Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2445
Peer-review started: August 30, 2023
First decision: September 13, 2023
Revised: September 22, 2023
Accepted: October 17, 2023
Article in press: October 17, 2023
Published online: November 27, 2023
Processing time: 89 Days and 1.6 Hours
Radical surgery is the most commonly used treatment for hepatocellular carcinoma (HCC). However, the surgical effect remains not ideal, and prognostic evaluation is insufficient. Furthermore, clinical intervention is rife with uncertainty and not conducive to prolonging patient survival.
To explore correlations between the systemic immune inflammatory index (SII) and geriatric nutritional risk index (GNRI) and HCC operation prognosis.
This retrospective study included and collected follow up data from 100 HCC. Kaplan–Meier survival curves were used to analyze the correlation between SII and GNRI scores and survival. SII and GNRI were calculated as follows: SII = neutrophil count × platelet count/lymphocyte count; GNRI = [1.489 × albumin (g/L) + 41.7 × actual weight/ideal weight]. We analyzed the predictive efficacy of the SII and GNRI in HCC patients using receiver operating characteristic (ROC) curves, and the relationships between the SII, GNRI, and survival rate using Kaplan–Meier survival curves. Cox regression analysis was utilized to analyze independent risk factors influencing prognosis.
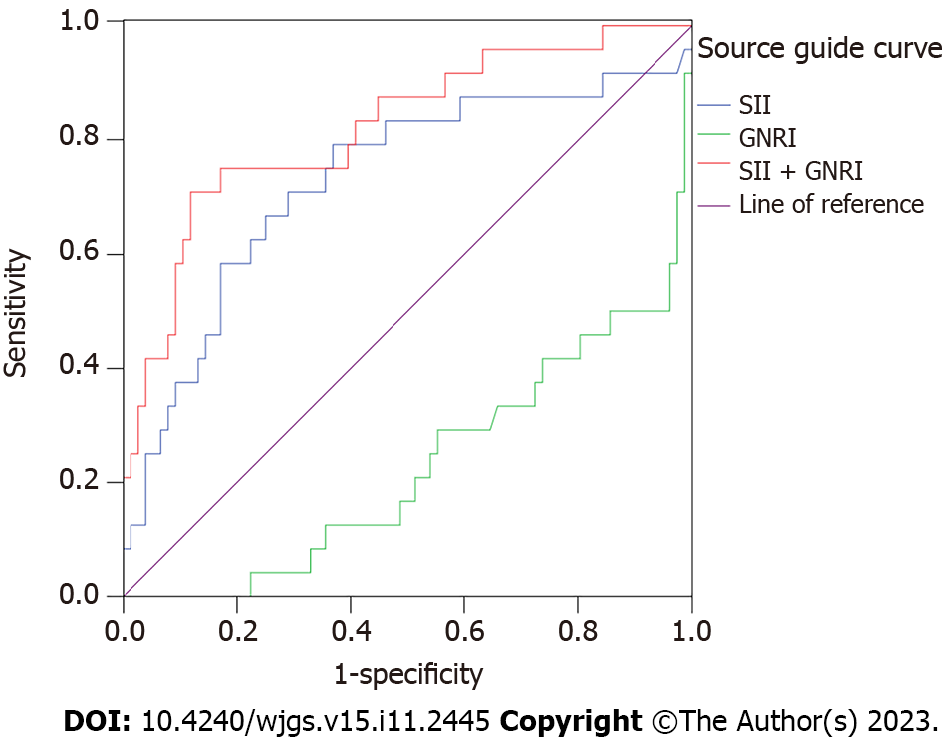
After 1 year of follow-up, 24 patients died and 76 survived. The area under the curve (AUC), sensitivity, specificity, and the optimal cutoff value of SII were 0.728 (95% confidence interval: 0.600-0.856), 79.2%, 63.2%, and 309.14, respectively. According to ROC curve analysis results for predicting postoperative death in HCC patients, the AUC of SII and GNRI combination was higher than that of SII or GNRI alone, and SII was higher than that of GNRI (P < 0.05). The proportion of advanced differentiated tumors, tumor maximum diameter (5–10 cm, > 10 cm), lymph node metastasis, and TNM stage III-IV in patients with SII > 309.14 was higher than that in patients with SII ≤ 309.14 (P < 0.05). The proportion of patients aged > 70 years was higher in patients with GNRI ≤ 98 than that in patients with GNRI > 98 (P < 0.05). The 1-year survival rate of the SII > 309.14 group (compared with the SII ≤ 309.14 group) and GNRI ≤ 98 group (compared with the GNRI > 98 group) was lower (P < 0.05).
The prognosis after radical resection of HCC is related to the SII and GNRI and poor in high SII or low GNRI patients.
Core Tip: Hepatocellular carcinoma (HCC) has a high incidence and mortality. We evaluated the systemic immune inflammatory index (SII), geriatric nutritional risk index (GNRI), and clinicopathological features of 100 patients undergoing radical HCC resection in this research. We analyzed the correlation between SII, GNRI, and clinicopathological characteristics and addressed the problem of weak prognostic assessment by studying the changes in survival rates of patients undergoing HCC treatment under different levels of SII and GNRI.
- Citation: Li J, Shi HY, Zhou M. Correlation between preoperative systemic immune inflammation index, nutritional risk index, and prognosis of radical resection of liver cancer. World J Gastrointest Surg 2023; 15(11): 2445-2455
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2445.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2445
Hepatocellular carcinoma (HCC) is the leading type of liver cancer, accounting for 90 percent of all liver tumors[1]. The prevalence and mortality of HCC are increasing annually, posing a significant threat to the health of residents. The onset of HCC is insidious, and the early symptoms are not obvious. Usually, when its clinical symptoms or signs appear, the disease has already progressed to the middle and late stages. The early diagnosis, treatment, and prognosis of HCC have received widespread attention. Clinical treatments for liver cancer are mainly surgical resection, radiofrequency ablation, and percutaneous hepatic arterial chemoembolization. As the primary treatment for resectable liver cancer, surgical resection can prolong the postoperative survival in patients; however, this is still not ideal. Early prognosis prediction and timely individualized therapeutic strategies are crucial for improving patient prognosis. Clinical indicators of prognosis include alpha-fetoprotein, tumor stage, vascular tumor thrombus, and tumor size[2,3]; however, these traditional clinicopathological features have limited predictive value. Recently, the systemic immune inflammatory index (SII) and geriatric nutritional risk index (GNRI) have become the focus of clinical research. They are easy to obtain and have been shown to be good predictors of prognosis of various solid tumors[4-7]. However, we found few reports on the application of the SII or GNRI in predicting the prognosis of HCC despite an urgent need to explore a new and widely used prognostic index of HCC after radical resection. Therefore, to guide clinical practice, we analyzed the clinical data of HCC patients undergoing radical resection with the aim to explore the relationship between the SII and GNRI and prognosis.
We screened 100 HCC patients who underwent radical resection in the Liuzhou Hospital of Traditional Chinese Medicine from January 2021 to December 2021. Among the included patients, there were 70 men and 30 women with the age of 68.78 ± 6.69 years old. According to the Child-Pugh classification there were 84 cases of grade A and 16 of grade B. Based on the Barcelona Clinic Liver Cancer staging there were 13 cases of stage 0, 35 of stage A, 42 of stage B, and 10 of stage C.
Inclusion criteria: (1) According to the relevant criteria in the “diagnostic criteria for primary liver cancer[8]”, HCC was clinically diagnosed and confirmed by pathology; (2) Age ≥ 60 years; (3) First onset; (4) No preoperative chemoradiotherapy; (5) Patients received radical resection of liver cancer and did not die during the perioperative period; (6) Preoperative SII, GNRI, and clinicopathological features were complete; and (7) Patients could be followed up normally for at least 1 year after surgery, and the clinical data were not missing. The exclusion criteria were as follows: (1) Previous liver surgery; (2) Combination with malignant tumors other than HCC; (3) Combination with other acute or chronic diseases or immune system diseases; (4) A history of drug allergy; and (5) An estimated survival time of < 6 mo.
The preoperative SII, GNRI, and clinicopathological features were obtained from electronic medical record system. SII calculation formula: SII = neutrophil count × platelet count/lymphocyte count[9]. It was determined that there were no infectious diseases, such as pulmonary or urinary tract infections, within 7 d before the radical resection of liver cancer. After special treatment without inhibition and/or promotion of bone marrow growth, the blood routine 3 d before the operation was defined to calculate the SII.
The source of GNRI was as follows: GNRI = [1.489 × albumin (g/L) + 41.7 × actual weight/ideal weight][10]. The ideal weight was calculated according to the Lorenz equation, male: Height − 100 − [(height − 150)/4]; female: Height − 100 − [(height − 150)/2.5]. When the patient's actual weight exceeded the ideal weight, the actual weight/ideal weight ratio was set at 1. GNRI > 98 was considered as normal nutrition, and GNRI ≤ 98 was considered at risk of malnutrition.
The clinicopathological features included sex, age, hepatitis B markers, degree of differentiation, maximum tumor diameter, number of tumors, ascites, lymph node metastasis, TNM stage, capsule integrity, portal vein tumor thrombus, Child-Pugh classification, and alpha-fetoprotein expression.
Patients were followed-up by outpatient, telephone, or readmission after the operation, and survival was calculated at the last follow-up. They were followed up every 1 m for 3 mo after the operation, and then every 3 mo for 1 year. The follow-up period ranged from 1 to 12 mo, and the last follow-up was on December 31, 2022.
All patients underwent conventional radical resection for liver cancer, with 62 undergoing regular hepatectomy and 38 limited hepatectomy. The clinical stage was identified on the basis of the American Cancer Diagnostic Criteria[11], and the degree of differentiation was distinguished in line with histopathological results. For the detection of alpha-fetoprotein, 3 mL of the morning fasting venous blood was centrifuged for 10 min at 3000 r/min. The supernatant was placed in an EP tube and then stored at -20 °C. Serum alpha-fetoprotein expression levels were detected using the cobas e 411 automatic electrochemiluminescence immunoassay analyzer (German Roche, Approval number: China Food and Drug Administration (Jin) Zi 2011 No. 3402843) and the supporting original kit. Alpha-fetoprotein expression > 20 μg/L was positive and ≤ 20 μg/L was negative.
Statistical software SPSS 23.0 and Excel 2016 were used for data analysis. The measurement data are presented as x ± s and compared using t-tests. The enumeration data are described by the number of cases and rate and analyzed using χ2 or corrected χ2 tests. The receiver operating characteristic (ROC) curve was used to observe the area under the curve (AUC) and analyze the efficacy of the SII and GNRI in predicting the death of HCC patients. We used the Kaplan–Meier model for the survival time cohort data and tested it by a log rank approach. Cox regression analysis was applied to analyze the independent risk factors affecting prognosis. Because these were bilateral tests, the statistical test level was α = 0.05.
In this study, 24 patients died, and 76 survived after 1 year of follow-up. The AUC, sensitivity, specificity, and the optimal cut-off value of SII were 0.728 (95% confidence interval: 0.600-0.856), 79.2%, 63.2%, and 309.14, respectively.
The AUC of the SII combined with the GNRI was higher than that of the SII or GNRI alone. Meanwhile, the AUC of the SII was higher than that of the GNRI (P < 0.05) (Table 1, Figure 1). Thus, the combined prediction ability of SII and GNRI is the highest for predicting mortality in patients undergoing radical hepatectomy, and the prediction ability of SII alone is higher than that of GNRI alone.
There were 47 patients with a SII > 309.14 and 53 with a SII ≤ 309.14. The proportion of well-differentiated tumors, maximum tumor diameter (5–10 cm, > 10 cm), lymph node metastasis, and TNM stage III-IV in patients with SII > 309.14 was higher than that in patients with SII ≤ 309.14 (all P < 0.05) (Table 2).
| Indexes | Number of cases | SII | χ2 | P value | |
| > 309.14 (47 cases) | ≤ 309.14 (53 cases) | ||||
| Gender | 0.002 | 0.965 | |||
| Male | 70 | 33 (70.21) | 37 (69.81) | ||
| Female | 30 | 14 (29.79) | 16 (30.19) | ||
| Age | 0.047 | 0.828 | |||
| ≤ 70 yr old | 33 | 15 (31.91) | 18 (33.96) | ||
| > 70 yr old | 67 | 32 (68.09) | 35 (66.04) | ||
| Hepatitis B markers | 0.097 | 0.755 | |||
| Negative | 27 | 12 (25.53) | 15 (28.30) | ||
| Positive | 73 | 35 (74.47) | 38 (71.70) | ||
| Degree of differentiation | 4.643 | 0.031 | |||
| Middle-low differentiation | 55 | 20 (42.55) | 35 (66.04) | ||
| High differentiation | 45 | 27 (57.45) | 18 (33.96) | ||
| Maximum tumor diameter | 6.807 | 0.033 | |||
| 5 cm | 28 | 8 (17.02) | 20 (37.74) | ||
| 5-10 cm | 54 | 27 (57.45) | 27 (50.94) | ||
| > 10 cm | 18 | 12 (25.53) | 6 (11.32) | ||
| Number of tumors | 0.004 | 0.948 | |||
| 3 | 13 | 6 (12.77) | 7 (13.21) | ||
| ≥ 3 | 87 | 41 (87.23) | 46 (86.79) | ||
| Ascites | 0.846 | 0.358 | |||
| No | 79 | 39 (82.98) | 40 (75.47) | ||
| Yes | 21 | 8 (17.02) | 13 (24.53) | ||
| Lymph node metastasis | 8.687 | 0.003 | |||
| Yes | 38 | 21 (44.68) | 17 (32.08) | ||
| No | 62 | 26 (55.32) | 36 (67.92) | ||
| TNM staging | 7.517 | 0.006 | |||
| I–II | 59 | 21 (44.68) | 38 (71.70) | ||
| III–IV | 41 | 26 (55.32) | 15 (28.30) | ||
| Envelope Integrity | 0.525 | 0.469 | |||
| Complete | 57 | 25 (53.19) | 32 (60.38) | ||
| Incomplete | 43 | 22 (46.81) | 21 (39.62) | ||
| Portal vein tumor thrombus | 0.200 | 0.655 | |||
| Yes | 32 | 14 (29.79) | 18 (33.96) | ||
| No | 68 | 33 (70.21) | 35 (66.04) | ||
| Child-Pugh classification | 0.424 | 0.515 | |||
| A | 54 | 27 (57.45) | 27 (50.94) | ||
| B | 46 | 20 (42.55) | 26 (49.06) | ||
| Alpha-fetoprotein expression | 0.102 | 0.749 | |||
| Negative | 43 | 21 (44.68) | 22 (41.51) | ||
| Positive | 57 | 26 (55.32) | 31 (58.49) | ||
There were 20 patients with a GNRI ≤ 98 and 80 with a GNRI > 9 8. The proportion of patients aged > 70 years was higher in patients with GNRI ≤ 98 than that in patients with GNRI > 98 (P < 0.05) (Table 3).
| Indexes | Number of cases | GNRI | χ2 | P value | |
| ≤ 98 (20 cases) | > 98 (80cases) | ||||
| Gender | 0.298 | 0.585 | |||
| Male | 70 | 15 (75.00) | 55 (68.75) | ||
| Female | 30 | 5 (25.00) | 25 (31.25) | ||
| Age | 4.752 | 0.029 | |||
| ≤ 70 yr old | 33 | 2 (10.00) | 31 (38.75) | ||
| > 70 yr old | 67 | 18 (90.00) | 49 (61.25) | ||
| Hepatitis B markers | 0.257 | 0.612 | |||
| Negative | 27 | 4 (20.00) | 23 (28.75) | ||
| Positive | 73 | 16 (80.00) | 57 (71.25) | ||
| Degree of differentiation | < 0.001 | > 0.999 | |||
| Middle-low differentiation | 55 | 11 (55.00) | 44 (55.00) | ||
| High differentiation | 45 | 9 (45.00) | 36 (45.00) | ||
| Maximum tumor diameter | 0.141 | 0.932 | |||
| < 5 cm | 28 | 5 (25.00) | 23 (28.75) | ||
| 5-10 cm | 54 | 11 (55.00) | 43 (53.75) | ||
| > 10 cm | 18 | 4 (20.00) | 14 (17.50) | ||
| Number of tumors | 0.174 | 0.677 | |||
| < 3 | 10 | 3 (15.00) | 7 (8.75) | ||
| ≥ 3 | 90 | 17 (85.00) | 73 (91.25) | ||
| Ascites | 1.221 | 0.269 | |||
| No | 79 | 14 (70.00) | 65 (81.25) | ||
| Yes | 21 | 6 (30.00) | 15 (18.75) | ||
| Lymph node metastasis | 0.042 | 0.837 | |||
| Yes | 38 | 8 (40.00) | 30 (37.50) | ||
| No | 62 | 12 (60.00) | 50 (62.50) | ||
| TNM staging | 0.056 | 0.812 | |||
| I–II | 77 | 15 (75.00) | 62 (77.50) | ||
| III–IV | 23 | 5 (25.00) | 18 (22.50) | ||
| Envelope Integrity | 0.092 | 0.762 | |||
| Complete | 57 | 12 (60.00) | 45 (56.25) | ||
| Incomplete | 43 | 8 (40.00) | 35 (43.75) | ||
| Portal vein tumor thrombus | 0.103 | 0.748 | |||
| Yes | 32 | 7 (35.00) | 25 (31.25) | ||
| No | 68 | 13 (65.00) | 55 (68.75) | ||
| Child-Pugh classification | < 0.001 | > 0.999 | |||
| A | 60 | 12 (60.00) | 48 (60.00) | ||
| B | 40 | 8 (40.00) | 32 (40.00) | ||
| Alpha-fetoprotein expression | 0.092 | 0.762 | |||
| Negative | 43 | 8 (40.00) | 35 (43.75) | ||
| Positive | 57 | 12 (60.00) | 45 (56.25) | ||
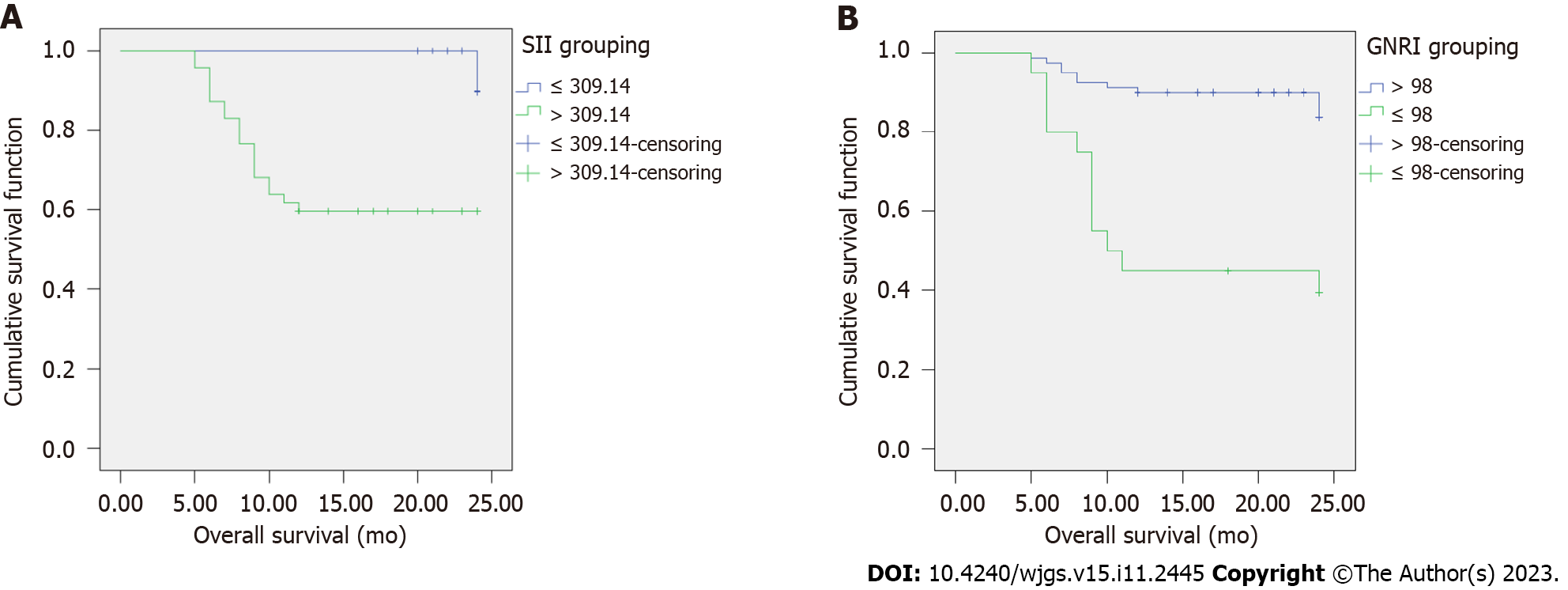
According to the Kaplan–Meier survival curve, the 1-year survival rates of the SII > 309.14 and GNRI ≤ 98 groups were 40.43% (19/47) and 60.00% (12/20), respectively, and those of the SII ≤ 309.14 and GNRI > 98 groups were 9.43% (5/53) and 15.00% (12/80), respectively. Compared with the SII ≤ 309.14 group, the 1-year survival rate of the SII > 309.14 group was lower; compared with the GNRI ≤ 98 group, the 1-year survival rate of the GNRI ≤ 98 group was lower (all P < 0.05) (Table 4, Figure 2).
| Indicators | Number of follow-up cases | 1-year survival (rate, %) | Log-rank test | |
| χ2 | P value | |||
| SII | 17.706 | < 0.001 | ||
| > 309.14 | 47 | 19 (40.43) | ||
| ≤ 309.14 | 53 | 5 (9.43) | ||
| GNRI | 21.624 | < 0.001 | ||
| > 98 | 80 | 12 (15.00) | ||
| ≤ 98 | 20 | 12 (60.00) | ||
Multivariate analysis of prognosis was performed by incorporating the SII, GNRI, and pathological features into the Cox proportional hazard regression model. The SII and GNRI were independent risk factors (P < 0.05) (Table 5).
| Variable | B | SE | Wald | P value | RR | 95%CI | |
| Lower limit | Upper limit | ||||||
| SII | 2.345 | 0.639 | 13.445 | < 0.001 | 10.429 | 2.978 | 36.518 |
| GNRI | 1.490 | 0.532 | 7.833 | 0.005 | 4.438 | 1.563 | 12.602 |
| Gender | 0.600 | 0.528 | 1.291 | 0.256 | 1.822 | 0.647 | 5.131 |
| Age | 0.041 | 0.025 | 2.760 | 0.097 | 1.042 | 0.993 | 1.093 |
| Hepatitis B markers | -0.339 | 0.527 | 0.414 | 0.520 | 0.713 | 0.254 | 2.000 |
| Degree of differentiation | 0.072 | 0.619 | 0.014 | 0.907 | 1.075 | 0.320 | 3.617 |
| Maximum tumor diameter | -0.056 | 0.053 | 1.091 | 0.296 | 0.946 | 0.852 | 1.050 |
| Number of tumors | -0.148 | 0.082 | 3.275 | 0.070 | 0.863 | 0.735 | 1.012 |
| Ascites | -0.020 | 0.554 | 0.001 | 0.971 | 0.980 | 0.331 | 2.902 |
| Lymph node metastasis | 0.226 | 0.495 | 0.209 | 0.647 | 1.254 | 0.475 | 3.309 |
| TNM staging | -0.478 | 0.568 | 0.709 | 0.400 | 0.620 | 0.204 | 1.886 |
| Envelope Integrity | -0.456 | 0.493 | 0.853 | 0.356 | 0.634 | 0.241 | 1.668 |
| Portal vein tumour thrombus | 0.581 | 0.479 | 1.470 | 0.225 | 1.788 | 0.699 | 4.575 |
| Child-Pugh classification | 0.321 | 0.467 | 0.472 | 0.492 | 1.378 | 0.552 | 3.440 |
| Alpha-fetoprotein expression | 0.095 | 0.504 | 0.036 | 0.851 | 1.100 | 0.409 | 2.955 |
The morbidity and mortality associated with HCC are at the forefront of malignant tumor research[12]. Radical resection of liver cancer is one of the main treatment methods and is associated with a high postoperative mortality rate, which can be confusing for surgeons. Tumor progression and invasion depend on the characteristics of tumor cells that are closely related to the tumor microenvironment[13]. Inflammatory cells are an integral part of the tumor microenvironment. These cells, including tumor necrosis factor-α and vascular endothelial growth factor, not only promote the formation of new blood vessels but also regulate the proliferation and invasion of tumor cells and affect their apoptosis[14,15]. In addition, due to factors such as insufficient nutritional intake and high metabolism in tumor cells, the probability of disease-related malnutrition is greatly increased[10], which substantially reduces the prognosis.
We found that the 1-year mortality rate in HCC patients undergoing radical resection was 24%, which was similar to previous studies[16]. The patients were classified into SII > 309.14 and SII ≤ 309.14 groups, and 47% were in the SII > 309.14 group. Approximately 20% of preoperative patients had abnormal GNRI (GNRI ≤ 98). Further statistical analysis indicated that the SII was related to tumor differentiation, maximum tumor diameter, lymph node metastasis, TNM stage, and other indicators reflecting the degree of malignancy of HCC. Our results showed a relationship between GNRI and age. Statistical analysis confirmed that the SII can be used as an index to evaluate the immune inflammatory state and malignant biological behavior in patients with HCC before radical resection. In addition, the GNRI can be used as an index to reflect nutritional risk and elderly status. Therefore, the SII and GNRI have guiding values for distinguishing high-risk liver cancer. Finally, the survival curve suggested that the survival rate in preoperative SII > 309.14 patients was significantly lower than that in SII ≤ 309.14 patients, and the survival rate in patients with normal GNRI (≤ 98) was significantly lower than that in patients with abnormal GNRI. This suggests that SII and GNRI can be used to estimate the survival status in patients with HCC after radical resection.
Cox multivariate analysis showed that high SII increased the risk of death in patients by approximately 10 times. SII is an efficient inflammatory immune index based on neutrophil, blood plate, and lymphocyte counts. This index comprehensively reflects the immune function and inflammatory responses. An increase in SII indicates an increase in platelets and neutrophils and a decrease in lymphocytes, suggesting that the body is in a state of enhanced inflammatory response and weak immune function[17]. Neutrophils are divided into N1 and N2 phenotypes, and their functions differ. In the early stages of the tumor, the antitumor effect is mainly exerted by the N1 type. In the middle and late tumor stages, the tumor microenvironment promotes the transformation of the N1 neutrophil phenotype into the N2 type and plays a role in promoting tumor development, tumor angiogenesis, and metastasis[18]. Platelets are a mass of cytoplasm shed from mature megakaryocyte cytoplasm in the bone marrow and are important members of the blood clotting system in the body. In recent years, tumors and tumor stromal cells have been found to secrete a large number of thrombogenic and platelet-activating factors. A large amount of angiogenic regulatory proteins in platelets can also promote tumor neovascular angiogenesis, thus participating in the occurrence and development of tumors[19]. Lymphocytes are the main executors of immune functions and participate in antitumor processes. These values reflect the immune functions of the body. Due to the long-term consumption of tumor cells, patients with HCC experience malnutrition and low immunity, and usually have lower lymphocyte counts. Neutrophil and platelet counts were increased, and the lymphocyte count decreased in patients with HCC, which jointly promoted an increase in SII.
The GNRI is a simple, accurate, and objective tool for assessing nutrition-related risks using indicators such as height, weight, and albumin. Changes in its value are accompanied by changes in the development of malignant tumors and overall survival rate in patients[20]. It can predict nutrition-related complications and mortality risk[21]. The GNRI is determined using only serum albumin level, height, and weight. Some scholars have proposed that the GNRI is related to perioperative and postoperative complications, postoperative recurrence, and the overall survival rate in patients with various malignant tumors. It can be used as an important predictor in the prognostic evaluation of gastric, stage I lung, and colorectal cancers[22]. Cox multivariate analysis showed that high GNRI increased the risk of death in patients by approximately 4 times. Serum albumin levels are routinely used to evaluate malnutrition. Scheufele et al[23] found that low preoperative serum albumin levels were associated with in-hospital mortality in patients undergoing esopha
In summary, the prognosis of patients with HCC after radical resection is related to the SII and GNRI. The prognosis was poor in patients with a high SII or low GNRI.
The prognostic effect of radical hepatocellular carcinoma (HCC) surgery is not ideal, and clinicians urgently need a reliable evaluation index to guide further clinical interventions.
Prognostic indicators for HCC after radical resection are lacking. The systemic immune inflammatory index (SII) and geriatric nutritional risk index (GNRI) are effective in predicting the prognosis of tumors; however, few attempts have been made to apply them to the prognosis of HCC.
To analyze the relationship between the SII and GNRI and the clinicopathological features in patients undergoing radical HCC resection, we further explored the correlation between the SII and GNRI and mortality and explained the possible causes.
This study retrospectively analyzed the SII, GNRI, and clinicopathological data in patients with HCC undergoing radical HCC resection at this research center, analyzed the relationship between the SII and GNRI and clinicopathological features, and further explored the relationship between the SII and GNRI and survival rate.
The SII > 309.14 group had a 1-year survival rate lower than that of the SII < 309.14 group. The 1-year survival rate was lower in the GNRI > 98 group than that in the GNRI < 98 group (P < 0.05).
After analysis, we put forward the theory of the correlation between SII and GNRI and the mortality of HCC radical operations in China. Using available independent early case reports, the difficult problem of postoperative prognosis assessment was resolved to a certain extent.
Based on the relationship between the SII and GNRI and the clinicopathological features in patients undergoing radical HCC surgery, the relationship between the SII and GNRI and the postoperative survival rate was further analyzed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aron-Wisnewsky J, France; Kabir A, Iran S-Editor: Lin C L-Editor: A P-Editor: Yu HG
| 1. | Alawyia B, Constantinou C. Hepatocellular Carcinoma: a Narrative Review on Current Knowledge and Future Prospects. Curr Treat Options Oncol. 2023;24:711-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 69] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 2. | Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:2439-2446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 3. | Borde T, Nezami N, Laage Gaupp F, Savic LJ, Taddei T, Jaffe A, Strazzabosco M, Lin M, Duran R, Georgiades C, Hong K, Chapiro J. Optimization of the BCLC Staging System for Locoregional Therapy for Hepatocellular Carcinoma by Using Quantitative Tumor Burden Imaging Biomarkers at MRI. Radiology. 2022;304:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Ji Y, Wang H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: a meta-analysis. World J Surg Oncol. 2020;18:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Nasr R, Shamseddine A, Mukherji D, Nassar F, Temraz S. The Crosstalk between Microbiome and Immune Response in Gastric Cancer. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Liu J, Gao D, Li J, Hu G, Liu J, Liu D. The Predictive Value of Systemic Inflammatory Factors in Advanced, Metastatic Esophageal Squamous Cell Carcinoma Patients Treated with Camrelizumab. Onco Targets Ther. 2022;15:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | Liu L, Nishihara R, Qian ZR, Tabung FK, Nevo D, Zhang X, Song M, Cao Y, Mima K, Masugi Y, Shi Y, da Silva A, Twombly T, Gu M, Li W, Hamada T, Kosumi K, Inamura K, Nowak JA, Drew DA, Lochhead P, Nosho K, Wu K, Wang M, Garrett WS, Chan AT, Fuchs CS, Giovannucci EL, Ogino S. Association Between Inflammatory Diet Pattern and Risk of Colorectal Carcinoma Subtypes Classified by Immune Responses to Tumor. Gastroenterology. 2017;153:1517-1530.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systematic Review and Meta-analysis. Ann Surg. 2021;273:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 9. | Polk N, Budai B, Hitre E, Patócs A, Mersich T. High Neutrophil-To-Lymphocyte Ratio (NLR) and Systemic Immune-Inflammation Index (SII) Are Markers of Longer Survival After Metastasectomy of Patients With Liver-Only Metastasis of Rectal Cancer. Pathol Oncol Res. 2022;28:1610315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Yan D, Shen Z, Zhang S, Hu L, Sun Q, Xu K, Jin Y, Sang W. Prognostic values of geriatric nutritional risk index (GNRI) and prognostic nutritional index (PNI) in elderly patients with Diffuse Large B-Cell Lymphoma. J Cancer. 2021;12:7010-7017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21:10327-10335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 131] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 12. | Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2021;74:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Zeng X, Wu Y, Liu Y, Zhang X, Song Z. Cuproptosis-Related Risk Score Predicts Prognosis and Characterizes the Tumor Microenvironment in Hepatocellular Carcinoma. Front Immunol. 2022;13:925618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 162] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 14. | Lim JS, Shi Y, Park SH, Jeon SM, Zhang C, Park YY, Liu R, Li J, Cho WS, Du L, Lee JH. Mutual regulation between phosphofructokinase 1 platelet isoform and VEGF promotes glioblastoma tumor growth. Cell Death Dis. 2022;13:1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Han L, Lin X, Yan Q, Gu C, Li M, Pan L, Meng Y, Zhao X, Liu S, Li A. PBLD inhibits angiogenesis via impeding VEGF/VEGFR2-mediated microenvironmental cross-talk between HCC cells and endothelial cells. Oncogene. 2022;41:1851-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Sheriff S, Madhavan S, Lei GY, Chan YH, Junnarkar SP, Huey CW, Low JK, Shelat VG. Predictors of mortality within the first year post-hepatectomy for hepatocellular carcinoma. J Egypt Natl Canc Inst. 2022;34:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Lee CH, Yen TH, Hsieh SY. Outcomes of Geriatric Patients with Hepatocellular Carcinoma. Curr Oncol. 2022;29:4332-4341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zhang P, Ono A, Fujii Y, Hayes CN, Tamura Y, Miura R, Shirane Y, Nakahara H, Yamauchi M, Uchikawa S, Uchida T, Teraoka Y, Fujino H, Nakahara T, Murakami E, Miki D, Kawaoka T, Okamoto W, Makokha GN, Imamura M, Arihiro K, Kobayashi T, Ohdan H, Fujita M, Nakagawa H, Chayama K, Aikata H. The presence of vessels encapsulating tumor clusters is associated with an immunosuppressive tumor microenvironment in hepatocellular carcinoma. Int J Cancer. 2022;151:2278-2290. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Cedervall J, Hamidi A, Herre M, Viitaniemi K, D'Amico G, Miao Z, Unnithan RVM, Vaccaro A, van Hooren L, Georganaki M, Thulin Å, Qiao Q, Andrae J, Siegbahn A, Heldin CH, Alitalo K, Betsholtz C, Dimberg A, Olsson AK. Platelet-Specific PDGFB Ablation Impairs Tumor Vessel Integrity and Promotes Metastasis. Cancer Res. 2020;80:3345-3358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Kinoshita A, Hagiwara N, Osawa A, Akasu T, Matsumoto Y, Ueda K, Saeki C, Oikawa T, Koike K, Saruta M. The Geriatric Nutritional Risk Index Predicts Tolerability of Lenvatinib in Patients With Hepatocellular Carcinoma. In Vivo. 2022;36:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Yamada S, Yamamoto S, Fukuma S, Nakano T, Tsuruya K, Inaba M. Geriatric Nutritional Risk Index (GNRI) and Creatinine Index Equally Predict the Risk of Mortality in Hemodialysis Patients: J-DOPPS. Sci Rep. 2020;10:5756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Ruan GT, Zhang Q, Zhang X, Tang M, Song MM, Zhang XW, Li XR, Zhang KP, Ge YZ, Yang M, Li QQ, Chen YB, Yu KY, Cong MH, Li W, Wang KH, Shi HP. Geriatric Nutrition Risk Index: Prognostic factor related to inflammation in elderly patients with cancer cachexia. J Cachexia Sarcopenia Muscle. 2021;12:1969-1982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Scheufele F, Vogel T, Gasiorek M, Novotny A, Friess H, Demir IE, Schorn S. Serum albumin at resection predicts in-hospital death, while serum lactate and aPTT on the first postoperative day anticipate anastomotic leakage after Ivor-Lewis-esophagectomy. Langenbecks Arch Surg. 2022;407:2309-2317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Nipper CA, Lim K, Riveros C, Hsu E, Ranganathan S, Xu J, Brooks M, Esnaola N, Klaassen Z, Jerath A, Arrington A, Wallis CJD, Satkunasivam R. The Association between Serum Albumin and Post-Operative Outcomes among Patients Undergoing Common Surgical Procedures: An Analysis of a Multi-Specialty Surgical Cohort from the National Surgical Quality Improvement Program (NSQIP). J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Yilma M, Saxena V, Mehta N. Models to Predict Development or Recurence of Hepatocellular Carcinoma (HCC) in Patients with Advanced Hepatic Fibrosis. Curr Gastroenterol Rep. 2022;24:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 26. | Fischer A, Fuchs J, Stravodimos C, Hinz U, Billeter A, Büchler MW, Mehrabi A, Hoffmann K. Influence of diabetes on short-term outcome after major hepatectomy: an underestimated risk? BMC Surg. 2020;20:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |