Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2294
Peer-review started: June 12, 2023
First decision: August 10, 2023
Revised: August 17, 2023
Accepted: September 4, 2023
Article in press: September 4, 2023
Published online: October 27, 2023
Processing time: 136 Days and 20.2 Hours
Given the poor prognosis of patients with lymph node metastasis, estimating the lymph node status in patients with early esophageal cancer is crucial. Indicators that could be used to predict lymph node metastasis in early esophageal cancer have been reported in many recent studies, but no recent studies have included a review of this subject.
To review indicators predicting lymph node metastasis in early esophageal squamous cell carcinoma (ESCC) and early esophageal adenocarcinoma (EAC).
We searched PubMed with “[early esophageal cancer (Title/Abstract)] and [lymph node (Title/Abstract)]” or “[early esophageal carcinoma (Title/Abstract)] and [lymph node (Title/Abstract)]” or “[superficial esophageal cancer (Title/Abstract)] and [lymph node (Title/Abstract)].” A total of 29 studies were eligible for analysis.
Preoperative imaging (size), serum markers (microRNA-218), postoperative pathology and immunohistochemical analysis (depth of invasion, tumor size, differentiation grade, lymphovascular invasion, neural invasion, expression of PIM-1 < 30%) were predictive factors for lymph node metastasis in both early ESCC and EAC. Serum markers (thymidine kinase 1 ≥ 3.38 pmol/L; cytokeratin 19 fragment antigen 21-1 > 3.30 ng/mL; stathmin-1) and postoperative pathology and immunohistochemical analysis (overexpression of cortactin, mixed-lineage leukaemia 2, and stanniocalcin-1) were predictive for lymph node metastasis in early ESCC. Transcription of CD69, myeloid differentiation protein 88 and toll-like receptor 4 and low expression of olfactomedin 4 were predictive of lymph node metastasis in early EAC. A total of 6 comprehensive models for early ESCC, including logistic regression model, nomogram, and artificial neural network (ANN), were reviewed. The areas under the receiver operating characteristic curve of these models reached 0.789-0.938, and the ANN performed best. As all these models relied on postoperative pathology, further models focusing on serum markers, imaging and immunohistochemical indicators are still needed.
Various factors were predictive of lymph node metastasis in early esophageal cancer, and present comprehensive models predicting lymph node metastasis in early ESCC mainly relied on postoperative pathology. Further studies focusing on serum markers, imaging and immunohistochemical indicators are still in need.
Core Tip: In this study, we reviewed factors predicting lymph node metastasis in early esophageal squamous cell carcinoma (ESCC) and early esophageal adenocarcinoma (EAC). Imaging (size), serum microRNA-218, postoperative pathology and immunohistochemical analysis (depth, size, differentiation grade, lymphovascular invasion, neural invasion, PIM-1) were predictive for both ESCC and EAC. Serum markers (thymidine kinase 1; cytokeratin 19 fragment antigen 21-1; stathmin-1) and overexpression of cortactin, mixed-lineage leukaemia 2, and stanniocalcin-1 were predictive for ESCC. Transcription of CD69, myeloid differentiation protein 88 and toll-like receptor 4 and low expression of olfactomedin 4 were predictive for EAC. Six comprehensive models for ESCC were reviewed, and the areas under the curve reached 0.789-0.938.
- Citation: Li Y, Wang JX, Yibi RH. Prediction of lymph node metastasis in early esophageal cancer. World J Gastrointest Surg 2023; 15(10): 2294-2304
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2294.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2294
Esophageal cancer was the seventh most commonly diagnosed cancer and the sixth leading cause of cancer death worldwide in 2018[1]. Squamous cell carcinoma (SCC) (85%) was the most common histological type, followed by adenocarcinoma (14%)[2]. Esophageal SCC (ESCC) and esophageal adenocarcinoma (EAC) differ greatly in tumor location and biological behaviour[3]. ESCC mainly occurs in the proximal two-thirds of the esophagus, while EAC mainly occurs in the distal third of the esophagus and the gastroesophageal junction. Alcohol and tobacco are risk factors for ESCC, and Barrett’s esophagus is correlated with EAC[4]. Early detection and treatment improve the prognosis[5,6]. According to the 8th edition of the Cancer Staging Manual for Esophagus and Esophagogastric Junctions developed by the American Joint Committee on Cancer and the Union for International Cancer Control, early esophageal cancer includes high-grade dysplasia or tumor in situ (Tis) and tumors limited to the mucosa (T1a) or submucosa (SM) (T1b), regardless of lymph node status[7]. As the risk of lymph node metastasis varied greatly due to the depth of invasion, Japanese investigators subclassified mucosal and SM esophageal cancer into 6 types (M1, limited to the epithelial layer; M2, invading the lamina propria; M3, invading into but not through the muscularis mucosa; SM1, penetrating the upper one-third of the SM; SM2, penetrating the middle one-third of the SM; SM3, penetrating the deepest one-third of the SM)[8,9]. Regarding the therapeutic strategy for early esophageal cancer, endoscopic resection is appropriate due to its fewer complications and similar survival compared with esophagectomy[10,11]. However, the incidence of lymph node metastasis in patients with newly diagnosed early esophageal cancer was reported as 20%-27%[12-14]. As lymph node involvement is related to poor prognosis[15], the lymph node status must be assessed when designing the therapeutic strategy, especially before surgery. To our knowledge, many recent studies have yielded reports of indicators that could predict lymph node metastasis in early esophageal cancer, but there has been no study in which this subject has been reviewed from the perspective of recent findings. Therefore, this study was designed to review predictive indicators for lymph node metastasis in patients with newly diagnosed early esophageal cancer.
We searched PubMed with “[early esophageal cancer (Title/Abstract)] and [lymph node (Title/Abstract)]” or “[early esophageal carcinoma (Title/Abstract)] and [lymph node (Title/Abstract)]” or “[superficial esophageal cancer (Title/Abstract)] and [lymph node (Title/Abstract)].” The last sought date of each resource was May 1, 2023. All studies were reviewed in detail, and only articles focusing on lymph node metastasis in T1 esophageal cancer were included. Case reports, reviews, systematic reviews, meta-analyses and articles without available full texts in English were excluded. Finally, a total of 29 studies were eligible for analysis, and all relevant factors are discussed below (Table 1).
| Category | ESCC | EAC | |
| Imaging | CT | The intrathoracic and abdominal nodes larger than 10 mm in the short axis, supraclavicular nodes greater than 5 mm and retrocrural nodes greater than 6 mm | |
| EUS | Regional lymph node metastasis: (1) Size greater than 10 mm, (2) a round shape, (3) sharply demarcated borders, and (4) hypoechoic structure | ||
| Serum markers | TK1 ≥ 3.38 pmol/L; CYFRA21-1 > 3.30 ng/mL; stathmin-1 | NA | |
| MicroRNA-218 | |||
| Postoperative pathology and immunohistochemical analysis | Depth of invasion; tumor size; histological differentiation grade; angiolymphatic invasion; neural invasion; expression of PIM-1 | ||
| Overexpression of cortactin; the protein levels of mixed-lineage leukaemia 2; the expression of stanniocalcin-1 | Transcriptions of CD69, MYD88 and TLR4; low expression of olfactomedin 4 | ||
The assessment of lymph node metastasis using computed tomography (CT) was mainly based on size. Intrathoracic and abdominal nodes larger than 10 mm in the short axis were generally suspected as lymph node metastasis, compared with supraclavicular nodes greater than 5 mm and retrocrural nodes greater than 6 mm[16]. This method might miss metastasis within normal-sized lymph nodes and misdiagnose inflammation within enlarged lymph nodes, given its sensitivity of 57% and specificity of 83%[17]. Moreover, these criteria were inappropriate for detecting lymph node metastasis in patients with early esophageal cancer [sensitivity of 1/18 (5%) and specificity of 25/31 (80%)][18]. Betancourt et al[18] considered lymph nodes adjacent to the primary tumor as positive for malignancy when they were round or ovoid with a mean size (short axis + long axis/2) > 5 mm or not adjacent when the mean size was > 7 mm. The sensitivity, specificity, and accuracy were 61% (11/18), 45% (14/31), and 51% (25/49), respectively[18].
Endoscopic ultrasonography was used to assess regional lymph node metastasis. The criteria were as follows: (1) Size greater than 10 mm; (2) a round shape; (3) sharply demarcated borders; and (4) hypoechoic structure[19]. Catalano et al[19] reported an accuracy of 100% if all four criteria were met, but other studies showed an accuracy of 66%-75%[20,21]. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) improved the sensitivity (14/22, 63% vs 28/30, 93%; P < 0.05) and accuracy (23/33, 70% vs 29/31, 93%; P < 0.05) of detecting nonperitumoral lymph node metastasis[22]. However, EUS-FNA must not traverse the malignancy to avoid tumor seeding from the primary site and false-positive results, which might decrease the sensitivity. With EUS-FNA, Betancourt et al[18] reported that 32.7%(16/49) of patients with positive lymph node metastasis were inappropriately designated as cN0.
Positron emission tomography and CT (PET/CT) are commonly used for tumor, node, and metastasis staging of malignancy. 18F-Fluorodeoxyglucose is the most commonly used contrast agent and is a radiolabelled glucose analogue mainly concentrated in tissues with high glucose consumption, such as malignant tissues[23]. However, the assessment of regional lymph node status might be affected by the signal uptake from the adjacent tumor. Cuellar et al[24] enrolled 79 patients with early esophageal cancer and performed N-staging using PET/CT. All 3 patients positive on PET/CT were negative on biopsy, and all 13 patients positive on biopsy were falsely negative on PET/CT. The sensitivity and positive predictive values were both 0%. Therefore, PET/CT might be inappropriate for the routine assessment of lymph node status in patients with early esophageal cancer.
Jiang et al[25] reported that patients with esophageal cancer (96 ESCC/10 EAC) with lymph node metastasis had a much lower concentration of serum microRNA-218 (0.64 ± 0.44 vs 0.33 ± 0.30, P < 0.05) than those with no lymph node metastasis. However, this study enrolled patients with esophageal cancer in both Tis-T1 (19.8%, 21/106) and T2-3 stages (80.2%, 85/106)[25]. Yang et al[26] also reported that microRNA-218 expression was lower in early esophageal cancer than in normal esophageal tissue. MicroRNA-218 might act as a suppressive miRNA in esophageal cancer, but the mechanism needs further clarification.
A high level of serum thymidine kinase 1 (TK1) (≥ 3.38 pmol/L) was more common in patients with lymph node metastasis of ESCC than in those without (21/29, 72.4% vs 21/51, 41.2%, P < 0.05)[27]. This study included patients with ESCC in both T1 stage and T2-4 stages, and no subgroup analysis was available. A high level of serum cytokeratin 19 fragment antigen 21-1 (CYFRA21-1) (> 3.30 ng/mL) was also more common in patients with lymph node metastasis of ESCC than in those without (9/15, 60% vs 5/42, 11.9%, χ2 = 11.33, P = 0.001)[28]. The levels of serum SCC antigen and carcinoembryonic antigen between patients with and without lymph node metastasis differed insignificantly. This study enrolled patients with ESCC in both Tis-T1 stage (n = 24) and T2 stage (n = 33)[28]. Further explorations are needed to investigate the correlations between the levels of serum TK1 and CYFRA21-1 and lymph node metastasis of early ESCC.
Stathmin-1 (STMN1) is a microtube regulatory protein that prevents the polymerization of tubulin heterodimers, destabilizing the microtubule cytoskeleton[29]. STMN1 was overexpressed in ESCC tissues[30], and serum STMN1 levels were significantly higher in patients with early ESCC than in healthy controls (P < 0.001)[29]. Patients with lymph node metastasis of early ESCC also had higher serum STMN1 levels than those not (P < 0.01)[29]. STMN1 might promote tumor cell metastasis by activating the integrin alpha5-focal adhesion kinase-extracellular signal-regulated kinase[31].
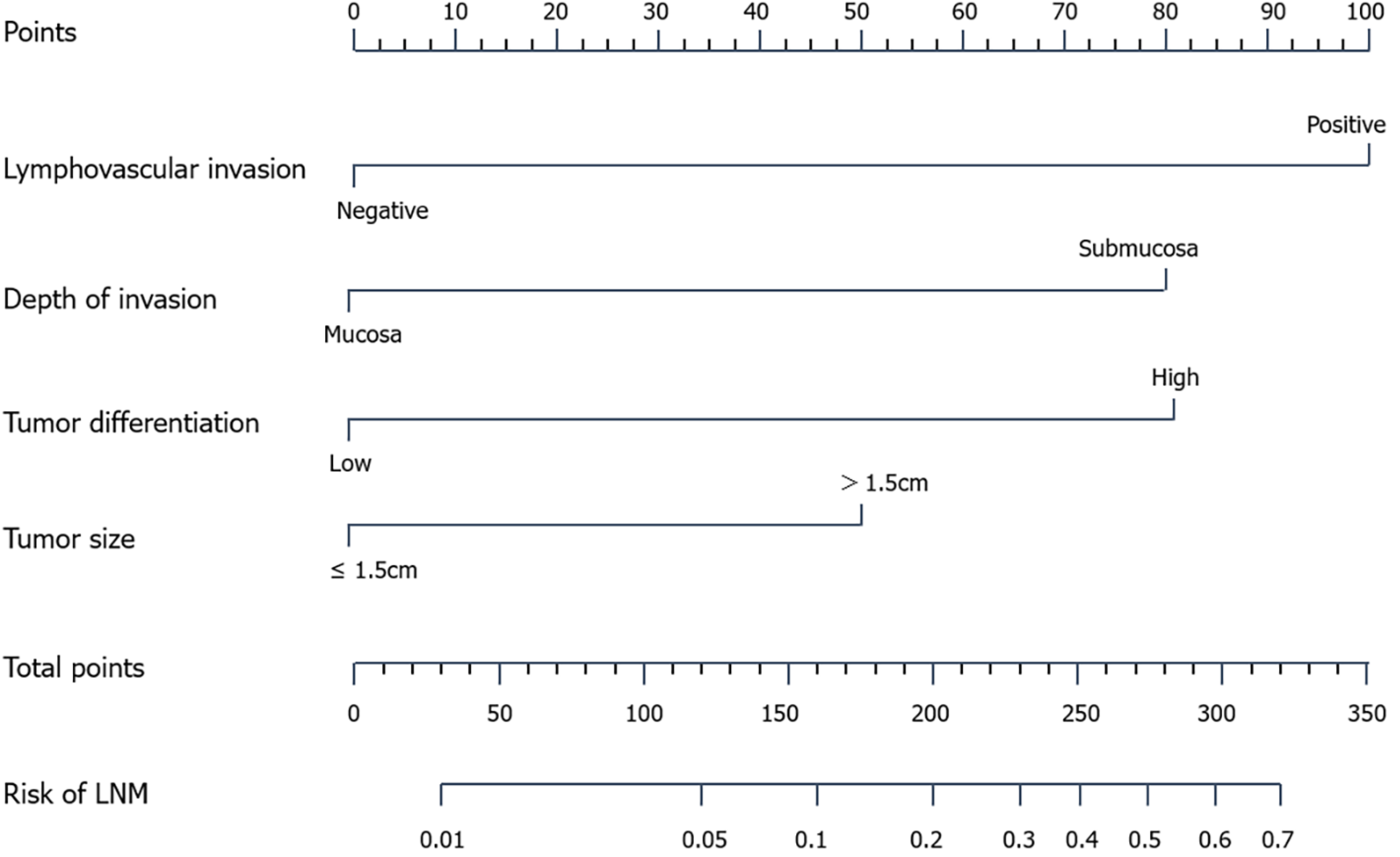
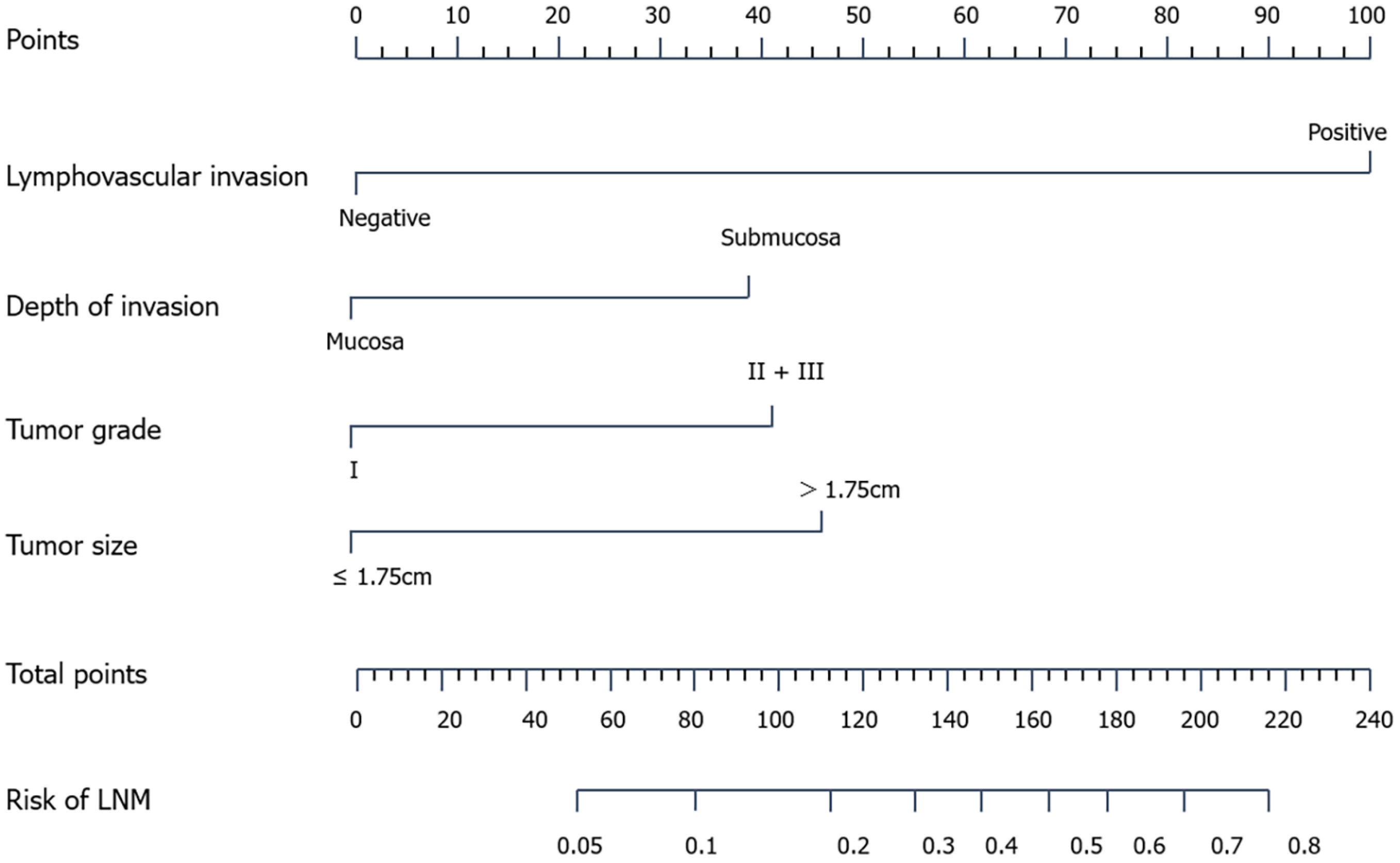
SM invasion was relevant to a higher risk of lymph node metastasis (SM 22.6%-45.5% vs mucosa 0%-7.9%; P < 0.05), reflecting common knowledge to most researchers[32-35]. Some studies showed that early esophageal cancer with SM2/3 invasion had a much higher rate of lymph node metastasis than the same with SM1 invasion (SM2/3 17/35, 48.6% vs SM1 3/36, 8.3%; P < 0.05)[33]. Other studies showed that early esophageal cancer with SM3 invasion had a much higher rate of lymph node metastasis than those with SM1/2 invasion (SM3 45.1%-55.6% vs SM1/2 8.7%-16.1%; P < 0.05)[34,36]. We believe that the risk of lymph node metastasis increased with increasing invasion depth. This might be related to the abundant lymphatic drainage of the SM and the direct connections of the SM to the central lymphatic channels[37]. Preoperative narrow band imaging and magnifying endoscopy contributed to the assessment of the invasion depth of early esophageal cancer. This therapeutic strategy has been widely adopted: esophageal cancer with a preoperative diagnosis of invasion into SM1 is first resected endoscopically, and the decision regarding subsequent surgery is informed by the depth of invasion and vascular invasion[38].
Tumor size was also relevant, but the cutoff value varied in different studies. Chen et al[39] reported that tumor size ≥ 1.85 cm was a risk factor (98/327, 30% vs 35/406, 8.6%; P < 0.05), and Zheng et al[40] suggested that patients with tumor size > 1.5 cm had a higher risk of lymph node metastasis (49/242, 20.2% vs 17/239, 7.1%; P < 0.05). Other cutoff values, such as 2.0 and 1.75 cm, were also reported to be significant in univariate and multivariate analyses[41,42]. The increase in tumor size was correlated with a higher risk of lymph node metastasis (P < 0.05)[32,36]. The values of incidence of lymph node metastasis in patients with tumor sizes ≤ 10, 11-20, 21-30, and ≥ 31 mm were 0% (0/26), 17.1% (6/35), 15% (3/20), and 33.3% (3/9) (P < 0.05), respectively[43]. Therefore, we might consider tumor size as a predictive factor. However, the cutoff-value selection should involve consideration of other confounding factors, and comprehensive modelling might be appropriate.
The histological differentiation grade was also related. Patients with moderately (G2) and poorly (G3) differentiated early ESCC had a higher risk of lymph node metastasis than those with high differentiation (G1) (19/89, 21.3% vs 2/39, 5.1%; P < 0.05)[32]. Patients with poorly differentiated and undifferentiated (G0) early esophageal cancer (67 ESCC/31 EAC) had a higher risk of lymph node metastasis than those with high and moderate differentiation (12/34, 35.3% vs 8/64, 12.5%; P < 0.05)[33]. Similarly, Chen et al[39] reported that patients with poorly differentiated early ESCC had a higher risk of lymph node metastasis than those with high and moderate differentiation (77/226, 34.1% vs 56/507, 11%; P < 0.05). This might be related to the highly progressive capacity of poorly differentiated and undifferentiated tumors[15].
Several studies showed that lymphovascular invasion (LVI) was related to a higher risk of lymph node metastasis (44.4%-60% vs 0-18.1%, P < 0.05), which might be the first step towards regional lymph node metastasis[32,33,36]. Ancona et al[33] revealed that neural invasion was also relevant to a higher risk of lymph node metastasis (8/14, 57.1% vs 12/84, 14.3%, P < 0.05). The sensitivity and specificity were 40% and 92%, respectively[33].
High expression of the proto-oncogene PIM-1 was detected in ESCC with lymph node metastasis[44], and PIM-1 siRNA inhibited the proliferation of ESCC cells and induced apoptosis[45]. Upregulation of PIM-1 was also found in gastric glands correlated with the lymph node metastasis of gastric cancer[46]. Plum et al[47] explored whether the expression of PIM-1 was associated with lymph node involvement in early esophageal cancer (28 ESCC/39 EAC). The expression of PIM-1 was insignificantly different between ESCC and EAC, and low-grade expression of PIM-1 (< 30%) was correlated with lymph node metastasis (low-grade 10/16, 62.5% vs. high-grade 16/51, 31.4%; P < 0.05)[47].
Kotsafti et al[48] analysed the tumor immune microenvironment in therapy-naïve EAC. The infiltration of CD8+ CD28+ T cells was lower in both tumoral and peritumoral mucosa for patients with lymph node metastasis. The transcription levels of CD69, myeloid differentiation protein 88 (MYD88) and toll-like receptor 4 (TLR4) were lower in the tumoral specimens from patients with lymph node metastasis. The areas under the receiver operating characteristic curve (AUROC) of CD69, MYD88 and TLR4 mRNA expression were 0.76 [95% confidence interval (CI): 0.47-0.93], 0.80 (95%CI: 0.52-0.95), and 0.80 (95%CI: 0.52-0.95), respectively (P < 0.05). In the peritumoral healthy mucosa, the levels of MYD88, TLR4, and CD69 mRNA levels were correlated with CD80 mRNA levels (Rho = 0.65, 0.47 and 0.82, respectively) (P < 0.05). In the external cohort (seven matched tumor and adjacent normal tissue samples), the expression levels of CD8A, CD8B and TBX21 were lower in the peritumoral mucosa for patients with lymph node involvement (P = 0.05). CD80 mRNA levels were correlated with CD38 mRNA (Rho = 0.85, P = 0.03) and CD69 mRNA (Rho = 0.77, P = 0.05) levels, confirming the possible role of CD80 in the pathway activating CD8 T cells. Moreover, the infiltration of CD8 T cells and M1 macrophages was also lower in patients with lymph node metastasis in the external cohort.
Olfactomedin 4 (OLFM4), formerly named hGC-1 or GW112, a secreted glycoprotein, could mediate cell adhesion by interacting with extracellular matrix proteins such as cadherins and lectins[49]. OLFM4-positive cells were found in Barrett’s esophagus, mainly confined to the base of metaplastic glands[50]. Low expression (< 30%) of OLFM4 was associated with nodal metastases in advanced EAC [odds ratio (OR) 2.7; 95%CI: 1.16-6.41; P = 0.022] but insignificantly in early EAC (OR 2.1; 95%CI: 0.46-9.84; P = 0.338)[51]. In this study, the sample size of early EAC (n = 44) was relatively small, and OLFM4 expression in early and advanced EAC with lymph node metastasis differed insignificantly (P = 0.844)[51]. Further exploration analysing the correlation between the expression of OLFM4 and lymph node metastasis in early EAC is still needed.
Lu et al[52] analysed the genome-wide gene expression profile of 10 primary ESCCs and their adjacent normal esophageal tissues. The overexpression of cortactin (CTTN) (dark brown staining in > 50% of normal or malignant esophageal squamous cells completely obscuring the cytoplasm) was associated with lymph node metastasis (N0 54/109, 49.5% vs N1 72/98, 80.9%; P < 0.05) and pathological stage (I + IIA 58/113, 51.3% vs IIB + III 68/85, 80.0%; P < 0.05). However, this study enrolled patients with ESCC in stages I-III, and no subgroup analysis was performed. The relationship between the overexpression of CTTN and lymph node metastasis of early ESCC needs further exploration.
Li et al[53] compared the positive staining of mixed-lineage leukaemia 2 (MLL2), also known as KMT2D, in 25 pairs of early ESCC (with and without lymph node metastasis). The MLL2 levels were much higher in tumors with lymph node metastasis (P < 0.001). In vitro, silencing MLL2 expression resulted in decreased migration of esophageal squamous carcinoma cells. Moreover, the expression of stanniocalcin-1 (STC1) was also higher in tumors with lymph node metastasis, which could be decreased with MLL2 siRNA treatment. Further investigations indicated that MLL2 was recruited to the STC1 promoter by p65 (RelA) and activated the expression of STC1[53].
Although a large number of factors were correlated, it was still difficult to assess the risk of lymph node metastasis with a single indicator. Several comprehensive models have been built to predict the risk of lymph node metastasis in early ESCC (Table 2).
| Number | Ref. | Year | Factors | Category | Training set | Validation set | ||
| Cutoff value | C-index (95%CI) | Cutoff value | C-index (95%CI) | |||||
| 1 | Jia et al[43] | 2016 | Depth of invasion and lymphovascular metastasis | Logistic regression model | NA | 0.858 (0.757-0.959) | NA | NA |
| 2 | Zhou et al[42] | 2019 | Dumor size, tumor grade, depth of invasion, and angiolymphatic invasion | Logistic regression model | 20% | 0.80 (0.737-0.862) | 20% | 0.814 (0.724-0.905) |
| 3 | Nomogram | NA | 0.80 (0.739-0.856) | NA | 0.814 (0.725-0.900) | |||
| 4 | Zheng et al[40] | 2018 | Depth of tumor invasion, grade of differentiation, tumor size, and lymphovascular invasion | Nomogram | 0.142 | 0.790 (0.717-0.864) | 0.224 | 0.789 (0.709-0.869) |
| 5 | Chen et al [39] | 2020 | Tumor size, histologic grade, invasion depth, lymphovascular invasion, CT-results, and alcohol taking | Logistic regression model | 3.735 | 0.857 (NA) | 3.735 | 0.881 (NA) |
| 6 | artificial neural network | NA | 0.904 (NA) | NA | 0.938 (NA) | |||
In 2016, Jia et al[43] built a logistic regression model using the depth of invasion and lymphovascular metastasis: p = ex/(1 + ex), and x = −5.469 + 0.839 × depth of invasion (M1 labelled 1; M2 labelled 2; M3 labelled 3; SM1 labelled 4, SM2 labelled 5, and SM3 labelled 6) + 1.992 × lymphovascular metastasis (negative 0, positive 1). The AUROC was 0.858 (95%CI: 0.757-0.959). However, the cutoff value and the calibration of this model were not reported.
In 2018, Zheng et al[40] built a nomogram using depth of tumor invasion, grade of differentiation, tumor size, and LVI (Figure 1). The Harrell’s concordance index (C-index) was 0.790 (95%CI: 0.717-0.864) and 0.789 (95%CI: 0.709-0.869) in the training and validation sets, respectively. The corresponding cutoff values were 0.142 and 0.224 in the training and validation cohorts, respectively. The P values in the Hosmer-Lemeshow test of the derivation and validation cohorts were 0.966 and 0.754, respectively.
In 2019, Zhou et al[42] built a logistic regression model using tumor size, tumor grade, depth of invasion, and presence of angiolymphatic invasion: ŷ = 1/[1 + exp(- xβ)], xβ = -4.339 + 1.211 × tumor size (≤ 1.75 cm labelled 0, > 1.75 cm labelled 1) + 1.078 × tumor grade (G1 labelled 0, G2/3 labelled 1) + 1.036 × depth of invasion (M1-3 labelled 0, SM1-3 labelled 1) + 2.661 × angiolymphatic invasion (absent labelled 0, present labelled 1). The AUROCs in the training and validation sets were 0.80 (95%CI: 0.737-0.862) and 0.814 (95%CI: 0.724-0.905), respectively. The predicted value ranged from 3.33% to 86.67%, and the optimal cutoff value of the estimated risk was 20%. Meanwhile, they built a nomogram to predict the risk of lymph node metastasis (Figure 2), with an AUROC of 0.80 (95%CI: 0.739-0.856) in the training cohort and 0.814 (95%CI: 0.725-0.900) in the validation cohort. Whether the Hosmer-Lemeshow test was performed and whether the P was > 0.05 were not reported.
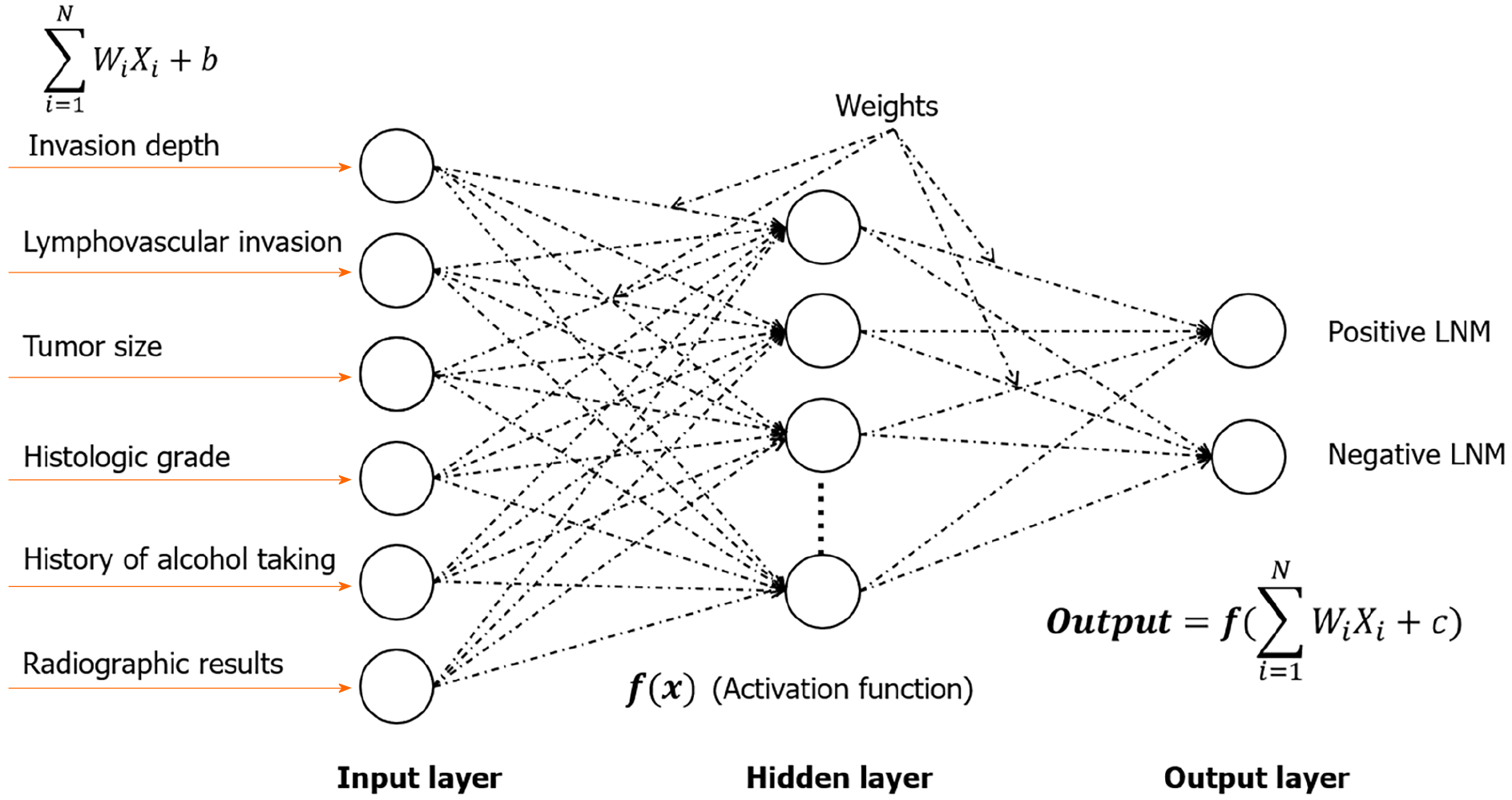
In 2020, Chen et al[39] built another logistic regression model with a previous history of alcohol consumption, tumourtumor size, SM invasion, histologic grade, LVI, and preoperative CT result: ŷ = 1/(1 + e–z), z = - 5.213 + 2.061 × invasion depth (M1/M2/M3 labelled 0, SM1/SM2 or deeper labelled 1) + 3.216 × LVI (negative labelled 0, positive labelled 1) + 0.956 × histologic grade (G1 and G2 labelled 0, G3 labelled 1) + 1.107 × CT results (negative labelled 0, positive labelled 1) + 0.594 × alcohol consumption (no labelled 0, yes labelled 1) + 1.327 × tumor size (< 1.85 cm labelled 0, ≥ 1.85 cm labelled 1) (Table 1). The optimal cutoff value of z was 3.735. The total AUROC and accuracy of the training, validation and test cohorts were 0.868 (95%CI: 0.837-0.900) and 74.49% (95%CI: 71.17-77.61), respectively. They further built an artificial neural network (ANN) model using these factors (Figure 3), with a much higher total AUROC of 0.915 (95%CI: 0.887-0.943) and a much higher total accuracy of 90.72% (95%CI: 88.39-92.72%) (P < 0.05).
Esophageal cancer is a malignancy with high morbidity and mortality. Endoscopic resection was applied to patients with early esophageal cancer owing to its lower trauma and fewer complications. However, lymph node metastasis is not rare and is often treated with additional surgery. In this study, we reviewed predictive indicators of lymph node metastasis in patients with early esophageal cancer, especially as observed in recent findings about serum markers, immunohistochemical indicators and comprehensive models. Preoperative imaging (size), serum markers (microRNA-218, TK1, CYFRA21-1, STMN1), postoperative pathology and immunohistochemical analysis (depth of invasion, tumor size, differentiation grade, angiolymphatic invasion, and neural invasion; PIM-1 expression < 30%; transcription of CD69, MYD88 and TLR4; low expression of OLFM 4; overexpression of CTTN, MLL 2, and STC1) were related. The sensitivity and specificity of a single criterion were relatively low, and comprehensive models, including the logistic regression model, nomogram, and ANN, performed much better. This helped clinical decision-making regarding whether endoscopic resection or radical surgery was appropriate and whether additional radical surgery was needed in patients with initial endoscopic resection. In this study, we mainly reviewed studies from PubMed, possibly missing some meaningful reports from other databases. In addition, all these comprehensive models relied on postoperative pathology. The present therapeutic strategy involves suggested initial endoscopic resection before subsequent surgery based on the depth of invasion and vascular invasion in patients with a preoperative diagnosis of SM1 invasion. If we could predict the lymph node status preoperatively, those with lymph node metastasis would not have to undergo endoscopic resection before radical surgery. Therefore, further studies using preoperative indicators such as imaging and serum markers are needed to predict lymph node status in patients with early esophageal cancer.
Various factors, including preoperative imaging, serum markers, preoperative pathology and immunohistochemical indicators, were predictive of lymph node metastasis in early ESCC and EAC. Several comprehensive models predicting lymph node metastasis in early ESCC performed well, but these models relied on postoperative pathology. Further studies focusing on serum markers, imaging and immunohistochemical indicators are still needed.
Given the poor prognosis of patients with lymph node metastasis, estimating the lymph node status in patients with early esophageal cancer is crucial. Indicators that could be used to predict lymph node metastasis in early esophageal cancer have been reported in many recent studies, but no recent studies have included a review of this subject.
This study aimed to review indicators predicting lymph node metastasis in early esophageal cancer.
This study was designed to review indicators predicting lymph node metastasis in early esophageal squamous cell carcinoma (ESCC) and early esophageal adenocarcinoma (EAC).
We searched PubMed with “[early esophageal cancer (Title/Abstract) and (lymph node (Title/Abstract)]” or “[early esophageal carcinoma (Title/Abstract)] and [lymph node (Title/Abstract)]” or “[superficial esophageal cancer (Title/Abstract)] and [lymph node (Title/Abstract)].” All studies were reviewed in detail, and a total of 29 studies were eligible for analysis.
Preoperative imaging, serum microRNA-218, depth of invasion, tumor size, differentiation grade, lymphovascular invasion, neural invasion, expression of PIM-1 < 30% were predictive factors for lymph node metastasis in both early ESCC and EAC. Serum thymidine kinase 1 ≥ 3.38 pmol/L, cytokeratin 19 fragment antigen 21-1 > 3.30 ng/mL, stanniocalcin-1 and overexpression of cortactin, mixed-lineage leukaemia 2, stathmin-1 were predictive for lymph node metastasis in early ESCC. Transcription of CD69, myeloid differentiation protein 88, toll-like receptor 4 and low expression of olfactomedin 4 were predictive of lymph node metastasis in early EAC. A total of 6 comprehensive models for early ESCC were reviewed. The areas under the receiver operating characteristic curve reached 0.789-0.938.
Various factors were predictive of lymph node metastasis in early ESCC and EAC. Several comprehensive models performed well, but these models relied on postoperative pathology. Further studies focusing on serum markers, imaging and immunohistochemical indicators are still in need.
Further studies focusing on serum markers, imaging and immunohistochemical indicators are still needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Kinami S, Japan S-Editor: Qu XL L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology. 2022;163:649-658.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 550] [Article Influence: 183.3] [Reference Citation Analysis (0)] |
| 3. | Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, West AB, Blaser M, Blot WJ, Gail MH, Fraumeni JF Jr. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 4. | Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 434] [Article Influence: 86.8] [Reference Citation Analysis (1)] |
| 5. | Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TÅ, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Walsh PM, Jackson C, Vernon S, Morgan E, Gavin A, Morrison DS, Huws DW, Porter G, Butler J, Bryant H, Currow DC, Hiom S, Parkin DM, Sasieni P, Lambert PC, Møller B, Soerjomataram I, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 730] [Article Influence: 121.7] [Reference Citation Analysis (0)] |
| 6. | Berry MF, Zeyer-Brunner J, Castleberry AW, Martin JT, Gloor B, Pietrobon R, D'Amico TA, Worni M. Treatment modalities for T1N0 esophageal cancers: a comparative analysis of local therapy versus surgical resection. J Thorac Oncol. 2013;8:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Mönig S, Chevallay M, Niclauss N, Zilli T, Fang W, Bansal A, Hoeppner J. Early esophageal cancer: the significance of surgery, endoscopy, and chemoradiation. Ann N Y Acad Sci. 2018;1434:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Endo M, Yoshino K, Kawano T, Nagai K, Inoue H. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus. 2000;13:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Shimada H, Nabeya Y, Matsubara H, Okazumi S, Shiratori T, Shimizu T, Aoki T, Shuto K, Akutsu Y, Ochiai T. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg. 2006;191:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Dunn JM, Reyhani A, Santaolalla A, Zylstra J, Gimson E, Pennington M, Baker C, Kelly M, Van Hemelrijck M, Lagergren J, Zeki SS, Gossage JA, Davies AR. Transition from esophagectomy to endoscopic therapy for early esophageal cancer. Dis Esophagus. 2022;35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Zheng H, Kang N, Huang Y, Zhao Y, Zhang R. Endoscopic resection versus esophagectomy for early esophageal cancer: a meta-analysis. Transl Cancer Res. 2021;10:2653-2662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Akutsu Y, Kato K, Igaki H, Ito Y, Nozaki I, Daiko H, Yano M, Udagawa H, Nakagawa S, Takagi M, Mizusawa J, Kitagawa Y. The Prevalence of Overall and Initial Lymph Node Metastases in Clinical T1N0 Thoracic Esophageal Cancer: From the Results of JCOG0502, a Prospective Multicenter Study. Ann Surg. 2016;264:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Chen H, Wu J, Guo W, Yang L, Lu L, Lin Y, Wang X, Zhang Y, Chen X. Clinical models to predict lymph nodes metastasis and distant metastasis in newly diagnosed early esophageal cancer patients: A population-based study. Cancer Med. 2023;12:5275-5292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Nentwich MF, von Loga K, Reeh M, Uzunoglu FG, Marx A, Izbicki JR, Bogoevski D. Depth of submucosal tumor infiltration and its relevance in lymphatic metastasis formation for T1b squamous cell and adenocarcinomas of the esophagus. J Gastrointest Surg. 2014;18:242-9; discussion 249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Zhang Y, Peng L, Zhang L. Research Progress on the Predicting Factors and Coping Strategies for Postoperative Recurrence of Esophageal Cancer. Cells. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 16. | Old OJ, Isabelle M, Barr H. Staging Early Esophageal Cancer. Adv Exp Med Biol. 2016;908:161-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Betancourt Cuellar SL, Sabloff B, Carter BW, Benveniste MF, Correa AM, Maru DM, Ajani JA, Erasmus JJ, Hofstetter WL. Early clinical esophageal adenocarcinoma (cT1): Utility of CT in regional nodal metastasis detection and can the clinical accuracy be improved? Eur J Radiol. 2017;88:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Choi J, Kim SG, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc. 2010;24:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Rösch T. Endosonographic staging of esophageal cancer: a review of literature results. Gastrointest Endosc Clin N Am. 1995;5:537-547. [PubMed] |
| 22. | Vazquez-Sequeiros E, Norton ID, Clain JE, Wang KK, Affi A, Allen M, Deschamps C, Miller D, Salomao D, Wiersema MJ. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc. 2001;53:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Lin J, Kligerman S, Goel R, Sajedi P, Suntharalingam M, Chuong MD. State-of-the-art molecular imaging in esophageal cancer management: implications for diagnosis, prognosis, and treatment. J Gastrointest Oncol. 2015;6:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 24. | Cuellar SL, Carter BW, Macapinlac HA, Ajani JA, Komaki R, Welsh JW, Lee JH, Swisher SG, Correa AM, Erasmus JJ, Hofstetter WL. Clinical staging of patients with early esophageal adenocarcinoma: does FDG-PET/CT have a role? J Thorac Oncol. 2014;9:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Jiang Z, Song Q, Yang S, Zeng R, Li X, Jiang C, Ding W, Zhang J, Zheng Y. Serum microRNA-218 is a potential biomarker for esophageal cancer. Cancer Biomark. 2015;15:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yang M, Liu R, Sheng J, Liao J, Wang Y, Pan E, Guo W, Pu Y, Yin L. Differential expression profiles of microRNAs as potential biomarkers for the early diagnosis of esophageal squamous cell carcinoma. Oncol Rep. 2013;29:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Ji Y, Wu XB, Chen JY, Hu B, Zhu QK, Zhu XF, Zheng MF. Serum thymidine kinase 1 levels correlate with clinical characteristics of esophageal squamous cell carcinoma. Int J Clin Exp Med. 2015;8:12850-12857. [PubMed] |
| 28. | Mei X, Zhu X, Zuo L, Wu H, Guo M, Liu C. Predictive significance of CYFRA21-1, squamous cell carcinoma antigen and carcinoembryonic antigen for lymph node metastasis in patients with esophageal squamous cancer. Int J Biol Markers. 2019;34:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Yan L, Dong X, Gao J, Liu F, Zhou L, Sun Y, Zhao X. A novel rapid quantitative method reveals stathmin-1 as a promising marker for esophageal squamous cell carcinoma. Cancer Med. 2018;7:1802-1813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Liu F, Sun YL, Xu Y, Liu F, Wang LS, Zhao XH. Expression and phosphorylation of stathmin correlate with cell migration in esophageal squamous cell carcinoma. Oncol Rep. 2013;29:419-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Han G, Wu Z, Zhao N, Zhou L, Liu F, Niu F, Xu Y, Zhao X. Overexpression of stathmin plays a pivotal role in the metastasis of esophageal squamous cell carcinoma. Oncotarget. 2017;8:61742-61760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Gong L, Yue J, Duan X, Jiang H, Zhang H, Zhang X, Yu Z. Comparison of the therapeutic effects of endoscopic submucosal dissection and minimally invasive esophagectomy for T1 stage esophageal carcinoma. Thorac Cancer. 2019;10:2161-2167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15:3278-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 34. | Hölscher AH, Bollschweiler E, Schröder W, Metzger R, Gutschow C, Drebber U. Prognostic impact of upper, middle, and lower third mucosal or submucosal infiltration in early esophageal cancer. Ann Surg. 2011;254:802-7; discussion 807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Bollschweiler E, Baldus SE, Schröder W, Prenzel K, Gutschow C, Schneider PM, Hölscher AH. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy. 2006;38:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Raja S, Rice TW, Goldblum JR, Rybicki LA, Murthy SC, Mason DP, Blackstone EH. Esophageal submucosa: the watershed for esophageal cancer. J Thorac Cardiovasc Surg. 2011;142:1403-11.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Yajin S, Murakami G, Takeuchi H, Hasegawa T, Kitano H. The normal configuration and interindividual differences in intramural lymphatic vessels of the esophagus. J Thorac Cardiovasc Surg. 2009;137:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Ishihara R, Arima M, Iizuka T, Oyama T, Katada C, Kato M, Goda K, Goto O, Tanaka K, Yano T, Yoshinaga S, Muto M, Kawakubo H, Fujishiro M, Yoshida M, Fujimoto K, Tajiri H, Inoue H; Japan Gastroenterological Endoscopy Society Guidelines Committee of ESD/EMR for Esophageal Cancer. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. 2020;32:452-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 39. | Chen H, Zhou X, Tang X, Li S, Zhang G. Prediction of Lymph Node Metastasis in Superficial Esophageal Cancer Using a Pattern Recognition Neural Network. Cancer Manag Res. 2020;12:12249-12258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Zheng H, Tang H, Wang H, Fang Y, Shen Y, Feng M, Xu S, Fan H, Ge D, Wang Q, Tan L. Nomogram to predict lymph node metastasis in patients with early oesophageal squamous cell carcinoma. Br J Surg. 2018;105:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Zhou Y, Du J, Li H, Luo J, Chen L, Wang W. Clinicopathologic analysis of lymph node status in superficial esophageal squamous carcinoma. World J Surg Oncol. 2016;14:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Zhou Y, Du J, Wang Y, Li H, Ping G, Luo J, Chen L, Zhang S, Wang W. Prediction of lymph node metastatic status in superficial esophageal squamous cell carcinoma using an assessment model combining clinical characteristics and pathologic results: A retrospective cohort study. Int J Surg. 2019;66:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Jia R, Luan Q, Wang J, Hou D, Zhao S. Analysis of Predictors for Lymph Node Metastasis in Patients with Superficial Esophageal Carcinoma. Gastroenterol Res Pract. 2016;2016:3797615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Liu HT, Wang N, Wang X, Li SL. Overexpression of Pim-1 is associated with poor prognosis in patients with esophageal squamous cell carcinoma. J Surg Oncol. 2010;102:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Li S, Xi Y, Zhang H, Wang Y, Wang X, Liu H, Chen K. A pivotal role for Pim-1 kinase in esophageal squamous cell carcinoma involving cell apoptosis induced by reducing Akt phosphorylation. Oncol Rep. 2010;24:997-1004. [PubMed] |
| 46. | Warnecke-Eberz U, Bollschweiler E, Drebber U, Metzger R, Baldus SE, Hölscher AH, Mönig S. Prognostic impact of protein overexpression of the proto-oncogene PIM-1 in gastric cancer. Anticancer Res. 2009;29:4451-4455. [PubMed] |
| 47. | Plum PS, Warnecke-Eberz U, Dhaouadi O, Alakus H, Drebber U, Metzger R, Prenzel KL, Hölscher AH, Bollschweiler E. Molecular markers predicting lymph node metastasis in early esophageal cancer. Histol Histopathol. 2015;30:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 48. | Kotsafti A, Fassan M, Cavallin F, Angerilli V, Saadeh L, Cagol M, Alfieri R, Pilati P, Castoro C, Castagliuolo I, Scarpa M. Tumor immune microenvironment in therapy-naive esophageal adenocarcinoma could predict the nodal status. Cancer Med. 2023;12:5526-5535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Liu W, Chen L, Zhu J, Rodgers GP. The glycoprotein hGC-1 binds to cadherin and lectins. Exp Cell Res. 2006;312:1785-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Jang BG, Lee BL, Kim WH. Intestinal Stem Cell Markers in the Intestinal Metaplasia of Stomach and Barrett's Esophagus. PLoS One. 2015;10:e0127300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Suzuki L, Ten Kate FJC, Gotink AW, Stoop H, Doukas M, Nieboer D, Spaander MCW, van Lanschot JJB, van Wijnhoven BPL, Koch AD, Bruno MJ, Looijenga LHJ, Biermann K. Olfactomedin 4 (OLFM4) expression is associated with nodal metastases in esophageal adenocarcinoma. PLoS One. 2019;14:e0219494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Lu P, Qiao J, He W, Wang J, Jia Y, Sun Y, Tang S, Fu L, Qin Y. Genome-wide gene expression profile analyses identify CTTN as a potential prognostic marker in esophageal cancer. PLoS One. 2014;9:e88918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Li H, Li Q, Lian J, Chu Y, Fang K, Xu A, Chen T, Xu M. MLL2 promotes cancer cell lymph node metastasis by interacting with RelA and facilitating STC1 transcription. Cell Signal. 2020;65:109457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |