Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2142
Peer-review started: March 11, 2023
First decision: June 14, 2023
Revised: July 4, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: October 27, 2023
Processing time: 230 Days and 0.2 Hours
Robotic resection using the natural orifice specimen extraction surgery I-type F method (R-NOSES I-F) is a novel minimally invasive surgical strategy for the treatment of lower rectal cancer. However, the current literature on this method is limited to case reports, and further investigation into its safety and feasibility is warranted.
To evaluate the safety and feasibility of R-NOSES I-F for the treatment of low rectal cancer.
From September 2018 to February 2022, 206 patients diagnosed with low rectal cancer at First Affiliated Hospital of Nanchang University were included in this retrospective analysis. Of these patients, 22 underwent R-NOSES I-F surgery (R-NOSES I-F group) and 76 underwent conventional robotic-assisted low rectal cancer resection (RLRC group). Clinicopathological data of all patients were collected and analyzed. Postoperative outcomes and prognoses were compared between the two groups. Statistical analysis was performed using SPSS software.
Patients in the R-NOSES I-F group had a significantly lower visual analog score for pain on postoperative day 1 (1.7 ± 0.7 vs 2.2 ± 0.6, P = 0.003) and shorter postoperative anal venting time (2.7 ± 0.6 vs 3.5 ± 0.7, P < 0.001) than those in the RLRC group. There were no significant differences between the two groups in terms of sex, age, body mass index, tumor size, TNM stage, operative time, intrao
R-NOSES I-F is a safe and effective minimally invasive procedure for the treatment of lower rectal cancer. It improves pain relief, promotes gastrointestinal function recovery, and helps avoid incision-related complications.
Core Tip: This retrospective study examined the efficacy and safety of a novel surgical procedure called robotic resection using the natural orifice specimen extraction surgery I-type F method (R-NOSES I-F) for lower rectal cancer. Through a comparison with robotic-assisted low rectal cancer resection, the study demonstrates that R-NOSES I-F is a safe and effective minimally invasive surgical approach for low rectal cancer. It offers several benefits, including decreased postoperative pain, improved gastrointestinal function recovery, reduced abdominal wall dysfunction, and avoidance of complications associated with abdominal wall incisions. Furthermore, R-NOSES I-F does not negatively impact anal and urinary functions and does not increase the risk of local recurrence or distant metastasis.
- Citation: Tao F, Liu DN, He PH, Luo X, Xu CY, Li TY, Duan JY. Robotic natural orifice specimen extraction surgery I-type F method vs conventional robotic resection for lower rectal cancer. World J Gastrointest Surg 2023; 15(10): 2142-2153
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2142.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2142
Colorectal cancer is a highly prevalent malignancy, ranking third in terms of incidence and second in terms of mortality worldwide in 2020[1]. The most recent cancer statistics in China indicate a significant increase in the incidence and mortality rates of colorectal cancer[2]; it ranks second in incidence and fifth in mortality rates in China[3,4]. Therefore, enhancing surgical techniques to improve the postoperative quality of life for patients with rectal cancer is crucial, especially for those with lower rectal cancer. In recent years, the combination of robotic surgery and natural orifice specimen extraction surgery (NOSES) has gained attention in the management of colorectal cancer[5-8].
The robotic surgical platform has a magnified 3D high-definition field of view, a flexible robotic arm capable of 540° free rotation in seven directions, a stable camera platform, and enhanced depth perception, mitigating the challenges of hand-eye coordination. These features enable surgeons to operate with greater precision within the limited space of the pelvic cavity[9]. Robotic-assisted total rectal mesenteric resection has played a pivotal role in the minimally invasive treatment of lower rectal cancer[10,11]. Therefore, robotic NOSES has garnered increasing attention as a surgical approach for the treatment of lower rectal cancer.
The introduction of the concept of NOSES has ushered in a new era of “no incision” in minimally invasive surgery[12]. Expert consensus on NOSES in colorectal neoplasms was initially published by the China NOSES Alliance in 2017[13], and was later updated and improved in 2019[14]. Additionally, an international consensus on NOSES for colorectal can
Previous studies[5,7,8,17] have confirmed the safety and feasibility of robotic NOSES surgery as a minimally invasive procedure, enhancing surgical quality and expediting postoperative recovery. Robotic resection using the NOSES I-type F method (R-NOSES I-F) represents a novel approach characterized by intussusception to achieve transanal specimen eversion. This technique involves resecting the specimen and placing the anvil into the proximal bowel extra-abdominally[16]. However, existing studies related to this surgical approach are limited to case reports[18,19], with a lack of long-term follow-up results. Therefore, this study aimed to compare the postoperative outcomes of R-NOSES I-F with those of conventional robotic low rectal cancer resection (RLRC) through retrospective analysis, thereby evaluating the effectiveness and safety of R-NOSES I-F in the treatment of low rectal cancer.
In this retrospective analysis, we collected and analyzed clinicopathological data of patients diagnosed with rectal cancer at our hospital from September 2018 to February 2022. The following inclusion criteria were applied: (1) Pathologically confirmed rectal malignancy through preoperative assessment; (2) Tumor located 3–7 cm from the anal verge; (3) Age between 18 and 80 years; (4) Body mass index (BMI) ≤ 30 kg/m2; and (5) Absence of distant metastasis. The exclusion criteria were as follows: (1) Patients who received preoperative neoadjuvant therapy; (2) TNM stage IV; (3) Requirement for multiorgan resection; (4) Presence of concomitant primary malignancies in other organs or multi-origin colorectal malignancies; (5) Emergency surgery due to acute intestinal obstruction, perforation, or bleeding; and (6) Major comorbidities such as coronary heart disease and cerebral infarction. According to the above criteria, patients who underwent R-NOSES I-F surgery were included in the R-NOSES I-F group, while patients who underwent RLRC surgery were included in the RLRC group. All patients provided informed consent before surgery. The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University and conducted following the principles outlined in the Declaration of Helsinki.
All patients underwent a comprehensive preoperative evaluation, including physical examination, blood tests for tumor markers, colonoscopy, pathological biopsy, chest computed tomography or radiography, abdominal computed tomography, and rectal magnetic resonance imaging. Bowel preparation was performed using 2 L of polyethylene glycol solution 1 d before surgery, and postoperative self-administered analgesia was employed. Postoperative pain was assessed using a visual analog scale (VAS) calibrated from 0 to 10, with 0 indicating no pain and 10 representing the most intense pain imaginable. Pain scores were recorded on postoperative days 1, 3, and 5. The postoperative inflammatory response was evaluated using global white blood cell and neutrophil counts (on postoperative days 1, 3, and 5), and body temperature (from postoperative days 1 to 5).
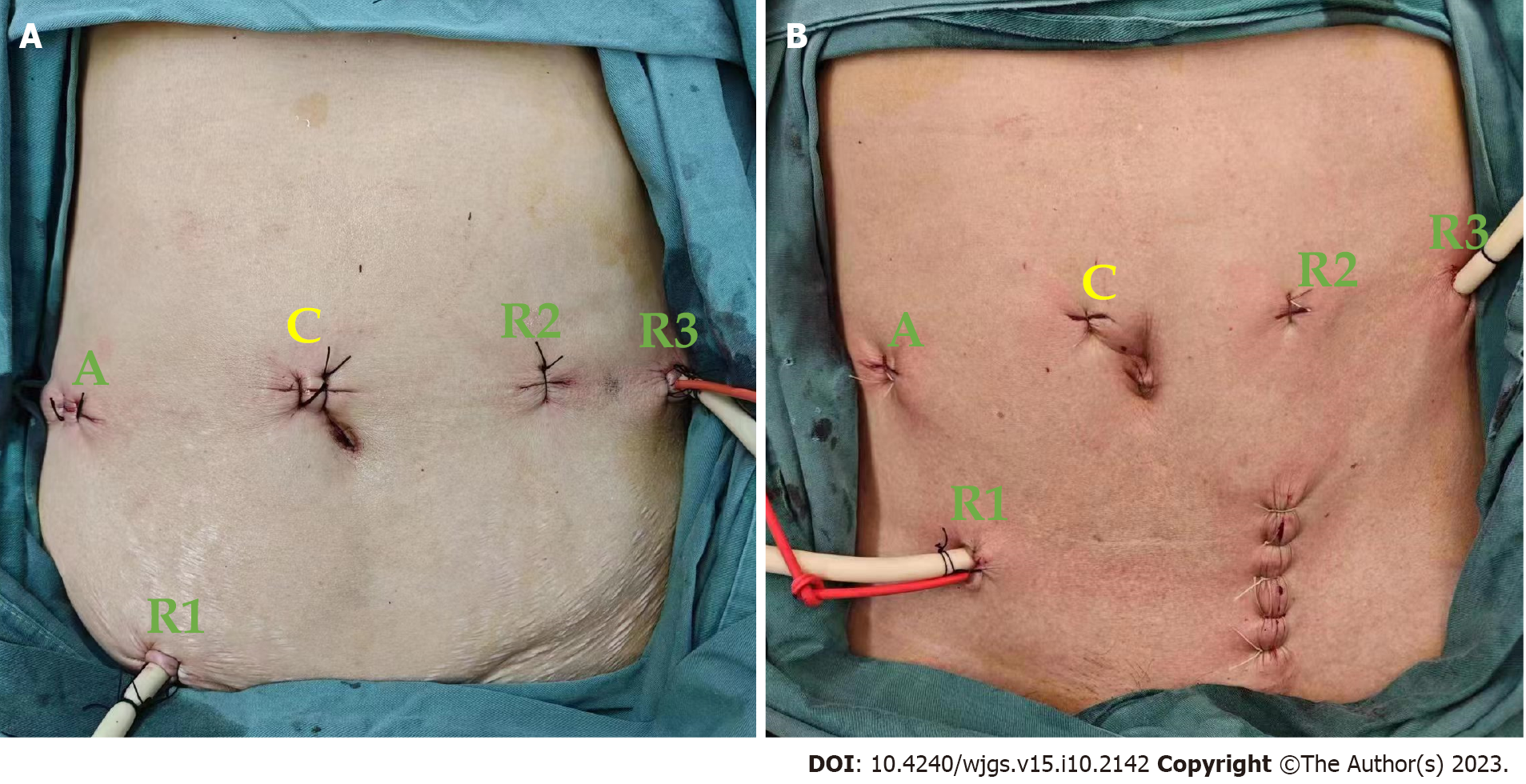
Following successful endotracheal intubation and general anesthesia, the patient was positioned in a lithotomy position with the head lowered and feet elevated between 15° and 30° and tilted to the right between 10° and 15°. The procedure was performed using a five-port approach with five trocar placements. The specific port locations were as follows: (1) Camera port C (12 mm), positioned 3-4 cm above the right side of the umbilicus; (2) Robotic operating port R1 (ultrasonic knife; 8 mm), located at one-third of the distance between the umbilicus and the right anterior superior iliac spine; (3) Robotic operating port R2 (bipolar electrocoagulation; 8 mm), placed 4–5 cm above the left side of the umbilicus; (4) Robotic operating port R3 (noninvasive grasping clamp; 8 mm), positioned 2 cm below the left anterior axillary line rib margin; and (5) Auxiliary port A (12 mm), medial to the right midclavicular line, corresponding to the position of the flat camera port (Figure 1). After establishing pneumoperitoneum, laparoscopic exploration was conducted to confirm the absence of tumor implantation and metastasis within the abdominal cavity and determine the precise location of the tumor.
The first incision was made below the sacral promontory, and dissection was carried out along Toldt’s space. The inferior mesenteric arteries and veins were ligated at the level of the duodenum. The rectal mesentery was freed to expose the bilateral seminal vesicles (men) or the posterior vaginal wall (women). The left and right intestinal walls of the rectum were further exposed 2–3 cm below the lower edge of the tumor, and the pre-cut line was determined approximately 10 cm above the tumor. The sigmoid mesentery was dissected, and the intestinal canal was exposed.
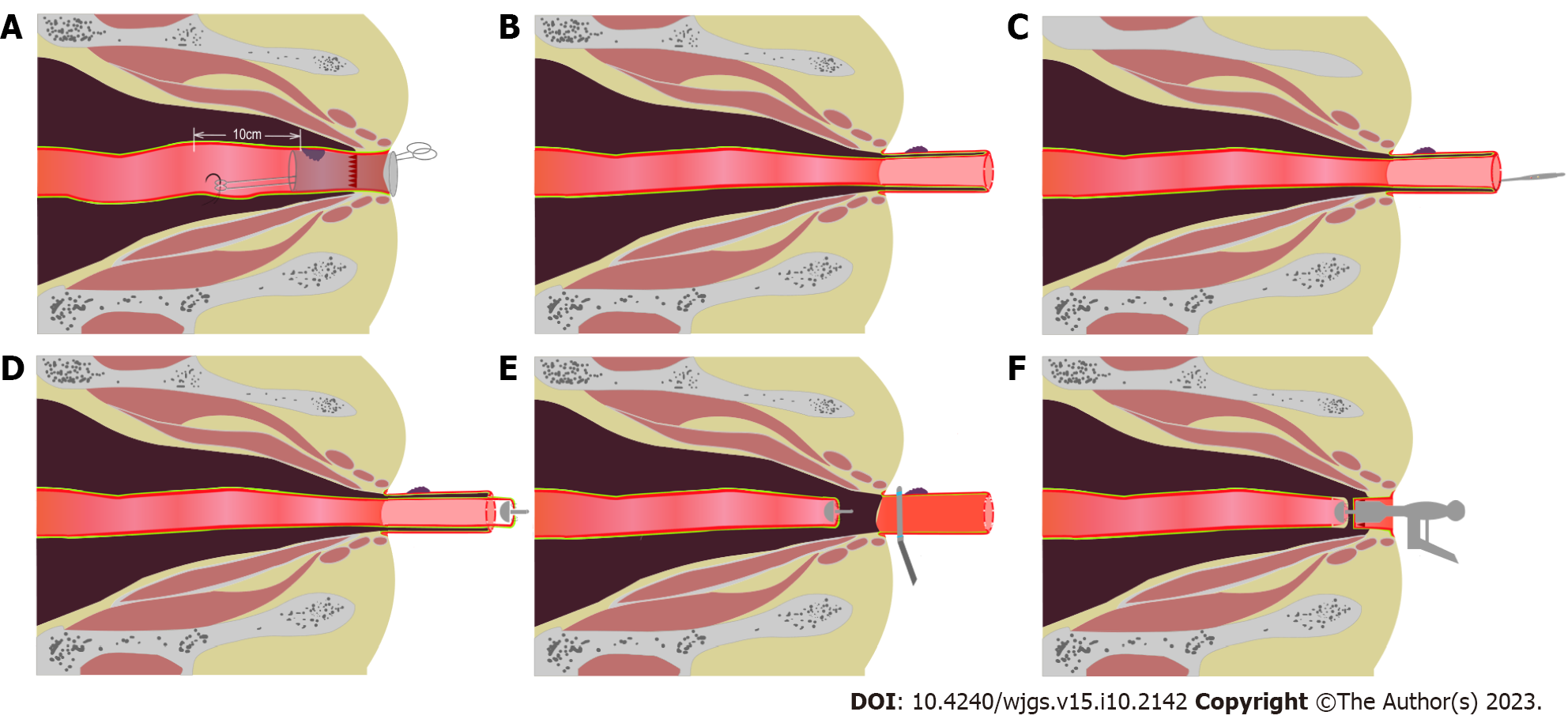
For the R-NOSES I-F group, the specimen resection and digestive tract reconstruction were performed as follows (Figure 2 and Video 1): The anus was fully dilated to accommodate the passage of six fingers, and a sterile plastic protective sleeve was inserted through the anus, extending 5 cm above the tumor. The oval forceps was introduced through the protective sleeve to the pre-excision site of the bowel lumen, approximately 10 cm from the upper edge of the tumor, and, under robotic view, it was secured to the bowel lumen with sutures (Figure 2A); then, the pre-excision bowel and mesentery were pulled out of the anus (Figure 2B). The tumor location was determined, and the tumor was flushed with iodophor water. The bowel was incised by the site of the oval forceps fixation, and the pre-exposed bowel was identified and disconnected (Figure 2C); after placing the anvil, it was secured at the sigmoid colon break and returned to the abdominal cavity (Figure 2D). The rectum was transected under direct vision, approximately 0.5–2 cm above the lower edge of the tumor, depending on the distance of the tumor from the anal edge (Figure 2E). The specimen was removed, and the distal rectal section was returned to the abdominal cavity. The anus was disinfected with iodophor water, and a circular stapler was used to perform a sigmoid-rectal end-to-end anastomosis (Figure 2F).
Specimen resection and digestive tract reconstruction in the RLRC group involved the following steps: The rectum was transected at a distance of 0.5-2 cm distal to the tumor, using a linear cutting closure device under the robotic system. Subsequently, a 6 cm incision was made adjacent to the rectus abdominis muscle in the left lower abdomen. The incision was protected with a protective sleeve. The proximal rectum and sigmoid colon containing the tumor were exteriorized from the abdominal cavity, and the affected intestinal segment was excised. An anvil was inserted into the proximal colon, pneumoperitoneum was re-established, and sigmoid-rectal end-to-end anastomosis was performed transanally using an anastomotic clutch while visualized through direct laparoscopy.
After thorough rinsing of the abdominopelvic cavity with iodophor water and injection of iodophor saline through the anus to ensure no anastomotic leakage, certain postoperative measures were implemented. First, an anal tube was inserted through the anus. Second, a double-sleeve drainage tube was positioned on the left side of the anastomosis and drained through the presacral area. Finally, another drainage tube was placed on the right side of the anastomosis and drained through the trocar orifice on the right side of the abdomen. These steps were performed at the conclusion of the surgery.
Patients with postoperative pathological TNM stage I or II without risk factors did not receive chemotherapy, a few patients with stage II with risk factors underwent fluorouracil single-agent oral chemotherapy, and those with stage III underwent XELOX regimen chemotherapy. After the surgical procedure, patients were scheduled for outpatient clinic visits every 3 mo for a period of 2 years. Subsequently, the follow-up frequency was adjusted to every 6 mo. During each visit, patients underwent a comprehensive physical examination and tumor marker analysis. Additionally, chest and whole abdominal computed tomography scans were performed to monitor their condition. Regular communication with patients via WeChat or telephone was also maintained to ensure continuous follow-up. At 6 mo after surgery, the postoperative anal function was evaluated using the low anterior resection syndrome (LARS) rating scale and Wexner Incontinence Score. The postoperative urinary function was assessed using the International Prostate Symptom Scale (IPSS). Owing to the impact of the novel coronavirus epidemic, some patients were followed up remotely through phone calls or WeChat. The final follow-up was conducted in June 2023.
All data analyses were performed using SPSS software (version 23.0; IBM, Armonk, NY, United States). Continuous variables were presented as mean ± SD and compared using the Mann-Whitney U test. Categorical variables were expressed as percentages and compared using the χ2 test or Fisher’s exact test, as appropriate. P values were two-tailed and differences were considered statistically significant at P < 0.05.
A total of 22 patients were included in the R-NOSES I-F group and 76 in the RLRC group. The clinical and pathological characteristics of the patients are summarized in Table 1. No significant differences were observed between the two groups in terms of sex, age, BMI, tumor size, distance of the lower margin of the tumor from the anal verge, CEA level, or TNM stage of the tumor (P > 0.05).
| Baseline characteristics | R-NOSES I-F (n = 22) | RLRC (n = 76) | Ρ value |
| Age (year) | 56.5 ± 8.9 | 59.5 ± 11.1 | 0.107 |
| BMI (kg/m2) | 21.8 ± 2.5 | 22.6 ± 2.0 | 0.136 |
| Gender | 0.580 | ||
| Male | 8 (36.4) | 45 (59.2) | |
| Female | 14 (63.6) | 31 (40.8) | |
| ASA score | 0.552 | ||
| I, II | 5 (22.7) | 11 (14.5) | |
| III | 17 (77.3) | 65 (85.5) | |
| Previous history of abdominal surgery | 4 (18.2) | 13 (17.1) | 1.000 |
| Maximum circumferential diameter of specimen (cm) | 0.217 | ||
| < 5 | 19 (86.4) | 56 (73.7) | |
| ≥ 5 | 3 (13.6) | 20 (26.3) | |
| Tumour location from anal verge (cm) | 4.3 ± 1.2 | 4.5 ± 0.9 | 0.278 |
| Abnormal serum CEA (ng/mL) | 0.700 | ||
| ≤ 5 | 16 (72.7) | 52 (68.4) | |
| > 5 | 6 (27.3) | 24 (31.6) | |
| Specimen length (cm) | 11.4 ± 2.2 | 12.8 ± 3.1 | 0.068 |
| Distal margin (cm) | 1.1 ± 0.7 | 1.1 ± 0.8 | 0.737 |
| Grade of differentiation | 0.976 | ||
| Well | 3 (13.6) | 12 (15.8) | |
| Moderate | 16 (72.7) | 52 (68.4) | |
| Poor | 2 (9.1) | 7 (9.2) | |
| Mucinous | 1 (4.5) | 5 (6.6) | |
| T stage | 0.376 | ||
| T0, Tis, T1 | 6 (27.3) | 12 (15.8) | |
| T2 | 7 (31.8) | 17 (22.4) | |
| T3 | 5 (22.7) | 27 (35.5) | |
| T4 | 4 (18.2) | 20 (26.3) | |
| N Stage | 0.511 | ||
| N0 | 14 (63.6) | 46 (60.5) | |
| N1 | 7 (31.8) | 20 (26.3) | |
| N2 | 1 (4.5) | 10 (13.2) | |
| pTNM | 0.110 | ||
| 0, I | 12 (54.5) | 23 (30.3) | |
| II | 4 (18.2) | 23 (30.3) | |
| III | 6 (27.3) | 30 (39.5) | |
| Number of lymph nodes harvested (n) | 14.2 ± 7.3 | 13.7 ± 6.0 | 0.759 |
| Nerve violation | 9 (40.9) | 30 (39.5) | 0.904 |
| Lymphovascular violation | 7 (31.8) | 17 (22.4) | 0.364 |
Perioperative results are summarized in Table 2. All procedures were performed using the da Vinci Surgical System (Da Vinci® Si System, Intuitive Surgical, Sunnyvale, CA, United States) and were performed by the same surgeon following the principles of total rectal mesenteric resection. None of the patients in the R-NOSES I-F or RLRC groups underwent open surgery. The operative time (173.0 ± 39.5 min vs 187.3 ± 50.9 min, P = 0.389) and intraoperative blood loss were comparable between the two groups (89.6 ± 47.9 mL vs 74.5 ± 62.8 mL, P = 0.068). No significant difference in the proportion of patients with a prophylactic stoma was observed between the two groups (31.8% vs 47.4%, P = 0.196). Regarding postoperative recovery, VAS scores on postoperative day 1 were significantly lower for patients in the R-NOSES I-F than for those in the RLRC group (1.7 ± 0.7 vs 2.2 ± 0.6, P = 0.003), and no significant difference in VAS scores on postoperative days 3 and 5 was observed (P > 0.05). The postoperative venting time was significantly shorter in the R-NOSES I-F group than in the RLRC group (2.7 ± 0.6 d vs 3.5 ± 0.7 d, P < 0.001). Regarding postoperative complications, three complications in the R-NOSES I-F group and 12 in the RLRC group occurred, with no significant difference between the two groups (13.6% vs 15.8%, P = 0.632). Regarding postoperative inflammation, no significant differences in global white blood cell and neutrophil counts were observed between the two groups on postoperative days 1, 3, and 5 (P > 0.05). Furthermore, no significant difference in the body temperature of the patients between postoperative days 1 and 5 was observed (P > 0.05).
| Outcomes | R-NOSES I-F (n = 22) | RLRC (n = 76) | Ρ value |
| Surgery time (min) | 173.0 ± 39.5 | 187.3 ± 50.9 | 0.389 |
| Intraoperative blood loss (mL) | 89.6 ± 47.9 | 74.5 ± 62.8 | 0.068 |
| Prophylactic stoma, n (%) | 7 (31.8) | 36 (47.4) | 0.196 |
| VAS score | |||
| POD1 | 1.7 ± 0.7 | 2.2 ± 0.6 | 0.003 |
| POD3 | 1.5 ± 0.6 | 1.6 ± 0.6 | 0.411 |
| POD5 | 1.1 ± 0.6 | 1.2 ± 0.4 | 0.247 |
| Time to pass flatus (d) | 2.7 ± 0.6 | 3.5 ± 0.7 | < 0.001 |
| Postoperative hospital stay (d) | 11.1 ± 5.2 | 9.9 ± 5.1 | 0.091 |
| Hospitalization costs ($) | 85098.7 ± 11067.9 | 82267.9 ± 14993.9 | 0.130 |
| Postoperative complications, n (%) | 0.632 | ||
| Anastomosis Leakage, n (%) | 2 (9.1) | 2 (2.6) | |
| Pelvic hemorrhage | 0 | 1 (1.3) | |
| Abdominal infection | 0 | 1 (1.3) | |
| Ileus, n (%) | 1 (4.5) | 1 (1.3) | |
| Incision infection, n (%) | 0 | 2 (2.6) | |
| Incisional hernia of the abdominal wall, n (%) | 0 | 4 (5.3) | |
| Urinary retention | 0 | 1 (1.3) | |
| White blood cell count (× 109/L) | |||
| POD1 | 9.0 ± 2.8 | 9.4 ± 2.9 | 0.462 |
| POD3 | 7.6 ± 2.2 | 8.5 ± 3.0 | 0.321 |
| POD5 | 6.8 ± 2.1 | 8.1 ± 4.3 | 0.112 |
| Neutrophil count (× 109/L) | |||
| POD1 | 7.8 ± 2.6 | 8.1 ± 2.7 | 0.579 |
| POD3 | 6.0 ± 2.0 | 6.6 ± 3.0 | 0.563 |
| POD5 | 5.1 ± 2.0 | 5.9 ± 2.7 | 0.266 |
| Body temperature (℃) | |||
| POD1 | 36.9 ± 0.4 | 37.0 ± 0.4 | 0.600 |
| POD2 | 37.1 ± 0.6 | 36.9 ± 0.4 | 0.057 |
| POD3 | 37.0 ± 0.4 | 36.9 ± 0.4 | 0.295 |
| POD4 | 36.8 ± 0.4 | 36.7 ± 0.4 | 0.300 |
| POD5 | 36.9 ± 0.7 | 36.7 ± 0.5 | 0.166 |
As shown in Table 3, no significant difference in the proportion of patients receiving postoperative chemotherapy between the R-NOSES I-F and RLRC groups (P = 0.995) was observed. The Wexner, LARS, and IPSS scores in both groups were not significantly different (P > 0.05), indicating a similar degree of damage to the anal and urinary systems for both surgical procedures. Until the last follow-up in June 2023, the median follow-up time was 26 and 36 mo (range 16-57 mo) in the R-NOSES I-F and RLRC groups, respectively. No deaths were reported for the R-NOSES I-F group, while two were reported for the RLRC group. One local anastomotic recurrence occurred in the R-NOSES I-F group, while nine distant metastases occurred in the RLRC group (four liver metastases, three lung metastases, and two pelvic metastases). However, no significant difference between the two groups (P = 0.291) was observed.
| Outcomes | R-NOSES I-F (n = 22) | RLRC (n = 76) | Ρ value |
| Postoperative chemotherapy | 0.995 | ||
| XELOX | 7 (31.8) | 24 (31.6) | |
| Fluorouracil monotherapy | 3 (13.6) | 11 (14.5) | |
| Defecation and urination function scores | |||
| Wexner | 4.9 ± 2.6 | 5.2 ± 3.1 | 0.817 |
| LARS | 15.3 ± 9.1 | 12.8 ± 10.1 | 0.177 |
| IPSS | 3.7 ± 4.6 | 3.5 ± 2.9 | 0.255 |
| Status at last follow-up | 0.291 | ||
| Local recurrence | 1 (4.5) | 0 | |
| Liver metastasis | 0 | 4 (5.3) | |
| Lung metastasis | 0 | 3 (3.9) | |
| Pelvic metastasis | 0 | 2 (2.6) | |
| Dead | 0 | 2 (2.6) |
Robotic technology combined with the NOSES concept has revolutionized minimally invasive surgeries by offering new possibilities. This retrospective cohort study represents the first published comparison between R-NOSES I-F and conventional laparoscopic RLRC. The study findings indicate that R-NOSES I-F is a safe and effective minimally invasive surgical technique for the treatment of lower rectal cancer.
In 2010, our center performed an improved laparoscopic transanal pull-through (ILTPT) technique for lower rectal cancer, which eliminated the need for auxiliary incisions in four patients with rectal cancer. This technique was the first of its kind on an international scale and the study represented the first investigation of laparoscopic R-NOSES I-F for lower rectal cancer. The results of this study demonstrated favorable short-term outcomes, with no instances of surgical site infections or complications in any of the cases. These findings provide substantial evidence that ILTPT is a safe and feasible approach for anus-preserving surgery in the treatment of lower rectal cancer[20]. Expert consensus[13] supports the notion that the anus serves as an ideal natural passage for extracting colorectal specimens, aligning with the requirements of minimally invasive surgery. Leveraging the clinical use of the Da Vinci robot, our center has also published a case report on R-NOSES I-F for low rectal cancer[18,19]. Although the above studies have shown good short-term results, they had the limitations of small sample sizes, lack of controlled trials, and lack of long-term follow-up results.
In this study, the R-NOSES I-F and RLRC groups had similar operative time (P = 0.389) and intraoperative blood loss (P = 0.068). However, the R-NOSES I-F group demonstrated significantly lower VAS scores on the first postoperative day (P = 0.003) and a significantly shorter postoperative anal venting time (P < 0.001) compared to those of the RLRC group. These findings are consistent with previous studies on laparoscopic NOSES[21,22]. Severe acute postoperative pain is reported as a risk factor for poor long-term prognosis[23]. Therefore, effective postoperative analgesia is crucial. By avoiding a long abdominal incision, patients in the R-NOSES I-F group experienced reduced postoperative abdominal pain, earlier mobilization, and faster recovery of gastrointestinal function, leading to a shorter postoperative anal venting time.
Regarding postoperative complications, our results revealed no significant difference in the incidence of complications between the R-NOSES I-F and RLRC groups (P = 0.632). The R-NOSES I-F group exhibited a 9.1% incidence of anasto
Inflammation is closely associated with the development, progression, and prognosis of cancer[28,29]. A growing array of evidence suggests that local and systemic inflammatory responses are important predictors of prognosis and recurrence in patients with colorectal cancer[30-32]. Previous animal experiments and clinical studies[22,33,34] have shown that transanal NOSES for colorectal cancer elicits a stronger systemic inflammatory response compared to conventional laparoscopic surgery. However, unlike previous studies, our study found that postoperative global white blood cell and neutrophil counts, and body temperature did not differ significantly between the patients in the two groups (P > 0.05). We conclude that the R-NOSES I-F group avoided the abdominal incision used to obtain the surgical specimen, thereby reducing surgical stress and decreasing the release of inflammatory mediators. Most importantly, the dissection and resection of specimens in the R-NOSES I-F group were performed entirely under direct in vitro vision, which shortens the time of intra-abdominal surgeries, avoids the potential risk of infection caused by dissecting the intestinal canal in the abdomen, minimizes the risk of contamination of the surgical area, and reduces the probability of intestinal bacteria entering the circulation. Additionally, the iodophor water used for irrigation before intestinal cutting and during the placement of the transanal circular stapler for digestive tract reconstruction ensured distal cleanliness. Consequently, in line with Efetov's findings[35], we believe that the R-NOSES I-F surgical approach does not exacerbate the postoperative inflammatory response.
The attainment of sterile and tumor-free standards in NOSES remains a substantial concern among surgeons. A recent multicenter study has shown that robotic NOSES had no adverse impact on the radical outcome of tumors[17]. Expert consensus[16] provides the following indications for R-NOSES I-F: (1) Appropriateness for low rectal cancer with the lower margin of the tumor located 2-5 cm from the dentate line; (2) Suitability for tumor invasion depth within T3; and (3) Applicability to tumors with a circumference of less than 5 cm. In our study, the R-NOSES I-F group included 81.8% of patients with a tumor infiltration depth within T3 and 86.4% of patients with a tumor circumference of < 5 cm. Adequate tumor size and proper bowel preparation facilitate conducive transanal specimen eversion. Moreover, the entire procedure was conducted following high standards. Before the specimen removal, a sterile protective sleeve was positioned, and the specimen underwent repeated rinsing with iodophor water before resection and reconstruction of the digestive tract. Additionally, resection of the specimen in the R-NOSES I-F group was performed entirely under direct extracorporeal vision with sufficient operating space, which provided favorable conditions for a more precise judgment of the surgical margins and allowed us to preserve more of the distal rectum while ensuring complete resection of the tumor. Finally, the perirectal circumferential resection margins were negative in both groups. The mean number of lymph nodes cleared in the R-NOSES I-F group was no less than that in the RLRC group (14.2 ± 7.3 vs 13.7 ± 6.0, P = 0.759) and exceeded the recommended threshold of at least 12 lymph nodes cleared, as outlined by the College of American Pathologists. Thus, we conclude that the R-NOSES I-F surgical approach adheres to aseptic and tumor-free principles.
The development and promotion of new approaches should prioritize the patient postoperative quality of life and long-term survival rates. Performing TME in the lower rectum is challenging owing to pelvic limitations, which can result in nerve injury. However, the magnified high-definition 3D view provided by robotic technology, along with the flexible and stable robotic arm, can help prevent permanent nerve injury during surgery[36]. In our study, we did not observe statistically significant differences in LARS and Wexner scores between patients in the R-NOSES I-F and RLRC groups (P > 0.05). This result is consistent with the findings of Tang et al[37]. No significant difference in the IPSS between the two groups (P = 0.207) was observed. Therefore, we believe that the R-NOSES I-F procedure does not cause more damage to the anal sphincter or urinary system during transanal specimen retrieval than the RLRC procedure. Furthermore, we did not find any difference in the incidence of local recurrence or distant metastasis between the two groups over the long follow-up period (P = 0.291).
However, it is important to acknowledge the limitations and disadvantages of R-NOSES I-F: (1) The method involves using intussusception to remove the required intestinal segments externally, which requires moving the descending colon upward during the surgery, increasing the operational complexity; and (2) In our study, we utilized single-point suture fixation to secure the oval forceps to the intestinal wall in the proximal pre-excision section. This approach places considerable tension on the intestinal wall amount of and carries a risk of intestinal tears. In future endeavors, we plan to improve this technique by employing a metal rod with a large head end and a small tail end (resembling the shape of a mushroom) as a replacement for the oval forceps. This modified approach involves binding the neck of the metal rod to the colon wall under robotic vision and then extracting the specimen, substantially reducing tension during specimen retrieval, and thereby mitigating surgical complexity and associated complications.
Furthermore, our study had several limitations. First, it was a single-center retrospective study, potentially introducing selection bias in patient enrollment. Second, the sample size was small, and the follow-up period was insufficient for some patients. A prospective multicenter randomized trial with a larger sample size and longer follow-up period is necessary to evaluate the advantages of R-NOSES I-F in the treatment of lower rectal cancer.
In conclusion, our findings support that R-NOSES I-F is a safe and effective, minimally invasive surgical approach for the treatment of lower rectal cancers. This procedure to did not lead to an increased postoperative inflammatory response compared to RLRC. It offers several advantages, including reduced postoperative pain, enhanced recovery of gastrointestinal function, minimized abdominal wall dysfunction, avoidance of complications associated with abdominal wall incisions, favorable cosmetic outcomes, and comparable rates of local recurrence and distant metastasis over a long follow-up period.
Robotic resection using the natural orifice specimen extraction surgery I-type F method (R-NOSES I-F) is a novel minimally invasive surgical strategy for the treatment of lower rectal cancer with robotic resection of rectal cancer and natural oral specimen extraction surgery. But its safety and feasibility are still worth exploring.
To evaluate the safety and feasibility of R-NOSES I-F for the treatment of lower rectal cancer by comparing R-NOSES I-F with traditional robotic lower rectal cancer resection. To provide a new minimally invasive surgical method for the treatment of lower rectal cancer.
To investigate the safety and feasibility of R-NOSES I-F surgery in the treatment of low rectal cancer.
We used retrospective analysis to include 22 patients who underwent R-NOSES I-F surgery into the R-NOSES I-F group and 76 patients who underwent robotic low rectal cancer resection (RLRC) surgery into the RLRC group. The clinicopathological data of all enrolled patients were analyzed to compare the postoperative outcomes and prognosis of the two groups.
Compared with the RLRC group, the R-NOSES I-F group had a lower visual analog scale of pain on day 1 after surgery (1.7 ± 0.7 vs 2.2 ± 0.6, P = 0.003) and a shorter postoperative ventilation time (2.7 ± 0.6 vs 3.5 ± 0.7, P < 0.001). After long-term follow-up, there was no significant difference in local recurrence rate and distant metastasis rate between the two groups (P > 0.05).
R-NOSES I-F is a safe and effective minimally invasive procedure for the treatment of lower rectal cancer, which has the advantages of relieving pain, promoting gastrointestinal function recovery, and avoiding incision complications.
The incidence and mortality of rectal cancer are increasing significantly, and it is particularly important to improve the postoperative quality of life of rectal cancer patients, especially those with low-grade rectal cancer, through improved surgical methods. In recent years, the combination of robotic surgery and NOSES has become one of the hot spots in rectal cancer surgery. R-NOSES I-F has the advantages of reducing postoperative pain, promoting gastrointestinal function recovery, reducing abdominal wall dysfunction, and avoiding complications of abdominal wall incision, and has certain cosmetic effects. It is a safe and effective minimally invasive surgical modality for the treatment of low-lying rectal cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cassell III AK, Liberia; Liang L, United States; Rosen SA, United States S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64702] [Article Influence: 16175.5] [Reference Citation Analysis (177)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13215] [Article Influence: 1468.3] [Reference Citation Analysis (3)] |
| 3. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2209] [Article Influence: 736.3] [Reference Citation Analysis (1)] |
| 4. | Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, Zhao R, Duan Y, Zeng Z, Li X, Li G, Xiong W, Zhou M. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 5. | Feng Q, Ng SSM, Zhang Z, Lin S, Niu Z, Wei Y, He G, Chang W, Zhu D, Xu J. Comparison between robotic natural orifice specimen extraction surgery and traditional laparoscopic low anterior resection for middle and low rectal cancer: A propensity score matching analysis. J Surg Oncol. 2021;124:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Cui B, Lei S, Liu K, Yao H. Robotic low anterior resection plus transanal natural orifice specimen extraction in a patient with situs inversus totalis. BMC Surg. 2018;18:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Gao G, Chen L, Luo R, Tang B, Li T. Short- and long-term outcomes for transvaginal specimen extraction versus minilaparotomy after robotic anterior resection for colorectal cancer: a mono-institution retrospective study. World J Surg Oncol. 2020;18:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Liu D, Luo R, Wan Z, Zhu W, He P, Ye S, Tang C, Lei X, Li T. Clinical outcomes and prognostic factors of robotic assisted rectal cancer resection alone versus robotic rectal cancer resection with natural orifice extraction: a matched analysis. Sci Rep. 2020;10:12848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Cheng CL, Rezac C. The role of robotics in colorectal surgery. BMJ. 2018;360:j5304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Kang J, Yoon KJ, Min BS, Hur H, Baik SH, Kim NK, Lee KY. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg. 2013;257:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 11. | Liu G, Zhang S, Zhang Y, Fu X, Liu X. Robotic Surgery in Rectal Cancer: Potential, Challenges, and Opportunities. Curr Treat Options Oncol. 2022;23:961-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 12. | Wang XS. [The present situation and prospects of colorectal tumor like-NOTES technique]. Zhonghua Yixue Zazhi. 2015;4:11-16. |
| 13. | China NOSES Alliance PCoNOSES; Colorectal Cancer Committee of Chinese Medical Doctor Association. [Expert consensus of natural orifice specimen extraction surgery in colorectal neoplasm (2017 edition)]. Zhonghua Jiezhichangjibing Dianzi Zazhi. 2017;6:266-272. |
| 14. | China NOSES Alliance PCoNOSES; Colorectal Cancer Committee of Chinese Medical Doctor Association. [Expert consensus of natural orifice specimen extraction surgery in colorectal neoplasm (2019)]. Zhonghua Jiezhichangjibing Dianzi Zazhi. 2019;8:336-342. |
| 15. | Guan X, Liu Z, Longo A, Cai JC, Tzu-Liang Chen W, Chen LC, Chun HK, Manuel da Costa Pereira J, Efetov S, Escalante R, He QS, Hu JH, Kayaalp C, Kim SH, Khan JS, Kuo LJ, Nishimura A, Nogueira F, Okuda J, Saklani A, Shafik AA, Shen MY, Son JT, Song JM, Sun DH, Uehara K, Wang GY, Wei Y, Xiong ZG, Yao HL, Yu G, Yu SJ, Zhou HT, Lee SH, Tsarkov PV, Fu CG, Wang XS; International Alliance of NOSES. International consensus on natural orifice specimen extraction surgery (NOSES) for colorectal cancer. Gastroenterol Rep (Oxf). 2019;7:24-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Professional Committee of Natural Orifice Specimen Extraction Surgery CCCoCMDA; Professional Committee of Robotic Surgery; Colorectal Cancer Committee of Chinese Medical Doctor Association. [Expert consensus on robotic natural orifice specimen extraction surgery for colorectal neoplasm]. Zhonghua Jiezhichangjibing Dianzi Zazhi. 2022;11:177-191. [DOI] [Full Text] |
| 17. | Liu GH, Yao HL, Wang XS, Wang GY, Xiong DH, She JJ, Hu JH, Yuan WT, Yang CK, Cai JC, Han FH, Zeng XF, He PH, Ye SP, Niu ZC, Liu KJ, Guan X, Tang QC, Huang R, Shi FY, Lian YG, Guan S, Jian JL, Wang ZF, Zhou SN, Zhao SF, Wei Y, Li TY. [Robotic natural orifice specimen extraction surgery of colorectal neoplasms in China: A nationwide muticenter analysis]. Zhonghua Jiezhichangjibing Dianzi Zazhi. 2022;11:474-481. [DOI] [Full Text] |
| 18. | Wang DQ, Zhu WQ, Liu DN, Zhong CH, Ju HQ, Li TY. [Application of extracorporeal resection of rectum in NOSES Ⅰ radical resection of low rectal cancer (report of 6 cases)]. Zhonghua Jiezhichangjibing Dianzi Zazhi. 2022;11:86-88. [DOI] [Full Text] |
| 19. | Tang LD, Zhu WQ, Luo R, He PH, Li TY. [A case report of robotic-assisted colorectal tumor natural orifice specimen extraction surgery Type I (NOSES I)]. Zhonghua Jiezhichangjibing Dianzi Zazhi. 2020;9:296-298. |
| 20. | Li T, Gong J, Duanmu J, Zhang H, Lei X. [Improved laparoscopic transanal pull-through technique for low-rectal cancer resection]. Chinese-German J Clin Oncol. 2010;9:606-609. [DOI] [Full Text] |
| 21. | Zhang Q, Wang M, Ma D, Zhang W, Wu H, Zhong Y, Zheng C, Ju H, Wang G. Short-term and long-term outcomes of natural orifice specimen extraction surgeries (NOSES) in rectal cancer: a comparison study of NOSES and non-NOSES. Ann Transl Med. 2022;10:488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Wolthuis AM, Fieuws S, Van Den Bosch A, de Buck van Overstraeten A, D'Hoore A. Randomized clinical trial of laparoscopic colectomy with or without natural-orifice specimen extraction. Br J Surg. 2015;102:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Peters ML, Sommer M, de Rijke JM, Kessels F, Heineman E, Patijn J, Marcus MA, Vlaeyen JW, van Kleef M. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg. 2007;245:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, Sohn DK, Oh JH. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann Surg. 2018;267:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 25. | Feroci F, Vannucchi A, Bianchi PP, Cantafio S, Garzi A, Formisano G, Scatizzi M. Total mesorectal excision for mid and low rectal cancer: Laparoscopic vs robotic surgery. World J Gastroenterol. 2016;22:3602-3610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Park EJ, Cho MS, Baek SJ, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg. 2015;261:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 27. | Guan X, Wang GY, Zhou ZQ, Zhou HT, Chen YZ, Tang QC, Song JM, Cai JC, Bao CQ, Zhang H, Liu YJ, Xiong ZG, Wu M, Song C, Zheng YC, Jiang JR, Yan S, Wang Y, Hu QL, Ma D, Ren K, Xiong DH, Zhang XH, Yang MR, Bai YK, Fu W, Li SH, Zhang SF, Liu JG, Mo XW, Gong HY, Jiang B, Wang T, Zhang AP, Zhu P, Fu T, Hu JH, Jia WZ, Qin CJ, Su Q, Wang DR, Wu WQ, Zhao ZG, Zhu HB, Jin WY, Jing CQ, Li DG, Liu WZ, Liu ZC, Pang LM, Tang D, Wang XQ, Yang GS, Yao KH, Zhang XM, Zhao L, Zhong XG, Zhou L, Zhu Z, Bai XF, Chen CW, Chen SW, Chen ZH, Dai L, Fu ZB, Gao F, Gao H, Gao L, Gong JF, Jiang Y, Jie JZ, Jin WS, Li DC, Li J, Lin HW, Liu BL, Liu CQ, Liu M, Meng JB, Qiu J, Rao GA, Sun DH, Sun XJ, Tai JD, Wang ZG, Xie GW, Xie M, Wei Y, Yan J, Yan LK, Yang F, Yang HM, Yang WJ, Chen LC, Ye ZZ, Yu ZG, Zhao ZH, Zhong M, Zhu YP, Fu CG, Wang XS. [Retrospective study of 718 colorectal neoplasms treated by natural orifice specimen extraction surgery in 79 hospitals]. Zhonghua Jiezhichangjibing Dianzi Zazhi. 2017;6:469-477. [DOI] [Full Text] |
| 28. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11285] [Article Influence: 490.7] [Reference Citation Analysis (2)] |
| 29. | Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1627] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 30. | Matsubara D, Arita T, Nakanishi M, Kuriu Y, Murayama Y, Kudou M, Konishi H, Komatsu S, Shiozaki A, Otsuji E. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int J Clin Oncol. 2020;25:602-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Yamamoto T, Kawada K, Obama K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 256] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 32. | Wesselink E, Balvers MGJ, Kok DE, Winkels RM, van Zutphen M, Schrauwen RWM, Keulen ETP, Kouwenhoven EA, Breukink SO, Witkamp RF, de Wilt JHW, Bours MJL, Weijenberg MP, Kampman E, van Duijnhoven FJB. Levels of Inflammation Markers Are Associated with the Risk of Recurrence and All-Cause Mortality in Patients with Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2021;30:1089-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Senft JD, Dröscher T, Gath P, Müller PC, Billeter A, Müller-Stich BP, Linke GR. Inflammatory response and peritoneal contamination after transrectal natural orifice specimen extraction (NOSE) versus mini-laparotomy: a porcine in vivo study. Surg Endosc. 2018;32:1336-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Zhou S, Wang X, Zhao C, Zhou H, Pei W, Liang J, Zhou Z. Can transanal natural orifice specimen extraction after laparoscopic anterior resection for colorectal cancer reduce the inflammatory response? J Gastroenterol Hepatol. 2020;35:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Efetov SK, Tulina IA, Kim VD, Kitsenko Y, Picciariello A, Tsarkov PV. Natural orifice specimen extraction (NOSE) surgery with rectal eversion and total extra-abdominal resection. Tech Coloproctol. 2019;23:899-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Tang B, Gao G, Ye S, Liu D, Jiang Q, Ai J, Lei X, Shi J, Li T. Male urogenital function after robot-assisted and laparoscopic total mesorectal excision for rectal cancer: a prospective cohort study. BMC Surg. 2022;22:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Tang Q, Zhu Y, Xiong H, Sheng X, Hu Z, Hu H, Huang R, Zhang Q, Yuan Z, Xie L, Gao Z, Wang Y, Wang G, Wang X. Natural Orifice Specimen Extraction Surgery versus Conventional Laparoscopic-Assisted Resection in the Treatment of Colorectal Cancer: A Propensity-Score Matching Study. Cancer Manag Res. 2021;13:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |