Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2280
Peer-review started: March 12, 2023
First decision: May 9, 2023
Revised: May 16, 2023
Accepted: June 12, 2023
Article in press: June 12, 2023
Published online: October 27, 2023
Processing time: 228 Days and 18.3 Hours
Gastric cancer is one of the leading causes of cancer burden and mortality, often resulting in peritoneal metastasis in advanced stages with negative survival outcomes. Staging laparoscopy has become standard practice for suspected cases before a definitive gastrectomy or palliation. This systematic review aims to compare the efficacy of other diagnostic modalities instead of staging laparoscopy as the alternatives are able to reduce cost and invasive staging procedures. Recently, a radiomic model based on computed tomography and positron emission tomography (PET) has also emerged as another method to predict peritoneal metastasis.
To determine if the efficacy of computed tomography, magnetic resonance imaging and PET is comparable with staging laparoscopy.
Articles comparing computed tomography, PET, magnetic resonance imaging, and radiomic models based on computed tomography and PET to staging laparoscopies were filtered out from the Cochrane Library, EMBASE, PubMed, Web of Science, and Reference Citations Analysis (https://www.referencecitationanalysis.com/). In the search for studies comparing computed tomography (CT) to staging laparoscopy, five retrospective studies and three prospective studies were found. Similarly, five retrospective studies and two prospective studies were also included for papers comparing CT to PET scans. Only one retrospective study and one prospective study were found to be suitable for papers comparing CT to magnetic resonance imaging scans.
Staging laparoscopy outperformed computed tomography in all measured aspects, namely sensitivity, specificity, positive predictive value and negative predictive value. Magnetic resonance imaging and PET produced mixed results, with the former shown to be only marginally better than computed tomography. CT performed slightly better than PET in most measured domains, except in specificity and true negative rates. We speculate that this may be due to the limited F-fluorodeoxyglucose uptake in small peritoneal metastases and in linitis plastica. Radiomic modelling, in its current state, shows promise as an alternative for predicting peritoneal metastases. With further research, deep learning and radiomic modelling can be refined and potentially applied as a preoperative diagnostic tool to reduce the need for invasive staging laparoscopy.
Staging laparoscopy was superior in all measured aspects. However, associated risks and costs must be considered. Refinements in radiomic modelling are necessary to establish it as a reliable screening technique.
Core Tip: This systematic review aimed to compare the efficacy of staging laparoscopy against computed tomography (CT) scanning in the diagnosis of peritoneal metastases, where staging laparoscopy was found to be unequivocally superior. We then proceeded to investigate the efficacy of CT scans against positron emission tomography (PET) and magnetic resonance imaging (MRI) scans. CT scans were marginally better than PET scans but were slightly inferior to MRI scans based on the measured domains. Radiomic modelling has also been shown to have the potential to become a promising alternative for predicting peritoneal metastases with further research.
- Citation: Ho SYA, Tay KV. Systematic review of diagnostic tools for peritoneal metastasis in gastric cancer-staging laparoscopy and its alternatives. World J Gastrointest Surg 2023; 15(10): 2280-2293
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2280.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2280
Gastric cancer is the fifth most common malignancy worldwide, the most common malignancy in many South-Central Asian countries and the fourth most common cause of cancer-related deaths, according to GLOBOCAN 2020 data[1]. Peritoneal carcinomatosis is the most common[2-4] type of metastasis secondary to gastric cancer, and its presence is associated with a higher risk of mortality, disease progression and poorer survival rates[4-6]. Currently, computed tomography (CT) scans and staging laparoscopy are the two most commonly utilised modalities for detecting peritoneal metastases[7,8]. This is attributed to CT scans having high rates of sensitivity and specificity, short scanning time, as compared to other imaging modalities such as positron emission tomography (PET) and magnetic resonance imaging (MRI)[9-11]. This review aims to investigate the efficacy of multi-imaging modalities in detecting peritoneal metastasis prior to management planning and during surveillance to detect disease recurrence.
The identification of peritoneal carcinomatosis is difficult as its presentation is commonly asymptomatic and hence, discovered late[12]. The detection of peritoneal metastasis on CT is dependent on visualising unique features such as ascites, omental caking and peritoneal thickening[13], but smaller deposits (< 5 mm) such as peritoneal nodules may be missed on imaging[14]. To rule out peritoneal metastasis prior to oncological gastrectomy, staging laparoscopy is commonly performed, especially in advanced gastric cancer patients. In this systematic review, we aim to investigate current evidence to determine if CT scans can yield comparable detection rates of peritoneal metastasis secondary to gastric cancer, with the goal of reducing the routine practice of staging laparoscopy.
PET and MRI scans are valuable non-invasive imaging techniques that are often used as alternatives to CT scans. PET scans detect cancer dissemination by mapping radioactive tracers, usually F-fluorodeoxyglucose (FDG)[15]. They are frequently used in oncological staging in primaries such as lymphoma and lung cancer[16], due to the high metabolic rate of these malignancies, which tend to have increased uptake of the tracers[15]. However, it is important to note that PET scans may not be as suitable for malignancies with low avidity for FDG, and infective or inflammatory sites may be mistaken for malignancies[17]. Since up to 90% of primary gastric malignancies have significant FDG uptake visible on PET[18], excluding patients with tumours of low FDG avid signet ring cells histology[19], we intend to find out if PET scans can be a useful modality in the diagnosis of peritoneal metastases.
As for MRI scans, its main advantage lies in its superior sensitivity, which provides better soft tissue definition, particularly when coupled with non-radioactive contrast[20]. Disadvantages to using MRI include its inability to be used in patients with magnetic implants, allergy to gadolinium, its high cost, longer scanning duration and high rate of motion artifacts[21-23]. Hence, we also evaluated the efficacy of PET and MRI scans as an alternative to CT scans in the detection of peritoneal metastasis secondary to gastric cancer. Although other forms of diagnostic modalities, such as ultrasound scanning, have been considered, it has proven to be inferior in sensitivity and specificity for the detection of peritoneal metastases. This has been attributed to the acoustic impedance of gas and fat, which decreases visualisation through bowel, omentum, mesentery and adipose tissues[7,24]. Endoscopic ultrasound has shown to be a valuable tool in predicting the T stage of tumours and local invasion. However, its efficacy in detecting distant peritoneal metastasis remains limited. Recent animal studies conducted in 2022 have shown promising results in imaging and scoring peritoneal metastasis, but this is still in the trial phase and requires further validation.
The use of PET/MRI scans have also gained attention in recent years as it has shown better detection of peritoneal metastasis secondary to primary abdominopelvic malignancies compared to PET/CT. A recent systematic review demonstrated that PET/MRI scans exhibited better sensitivity for detecting peritoneal metastasis in gastric cancer than PET/CT scans. However, this systematic review was limited to five papers, each assessing a small cohort of 10-15 patients, thereby making it difficult to draw definitive conclusions regarding the efficacy of peritoneal metastasis detection until further studies are conducted.
In recent years, there has been a growing interest in the use of big data and artificial intelligence, to develop radiomic models that can accurately predict peritoneal metastasis based on preoperative CT and PET scans[25]. These models may also have the potential to prognosticate and estimate recurrence rates based on deep learning of retrospective cohorts[26]. As such, we have also included radiomic analysis in our search strategy in order to see the latest development in the diagnosis of peritoneal metastases.
In this paper, we investigate whether there are non-invasive alternatives that can provide comparable accuracy to the current standard of peritoneal metastases detection, staging laparoscopy. This is an important question, as staging laparoscopy is an invasive procedure that carries risks for patients. Identifying non-invasive alternatives that are equally effective would represent a significant advance in the field of peritoneal metastases detection and could improve patients’ management and safety outcomes.
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The Cochrane Library, EMBASE, PubMed, Web of Science libraries and Reference Citations Analysis (https://www.referencecitationanalysis.com/) were searched using the following search terms: Stomach neoplasms, gastric cancer, peritoneal neoplasms, peritoneal metastasis, peritoneal carcinomatosis, laparoscopy, CT, PET, and MRI. The respective search terms are detailed in the appendix as Supplementary Tables 1 and 2. After reviewing the references in the reports and articles for CT and staging laparoscopy, no additional relevant studies were identified.
Studies were considered in the review if they met the inclusion criteria: (1) Prospective or retrospective comparative papers; (2) Diagnosis of peritoneal metastases secondary to gastric cancer; and (3) Compared CT against PET or MRI or staging laparoscopy.
Studies were excluded if the following were met: (1) Articles were not in English; (2) Articles were case reports, guidelines, letters, non-comparative studies, protocols; or meta-analyses; (3) Patients were already diagnosed with metastatic disease; (4) Diagnostic modality used was none of laparoscopy, CT, PET nor MRI; and (5) Comparison was not made for peritoneal metastases.
The risk of bias was assessed as low, moderate, or high based on the Newcastle-Ottawa scale (NOS)[27] for non
Data collection: The following data were extracted from the included studies: (1) Patient demographics; (2) Tumour characteristics; (3) The specific type of diagnostic modality used; and (4) Statistical outcomes in the detection of peritoneal carcinomatosis.
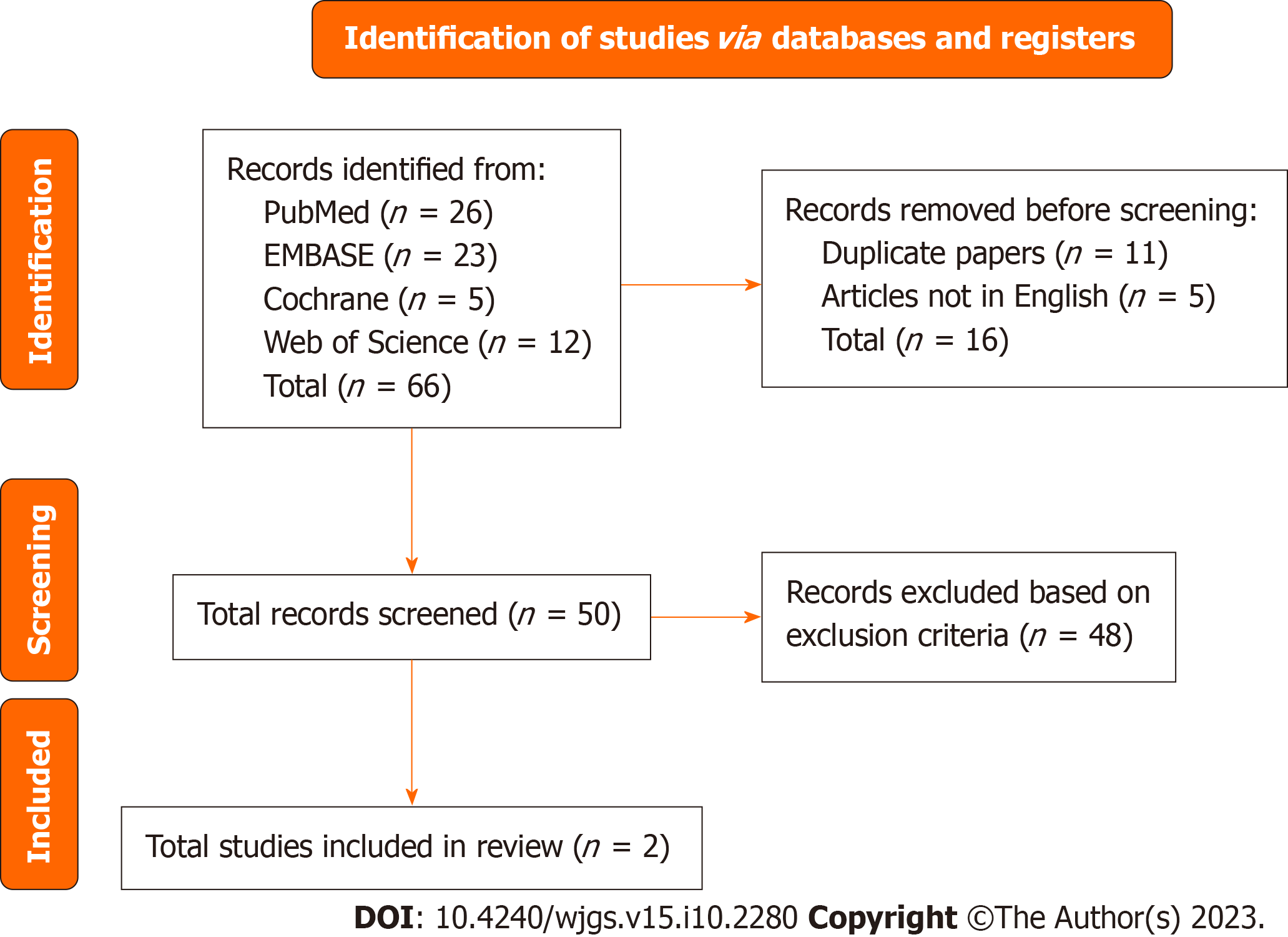
Figure 1 shows the flow chart for the article selection process. Eight relevant articles for CT and staging laparoscopy were identified and included for review. Search details are reflected in Supplementary Table 1.
A total of five retrospective studies and three prospective studies were included. A summary of the patient demographics (Table 1), tumour characteristics and type of CT used (Table 2) and outcome parameters (Table 3) are included below.
| Ref. | Total number of patients | Mean age in years (range) | Gender ratio (M:F) |
| Burbidge et al[28], 2013 | 220 | 69 (41-96) | 136:84 |
| Li et al[29], 2020 | 385 | - | - |
| Davies et al[30], 1997 | 105 | 69 (33-92) | 68:37 |
| Kim et al[31], 2009 | 498 | 59.6 (27-89) | 332:166 |
| Stell et al[32], 1996 | 103 | 65 (33-91) | 68:35 |
| Asencio et al[58], 1997 | 71 | 65.8 (47-81) | 43:27 |
| Fujimura et al[59], 2002 | 39 | (26-80) | 17:22 |
| Leeman et al[60], 2017 | 74 | 67.6 (29-84) | 54:20 |
| Ref. | Primary tumour location | Tumour histology | CT modality |
| Burbidge et al[28], 2013 | - | Adenocarcinoma (220, 100%) | Multidetector CT with gastric staging protocol |
| Li et al[29], 2020 | - | - | Unenhanced, two-phase dynamic enhanced CT |
| Davies et al[30], 1997 | - | Adenocarcinoma (105, 100%) | Philips Tomoscan SR 7000 scanner (120 Kvp and 225-300 mAs), contrast enhanced spiral CT |
| Kim et al[31], 2009 | - | Intestinal (162, 32.5%) | 16-detector row (n = 427) or 64-detector row (n = 71) scanners |
| Diffuse (336, 67.5%) | |||
| Stell et al[32], 1996 | Proximal third (60, 58.3%) | Adenocarcinoma (103, 100%) | Contrast-enhanced CT using a GE model 9800 Hilight whole-body scanner (GEC, Milwaukee, Wisconsin, United States) |
| Body (24, 23.3%) | |||
| Antrum (10, 9.7%) | |||
| Body and antrum (6, 5.8%) | |||
| Fundus (2, 1.9%) | |||
| Linitis plastica (1, 1%) | |||
| Asencio et al[58], 1997 | Upper third (12, 17%) | Adenocarcinoma (71, 100%) | Dynamic contrast-enhanced CT |
| Middle third (21, 30%) | |||
| Lower third (19, 27%) | |||
| Fujimura et al[59], 2002 | Japanese classification of gastric carcinoma type 1 (1, 2.6%); type 2 (4, 10.3%); type 3 (14, 35.9%); type 4 (20, 51.3%) | Differentiated (16, 41%) | CT |
| Undifferentiated (23, 59%) | |||
| Leeman et al[60], 2017 | Proximal (7, 9.5%) | Adenocarcinoma (74, 100%) | Toshiba Aquilion 16 (16 slice), Siemens Somatom Sensation 16 (16 slice), Toshiba Aquilion Multi (4 slice) |
| Body (23, 31.1%) | |||
| Distal (10, 13.5%) | |||
| Linitis plastica (6, 8.1%) |
| Ref. | Sensitivity | Specificity | Positive predictive value | Negative predictive value | ||||
| CT | Staging laparoscopy | CT | Staging laparoscopy | CT | Staging laparoscopy | CT | Staging laparoscopy | |
| Burbidge et al[28], 2013 | 25% | - | 99% | - | 83% | - | 82% | - |
| Li et al[29], 2020 | 87.5% | - | 76.4% | - | 31.8% | - | - | - |
| Davies et al[30], 1997 | 71% | - | 93% | - | 67% | - | 94% | - |
| Kim et al[31], 2009 | 28.3% | - | 98.9% | - | - | - | - | - |
| Asencio et al[58], 1997 | 0% | 88.9% | - | 100% | - | 100% | - | 95.5% |
| Fujimura et al[59], 2002 | 38% | 86% | 100% | 100% | 67% | 92% | - | - |
| Leeman et al[60], 2017 | 58.8% | 94.1% | 89.6% | 100% | 66.7% | 100% | 86% | 98% |
| False positives | False negatives | |||||||
| Stell et al[32], 1996 | 8% | 69% | 100% | 100% | 0% | 0% | 12% | 4% |
A total of 1495 patients with synchronous peritoneal metastases were included in the studies. Excluding the participants from Li et al[29] as the gender proportion was not provided, 718 (64.7%) were male and 391 (35.2%) were female. The age distributions of the participants are also described in Table 1.
The details of the distribution and histological subtypes of the tumours are listed in Table 2, along with the type of CT scanner used. The findings of the study indicate that the source of the primary tumour does not exhibit any discernible pattern, although the histological subtype of lesion typically presents as an adenocarcinoma. The anatomical distribution of the primary lesion was not listed by Burbidge et al[28], Davies et al[30], Kim et al[31] and Li et al[29] Histological subtypes were not mentioned by Li et al[29].
Among the studies that compared the sensitivities of both CT and laparoscopy, staging laparoscopy consistently yielded better results at an average of 58.3%. Staging laparoscopy also demonstrated an average of 3.5% better specificity compared to CT in all studies that compared the two. Similarly, staging laparoscopy exhibited better positive and negative predictive values, with an average improvement of 29.2% and 12% respectively. Stell et al[32] reported false positive and false negative rates instead, in which staging laparoscopy had less or equal numbers of incorrect reporting. This study did not note any false positives, and CT reported an average of 8% more false negatives. In the papers by Burbidge et al[28], Davies et al[30], Kim et al[31] and Li et al[29], statistics for staging laparoscopy were not provided as laparoscopy was used to confirm the provisional diagnosis derived from CT scanning.
Following this discovery, we proceeded to evaluate whether PET or MRI scans are accurate enough to be used as alternatives to CT scans for the purpose of minimising the need for invasiveness of staging laparoscopy, while simultaneously maintaining high detection rates.
Figures 2 and 3 show the flow chart for the article selection process. Seven relevant articles comparing CT to PET and two articles comparing CT to MRI were identified and included for review. Search details are reflected in Supple
A total of five retrospective studies and two prospective studies were included for CT vs PET scans. One retrospective and one prospective study were included for CT vs MRI scans. A summary of the patient demographics (Table 4), tumour characteristics and type of CT used (Table 5), and outcome parameters (Tables 6 and 7) are included below. Following the review of the references in the reports and articles available, the study by Sim et al[33] was also included.
| Modality | Ref. | Total number of patients | Mean age in years (range) | Gender ratio (M:F) |
| PET | Lim et al[11], 2006 | 17 | 51.4 (32-74) | 12:5 |
| Sim et al[33], 2009 | 52 | 62 (median) (33-80) | 43:9 | |
| Turlakow et al[34], 2003 | 88 | 54 (28-84) | 50:38 | |
| Perlaza et al[36], 2018 | 50 | 65.7 ± 12.1 | 30:20 | |
| Kim et al[37], 2017 | 60 | 60.6 (29-80) | 16:44 | |
| Chen et al[38], 2005 | 68 | 54.8 (28-81) | 49:19 | |
| Kim et al[39], 2011 | 139 | 61.5 ± 11.6 | 88:51 | |
| MRI | Lin et al[35], 2021 | 62 (11 gastric) | 56 ± 12 (54 ± 13 in gastric) | 20:42 (6:5 in gastric) |
| De Vuysere et al[40], 2021 | 32 | (29-85) | 22:10 |
| Modality | Ref. | Tumour histology | Specific scanner used | |
| CT | PET/MRI | |||
| PET | Lim et al[11], 2006 | Moderate differentiation (n = 2) | Single-section spiral CT, HiSpeed CT/I or multi-detector CT scanning fourdetector row, LightSpeed Plus | GE advance PET scanner or Philips Allegro PET system |
| Mixed type of moderate and poor differentiation (n = 2) | ||||
| Signet cell differentiation (n = 4) | ||||
| Poor differentiation (n = 9) | ||||
| Sim et al[33], 2009 | Adenocarcinoma (n = 47) | Not mentioned | PET/CT system, Philips Gemini, DA best | |
| Signet ring cell (n = 4) | ||||
| Unknown (n = 1) | ||||
| Turlakow et al[34], 2003 | Gastric (n = 48) | Not mentioned | PET | |
| Ovarian (n = 13) | ||||
| Adrenocortical (n = 6) | ||||
| Mesothelioma (n = 21) | ||||
| Perlaza et al[36], 2018 | Well-differentiated (n = 4) | Somatom sensation 64 | Hybrid PET/CT biograph mCT 64S | |
| Moderately differentiated (n = 20) | ||||
| Poorly differentiated (n = 26) | ||||
| Kim et al[37], 2017 | Adenocarcinoma (n = 51) | 16 or 64-detector row CT scanner, LightSpeed 16 or LightSpeed VCT | Discovery ST PET/CT system | |
| Signet ring cell carcinoma (n = 5) | ||||
| Mucinous carcinoma (n = 4) | ||||
| Chen et al[38], 2005 | Adenocarcinoma (n = 13) | Somatom Plus-S or Tomoscan 310 or LightSpeed Plus | GE Advance | |
| Undifferentiated adenocarcinoma (n = 55) | ||||
| Kim et al[39], 2011 | Adenocarcinoma (n = 117) | Multi-detector row CT scanners, Somatom Volume Zoom | Cyclotron RDS-111 | |
| Signet ring cell carcinoma (n = 19) | ||||
| Mucinous adenocarcinoma (n = 1) | ||||
| Others (n = 2) | ||||
| MRI | Lin et al[35], 2021 | Appendiceal (n = 6) | Somatom sensation 64, Aquilion 64 or Aquilion ONE | MRI |
| Colon (n = 25) | ||||
| Ovarian (n = 20) | ||||
| Gastric (n = 11) | ||||
| De Vuysere et al[40], 2021 | Adenocarcinoma (n = 9) | Somatom Force | Aera 1.5 T scanner | |
| Adenocarcinoma with signet ring cell differentiation (n = 9) | ||||
| Ref. | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | True positive/true negative | False positive/false negative | |||||||
| CT | PET | CT | PET | CT | PET | CT | PET | CT | PET | CT | PET | CT | PET | |
| Lim et al[11], 2006 | 96.5% | 35.3% | 91.6% | 98.9% | - | - | - | - | 89.3% | 89.3% | 13/87 | 6/94 | 8/4 | 1/11 |
| Sim et al[33], 2009 | 86.6% | 46.6% | 91.9% | 94.2% | 82.3% | 80% | - | - | - | - | - | - | - | - |
| Turlakow et al[34], 2003 | 43% | 57% | - | - | 100% | 93% | - | - | - | - | - | - | - | - |
| Perlaza et al[36], 2018 | 64% | 68% | 93% | 100% | - | - | - | - | - | - | - | - | - | - |
| Kim et al[37], 2017 | 96% | 50% | 100% | 100% | 100% | 100% | 99% | 89% | 99% | 90% | - | - | - | - |
| Chen et al[38], 2005 | 80% | 30% | 91% | 98% | - | - | - | - | 89% | 88% | - | - | - | - |
| Kim et al[39], 2011 | 63.6% | 18.2% | 97.7% | 100% | - | - | - | - | 95% | 93.5% | - | - | - | - |
A total of 474 and 94 patients with metachronous peritoneal metastases identified by CT or PET and CT or MRI respectively were included in the studies. A total of 288 (60.8%) were male and 186 (39.2%) were female in the CT vs PET group. In the CT vs MRI group, 42 (44.7%) patients were male and 52 (55.3%) were female. The age distributions of the participants are also described in Table 4.
The origin of the primary tumour in these two populations also does not seem to exhibit a discernable pattern but among the studies that have reported histology, the histological subtype of the lesion is typically an adenocarcinoma. The details of the distribution and histological subtypes of the tumours are listed in Table 5, along with the type of CT, PET or MRI scanner used. The studies by Turlakow et al[34] and Lin et al[35] did not specify the histology of gastric cancer in their patients.
The sensitivity of CT seems to be superior to PET in all studies except in Perlaza et al[36] and Turlakow et al[34]. Conversely, the specificity of PET is superior to CT in every study except in Kim et al[37], where both had 100% specificity. This parameter was not measured by Turlakow et al[34]. Positive predictive value (PPV) was only calculated by Kim et al[37], Sim et al[33] and Turlakow et al[34] where CT slightly outperformed PET by an average of 3.1%. Negative predictive value (NPV) was only reported by Kim et al[37] where CT was better than PET by 10%. The accuracy of CT was also marginally greater than or equal to PET in the studies by Chen et al[38], Kim et al[39], Kim et al[37] and Lim et al[11] by an average of 2.875%. True positive, true negative, false positive, false negative values were only reported by Lim et al[11], where CT was better at picking up true positive cases (13 vs 6) and had lower false negative rates (4 vs 11). PET was better than CT at picking up true negative cases (87 vs 94) and had lower false positive rates (8 vs 1).
The values obtained by Lin et al[35] were the calculated average value between the two types of CT and MRI scanners used. The decimal values in Lin et al[35] were converted to percentages in the calculation of average scores.
The sensitivity of MRI was superior to CT in both papers by an average of 38.1%. Conversely, CT had better or equal specificity than MRI by an average of 6%. In the study by De Vuysere et al[40], PPV was equally high at 100% but MRI had much greater NPV at 68.7% better. MRI also had better accuracy by an average of 33.75%. Precision was only reported by Lin et al[35], where CT was 8.3% better. Iodinated contrast (Telebrix, Xenetix, Omnipaque) were used in both studies, and T2-weighted, diffusion-weighted images were obtained.
Radiomic modelling is a cutting-edge technique that employs artificial intelligence and a quantitative approach to augment medical imaging data, thereby improving diagnostic accuracy. The use of radiomic analysis and deep learning is gaining traction in the diagnosis of peritoneal metastases, with several preliminary papers demonstrating its potential. However, there is currently a lack of comparative studies that evaluate the efficacy of radiomic models. In the study by Jiang et al[14], the performance of radiomics was significantly better than conventional clinicopathological factors [Area under the curve (AUC) range, 0.51-0.63]. It also had value as an independent predictor of occult peritoneal metastases. In a separate paper by Jiang et al[41], radiomic modelling was able to predict peritoneal recurrence (AUC: 0.857, 0.856 and 0.843) and disease-free survival independently in all three cohorts stated (C-index 0.654, 0.668 and 0.610). The paper by Huang et al[26] affirms this possibility as radiomic modelling has been demonstrated to be an independent predictor of peritoneal metastases, with AUC values of 0.751, 0.802 and 0.745. Xue et al[42] have reported promising performance with the radiomic model, achieving an AUC of 0.90 in the training cohort and 0.88 in the validation cohort respectively. Ultimately, radiomic modelling is still in a developmental phase, requiring the multidisciplinary coordination of physicians, computers and data scientists in order to interpret the imaging data and analysis.
The mean age of participants falls between 58 to 69 years, which is in line with the peak age where gastric cancer and peritoneal metastasis are reported[2,43]. Most of the participants in the studies were male, except in those by Fujimura et al[59], Kim et al[37] and Lin et al[35], which is converse to current literature. In the 2021 systematic review by Rijken et al[44], peritoneal metastases were noted more frequently in females. Tan et al[45] also reported similar findings in their retrospective review[45].
Although the location of the primary tumour in the studies included did not show any distinct distribution pattern, most of the lesions arise from non-cardia areas, which corresponds to literature by Rijken et al[44] and Sanjeevaiah et al[46] It is frequently reported that signet ring cell or diffuse type tumours have a greater risk of peritoneal metastases but the majority of cases seen in the studies are adenocarcinomas[44,47,48]. This may be attributed to the vast majority of gastric cancers being adenocarcinomas or intestinal types[49,50].
The use of staging laparoscopy has demonstrated superior or comparable results to CT scans in all domains of measurement, namely sensitivity, specificity, PPV, NPV, false negative and false positive rates. These findings are consistent with current research indicating that staging laparoscopy more accurately reflects the actual M stage of patients, leading to a significant reduction in unnecessary laparotomies[51]. A systematic review by Giger et al[51] suggested the number of diagnostic laparotomies performed can be lowered by up to 63% by performing staging laparotomy prior.
However, the risks of staging laparoscopy were not properly assessed in the studies. The most significant risk appeared to be port-site metastasis as seen in the five cases reported by Shoup et al[52], one case by McCulloch et al[53] and one case by Davies et al[54] Despite these findings, all three papers suggest that the value of diagnostic laparoscopy far outweighs the risks, and such occurrences are rare and unlikely.
The results from comparing between CT and PET scans were not as clear cut. CT scans performed marginally better than PET scans in most aspects, namely sensitivity, PPV, NPV, accuracy, and the detection of true positive cases with lower false negative rates. However, PET scans offered slightly better specificity and greater identification of true negative cases with lower false positive rates. These findings are consistent with the study by Dromain et al[55], which found that PET scans did not perform as well as CT scans in detecting peritoneal metastases in gastrointestinal malignancies. The limited FDG uptake due to the small (< 2 mm) and even microscopic size of peritoneal metastasis could be a contributing factor to this discrepancy[55].
MRI scans had outperformed or performed equally well to CT scans in all areas of comparison except when comparing specificity and precision, where CT scans were superior. This includes sensitivity, PPV, NPV and accuracy. Similar results were also reported by Low et al[22], where MRI scans were found to be more successful than CT scans in detecting peritoneal metastasis in all cases of low, moderate and large tumour burden. However, it should be noted that the significant downsides of using MRI scans include the high cost and time necessary for the procedure, along with the motion artefacts that can compromise image quality.
The use of PET/MRI has become increasingly prevalent and has demonstrated its effectiveness in detecting peritoneal metastasis. The degree of peritoneal involvement is a crucial factor in determining the resectability and prognosis of the tumor. However, the detection rate of 18-fluorothymine-FDG in peritoneal metastasis is often poor due to its low level of FDG uptake, leading to potential underestimation of the degree of involvement. In a recent study by Wang et al[3,9], [68Ga]Ga-FAPI-04 PET MRI/CT was found to be 100% sensitive in detecting peritoneal metastasis in gastric cancer. This success may be attributed to the fibrotic reaction of tumor cells invading the peritoneum, and the targeting of fibroblast activating protein (FAP) by FAPI. By improving the detection rate of peritoneal metastasis, clinicians can more accurately assess disease involvement and evaluate treatment response.
In the same vein, recent advances in PET radiotracers have shown promise in addressing the limitations of imaging FDG non-avid tumours, such as early stage, diffuse type, and mucinous tumours. Some examples of novel PET radiotracers that have shown potential in this regard include 18-fluorothymine, FAPI, and DOTA-FAPI PET. Further research is needed to fully understand the potential of these radiotracers, but early results are encouraging.
The use of radiomic modelling has also emerged as a potential tool for diagnosing peritoneal metastasis with the aid of CT and PET scanning, as shown by the papers by Jiang et al[14,41], Huang et al[26], and Xue et al[42]. Chen et al[25] also reported encouraging results in their preliminary evaluation of radiomics in the use of non-invasive peritoneal metastases diagnosis by studying three types (R_IU model for iodine uptake images, R_MIX model for mixed images, R_comb model for the combined radiomics model) of radiomics models in dual-energy CT scanning. The retrospective paper by Kim et al[56] has further shown the possibility of using texture analysis and entropy in CT scans to detect occult peritoneal metastases. When the cut-off value for entropy was applied, the sensitivity and specificity were found to be 80% and 90% respectively. With further research, deep learning and radiomic modelling can be refined and potentially applied as a preoperative diagnostic modality, thereby reducing the need for invasive staging laparoscopy.
The lack of homogeneity in the methodology of studies included in the review is a key concern that could have contributed to some disparity as different types of data were reported. For instance, while the paper by Stell et al[32] reported false negative and false positive rates, other papers reported PPV and NPV instead. Additionally, papers by Burbidge et al[28], Davies et al[30], Kim et al[31] and Li et al[29], did not report the statistical values representing staging laparoscopies, as they utilized laparoscopy to confirm the preoperative diagnosis made by CT scans, basing the statistical values reported for CT scans on laparoscopy. This implied that staging laparoscopy was assumed to have maximum accuracy and remained the standard of care prior to definitive gastrectomy based on features such as poorly differentiated adenocarcinoma on histology, linitis plastica, large sized type 3, or equivocal CT findings for peritoneal dissemination[57]. However, it is important to acknowledge that heterogeneity among studies is a common occurrence, particularly when papers are produced by various institutions that adhere to different reporting guidelines and compare different methods of diagnostic tools.
The type of CT, PET and MRI scanners used also varied from study to study. With different types of scanners used, the cut of the images obtained will also vary, potentially affecting the accuracy. The quality of images obtained will also vary, which could result in inconsistencies in the level of human error when reading the scans.
Similarly, the staging laparoscopy procedures were performed by different surgeons with differing levels of competency and proficiency with the laparoscope. This difference in ability could have altered the accuracy rates yielded as well. However, considering that many of the statistics obtained from the papers are 100%, the margin of error in this aspect appears to be limited.
Included reviews comparing MRI and CT scans were only limited to two studies by Lin et al[35] and De Vuysere et al[40], reducing the quality of analysis obtained due to a small sample size. Some of the statistics obtained were non-overlapping, which impacted the data analysis, resulting in a less robust comparison. Additionally, the study by Lin et al[35] was not specific for gastric cancer. This is a clear indication for further studies specifically comparing MRI and CT scans in the detection of peritoneal metastases secondary to gastric cancer. Several studies evaluating the use of PET/MRI in detecting peritoneal metastasis in gastric cancer have been conducted, but due to the nascent nature of these studies, the use of PET/MRI may not be currently available for patients.
There were no studies comparing radiomic modelling to the conventional diagnostic modalities available at the time of the search. Hence, essential analysis of quantitative values could not be carried out and the efficacy of radiomic modelling cannot be fully assessed. Due to its high potential based on preliminary investigations, more research is necessary to provide patients with a possible non-invasive alternative to staging laparoscopy in the diagnosis of peritoneal metastases.
Overall, staging laparoscopy outperformed CT scans in every measured aspect. These findings indicate that staging laparoscopy is statistically the superior modality for the diagnosis of peritoneal metastases in patients with gastric cancer or to rule out peritoneal metastases in other patients. It is important to note, however, that staging laparoscopies are still considered an invasive surgical procedure where general anaesthesia is necessary and multiple surgeons are involved. This would implicate the risks of anaesthesia, infection, and require more time and resources, and as a result, cost per patient may increase.
As such, non-invasive imaging remains invaluable in the work-up of gastric cancer patients. Among the commonly available scanning modalities, MRI scans have demonstrated superior performance in detecting peritoneal metastases compared to CT scans, which in turn showed slightly better results than PET scans. Hence, there is potential in these scanning modalities to provide patients with a non-invasive yet accurate alternative to staging laparoscopy, especially with the addition of alternate radiotracers such as FAPI and Flurothyrmine. However, further research is imperative to enhance the sensitivity and specificity of these techniques in the diagnosis of peritoneal carcinomatosis, such that they may soon be comparable to staging laparoscopies. In the same vein, more research in radiomic modelling is pivotal in achieving the same goal, as it has shown great promise in attaining a comparable, non-invasive alternative to staging laparoscopies.
Staging laparoscopy is currently the gold standard for diagnosing peritoneal metastasis in gastric cancer patients. However, this procedure comes with risks of general anaesthesia and surgery which are of importance in elderly and frail patients, the demographic most affected by gastric cancer. Hence, we sought to evaluate non-invasive alternatives to staging laparoscopy with comparable accuracy.
Staging laparoscopy remains the gold standard for diagnosing peritoneal metastasis in gastric cancer patients, which comes with risks of general anaesthesia and surgery. Many non-invasive diagnostic modalities are available in the current day and age, hence, we sought to evaluate non-invasive alternatives to staging laparoscopy that may provide us with comparable accuracy. With further research in this field, along with newer developments such as radiomic modelling and new radiotracers, there is great potential for developing such a diagnostic tool with comparable or even greater accuracy than staging laparoscopy.
We sought to determine if computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) could be a potential non-invasive yet accurate alternative to staging laparoscopy.
Data from relevant studies that reported patients with peritoneal metastasis secondary to gastric cancer diagnosed by non-invasive scans were extracted and presented according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Significant data such as sensitivity, specificity, negative and positive predictive values were analysed and compared between diagnostic modalities in our systematic review.
Our findings suggested that staging laparoscopy still delivered the best results in terms of sensitivity, specificity, negative and positive predictive values when compared to CT scans in diagnosing peritoneal metastasis in advanced gastric cancer. MRI had generally outperformed CT scans which had in turn, generally performed better than PET scans. Despite this, the difference in performance between all the diagnostic modalities are marginal, suggesting that there is great potential for the development of the ideal diagnostic tool capable of providing us with the same or even better accuracy than staging laparoscopy, while remaining non-invasive. With additional tools such as radiomic modelling and new radiotracers, the development of such a diagnostic modality may be possible sooner than expected.
Although staging laparoscopy remains superior to other non-invasive diagnostic modalities in the detection of peritoneal metastasis in advanced gastric cancer, the potential for developing a comparable or even better diagnostic tool is great. This may be achieved with new technologies such as radiomic modelling and new radiotracers, on top of the already advanced capabilities of CT, MRI and PET scans. With further research, this breakthrough may be possible sooner than expected.
Given the rapid and enthusiastic development of new technologies in diagnostic tools, the development of a highly sensitive and specific non-invasive alternative to staging laparoscopy in peritoneal metastasis detection is highly likely with further research. On top of the already cutting edge diagnostic modalities, additional improvements and developments may bring us closer than ever to this goal.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W, China; Liu Q, China; Luo W, China S-Editor: Fan JR L-Editor: A P-Editor: Xu ZH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64695] [Article Influence: 16173.8] [Reference Citation Analysis (177)] |
| 2. | Yarema R, Оhorchak М, Hyrya P, Kovalchuk Y, Safiyan V, Karelin I, Ferneza S, Fetsych M, Matusyak M, Oliynyk Y, Fetsych Т. Gastric cancer with peritoneal metastases: Efficiency of standard treatment methods. World J Gastrointest Oncol. 2020;12:569-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Wang Z, Chen JQ, Liu JL, Tian L. Issues on peritoneal metastasis of gastric cancer: an update. World J Surg Oncol. 2019;17:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Wei J, Wu ND, Liu BR. Regional but fatal: Intraperitoneal metastasis in gastric cancer. World J Gastroenterol. 2016;22:7478-7485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Leiting JL, Grotz TE. Optimizing outcomes for patients with gastric cancer peritoneal carcinomatosis. World J Gastrointest Oncol. 2018;10:282-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Montori G, Coccolini F, Ceresoli M, Catena F, Colaianni N, Poletti E, Ansaloni L. The treatment of peritoneal carcinomatosis in advanced gastric cancer: state of the art. Int J Surg Oncol. 2014;2014:912418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Healy JC. Detection of peritoneal metastases. Cancer Imaging. 2001;1:4-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Saiz Martínez R, Dromain C, Vietti Violi N. Imaging of Gastric Carcinomatosis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Wang Z, Chen JQ. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: a systematic review. BMC Gastroenterol. 2011;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Borggreve AS, Goense L, Brenkman HJF, Mook S, Meijer GJ, Wessels FJ, Verheij M, Jansen EPM, van Hillegersberg R, van Rossum PSN, Ruurda JP. Imaging strategies in the management of gastric cancer: current role and future potential of MRI. Br J Radiol. 2019;92:20181044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, Park MS, Cha SW, Lee JD, Noh SH, Yoo HS, Kim KW. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006;7:249-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Irino T, Sano T, Hiki N, Ohashi M, Nunobe S, Kumagai K, Ida S, Yamaguchi T. Diagnostic staging laparoscopy in gastric cancer: a prospective cohort at a cancer institute in Japan. Surg Endosc. 2018;32:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Kim M, Jeong WK, Lim S, Sohn TS, Bae JM, Sohn IS. Gastric cancer: development and validation of a CT-based model to predict peritoneal metastasis. Acta Radiol. 2020;61:732-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Jiang Y, Liang X, Wang W, Chen C, Yuan Q, Zhang X, Li N, Chen H, Yu J, Xie Y, Xu Y, Zhou Z, Li G, Li R. Noninvasive Prediction of Occult Peritoneal Metastasis in Gastric Cancer Using Deep Learning. JAMA Netw Open. 2021;4:e2032269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Hess S, Blomberg BA, Zhu HJ, Høilund-Carlsen PF, Alavi A. The pivotal role of FDG-PET/CT in modern medicine. Acad Radiol. 2014;21:232-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Kapoor M, Kasi A. PET Scanning. StatPearls. Treasure Island (FL), 2022. |
| 17. | Griffeth LK. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc (Bayl Univ Med Cent). 2005;18:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Kim HW, Won KS, Song BI, Kang YN. Correlation of Primary Tumor FDG Uptake with Histopathologic Features of Advanced Gastric Cancer. Nucl Med Mol Imaging. 2015;49:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Arslan E, Cermik T, Sen C, Yilmaz S, Gundogan C, Dursun N, Bayrak S. Diagnostic value of 18 F-FDG PET/CT for staging of gastric signet ring cell carcinomas. Soc Nuclear Med. 2018;. |
| 20. | Seemann MD. Whole-body PET/MRI: the future in oncological imaging. Technol Cancer Res Treat. 2005;4:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Havsteen I, Ohlhues A, Madsen KH, Nybing JD, Christensen H, Christensen A. Are Movement Artifacts in Magnetic Resonance Imaging a Real Problem?-A Narrative Review. Front Neurol. 2017;8:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2015;22:1708-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Ghadimi M, Sapra A. Magnetic Resonance Imaging Contraindications. StatPearls. Treasure Island (FL), 2022. |
| 24. | Szadkowska MA, Pałucki J, Cieszanowski A. Diagnosis and treatment of peritoneal carcinomatosis - a comprehensive overview. Pol J Radiol. 2023;88:e89-e97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Xi W, Yao W, Wang L, Xu Z, Wels M, Yuan F, Yan C, Zhang H. Dual-Energy Computed Tomography-Based Radiomics to Predict Peritoneal Metastasis in Gastric Cancer. Front Oncol. 2021;11:659981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Huang W, Zhou K, Jiang Y, Chen C, Yuan Q, Han Z, Xie J, Yu S, Sun Z, Hu Y, Yu J, Liu H, Xiao R, Xu Y, Zhou Z, Li G. Radiomics Nomogram for Prediction of Peritoneal Metastasis in Patients With Gastric Cancer. Front Oncol. 2020;10:1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Chacón-Moscoso S, Sanduvete-Chaves S, Sánchez-Martín M. The Development of a Checklist to Enhance Methodological Quality in Intervention Programs. Front Psychol. 2016;7:1811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Burbidge S, Mahady K, Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin Radiol. 2013;68:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Li ZY, Tang L, Li ZM, Li YL, Fu J, Zhang Y, Li XT, Ying XJ, Ji JF. Four-Point Computed Tomography Scores for Evaluation of Occult Peritoneal Metastasis in Patients with Gastric Cancer: A Region-to-Region Comparison with Staging Laparoscopy. Ann Surg Oncol. 2020;27:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Davies J, Chalmers AG, Sue-Ling HM, May J, Miller GV, Martin IG, Johnston D. Spiral computed tomography and operative staging of gastric carcinoma: a comparison with histopathological staging. Gut. 1997;41:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Kim SJ, Kim HH, Kim YH, Hwang SH, Lee HS, Park DJ, Kim SY, Lee KH. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology. 2009;253:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Stell DA, Carter CR, Stewart I, Anderson JR. Prospective comparison of laparoscopy, ultrasonography and computed tomography in the staging of gastric cancer. Br J Surg. 1996;83:1260-1262. [PubMed] |
| 33. | Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, Im SA, Kim TY, Kim WH, Heo DS, Bang YJ. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer. 2009;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Turlakow A, Yeung HW, Salmon AS, Macapinlac HA, Larson SM. Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nucl Med. 2003;44:1407-1412. [PubMed] |
| 35. | Lin CN, Huang WS, Huang TH, Chen CY, Huang CY, Wang TY, Liao YS, Lee LW. Adding Value of MRI over CT in Predicting Peritoneal Cancer Index and Completeness of Cytoreduction. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 36. | Perlaza P, Ortín J, Pagès M, Buxó E, Fernández-Esparrach G, Colletti PM, Rubello D, Mayoral M, Sánchez N, Ruiz C, Ginés A, Fuster D. Should 18F-FDG PET/CT Be Routinely Performed in the Clinical Staging of Locally Advanced Gastric Adenocarcinoma? Clin Nucl Med. 2018;43:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Kim JH, Heo SH, Kim JW, Shin SS, Min JJ, Kwon SY, Jeong YY, Kang HK. Evaluation of recurrence in gastric carcinoma: Comparison of contrast-enhanced computed tomography and positron emission tomography/computed tomography. World J Gastroenterol. 2017;23:6448-6456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, Noh SH. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Kim DW, Park SA, Kim CG. Detecting the recurrence of gastric cancer after curative resection: comparison of FDG PET/CT and contrast-enhanced abdominal CT. J Korean Med Sci. 2011;26:875-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 40. | De Vuysere S, Vandecaveye V, De Bruecker Y, Carton S, Vermeiren K, Tollens T, De Keyzer F, Dresen RC. Accuracy of whole-body diffusion-weighted MRI (WB-DWI/MRI) in diagnosis, staging and follow-up of gastric cancer, in comparison to CT: a pilot study. BMC Med Imaging. 2021;21:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Jiang Y, Zhang Z, Yuan Q, Wang W, Wang H, Li T, Huang W, Xie J, Chen C, Sun Z, Yu J, Xu Y, Poultsides GA, Xing L, Zhou Z, Li G, Li R. Predicting peritoneal recurrence and disease-free survival from CT images in gastric cancer with multitask deep learning: a retrospective study. Lancet Digit Health. 2022;4:e340-e350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 42. | Xue B, Jiang J, Chen L, Wu S, Zheng X, Tang K. Development and Validation of a Radiomics Model Based on (18)F-FDG PET of Primary Gastric Cancer for Predicting Peritoneal Metastasis. Front Oncol. 2021;11:740111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Rau B, Brandl A, Piso P, Pelz J, Busch P, Demtröder C, Schüle S, Schlitt HJ, Roitman M, Tepel J, Sulkowski U, Uzunoglu F, Hünerbein M, Hörbelt R, Ströhlein M, Beckert S, Königsrainer I, Königsrainer A; Peritoneum Surface Oncology Group and members of the StuDoQ|Peritoneum Registry of the German Society for General and Visceral Surgery (DGAV). Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2020;23:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 44. | Rijken A, Lurvink RJ, Luyer MDP, Nieuwenhuijzen GAP, van Erning FN, van Sandick JW, de Hingh IHJT. The Burden of Peritoneal Metastases from Gastric Cancer: A Systematic Review on the Incidence, Risk Factors and Survival. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 45. | Tan HL, Chia CS, Tan GH, Choo SP, Tai DW, Chua CW, Ng MC, Soo KC, Teo MC. Gastric peritoneal carcinomatosis - a retrospective review. World J Gastrointest Oncol. 2017;9:121-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 46. | Sanjeevaiah A, Park H, Fangman B, Porembka M. Gastric Cancer with Radiographically Occult Metastatic Disease: Biology, Challenges, and Diagnostic Approaches. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Fanelli MF, de Paiva TF Jr, Silva MJ, Benevides CF, Guimarães AP, Gimenes DL, Pinheiro ED, Rinck JA Jr, Nicolau UR, Sanches SM, Mello CA, Dettino AL, Cruz MR, de Melo LM, Formiga MN, de Lima VC, Chinen LT. Predictors of peritoneal carcinomatosis in patients with gastric cancer treated at a single institution in Brazil. J Surg Oncol. 2009;100:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Fanelli MF, Silva MJ, de Paiva TF Jr, Chinen LT, Guimarães AP, Gimenes DL, Pinheiro ED, Rinck JA Jr, Nicolau UR, Sanches SM, Melo CA, Dettino AL, Cruz MR, de Melo LM, Formiga MN, de Lima VC. Factors correlated with peritoneal carcinomatosis and survival in patients with gastric cancer treated at a single institution in Brazil. Int J Clin Oncol. 2009;14:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 858] [Article Influence: 171.6] [Reference Citation Analysis (0)] |
| 50. | Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 256] [Reference Citation Analysis (0)] |
| 51. | Giger U, Schäfer M, Krähenbühl L. Technique and value of staging laparoscopy. Dig Surg. 2002;19:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Shoup M, Brennan MF, Karpeh MS, Gillern SM, McMahon RL, Conlon KC. Port site metastasis after diagnostic laparoscopy for upper gastrointestinal tract malignancies: an uncommon entity. Ann Surg Oncol. 2002;9:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | McCulloch P, Johnson M, Jairam R, Fischer W. Laparoscopic staging of gastric cancer is safe and affects treatment strategy. Ann R Coll Surg Engl. 1998;80:400-402. [PubMed] |
| 54. | Davies AR, Ahmed W, Purkiss SF. Port site metastasis following diagnostic laparoscopy for a malignant Gastro-intestinal stromal tumour. World J Surg Oncol. 2008;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Dromain C, Leboulleux S, Auperin A, Goere D, Malka D, Lumbroso J, Schumberger M, Sigal R, Elias D. Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT. Abdom Imaging. 2008;33:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 56. | Kim HY, Kim YH, Yun G, Chang W, Lee YJ, Kim B. Could texture features from preoperative CT image be used for predicting occult peritoneal carcinomatosis in patients with advanced gastric cancer? PLoS One. 2018;13:e0194755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Fukagawa T. Role of staging laparoscopy for gastric cancer patients. Ann Gastroenterol Surg. 2019;3:496-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 58. | Asencio F, Aguiló J, Salvador JL, Villar A, De la Morena E, Ahamad M, Escrig J, Puche J, Viciano V, Sanmiguel G, Ruiz J. Video-laparoscopic staging of gastric cancer. A prospective multicenter comparison with noninvasive techniques. Surg Endosc. 1997;11:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Fujimura T, Kinami S, Ninomiya I, Kitagawa H, Fushida S, Nishimura G, Kayahara M, Shimizu K, Ohta T, Miwa K. Diagnostic laparoscopy, serum CA125, and peritoneal metastasis in gastric cancer. Endoscopy. 2002;34:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Leeman MF, Patel D, Anderson J, OʼNeill JR, Paterson-Brown S. Multidetector Computed Tomography Versus Staging Laparoscopy for the Detection of Peritoneal Metastases in Esophagogastric Junctional and Gastric Cancer. Surg Laparosc Endosc Percutan Tech. 2017;27:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |