]. Research on noninvasive portal vein manometry also considers the hepatic vein pressure as a standard. There are two principal ways to measure the hepatic vein pressure: Intubating the hepatic vein through the jugular or femoral vein to measure WHVP or FHVP, respectively. HVPG is calculated by subtracting FHVP from WHVP. WHVP can be measured using two methods: Measuring the pressure after the balloon blocks the hepatic vein or obtaining the pressure by inserting an end-hole catheter into the end of the branch of the hepatic vein. The former method is more accurate and is commonly used[13]. In normal liver hemodynamics, PVP is equal to or greater than the hepatic sinus pressure, WHVP is equal to the hepatic sinus pressure, and FHVP is 0.5–1.0 mmHg higher than IVCP[7]. Therefore, HVPG indirectly represents PPG and the portal vein perfusion pressure[8,14]. It is more meaningful to use PPG than PVP to predict the risk of various PHT complications[8,15]. Significant pathological changes occur in the hepatic tissue structure of patients with alcoholic cirrhosis and PHT, resulting in significant changes in liver hemodynamics. Regarding whether WHVP is representative of PVP, some scholars believe that WHVP and PVP are correlated in patients with alcoholic cirrhosis[16,17]. However, this remains controversial, as the number of cases is limited. Difference in WHVP and PVP have been reported in patients with cirrhosis with the same etiology but different pathology types. In macronodular cirrhosis, WHVP and PVP are poorly correlated[18], possibly due to the existence of normal tissues between macro
The factors influencing the pressure measurements were strictly controlled before the operation. Patients who received preoperative health education and those who underwent elective surgery were selected, and patients were given adequate psychological preparation to allow for active compliance with the procedure. Suitable paracentesis was performed for patients with large ascites, and the use of drugs that affect the venous pressure, such as non-selective β-receptor blockers that influence PVP[12,20-22], or propofol deep sedation, which can affect PPG[19,23], were avoided. During the TIPS procedure and pressure measurement, clinicians should perform local anesthesia, measure the pressure after the patient is stable, repeat the measurement several times, and ensure that the catheter is at the same location for each measurement. Gallbladder-heart reflections and incomplete balloon occlusion shoud be considered during the procedure. If these conditions are not corrected, patients should be excluded.
Our study was limited by the fact that this was a retrospective study with a small sample size. Although all study patients had alcoholic cirrhosis patients, the degree of progression of cirrhosis and the pathological type varied, with some cases progressing to hepatocellular carcinoma, resulting in different hepatic structural and hemodynamic changes, the potential impact of which is unclear. Considering these preliminary findings, prospective studies are necessary to validate our findings.
In conclusion, the correlation is very poor between WHVP and PVP as well as that between HVPG and PPG in patients with alcoholic cirrhosis and complications of PHT. HVPG cannot accurately represent PPG in most patients, and the former is lower than the latter. The formation of hepatic collaterals is a vital reason for the strong underestimation of HVPG. Except for the influencing factors of hepatic collateral branches in a few patients, the correlation in most cases remained poor. The cause remains unknown, and further investigations are required in this area to elucidate these mechanisms.
The hepatic venous pressure gradient (HVPG), rather than the portal venous pressure gradient (PPG), is regarded as the gold standard for diagnosing portal hypertension (PHT).
The relationship between HVPG and PPG is controversial and lacks substantial research to prove it.
This study aimed to classify the correlation between HVPG and PPG in patients with alcoholic cirrhosis and PHT.
This retrospective analysis of various pressures during transjugular intrahepatic portosystemic shunt (TIPS) procedures explored the relationship between HVPG and PPG in patients with alcoholic cirrhosis and PHT.
The correlation coefficient (r) and determination coefficient (R2) between HVPG and PPG were 0.201 and 0.040, respectively (P = 0.020). Hepatic collaterals were identified in 26 patients with balloon occlusion hepatic venography (19.4%), while the average PPG was significantly higher than the average HVPG (25.94 ± 7.42 mmHg vs 9.86 ± 7.44 mmHg; P < 0.001). The collateral versus no collateral branches groups had 3 (11.54%) and 44 (40.74%) patients, respectively, with differences of < 5 mmHg between HVPG and PPG.
HVPG cannot accurately represent PPG in most patients. The formation of hepatic collaterals is a vital reason for the strong underestimation of HVPG.
Based on different pressures during TIPS procedures, the correlation and differences between HVPG and PPG of patients were explored.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MHF, Egypt; Mahmoud MZ, Saudi Arabia S-Editor: Lin C L-Editor: A P-Editor: Zhang YL
| 1. | Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20:37-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 265] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 2. | Hyun J, Han J, Lee C, Yoon M, Jung Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 3. | Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. 2022;75:473-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 260] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 4. | Lucey MR. Alcohol-Associated Cirrhosis. Clin Liver Dis. 2019;23:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Vilela EG, Thabut D, Rudler M, Bittencourt PL. Management of Complications of Portal Hypertension. Can J Gastroenterol Hepatol. 2019;2019:6919284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 219] [Article Influence: 54.8] [Reference Citation Analysis (1)] |
| 7. | Allaire M, Rudler M, Thabut D. Portal hypertension and hepatocellular carcinoma: Des liaisons dangereuses…. Liver Int. 2021;41:1734-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Turco L, Garcia-Tsao G. Portal Hypertension: Pathogenesis and Diagnosis. Clin Liver Dis. 2019;23:573-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Masuda Y, Yoshizawa K, Ohno Y, Mita A, Shimizu A, Soejima Y. Small-for-size syndrome in liver transplantation: Definition, pathophysiology and management. Hepatobiliary Pancreat Dis Int. 2020;19:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Wan S, Wei Y, Zhang X, Yang C, Hu F, Song B. Computed Tomography-Based Texture Features for the Risk Stratification of Portal Hypertension and Prediction of Survival in Patients With Cirrhosis: A Preliminary Study. Front Med (Lausanne). 2022;9:863596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | La Mura V, Garcia-Guix M, Berzigotti A, Abraldes JG, García-Pagán JC, Villanueva C, Bosch J. A Prognostic Strategy Based on Stage of Cirrhosis and HVPG to Improve Risk Stratification After Variceal Bleeding. Hepatology. 2020;72:1353-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Zhang W, Peng C, Zhang S, Huang S, Shen S, Xu G, Zhang F, Xiao J, Zhang M, Zhuge Y, Wang L, Zou X, Lv Y. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest Endosc. 2021;93:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Li L, Liu S, Wu H, Qi X. Standardized HVPG measurement: call for action. Hepatol Int. 2022;16:737-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Reiniš J, Petrenko O, Simbrunner B, Hofer BS, Schepis F, Scoppettuolo M, Saltini D, Indulti F, Guasconi T, Albillos A, Téllez L, Villanueva C, Brujats A, Garcia-Pagan JC, Perez-Campuzano V, Hernández-Gea V, Rautou PE, Moga L, Vanwolleghem T, Kwanten WJ, Francque S, Trebicka J, Gu W, Ferstl PG, Gluud LL, Bendtsen F, Møller S, Kubicek S, Mandorfer M, Reiberger T. Assessment of portal hypertension severity using machine learning models in patients with compensated cirrhosis. J Hepatol. 2023;78:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Karagiannakis DS, Voulgaris T, Siakavellas SI, Papatheodoridis GV, Vlachogiannakos J. Evaluation of portal hypertension in the cirrhotic patient: hepatic vein pressure gradient and beyond. Scand J Gastroenterol. 2018;53:1153-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Osada Y, Kanazawa H, Narahara Y, Mamiya Y, Nakatsuka K, Sakamoto C. Wedged hepatic venous pressure does not reflect portal pressure in patients with cirrhosis and hepatic veno-venous communications. Dig Dis Sci. 2008;53:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Ferrusquía-Acosta J, Bassegoda O, Turco L, Reverter E, Pellone M, Bianchini M, Pérez-Campuzano V, Ripoll E, García-Criado Á, Graupera I, García-Pagán JC, Schepis F, Senzolo M, Hernández-Gea V. Agreement between wedged hepatic venous pressure and portal pressure in non-alcoholic steatohepatitis-related cirrhosis. J Hepatol. 2021;74:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | Pomier-Layrargues G, Kusielewicz D, Willems B, Villeneuve JP, Marleau D, Côté J, Huet PM. Presinusoidal portal hypertension in non-alcoholic cirrhosis. Hepatology. 1985;5:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Reverter E, Blasi A, Abraldes JG, Martínez-Palli G, Seijo S, Turon F, Berzigotti A, Balust J, Bosch J, García-Pagán JC. Impact of deep sedation on the accuracy of hepatic and portal venous pressure measurements in patients with cirrhosis. Liver Int. 2014;34:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Simonetto DA, Liu M, Kamath PS. Portal Hypertension and Related Complications: Diagnosis and Management. Mayo Clin Proc. 2019;94:714-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (34)] |
| 21. | Giannitrapani L, Granà W, Licata A, Schiavone C, Montalto G, Soresi M. Nontumorous Portal Vein Thrombosis in Liver Cirrhosis: Possible Role of β-Blockers. Med Princ Pract. 2018;27:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Danielsen KV, Nabilou P, Wiese SS, Hove JD, Bendtsen F, Møller S. Effect of beta-blockers on multiple haemodynamics in cirrhosis: A cross-over study by MR-imaging and hepatic vein catheterization. Liver Int. 2023;43:2245-2255. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Ebrahimi F, Semela D, Heim M. Impact of propofol sedation on the diagnostic accuracy of hepatic venous pressure gradient measurements in patients with cirrhosis. Hepatol Int. 2022;16:817-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |