Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2320
Peer-review started: March 2, 2023
First decision: May 16, 2023
Revised: June 4, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: October 27, 2023
Processing time: 239 Days and 8.6 Hours
Intra-abdominal infections (IAIs) is the most common type of surgical infection, with high associated morbidity and mortality rates. In recent years, due to the use of antibiotics, various drug-resistant bacteria have emerged, making the treatment of abdominal infections more challenging. Early surgical exploration can reduce the mortality of patients with abdominal infection and the occurrence of complications. However, available evidence regarding the optimal timing of IAI surgery is still weak. In study, we compared the effects of operation time on patients with abdominal cavity infection and tried to confirm the best timing of surgery.
To assess the efficacy of early vs delayed surgical exploration in the treatment of IAI, in terms of overall mortality.
A systematic literature search was performed using PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Ovid, and ScienceDirect. The systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses method. Based on the timing of the surgical operation, we divided the literature into two groups: Early surgery and delayed surgery. For the early and delayed surgery groups, the intervention was per
We identified nine eligible trial comparisons. Early surgical exploration of patients with IAIs (performed within 12 h) has significantly reduced the mortality and complications of patients, improved the survival rate, and shortened the hospital stay.
Early surgical exploration within 12 h may be more effective for the treatment of IAIs relative to a delayed operation.
Core Tip: The available evidence regarding the optimal timing of intra-abdominal infection surgery is still limited. In a systematic review and meta-analysis of the cohort study, we compared the effects of operation time on patients with abdominal cavity infection and aimed to confirm the best timing of surgery.
- Citation: Song SR, Liu YY, Guan YT, Li RJ, Song L, Dong J, Wang PG. Timing of surgical operation for patients with intra-abdominal infection: A systematic review and meta-analysis. World J Gastrointest Surg 2023; 15(10): 2320-2330
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2320.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2320
Intra-abdominal infections (IAIs) include any infection of the internal organs in the abdominal cavity (including the peritoneum) as well as infectious diseases caused by pathogens invading the host's abdominal cavity, retroperitoneal cavity, or internal organs of the abdominal cavity and causing obvious damage[1,2]. An IAI is usually a mixed infection of a variety of intestinal microbes. The pathogenic bacteria are mainly intestinal flora and primarily Escherichia coli[3,4]. An IAI is the most common type of surgical infection, with high associated morbidity and mortality rates. The mortality rate can be as high as 23%-38%[5-7]. The mortality rate associated with severe IAIs is even higher, representing the second most common cause of sepsis in critically ill patients[8].
In recent years, due to the use of antibiotics, various drug-resistant bacteria have emerged, making the treatment of abdominal infections more challenging. The cornerstone of IAI management is timely and adequate control of the source of anatomical infection[8,9]. Regarding the control of the source of infection, each guide mentions that treatment first requires surgical control of the source of infection. Guidelines recommend initiating source control as soon as possible in cases of well-defined IAIs[10]. In 2017, the Surgical Infection Society issued a guideline recommending that the source of infection be controlled within 24 h after an IAI diagnosis[11]. If the initial source of infection control is delayed 24 h after the diagnosis is clear, the case fatality rate will increase significantly[1,11,12]. Some authors agree that reopening should be performed within 48 h after the initial operation[13]. However, the exact time has not yet been clearly determined[10,14,15].
An operation can reverse the multiorgan failure of some patients with intra-abdominal sepsis after surgery and reduce their mortality rate[16]. Although the mechanism is not fully understood, early surgical exploration can reduce the mortality of patients with abdominal infection and the occurrence of complications[13,17]. However, available evidence regarding the optimal timing of IAI surgery is still weak. In abdominal infections, clinical studies on the timing of surgery tend to have small sample sizes and be of relatively low levels of quality. In a systematic review and meta-analysis of the cohort study, we compared the effects of operation time on patients with abdominal cavity infection and tried to confirm the best timing of surgery.
PRISMA statement guidelines were followed for conducting and reporting meta-analysis data[18-20]. The PICOS scheme was followed for reporting inclusion criteria. A systematic literature search was performed independently by two of the authors (Shurui Song and Yangyang Liu) using PubMed, EMBASE, Cochrane Central Register of Controlled Trials, via Ovid, Reference Citation Analysis (https://www.referencecitationanalysis.com/) database and ScienceDirect. The search was limited to studies on humans and to articles reported in the English language. No restriction was set for type of publication, date, or publication status. This study has been registered with the International Prospective Register of Systematic Reviews (no. CRD42021255402). PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Ovid, and ScienceDirect were systematically searched. The complete search used for PubMed was as follows: (((((((((((((intraabdominal infections[MeSH Terms]) OR intraabdominal infection*[Title/Abstract]) OR intra-abdominal infection*[Title/Abstract]) OR intra-abdominal infection*[Title/Abstract]) OR appendicitis[Title/Abstract]) OR diverticulitis[Title/Abstract]) OR peritonitis[Title/Abstract]) OR typhlitis[Title/Abstract]) OR peritonitis tuberculous[Title/Abstract]) OR subphrenic abscess[Title/Abstract]) OR abdominal sepsis[Title/Abstract]) OR intraabdominal sepsis[Title/Abstract]) OR intra-abdominal sepsis[Title/Abstract]) AND (((((((((((((surgery[MeSH Terms]) OR operative therapy[Title/Abstract]) OR invasive procedure*[Title/Abstract]) OR operative procedure*[Title/Abstract]) OR operation*[Title/Abstract]) OR perioperative procedure*[Title/Abstract]) OR intraoperative procedure*[Title/Abstract]) OR perioperative procedure*[Title/Abstract]) OR preoperative procedure*[Title/Abstract]) OR Surgical Procedure*[Title/Abstract]) OR Operative Surgical Procedure* [Title/Abstract]) OR Surgical Procedure[Title/Abstract]) OR Operative Surgical[Title/Abstract] OR laparoscopy[Title/Abstract]) OR laparotomy [Title/Abstract]) AND ((((((((((((((((time) OR Operative Times) OR Time, Operative) OR Times, Operative) OR Time Length of Surgery) OR Surgery Time Length) OR Surgery Time Lengths) OR Length of Operative Time) OR Operative Time Length) OR Operative Time Lengths) OR Time Length, Operative) OR Time Lengths, Operative) OR Surgical Time) OR Surgical Times) OR Time, Surgical) OR Times, Surgical)) AND ((((((((((((time) OR Time-to-Treatments) OR Time to Treatment) OR Time to Treatments) OR Door-to-Treatment Time) OR Door to Treatment Time) OR Delayed Treatment) OR Delayed Treatments) OR Treatment, Delayed) OR Treatment Delay) OR Delay, Treatment) OR Treatment Delays)). The search was performed from May 5 to 20, 2021. No language restrictions were applied.
The same two authors independently screened the titles and abstracts of the primary studies that were identified in the electronic search. Duplicate studies were excluded. The inclusion criteria were: (1) Human subjects; (2) inclusion of mean values with standard deviation values of related indices of information ratios; and (3) a clear indication that the study subjects have symptoms of abdominal infections or diseases mentioned in the guidelines.
The exclusion criteria were: (1) Case reports, reviews, comments, protocols, meeting abstracts, and meta-analyses; (2) studies of other interventions; and (3) studies where it was impossible to retrieve or calculate data of interest or without full-text versions available.
The Cohen kappa statistic was used to quantify the agreement between the investigators.
The same two authors extracted the following main data (Tables 1 and 2): (1) First author, year of publication and study type; (2) number and characteristics of patients of both early surgical exploration and delayed operation groups; and (3) treatment outcomes, including mortality rate, length of hospital stay, survival rate, and the occurrence of procedure-related complications. All relevant texts, tables and figures were reviewed for data extraction; whenever further information was required, the corresponding authors of the papers were contacted by e-mail. Discrepancies between the two reviewers were resolved by consensus discussion.
| Ref. | Design | Sample size, n | Age in year | Sex, male/female | Start of time calculation | Primary end-point(s) | Follow-up time |
| Elramah et al[24] | RC | 523 | 61 | - | Admission time | Mortality rate; Cure rate | 9 year |
| Giraudo et al[23] | RC | 746 | 28.8 | 343/380 | Admission time | Complications rate; Mortality rate | 6 year |
| Lee et al[25] | RC | 1076 | 35.2 ± 17.1 | 612/464 | Time of symptom onset | Cure rate; Complications rate | 3 year |
| Maroju et al[28] | PC | 114 | 28.3 | 86/28 | Time of symptom onset | Length of hospital stay | 1 year |
| Kim et al[29] | RC | 192 | 33.6 ± 19.5 | 99/93 | Time of symptom onset | Complications rate; Length of hospital stay | 1 year |
| Saar et al[30] | PC | 270 | 35.4 ± 14.8 | 136/130 | Time of symptom onset | Cure rate | 1 year |
| Rause et al[22] | RC | 197 | 68 | 51%/49% | Admission time | Mortality rate | 8 year |
| Azuhata et al[26] | PC | 154 | 66.5 ± 13.9 | 88/66 | Admission time | Survival rate | 4 year |
| Wen and Tang[27] | RC | 101 | 56.26 ± 15.7 | 65/36 | Admission time | Mortality rate; Length of hospital stay | 3.5 year |
| Ref. | Selection | Comparability | Exposure | Quality score | |||||
| Adequate definition of cases | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-Response rate | ||
| Elramah et al[24] | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 7 |
| Giraudo et al[23] | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 7 |
| Lee et al[25] | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 7 |
| Maroju et al[28] | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 7 |
| Kim et al[29] | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 | 7 |
| Saar et al[30] | 1 | 1 | - | - | 2 | 1 | 1 | 1 | 7 |
| Rause et al[22] | 1 | 1 | - | - | 1 | 1 | 1 | 1 | 6 |
| Azuhata et al[26] | 1 | 1 | - | 1 | 2 | 1 | 1 | 1 | 8 |
| Wen and Tang[27] | 1 | 1 | - | 1 | 2 | 1 | 1 | 1 | 8 |
The Newcastle-Ottawa Scale was used for retrospective studies to assess quality. Funnel plots were constructed to assess the risk of publication bias across series for all outcome measures.
Review Manager version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used for primary data analyses. Data were presented using odds ratios (ORs) for categorical outcomes and mean differences (MDs) for continuous outcomes, with 95%CIs calculated for all estimates; in the random-effects model, P < 0.05 was considered to be statistically significant. Interstudy heterogeneity was evaluated using Cochran’s Q statistic and quantified using the I2 statistic; a value greater than 50%[21] indicated substantial heterogeneity, and statistical significance was indicated by P < 0.10. The source of heterogeneity was explored using sensitivity and subgroup analyses. Publication bias was assessed by a visual inspection of funnel plots.
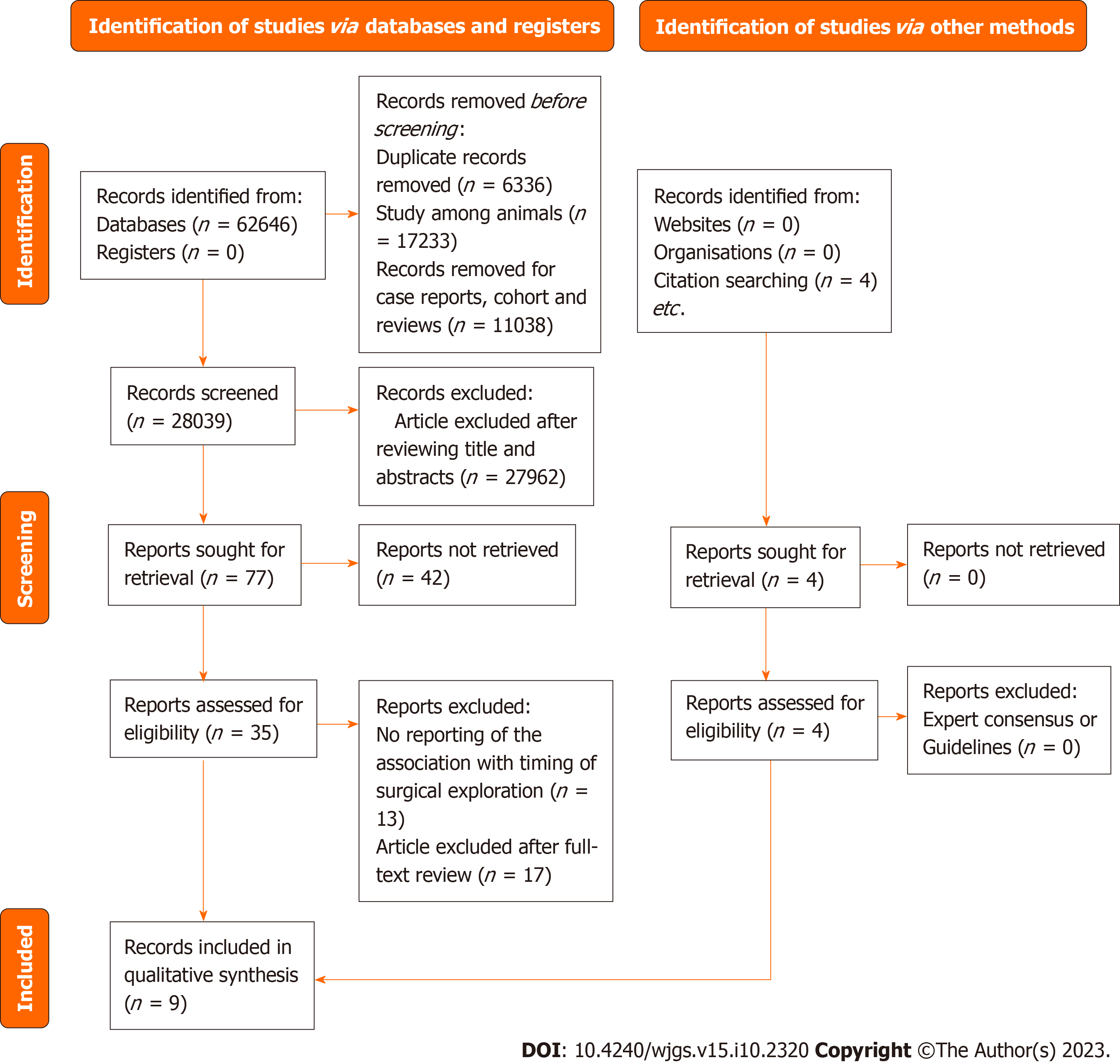
The literature search returned a total of 62646 records, of which 35 articles underwent full-text review and nine were finally included in our meta-analysis (Table 1)[22-30]. The details of the study selection process are presented as a literature search flow diagram (Figure 1). The nine studies included a total of 3373 patients who had acute diverticulitis, gastrointestinal perforation, appendicitis complicated by abdominal cavity infection manifestations, and/or bacterial inflammation in the abdominal cavity caused by other hollow organs. All nine selected studies were observational studies, with seven being retrospective cohort studies and two being prospective cohort studies; all nine included studies were full-text articles published in journals, meeting the inclusion criteria. Participants in the included studies were adults from seven countries (China, South Korea, India, the United States, Italy, Germany, and Japan). The six retrospective cohort studies reported follow-up periods ranging from 1 year to 9 years in length, and the three prospective cohort studies[26,28,30] had follow-up periods ranging from 1 year to 4 years in length.
Based on the Newcastle-Ottawa scale, all included observational studies were of high quality (Table 2) but did not indicate whether or not they had received funding.
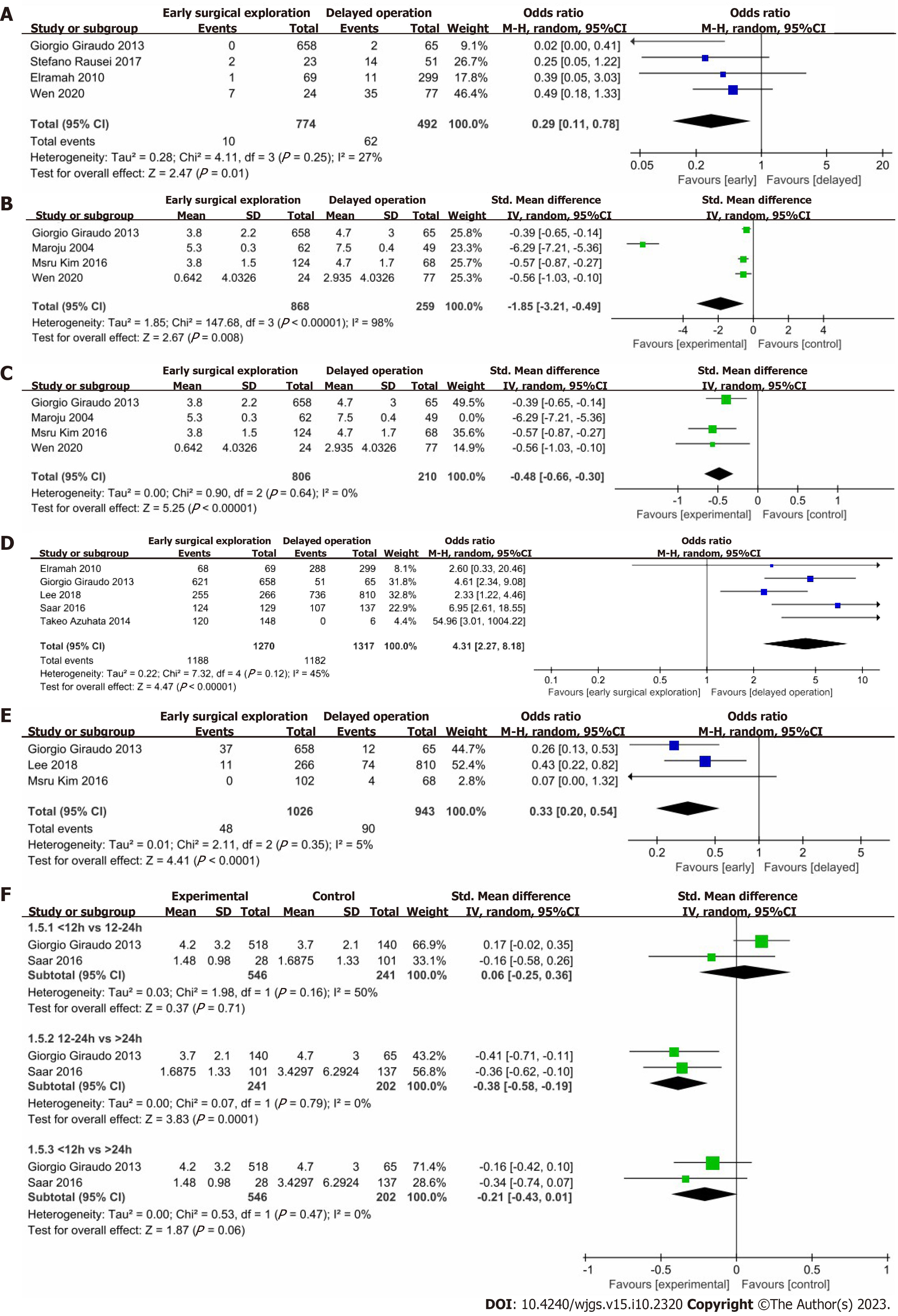
Figure 2A shows the difference in mortality rate between the early surgical exploration group and the control group. The risk of mortality rate in the early surgical exploration group was significantly lower than in the control group (OR: 0.29; 95%CI: 0.11-0.78; P = 0.01), with no evidence of significant heterogeneity (I2 = 27%; P = 0.25). According to Rausei et al[22], for patients having severe IAIs, the mortality rate increased linearly for each 6-hour delay in the timing of surgery. Notably, most patients in the 6-12 h delayed group had the highest prognostic scores.
Figure 2B shows that patients in the early surgical exploration group had significantly shorter hospital stays (SMD: -1.85 d; 95%CI: -3.21 to -0.49 d; P = 0.008), with evidence of high heterogeneity (I2 = 98%, P < 0.00001). A systematic removal of individual studies can change the results or account for the findings (SMD: -0.48 d; 95%CI: -0.66 to -0.30 d; P < 0.00001), with no evidence of significant heterogeneity (I2 = 0%; P = 0.64) (Figure 2C). The reason for removal is because the time standard for inclusion in this study was from the onset of symptoms to the beginning of surgery.
Figure 2D shows the difference in the survival rate between the early surgical exploration within the 6 h group and the control group. The risk of survival rate in the early surgical exploration group was significantly higher than that in the control group (OR: 4.31; 95%CI: 2.27-8.18; P < 0.00001), with evidence of moderate heterogeneity (I2 = 45%; P = 0.12). Azuhata et al[26] found that for patients with gastrointestinal perforation combined with septic shock, the survival rate decreased with delay in the operation start time, and the survival rate of patients who waited for more than 6 h was 0%.
Figure 2E shows the differences in the occurrence of procedure-related complications between the early surgical exploration group and the control group. The risk of occurrence of procedure-related complications in the early surgical exploration group was significantly lower than that in the control group (OR: 0.33; 95%CI: 0.20-0.54; P < 0.0001), with no evidence of significant heterogeneity (I2 = 5%; P = 0.35).
Figure 2F shows that patients in the surgery within 12 h group had significantly shorter hospital stays than those of patients in the surgery after 24 h group (SMD: -0.21 d; 95%CI: -0.43 to -0.01 d; P = 0.06), with no evidence of significant heterogeneity (I2 = 0%; P = 0.47). Patients in the surgery within 12 h group had significantly shorter hospital stays than those of patients in the surgery within 12-24 h group [SMD: -0.06 d (95%CI: -0.25 to -0.36 d; P = 0.71)], with evidence of medium heterogeneity (I2 = 50%; P = 0.16). Patients in the surgery within 12-24 h group had significantly shorter hospital stays than those of patients in the surgery after 24 h group [SMD: -0.38 d (95%CI: -0.58 to -0.19; P = 0.0001)], with no evidence of significant heterogeneity (I2 = 0%; P = 0.79). A systematic removal of individual studies did not change the results.
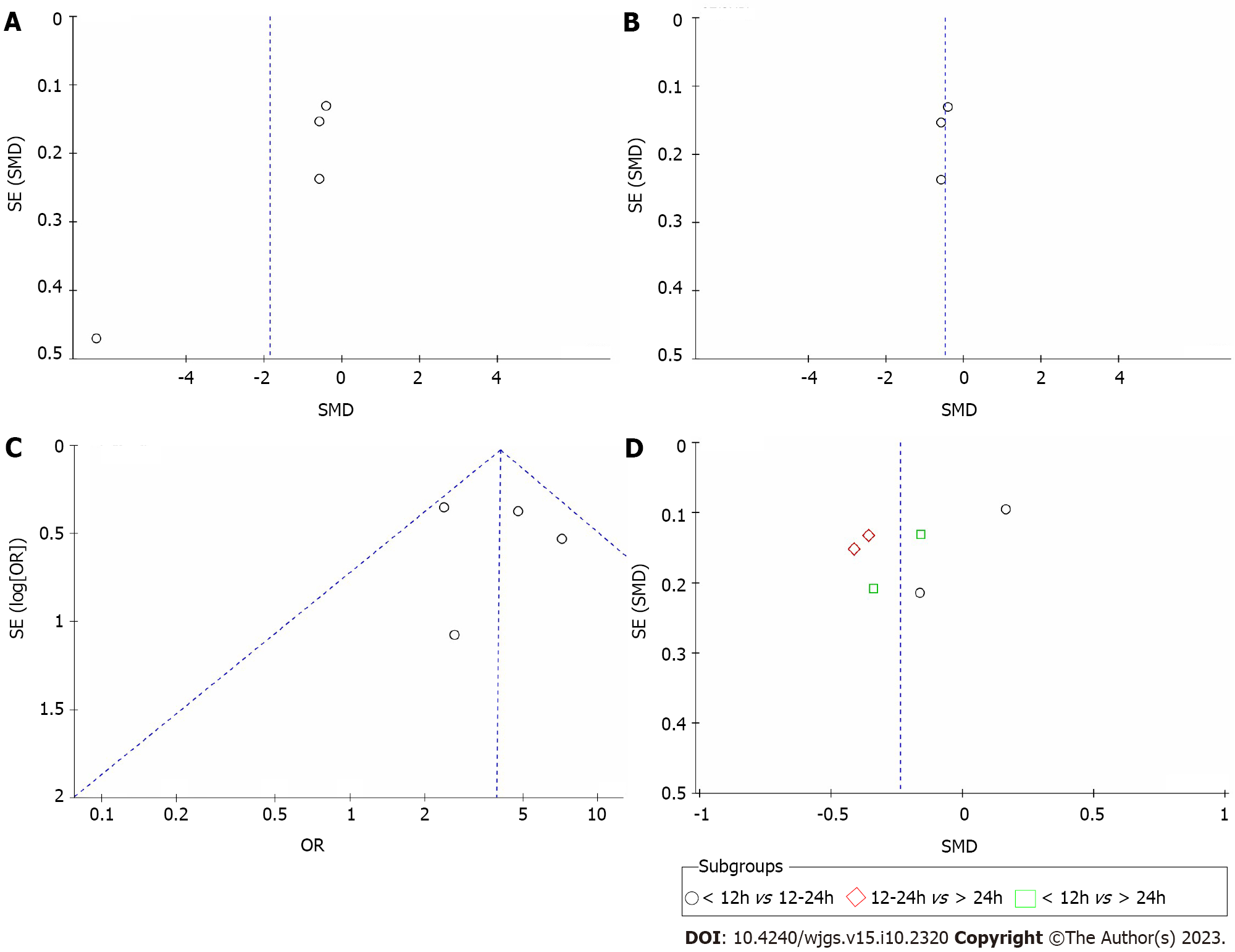
A sensitivity analysis of the included observational studies was performed by excluding the abstracts with no available published full-text versions and the studies with the highest risk of bias; however, this did not affect the findings. Publication bias was evaluated by funnel plot analysis (Figure 3). Figure 3A shows a funnel plot of hospital stay length; when a system of individual studies was removed, the funnel chart indicated that there was no asymmetry (Figure 3B). In addition, funnel plots of the survival rate (Figure 3C) and length of hospital stay by surgical timing subgroup (Figure 3D) are presented and similarly suggested no asymmetry. Publication bias was not assessed for other components as there were less than four trial comparisons available.
Abdominal infection is the most common surgical infection[14,15]. IAI is a common postoperative complication after surgery, causing pain and suffering to patients. In addition, this complication has been associated with negative economic impact including increased morbidity, extended postoperative hospital stay, readmission, sepsis, and death[31]. Concerning the role of the surgical technique (i.e. open vs laparoscopic approach) on IAI, studies have shown that the technique of surgery does not appear to affect the incidence of IAI[32]. In the current research, the optimal duration of postoperative IAI in the treatment of critically ill patients is unclear[33]. In addition, our data show that, compared with delayed surgery, early surgical exploration reduces mortality and may reduce both hospital stay length and adverse events. In fact, the timing of infection control is critical for patients with IAIs, but the definition of ‘early’ control in the literature varies.
In recent years, scholars have performed a series of studies on the timing of surgery and offered their own opinions. Our results suggest that early surgery within 6 h can improve the survival rate of patients, which is consistent with the results of previous studies[22,27]. According to Rausei’s observations[22], for every 6 h of delay in source control, the mortality rate will directly increase, and the rate of abdominal wound closure will also decrease. Azuhata's analysis[26] also supports that the target time for a good prognosis may be within 6 h after admission. When the start of surgery was delayed, the survival rate was decreased, and the survival rate for those treated after more than 6 h was 0%. However, this extreme result may be because their study only included a small group of patients and was an observational study conducted at a single institution.
Some studies have suggested that surgery performed within 12 h has a critical effect on reducing mortality and complications. The research by Lee et al[25] is biased toward performing surgery within 18 h, which will reduce the incidence of complications. Under certain conditions, surgery can be performed earlier, which will not adversely affect the outcome of the operation. A study by Saar et al[30] indicated that delaying surgery for 12 h will gradually increase the number of complications per comprehensive complication index and will also significantly lengthen the operation time and the hospital stay. Colson et al[34] also offered similar results. Patients who undergo surgery more than 12 h after the onset of their symptoms have a greater incidence of perforation. Interestingly, a study[35] found that early surgery within 6 h increased the abdominal closure rate but also the length of hospital stay. This may be due to insufficient preparations made for early surgery and longer inter-hospital observation and nursing times after surgery. In addition, for patients with abdominal cavity infections, the emergency may occur at night. At this time, a shortage of doctors and incomplete inspection may render preoperative preparations incomplete. Therefore, premature intervention can lead to a series of adverse effects not seen in concert with adequate preparation for surgery. “Most sepsis patients require surgical treatment and an emergency procedure, that is carried out when the patient's condition is severe and rapidly worsening. Furthermore, there is often multiorgan damage and a combination of chronic diseases. As a result, anesthesiologists should exercise caution when choosing anesthetics, and the optimization of anesthesia strategies is necessary. However, not all patients with IAIs develop sepsis, and not all patients with sepsis are also infected in the abdominal cavity”. “In summary, for patients with non-emergency conditions, as well as those with obvious contraindications to surgery, it is beneficial to delay surgery, complete the relevant investigations, or optimize the anaesthetic protocol”.
The results of our subgroup analysis showed that early surgical exploration (within 12 h) can shorten the hospital stay. When the operation is delayed, the abdominal cavity infection may be aggravated and thus become more difficult to control effectively. This may be mainly because early surgery can not only remove abdominal necrosis on time but can also further control the source of infection through surgical exploration. Moreover, compared with delayed treatment, the incidence of adverse events in early surgery is lower, which may also be related to this.
In short, most of the included studies were observational, with high-quality evidence and a moderate risk of bias. The conclusions of this study are limited by the heterogeneity between the included trials, our inability to know the study blinding status, the starting point of the operation time, and the difference in treatment standards. First, each country and hospital has a different standard for the definition of patients with abdominal cavity infection. So, the standards are not uniform.
In addition, different countries have varying indications for surgical intervention. Whether all patients need surgical intervention and whether surgical intervention is directly performed after diagnosis are controversial, which may lead to potential deviations between studies.
Furthermore, there is no uniform standard for calculating the start time of surgery. Some hospitals start the calculation based on the time of admission[23,26,27], while some hospitals start the calculation based on the time at which the patient shows symptoms[22,29]. Subjective factors such as time calculation will also affect the research results. In our research, for studies that did not indicate the starting point of time, we uniformly defined such as the time from the point of admission to the beginning of the operation.
Overall, due to a lack of research on the timing of abdominal infection surgery at home and abroad and the varying standards of observation methods between studies, we included fewer research projects, and the quality of the data obtained is not very high, which renders our results not universally applicable.
Our study results do support that, for patients with IAI, surgical intervention should be carried out within 12 h as much as possible, or even preferably within 6 h if possible. Although each doctor attempts to avoid delays in surgery, early surgery requires accurate judgment and precise surgical skills, which places greater demands on doctors. Moreover, it is necessary to improve preoperative preparations, closely monitor the vital signs of patients, and cooperate with each department involved in the procedure. Because studies spanned long periods and had differences in IAI and the data available from the included studies were limited, we did not analyze the time of surgical intervention for IAIs caused by various means. Attempting full preparation before surgery with a series of related inspections can help to carry out the procedure early.
Although further high-quality randomized controlled trials are still needed to determine the optimal surgical exploration time, our findings clearly show that in IAI patients, early surgical exploration within 12 h or even 6 h can reduce the mortality rate and adverse events, shorten the hospital stay, and improve the survival rate.
Intra-abdominal infections (IAIs) is the most common type of surgical infection, with high associated morbidity and mortality rates. In recent years, due to the use of antibiotics, various drug-resistant bacteria have emerged, making the treatment of abdominal infections more challenging. Early surgical exploration can reduce the mortality of patients with abdominal infection and the occurrence of complications. However, available evidence regarding the optimal timing of IAI surgery is still weak.
We compared the effects of operation time on patients with abdominal cavity infection and tried to confirm the best timing of surgery.
This study aimed to assess the efficacy of early vs delayed surgical exploration in the treatment of IAI, in terms of overall mortality.
A systematic literature search was performed using PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Ovid, and ScienceDirect. The systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses method. Based on the timing of the surgical operation, we divided the literature into two groups: early surgery and delayed surgery. For the early and delayed surgery groups, the intervention was performed with and after 12 h of the initial surgical intervention, respectively. The main outcome measure was the mortality rate. The literature search was performed from May 5 to 20, 2021. We also searched the World Health Organization International Clinical Trials Registry Platform search portal and ClinicalTrials.gov on May 20, 2021, for ongoing trials. This study was registered with the International Prospective Register of Systematic Reviews.
We identified nine eligible trial comparisons. Early surgical exploration of patients with IAIs (performed within 12 h) has significantly reduced the mortality and complications of patients, improved the survival rate, and shortened the hospital stay.
Early surgical exploration within 12 h may be more effective for the treatment of IAIs relative to a delayed operation.
In this study, we developed a strict and reasonable study design, literature search strategy and screening and quality evaluation, selected appropriate statistical analysis methods, and analyzed the results’ scientificity and sensitivity.
We thank the patient advisers for their efforts and the patients for their participation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Mynster T, Denmark; Tanpowpong P, Thailand S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Chinese Society of Surgery of Chinese Medical Association; Infectious Diseases Society for Evidence-based and Translational Medicine of Chinese Research Hospital Association; Editorial Board of Chinese Journal of Surgery. [Expert consensus on multidisciplinary management of intra-abdominal infections]. Zhonghua Wai Ke Za Zhi. 2021;59:161-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Liu C, Zhang JY. [New concept of diagnosis and treatment of abdominal infection: consensus and controversy]. Zhongguo Shiyong Waike Zazhi. 2019;39. [DOI] [Full Text] |
| 3. | Hawser S, Hoban DJ, Badal RE, Bouchillon SK, Biedenbach D, Hackel M, Morrissey I. Epidemiology and antimicrobial susceptibility of Gram-negative aerobic bacteria causing intra-abdominal infections during 2010-2011. J Chemother. 2015;27:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Montravers P, Lepape A, Dubreuil L, Gauzit R, Pean Y, Benchimol D, Dupont H. Clinical and microbiological profiles of community-acquired and nosocomial intra-abdominal infections: results of the French prospective, observational EBIIA study. J Antimicrob Chemother. 2009;63:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Volakli E, Spies C, Michalopoulos A, Groeneveld AB, Sakr Y, Vincent JL. Infections of respiratory or abdominal origin in ICU patients: what are the differences? Crit Care. 2010;14:R32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Barie PS, Hydo LJ, Eachempati SR. Longitudinal outcomes of intra-abdominal infection complicated by critical illness. Surg Infect (Larchmt). 2004;5:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | De Waele J, Lipman J, Sakr Y, Marshall JC, Vanhems P, Barrera Groba C, Leone M, Vincent JL; EPIC II Investigators. Abdominal infections in the intensive care unit: characteristics, treatment and determinants of outcome. BMC Infect Dis. 2014;14:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Wu XW, Zheng T, Hong ZW, Ren HJ, Wu L, Wang GF, Gu GS, Ren JA. Current progress of source control in the management of intra-abdominal infections. Chin J Traumatol. 2020;23:311-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, Ansaloni L, Bala M, Balogh ZJ, Beltrán MA, Ben-Ishay O, Biffl WL, Birindelli A, Cainzos MA, Catalini G, Ceresoli M, Che Jusoh A, Chiara O, Coccolini F, Coimbra R, Cortese F, Demetrashvili Z, Di Saverio S, Diaz JJ, Egiev VN, Ferrada P, Fraga GP, Ghnnam WM, Lee JG, Gomes CA, Hecker A, Herzog T, Kim JI, Inaba K, Isik A, Karamarkovic A, Kashuk J, Khokha V, Kirkpatrick AW, Kluger Y, Koike K, Kong VY, Leppaniemi A, Machain GM, Maier RV, Marwah S, McFarlane ME, Montori G, Moore EE, Negoi I, Olaoye I, Omari AH, Ordonez CA, Pereira BM, Pereira Júnior GA, Pupelis G, Reis T, Sakakhushev B, Sato N, Segovia Lohse HA, Shelat VG, Søreide K, Uhl W, Ulrych J, Van Goor H, Velmahos GC, Yuan KC, Wani I, Weber DG, Zachariah SK, Catena F. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Sartelli M, Catena F, Coccolini F, Pinna AD. Antimicrobial management of intra-abdominal infections: literature's guidelines. World J Gastroenterol. 2012;18:865-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (3)] |
| 11. | Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, Chang PK, O'Neill PJ, Mollen KP, Huston JM, Diaz JJ Jr, Prince JM. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg Infect (Larchmt). 2017;18:1-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 12. | Sartelli M, Catena F, Ansaloni L, Leppaniemi A, Taviloglu K, van Goor H, Viale P, Lazzareschi DV, Coccolini F, Corbella D, de Werra C, Marrelli D, Colizza S, Scibè R, Alis H, Torer N, Navarro S, Sakakushev B, Massalou D, Augustin G, Catani M, Kauhanen S, Pletinckx P, Kenig J, Di Saverio S, Jovine E, Guercioni G, Skrovina M, Diaz-Nieto R, Ferrero A, Rausei S, Laine S, Major P, Angst E, Pittet O, Herych I, Agresta F, Vettoretto N, Poiasina E, Tepp J, Weiss G, Vasquez G, Vladov N, Tranà C, Delibegovic S, Dziki A, Giraudo G, Pereira J, Tzerbinis H, van Dellen D, Hutan M, Vereczkei A, Krasniqi A, Seretis C, Mesina C, Rems M, Campanile FC, Coletta P, Uotila-Nieminen M, Dente M, Bouliaris K, Lasithiotakis K, Khokha V, Zivanovic D, Smirnov D, Marinis A, Negoi I, Ney L, Bini R, Leon M, Aloia S, Huchon C, Moldovanu R, de Melo RB, Giakoustidis D, Ioannidis O, Cucchi M, Pintar T, Krivokapic Z, Petrovic J. Complicated intra-abdominal infections in Europe: a comprehensive review of the CIAO study. World J Emerg Surg. 2012;7:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Koperna T, Schulz F. Relaparotomy in peritonitis: prognosis and treatment of patients with persisting intraabdominal infection. World J Surg. 2000;24:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Pieracci FM, Barie PS. Management of severe sepsis of abdominal origin. Scand J Surg. 2007;96:184-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Wu XW, Ren JA. [Interpretation of domestic and foreign guidelines on diagnosis and treatment of abdominal infection]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Wacha H, Hau T, Dittmer R, Ohmann C. Risk factors associated with intraabdominal infections: a prospective multicenter study. Peritonitis Study Group. Langenbecks Arch Surg. 1999;384:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Koperna T, Schulz F. Prognosis and treatment of peritonitis. Do we need new scoring systems? Arch Surg. 1996;131:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2931] [Article Influence: 488.5] [Reference Citation Analysis (0)] |
| 19. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8255] [Article Influence: 825.5] [Reference Citation Analysis (0)] |
| 20. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11041] [Article Influence: 690.1] [Reference Citation Analysis (0)] |
| 21. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25814] [Article Influence: 1122.3] [Reference Citation Analysis (0)] |
| 22. | Rausei S, Pappalardo V, Ruspi L, Colella A, Giudici S, Ardita V, Frattini F, Rovera F, Boni L, Dionigi G. Early Versus Delayed Source Control in Open Abdomen Management for Severe Intra-abdominal Infections: A Retrospective Analysis on 111 Cases. World J Surg. 2018;42:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Giraudo G, Baracchi F, Pellegrino L, Dal Corso HM, Borghi F. Prompt or delayed appendectomy? Influence of timing of surgery for acute appendicitis. Surg Today. 2013;43:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Elramah MM, Affi A, Horwitz J, Einstein M, Omballi M, Leo J, Qureshi J, Noor M, Vakil N. Acute Diverticulitis: High Mortality in Patients Undergoing Delayed Surgery: A Case-control Study. Am J Gastroenterol. 2010;389. [DOI] [Full Text] |
| 25. | Lee JM, Kwak BS, Park YJ. Is a One Night Delay of Surgery Safe in Patients With Acute Appendicitis? Ann Coloproctol. 2018;34:11-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Azuhata T, Kinoshita K, Kawano D, Komatsu T, Sakurai A, Chiba Y, Tanjho K. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 2014;18:R87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Wen J, Tang ZH. The relationship between the timing of infection source control and prognosis in patients with abdominal cavity infection. Medical Information Jun. 2020;33:11. [DOI] [Full Text] |
| 28. | Maroju NK, Robinson Smile S, Sistla SC, Narasimhan R, Sahai A. Delay in surgery for acute appendicitis. ANZ J Surg. 2004;74:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Kim M, Kim SJ, Cho HJ. Effect of surgical timing and outcomes for appendicitis severity. Ann Surg Treat Res. 2016;91:85-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Saar S, Talving P, Laos J, Põdramägi T, Sokirjanski M, Lustenberger T, Lam L, Lepner U. Delay Between Onset of Symptoms and Surgery in Acute Appendicitis Increases Perioperative Morbidity: A Prospective Study. World J Surg. 2016;40:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Mulita F, Liolis E, Akinosoglou K, Tchabashvili L, Maroulis I, Kaplanis C, Vailas M, Panos G. Postoperative sepsis after colorectal surgery: a prospective single-center observational study and review of the literature. Prz Gastroenterol. 2022;17:47-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Mulita F, Plachouri KM, Liolis E, Kehagias D, Kehagias I. Comparison of intra-abdominal abscess formation after laparoscopic and open appendectomy for complicated and uncomplicated appendicitis: a retrospective study. Wideochir Inne Tech Maloinwazyjne. 2021;16:560-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Montravers P, Tubach F, Lescot T, Veber B, Esposito-Farèse M, Seguin P, Paugam C, Lepape A, Meistelman C, Cousson J, Tesniere A, Plantefeve G, Blasco G, Asehnoune K, Jaber S, Lasocki S, Dupont H; DURAPOP Trial Group. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med. 2018;44:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 34. | Colson M, Skinner KA, Dunnington G. High negative appendectomy rates are no longer acceptable. Am J Surg. 1997;174:723-6; discussion 726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12668] [Article Influence: 844.5] [Reference Citation Analysis (0)] |