Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2201
Peer-review started: July 12, 2023
First decision: August 2, 2023
Revised: August 9, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: October 27, 2023
Processing time: 107 Days and 6.7 Hours
Anastomotic leakage (AL) occurs frequently after sphincter-preserving surgery for rectal cancer and has a significant mortality rate. There are many factors that influence the incidence of AL, and each patient’s unique circumstances add to this diversity. The early identification and prediction of AL after sphincter-preserving surgery are of great significance for the application of clinically targeted preven
To develop nomogram, decision tree, and random forest prediction models for AL following sphincter-preserving surgery for rectal cancer and to evaluate the pre
The clinical information of 497 patients with rectal cancer who underwent sphinc
AL occurred in 10.26% of the 497 patients with rectal cancer. The nomogram model had an AUC of 0.922, sen
The random forest model may be used to identify patients at high risk of AL after sphincter-preserving surgery for rectal cancer owing to its strong predictive effect and stability.
Core Tip: Anastomotic leakage (AL) is a very dangerous complication of rectal cancer surgery, which not only increases the recurrence rate of the tumor but also lowers the quality of life of affected patients. We examined the clinical data of 497 patients with rectal cancer to determine variables that influence AL. We established nomogram, decision tree, and random forest models to identify a prediction model tool for forecasting AL after rectal cancer surgery.
- Citation: Li HY, Zhou JT, Wang YN, Zhang N, Wu SF. Establishment and application of three predictive models of anastomotic leakage after rectal cancer sphincter-preserving surgery. World J Gastrointest Surg 2023; 15(10): 2201-2210
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2201.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2201
According to the most recent data from 2020, colorectal cancer has become the third most common cancer worldwide. In China, colorectal cancer has the third highest incidence and fatality rate among all malignancies. Cases of rectal cancer account for 39% of total colorectal cancer cases, making it a serious public health issue in China[1,2]. Radical surgery remains the first choice of treatment for rectal cancer, both for primary and secondary tumors[3]. With advances in medical technology, the prognosis of patients undergoing rectal cancer surgery has significantly improved, and the sph
Nevertheless, anastomotic leakage (AL) remains the most common complication following sphincter-preserving sur
For this retrospective analysis, we collected clinical data from 497 patients with rectal cancer who underwent sphincter-preserving surgery at Jincheng People’s Hospital of Shanxi Province between January 2017 and September 2022. The inclusion criteria were: Rectal cancer diagnosed by colonoscopy or anal biopsy; tumor within 12 cm of the anal margin; age ≥ 18 years old; and complete clinical data. The exclusion criteria were: Extensive tumor metastasis or the presence of other malignant tumors; a history of rectal surgery, anal stenosis or anal fistula; and conversion to laparotomy. The patients were divided into two groups, those with AL and those without (no AL). The study was approved by the Jincheng People’s Hospital of Shanxi Province (JCPH.No20230407001) and written informed consent was obtained from all study participants or their legal guardians.
The following variables were analyzed: Sex, age, body mass index, diabetes mellitus, hypertension, smoking history, neoadjuvant treatment, hemoglobin level, albumin (Alb) level, tumor size, tumor-node-metastasis stage, American Society of Anesthesiologists score, tumor location, surgical approach, operative time, and blood loss.
The diagnosis of AL was based on clinical manifestations (pain, persistent body temperature of > 38 °C, peritonitis, watery fecal matter, food residue in fecal matter, or pus in the drainage fluid), laboratory tests (elevated white blood cell count and neutrophil percentage), and imaging findings (computed tomography following an enema with a liquid, gas, or water-soluble contrast agent)[9].
Statistical analyses were performed using SPSS for Windows version 26.0 (IBM Corp., Armonk, NY, United States). All continuous variable data are presented as mean ± SD, and student’s t-tests were used to compare differences. Data from discrete variables are presented as numbers and percentages, and the χ2 test was used to assess differences between groups. Variables associated with AL were identified using univariate and multivariate logistic regression analyses.
The prediction models were constructed using the R software, and the data were randomly divided between a training set and a verification set in a 7:3 ratio. The nomogram was created using the ‘rms’ package, the decision tree with the ‘rpart’ package, and the random forest using the ‘random Forest’ package. The model with the best predictive effect was selected by comparing the sensitivity, specificity, accuracy, recall rate, precision rate, and area under the receiver ope
There were 271 men and 226 women among the 497 patients. The incidence of AL was 10.26% (51/497). Patients in the AL group had a mean age of 60.98 ± 10.83 years. The no AL group included 446 patients with a mean age of 59.27 ± 10.76 years.
Univariate analysis revealed statistically significant differences between the AL and no AL groups for the following variables: Sex, diabetes mellitus, smoking history, neoadjuvant treatment, Alb level, tumor size, and tumor location (Table 1).
| Patient characteristics | AL (n = 51) | No AL (n = 446) | t/χ² | P value |
| Sex | 6.921 | 0.009 | ||
| Male | 33 (64.71) | 244 (54.71) | ||
| Female | 18 (35.29) | 202 (45.29) | ||
| Age, year (mean ± SD) | 60.98 ± 10.83 | 59.27 ± 10.76 | 1.073 | 0.284 |
| BMI, kg/m² (mean ± SD) | 22.30 ± 2.91 | 21.45 ± 3.06 | 1.896 | 0.058 |
| Diabetes mellitus | 14.164 | < 0.001 | ||
| No | 21 (41.18) | 302 (67.71) | ||
| Yes | 30 (58.82) | 144 (32.29) | ||
| Hypertension | 0.232 | 0.630 | ||
| No | 16 (31.37) | 155 (34.75) | ||
| Yes | 35 (68.63) | 291 (65.25) | ||
| Smoking history | 6.970 | 0.008 | ||
| No | 14 (27.45) | 209 (46.86) | ||
| Yes | 37 (72.55) | 237 (53.14) | ||
| Neoadjuvant treatment | 7.973 | 0.005 | ||
| No | 16 (31.37) | 233 (52.24) | ||
| Yes | 35 (68.63) | 213 (47.76) | ||
| Hb, g/L (mean ± SD) | 135.60 ± 10.46 | 136.75 ± 10.41 | 0.746 | 0.456 |
| Alb, g/L (mean ± SD) | 33.23 ± 6.59 | 37.13 ± 7.25 | 3.664 | < 0.001 |
| Tumor size, cm (mean ± SD) | 4.73 ± 1.22 | 3.42 ± 1.26 | 7.009 | < 0.001 |
| Tumor location, cm (mean ± SD) | 4.32 ± 1.28 | 6.13 ± 1.30 | 9.378 | < 0.001 |
| TNM stage | 0.010 | 0.995 | ||
| I | 32 (62.75) | 281 (63.00) | ||
| II | 12 (23.53) | 106 (23.77) | ||
| III | 7 (13.72) | 59 (13.23) | ||
| ASA score | 0.289 | 0.866 | ||
| I | 34 (66.67) | 282 (63.23) | ||
| II | 12 (23.53) | 111 (24.89) | ||
| III | 5 (9.80) | 53 (11.88) | ||
| Surgical approach | 1.676 | 0.195 | ||
| Open | 13 (25.49) | 154 (34.53) | ||
| Laparoscopic | 38 (74.51) | 292 (65.47) | ||
| Operation time, min (mean ± SD) | 182.19 ± 6.25 | 181.87 ± 5.79 | 0.378 | 0.705 |
| Blood loss, mL (mean ± SD) | 230.45 ± 17.62 | 232.74 ± 20.58 | 0.761 | 0.447 |
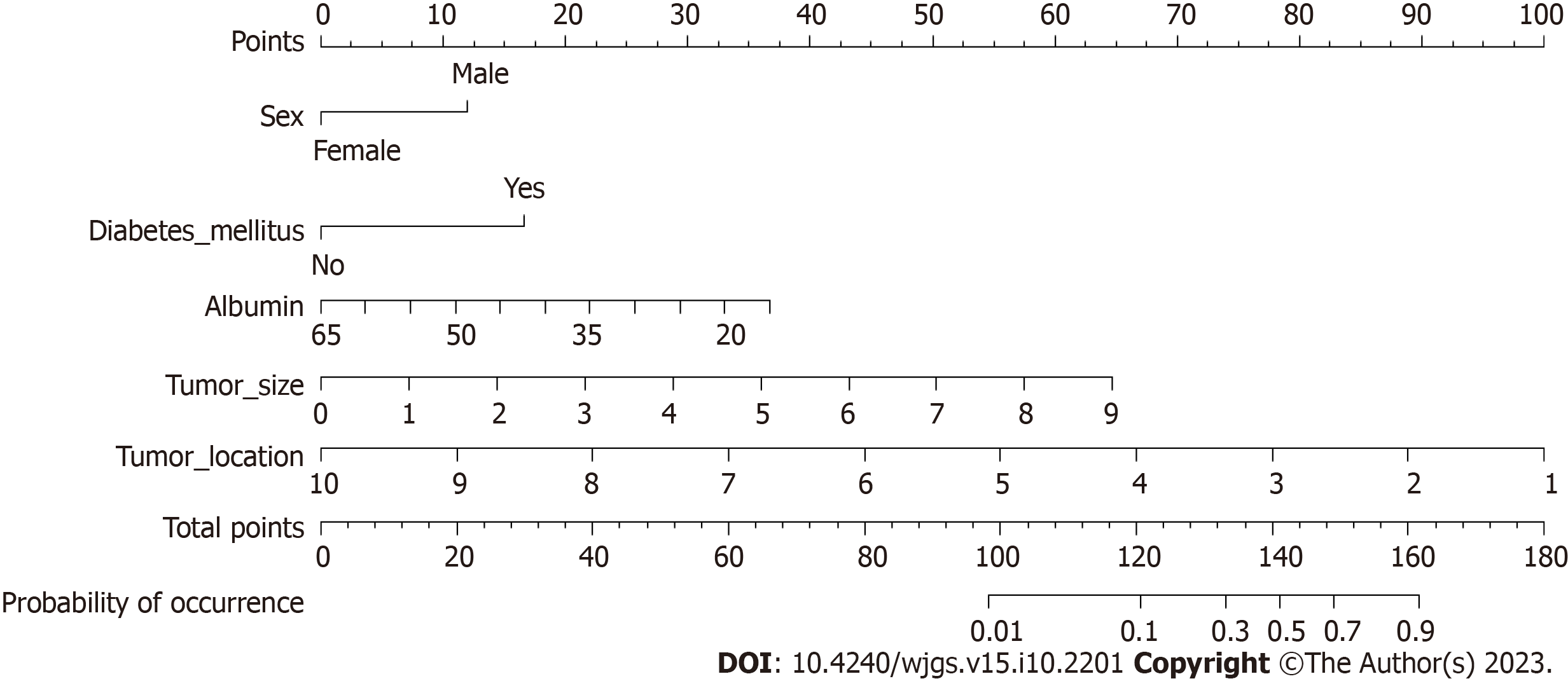
Whether the patient had AL after surgery (not occurring = 0, occurring = 1) was used as the dependent variable, and the statistically significant factors identified by univariate analysis and shown in Table 1 (sex, diabetes mellitus, smoking history, neoadjuvant treatment, Alb level, tumor size, and tumor location) were used as independent variables. Table 2 lists the assignments of the indicators. A multivariate logistic regression analysis revealed sex [odds ratio (OR) = 3.656, 95% confidence interval (CI): 1.538-8.264, P = 0.003), diabetes mellitus (OR = 5.669, 95%CI: 2.455-13.092, P < 0.001), Alb level (OR = 0.898, 95%CI: 0.846-0.953, P < 0.001), tumor size (OR = 2.604, 95%CI: 1.840-3.684, P < 0.001), and tumor location (OR = 0.272, 95%CI: 0.180-0.413, P < 0.001) as factors that influence AL in patients with rectal cancer following sphincter-preserving surgery (Table 3).
| Factor | Assignment |
| Sex | Female = 0, male = 1 |
| Diabetes mellitus | No = 0, yes = 1 |
| Smoking history | No = 0, yes = 1 |
| Neoadjuvant treatment | No = 0, yes = 1 |
| Albumin level | Enter the original value |
| Tumor size | Enter the original value |
| Tumor location | Enter the original value |
| Factor | β | SE | Wald χ² | P value | OR (95%CI) |
| Sex | 1.271 | 0.429 | 8.778 | 0.003 | 3.656 (1.538-8.264) |
| Diabetes mellitus | 1.735 | 0.427 | 16.504 | < 0.001 | 5.669 (2.455-13.092) |
| Smoking history | 1.758 | 0.967 | 3.309 | 0.069 | 5.801 (0.873-38.572) |
| Neoadjuvant treatment | -0.947 | 0.940 | 1.015 | 0.314 | 0.388 (0.062-2.448) |
| Albumin level | -0.108 | 0.030 | 12.627 | < 0.001 | 0.898 (0.846-0.953) |
| Tumor size | 0.957 | 0.177 | 29.204 | < 0.001 | 2.604 (1.840-3.684) |
| Tumor location | -1.300 | 0.212 | 37.699 | < 0.001 | 0.272 (0.180-0.413) |
According to the results presented in Table 3, five variables (sex, diabetes mellitus, Alb level, tumor size, and tumor location) were used to construct a nomogram model for predicting AL after sphincter-preserving surgery for rectal cancer (Figure 1).
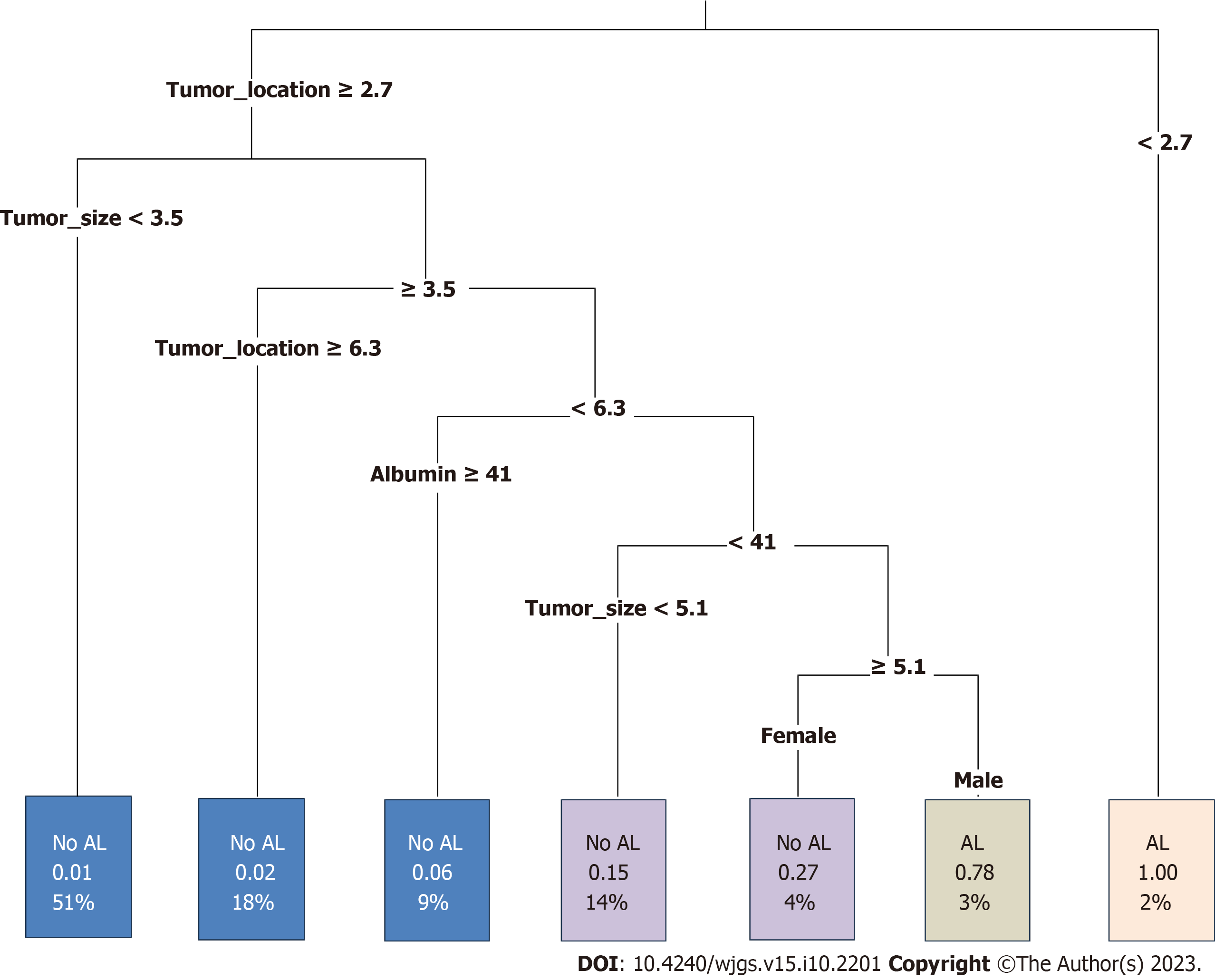
A decision tree prediction model for AL after sphincter-preserving surgery for rectal cancer was also constructed, and four explanatory variables were screened: Tumor location, tumor size, Alb level, and sex. The results of the model showed that tumor location was the first-level factor influencing AL in patients with rectal cancer after sphincter-preserving surgery. The incidence of AL was 100% in patients with a distance of the tumor from the anal verge < 2.7 cm, and 78% in male patients with tumor location < 6.3 cm, Alb level < 41 g/L, and tumor size ≥ 5.1 cm (Figure 2).
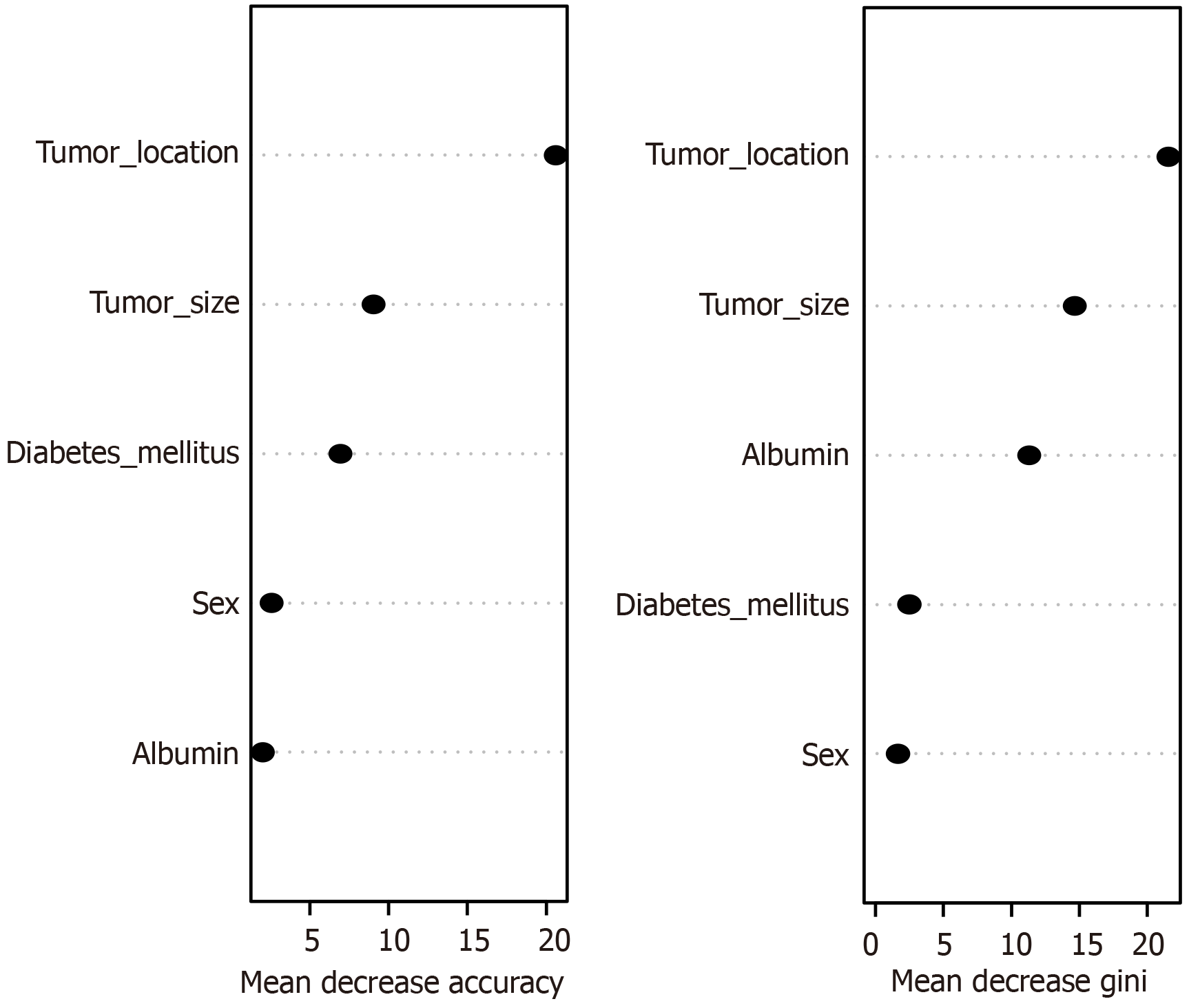
According to the change in the overall prediction accuracy of the best model, the variables affecting AL in patients with rectal cancer after sphincter-preserving surgery were tumor location, tumor size, diabetes mellitus, sex, and Alb level (Figure 3).
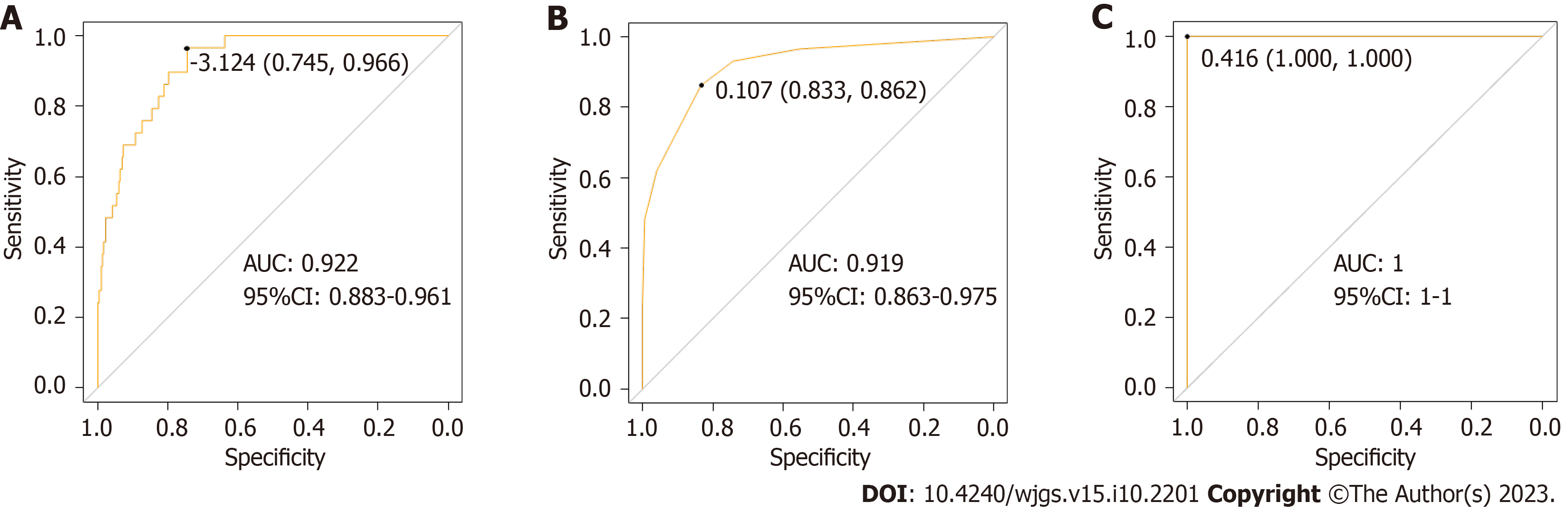
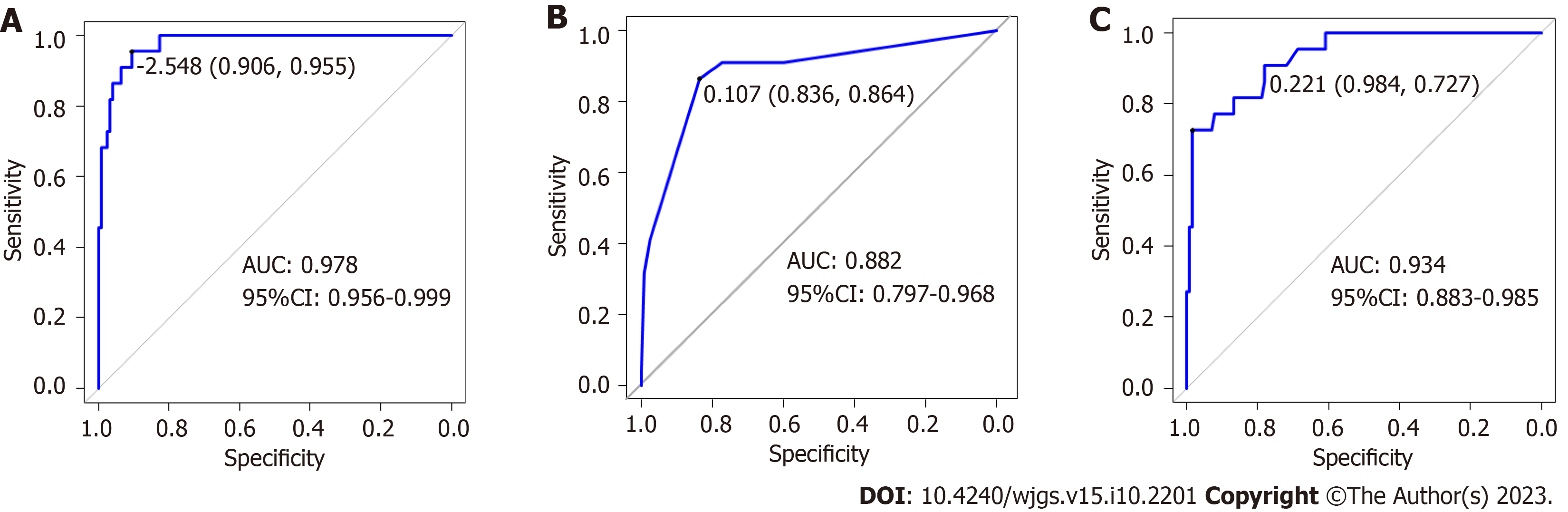
In the training dataset, the overall performance of the random forest model in predicting AL after sphincter-preserving surgery for rectal cancer was comparable to that of the decision tree model. The AUC of the random forest model was significantly higher than that of the decision tree model (Z = -2.836, P = 0.004) (Table 4 and Figure 4). In the validation dataset, the overall effectiveness of the three models was equivalent (Table 5 and Figure 5).
| Model | Sensitivity | Specificity | Accuracy | Recall | Precision | AUC (95%CI) |
| Nomogram | 0.745 | 0.966 | 0.936 | 0.987 | 0.946 | 0.922 (0.883-0.961) |
| Decision tree | 0.833 | 0.862 | 0.951 | 0.994 | 0.955 | 0.919 (0.863-0.975) |
| Random forest | 1.000 | 1.000 | 0.951 | 0.994 | 0.955 | 1.000 (1.000-1.000) |
| Model | Sensitivity | Specificity | Accuracy | Recall | Precision | AUC (95%CI) |
| Nomogram | 0.867 | 0.909 | 0.927 | 1.000 | 0.921 | 0.950 (0.908-0.992) |
| Decision tree | 0.836 | 0.864 | 0.951 | 0.994 | 0.955 | 0.882 (0.797-0.968) |
| Random forest | 0.984 | 0.727 | 0.893 | 1.000 | 0.889 | 0.934 (0.883-0.985) |
Rectal carcinoma is a prevalent cancer of the digestive system. Currently, the treatment of this disease is mainly surgical[10]. AL is one of the most common and dangerous complications associated with rectal cancer surgery. According to studies, the incidence of AL after rectal cancer surgery ranges from 2.6% to 19.0%[11]. AL not only affects recovery from surgery, but also leads to a variety of complications, such as intra-abdominal abscesses, diffuse peritonitis, and sepsis, and can even cause tumor recurrence. In severe cases, secondary surgery is required, which worsens patient survival rates[12]. Therefore, it is critical to identify the factors that influence the development of AL in patients with rectal cancer after sphincter-preserving surgery and provide targeted interventions to reduce its occurrence.
The incidence of postoperative AL was analyzed in 497 patients with rectal cancer admitted to our hospital. A total of 51 patients developed postoperative AL, representing an incidence of 10.26%. This is similar to the incidence of AL reported by Degiuli et al[13] in 5398 patients with rectal cancer (10.2%), but lower than that reported by Peltrini et al[14] in 367 patients with rectal cancer (17.4%). These differences may be related to factors such as inclusion criteria and different populations. AL after sphincter-preserving surgery for rectal cancer results from multiple factors, including patient characteristics, tumor status, and operation-related factors. The results of this study showed that sex, diabetes mellitus, Alb level, tumor size, and tumor location influence AL.
According to this study, males were 3.656 times more likely to have postoperative AL than women, which is consistent with the results of most studies[15,16]. As the male pelvis is narrow, the visual field is not fully exposed during the ope
Penna et al[18] confirmed that diabetes mellitus is an independent risk factor for AL after transanal total mesorectal excision. Additionally, this study reported that individuals with diabetes had an AL risk 5.669 times greater than that of non-diabetic patients. Diabetes mellitus can affect the anastomotic blood supply as uncontrolled hyperglycemia leads to vascular damage, reduced blood flow, and cellular accumulation of toxic glucose-derived metabolites, resulting in a sig
According to the findings of this study, which are consistent with those of Shimura et al[20], there is a direct association between preoperative Alb levels and the risk of developing postoperative AL. This may be due to a low perioperative nutritional status leading to reduced immune function and a greater risk of infection and the spread of infection[21]. Yasui et al[22] found that patients with tumor sizes ≥ 4 cm were more likely to develop AL. This study also found that the probability of postoperative AL increases with tumor size. As more tissue has to be removed during surgery, more dama
Both domestically and internationally, there is consensus on the impact of tumor location on AL following low anterior resection of rectal cancer; that is, the closer the tumor is to the anal margin, the higher the risk of AL[24-26]. The same conclusion was reached in the present study. This may be because the closer the tumor is to the anus, the larger the wound during resection. In addition, intraoperative electrocoagulation damage to the tissue and blood vessels causes exudation and bleeding, which reduces the blood supply to the anastomosis and increases the risk of postoperative AL[27].
Currently, there is no consensus regarding whether neoadjuvant therapy increases the incidence of AL. Arezzo et al[12] analyzed the effects of short- and long-term radiotherapy on AL and showed that the risk of postoperative AL signifi
With the development of computer software and artificial intelligence, machine learning has become a new direction for medical research. Studies have used machine learning models to predict the risk of anti-tuberculosis drug-induced liver injury in inpatients with tuberculosis[29], and machine learning can also predict the risk of essential hypertension[30]. In this study, five indicators with statistically significant differences in the multivariate analysis were used to establish three prediction models of AL after sphincter-preserving surgery for rectal cancer using machine learning algorithms. In the training dataset, the sensitivity, specificity, accuracy, recall rate, and precision rate of the nomogram were lower than those of the random forest and decision tree models, and the prediction efficacy of the random forest model was better than that of the decision tree. But the sensitivity, specificity, and AUC of the random forest model reached 1, indicating that the random forest model may have overfitting or the generalization effect may be poor due to insufficient data in the training set. In the validation dataset, the three models exhibited similar prediction performances. Although the random forest model has better predictive performance, it also has some disadvantages. As a result, in practice, each of the three prediction models has benefits and limitations, and the most appropriate method should be chosen based on the situation.
As this was a single-center retrospective study, it has several limitations. The representativeness of single-center research is limited, and there may be some bias owing to time constraints. This study did not analyze additional risk factors for AL after sphincter-preserving surgery for rectal cancer, and the constructed model may have been overfitted. Future research will use a larger and more comprehensive sample set and multicenter studies to verify and build a more complete prediction model.
Overall, AL is a serious complication of rectal cancer surgery, with a high incidence rate. In this study, nomogram, random forest, and decision tree prediction models of AL after sphincter-preserving surgery for rectal cancer were established using machine learning algorithms. The random forest model was found to have excellent predictive effect and stability, and might serve as a reference for the clinical identification of high-risk groups for AL following sphincter-preserving surgery for rectal cancer.
With advances in medical technology, the success rate of sphincter-preserving surgery in patients with rectal cancer is increasing. However, anastomotic leakage (AL) remains a devastating complication.
AL significantly lowers patients’ quality of life. This study examines the elements that influence AL and establishes models to help doctors predict whether patients will develop AL, allowing the timely adoption of preventive measures.
This study aimed to identify the characteristics that influence AL and utilize these factors to build a prediction model for AL after sphincter-preserving surgery for rectal cancer.
The clinical data of patients with rectal cancer who underwent sphincter-preserving surgery at our institution in the past five years were examined to analyze the factors influencing AL; nomogram, decision tree, and random forest prediction models were established; and the predictive efficacy of the three models was compared.
The factors influencing AL after sphincter-preserving surgery for rectal cancer were sex, diabetes mellitus, albumin level, tumor size, and tumor location. To predict the probability of postoperative AL, we constructed nomogram, decision tree, and random forest models.
This study compared the predictive efficacy of the three prediction models. The random forest model performed the best and may be a useful alternative tool for predicting patients at a high risk of AL.
Future research will include larger and more comprehensive cohorts across multiple centers, and build a more complete prediction model.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pazouki A, Iran; Pedziwiatr M, Poland S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Manceau G, Brouquet A, Chaibi P, Passot G, Bouché O, Mathonnet M, Regimbeau JM, Lo Dico R, Lefèvre JH, Peschaud F, Facy O, Volpin E, Chouillard E, Beyert-Berjot L, Verny M, Karoui M, Benoist S. Multicenter phase III randomized trial comparing laparoscopy and laparotomy for colon cancer surgery in patients older than 75 years: the CELL study, a Fédération de Recherche en Chirurgie (FRENCH) trial. BMC Cancer. 2019;19:1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64702] [Article Influence: 16175.5] [Reference Citation Analysis (177)] |
| 3. | Ghaem H, Amiri Z, Kianpour F, Rezaianzadeh A, Hosseini SV, Khazraei H. Comparing Recurrence and Complications After Laparoscopy and Laparotomy Surgery among Patients Suffering from Colorectal Cancer, Shiraz, Iran. Asian Pac J Cancer Prev. 2016;17:3111-3116. [PubMed] |
| 4. | Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA. 2017;318:1569-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 921] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 5. | Chiarello MM, Fransvea P, Cariati M, Adams NJ, Bianchi V, Brisinda G. Anastomotic leakage in colorectal cancer surgery. Surg Oncol. 2022;40:101708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 6. | Stommel MWJ, Ten Broek RPG, Strik C, Slooter GD, Verhoef C, Grünhagen DJ, van Duijvendijk P, Bemelmans MHA, den Dulk M, Sietses C, van Heek TNT, van den Boezem PB, de Wilt JHW, van Goor H. Multicenter Observational Study of Adhesion Formation After Open-and Laparoscopic Surgery for Colorectal Cancer. Ann Surg. 2018;267:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Kinugasa T, Nagasu S, Murotani K, Mizobe T, Ochi T, Isobe T, Fujita F, Akagi Y. Analysis of risk factors for anastomotic leakage after lower rectal Cancer resection, including drain type: a retrospective single-center study. BMC Gastroenterol. 2020;20:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Yu XN, Xu LM, Bin YW, Yuan Y, Tian SB, Cai B, Tao KX, Wang L, Wang GB, Wang Z. Risk Factors of Anastomotic Leakage After Anterior Resection for Rectal Cancer Patients. Curr Med Sci. 2022;42:1256-1266. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1033] [Article Influence: 68.9] [Reference Citation Analysis (4)] |
| 10. | Shiga M, Maeda H, Oba K, Okamoto K, Namikawa T, Fujisawa K, Yokota K, Kobayashi M, Hanazaki K. Safety of laparoscopic surgery for colorectal cancer in patients over 80 years old: a propensity score matching study. Surg Today. 2017;47:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Jannasch O, Klinge T, Otto R, Chiapponi C, Udelnow A, Lippert H, Bruns CJ, Mroczkowski P. Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget. 2015;6:36884-36893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Arezzo A, Migliore M, Chiaro P, Arolfo S, Filippini C, Di Cuonzo D, Cirocchi R, Morino M; REAL Score Collaborators. The REAL (REctal Anastomotic Leak) score for prediction of anastomotic leak after rectal cancer surgery. Tech Coloproctol. 2019;23:649-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 13. | Degiuli M, Elmore U, De Luca R, De Nardi P, Tomatis M, Biondi A, Persiani R, Solaini L, Rizzo G, Soriero D, Cianflocca D, Milone M, Turri G, Rega D, Delrio P, Pedrazzani C, De Palma GD, Borghi F, Scabini S, Coco C, Cavaliere D, Simone M, Rosati R, Reddavid R; collaborators from the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Risk factors for anastomotic leakage after anterior resection for rectal cancer (RALAR study): A nationwide retrospective study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Colorectal Dis. 2022;24:264-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 14. | Peltrini R, Carannante F, Costa G, Bianco G, Garbarino GM, Canali G, Mercantini P, Bracale U, Corcione F, Caricato M, Capolupo GT. Oncological outcomes of rectal cancer patients with anastomotic leakage: A multicenter case-control study. Front Surg. 2022;9:993650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 15. | Tsai KY, Huang SH, You JF, Tang R, Chiang JM, Yeh CY, Hsieh PS, Tsai WS, Chiang SF, Lai CC. Smoking cessation for less than 10 years remains a risk factor of anastomotic leakage in mid-to-low rectal cancer patients undergoing sphincter-preserving surgery. Langenbecks Arch Surg. 2022;407:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Zhang W, Lou Z, Liu Q, Meng R, Gong H, Hao L, Liu P, Sun G, Ma J, Zhang W. Multicenter analysis of risk factors for anastomotic leakage after middle and low rectal cancer resection without diverting stoma: a retrospective study of 319 consecutive patients. Int J Colorectal Dis. 2017;32:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Koyama M, Murata A, Sakamoto Y, Morohashi H, Hasebe T, Saito T, Hakamada K. Risk Factors for Anastomotic Leakage After Intersphincteric Resection Without a Protective Defunctioning Stoma for Lower Rectal Cancer. Ann Surg Oncol. 2016;23 Suppl 2:S249-S256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; International TaTME Registry Collaborative. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann Surg. 2019;269:700-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 19. | Feinberg AE, Chesney TR, Acuna SA, Sammour T, Quereshy FA. Oncologic Outcomes Following Laparoscopic versus Open Resection of pT4 Colon Cancer: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2017;60:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Shimura T, Toiyama Y, Hiro J, Imaoka H, Fujikawa H, Kobayashi M, Ohi M, Inoue Y, Mohri Y, Kusunoki M. Monitoring perioperative serum albumin can identify anastomotic leakage in colorectal cancer patients with curative intent. Asian J Surg. 2018;41:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Liu YT, Huang Y, Hao YG, Zhang PF, Yin X, Zhang JF, Hu XH, Li BK, Wang GY. [Current status of influencing factors for postoperative anastomotic leakage in low rectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Yasui M, Takemasa I, Miyake Y, Hata T, Ikeda M, Hasegawa J, Ota H, Matsuda C, Mizushima T, Doki Y, Mori M; Clinical Study Group of Osaka University (CSGO), Colorectal Group. Tumor Size as an Independent Risk Factor for Postoperative Complications in Laparoscopic Low Anterior Resection for Advanced Rectal Cancer: A Multicenter Japanese Study. Surg Laparosc Endosc Percutan Tech. 2017;27:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Yun JA, Cho YB, Park YA, Huh JW, Yun SH, Kim HC, Lee WY, Chun HK. Clinical manifestations and risk factors of anastomotic leakage after low anterior resection for rectal cancer. ANZ J Surg. 2017;87:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Kim CW, Baek SJ, Hur H, Min BS, Baik SH, Kim NK. Anastomotic Leakage After Low Anterior Resection for Rectal Cancer Is Different Between Minimally Invasive Surgery and Open Surgery. Ann Surg. 2016;263:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Shi WK, Qiu XY, Li YH, Lin GL. [Risk factor and early diagnosis of anastomotic leakage after rectal cancer surgery]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:981-986. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Zhou S, Pei W, Li Z, Zhou H, Liang J, Liu Q, Zhou Z, Wang X. Evaluating the predictive factors for anastomotic leakage after total laparoscopic resection with transrectal natural orifice specimen extraction for colorectal cancer. Asia Pac J Clin Oncol. 2020;16:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Zheng H, Wu Z, Wu Y, Mo S, Dai W, Liu F, Xu Y, Cai S. Laparoscopic surgery may decrease the risk of clinical anastomotic leakage and a nomogram to predict anastomotic leakage after anterior resection for rectal cancer. Int J Colorectal Dis. 2019;34:319-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Chang JS, Keum KC, Kim NK, Baik SH, Min BS, Huh H, Lee CG, Koom WS. Preoperative chemoradiotherapy effects on anastomotic leakage after rectal cancer resection: a propensity score matching analysis. Ann Surg. 2014;259:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Zhao P, Chen J, Yang YQ, Peng Y. [Nomogram model for predicting risk of anti-tuberculosis drug-induced liver injury among inpatients with tuberculosis]. Zhonghua Jie He He Hu Xi Za Zhi. 2022;45:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Pei Z, Liu J, Liu M, Zhou W, Yan P, Wen S, Chen Y. Risk-Predicting Model for Incident of Essential Hypertension Based on Environmental and Genetic Factors with Support Vector Machine. Interdiscip Sci. 2018;10:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |