Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2115
Peer-review started: July 6, 2023
First decision: July 27, 2023
Revised: August 5, 2023
Accepted: August 25, 2023
Article in press: August 25, 2023
Published online: October 27, 2023
Processing time: 113 Days and 1 Hours
During cirrhosis, the liver is impaired and unable to synthesize and clear thrombopoietin properly. At the same time, the spleen assumes the function of hemofiltration and storage due to liver dysfunction, resulting in hypersplenism and excessive removal of platelets in the spleen, further reducing platelet count. When liver function is decompensated in cirrhotic patients, the decrease of throm
To investigate the clinical efficacy of recombinant human TPO (rhTPO) in the treatment of perioperative thrombocytopenia during liver transplantation in cirrhotic mice with hypersplenism.
C57BL/6J mice and TPO receptor-deficient mice were used to establish models of cirrhosis with hypersplenism. Subsequently, these mice underwent orthotopic liver transplantation (OLT). The mice in the experimental group were given rhTPO treatment for 3 consecutive days before surgery and 5 consecutive days after surgery, while the mice in the control group received the same dose of saline at the same frequency. Differences in liver function and platelet counts were determined between the experimental and control groups. Enzyme-linked immunosorbent assay was used to assess the expression of TPO and TPO receptor (c-Mpl) in the blood.
Preoperative administration of rhTPO significantly improved peri-OLT thrombocytopenia in mice with cirrhosis and hypersplenism. Blocking the expression of TPO receptors exacerbated peri-OLT thrombocytopenia. The concentration of TPO decreased while the concentration of c-Mpl increased in compensation in the mouse model of cirrhosis with hypersplenism. TPO pre-treatment significantly increased the postoperative TPO concentration in mice, which in turn led to a decrease in the c-Mpl concentration. TPO pre-treatment also significantly enhanced the Janus kinase (Jak)/signal transducers and activators of transcription pathway protein expressions in bone marrow stem cells of the C57BL/6J mice. Moreover, the administration of TPO, both before and after surgery, regulated the levels of biochemical indicators, such as alanine aminotransferase, alkaline phosphatase, and aspartate aminotransferase in the C57BL/6J mice.
Pre-treatment with TPO not only exhibited therapeutic effects on perioperative thrombocytopenia in the mice with cirrhosis and hypersplenism, who underwent liver transplantation but also significantly enhanced the perioperative liver function.
Core Tip: Our research results confirm that thrombopoietin (TPO) can improve liver function after liver transplantation in mice by enhancing the effect of platelets. Pre-treatment with TPO not only exhibited therapeutic effects on perioperative thrombocytopenia in the mice with cirrhosis and hypersplenism, who underwent liver transplantation but also significantly enhanced the perioperative liver function.
- Citation: Liu ZR, Zhang YM, Cui ZL, Tong W. Effects of thrombopoietin pre-treatment on peri-liver transplantation thrombocytopenia in a mouse model of cirrhosis with hypersplenism. World J Gastrointest Surg 2023; 15(10): 2115-2122
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2115.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2115
Thrombocytopenia in cirrhosis is a rare condition with various causes including hypersplenism, reduced levels of thrombopoietin (TPO), presence of anti-platelet autoantibodies, suppression of bone marrow cells by hepatitis viruses, adverse reactions to excessive alcohol intake, liver dysfunction, and vitamin B12 and folic acid deficiencies. Of these, hypersplenism is the most common cause. However, for patients with cirrhosis in the decompensated stage, a decrease in TPO production by hepatocytes is the main cause for the reduced production of new platelets[1-3]. Most patients with cirrhosis and hypersplenism, who are candidates for orthotopic liver transplantation (OLT), are in the decompensated stage, with comorbidities, such as hepatitis or a long history of excessive alcohol consumption. As a result, these patients exhibit more pronounced hypersplenism and have a slower postoperative recovery compared to those with cirrhosis and hypersplenism alone. A study of patients with liver failure, before and after liver transplantation, found that all patients manifested prominent thrombocytopenia before transplantation, with serum levels so low that TPO was undetectable. In contrast, the serum TPO levels increased significantly 2 d after transplantation and reached their peaks at 4-6 d. At the same time, platelet counts began to rise and peaked at 14 d after transplantation. Furthermore, other cytokines affecting platelet production did not change significantly before and after transplantation[4,5]. These findings suggest that, in patients with liver failure, TPO plays a critical role in thrombocytopenia and in the restoration of platelet count after liver transplantation. Gollomp et al[6] found similar serum TPO levels before and after liver transplantation, as well as significantly lower TPO mRNA expressions in the liver tissues of patients with cirrhosis. Thrombocytopenia in patients with liver failure is challenging. Current research to improve the postoperative platelet counts of these patients and, thus, reduce the risk of bleeding and promote rapid recovery from hypersplenism is necessary.
A mouse model of liver cirrhosis with hypersplenism was established by weighing the mice and injecting them intraperitoneally with carbon tetrachloride (CCl4, 1 mL/kg body weight) three times a week for 6 wk. At the end of the 6 wk, blood was collected from the orbital vein to determine the serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and the liver tissues were stained with hematoxylin and eosin (HE) to determine whether the model was successfully developed.
Groups: C57BL/6J mice were used to establish a model of cirrhosis with hypersplenism. These mice subsequently underwent OLT. The experimental group received recombinant human TPO (rhTPO) treatment for 3 consecutive days before surgery and 5 consecutive days after surgery, while the control group received saline instead at the same dose and frequency.
Sample collection: Blood samples were collected 1 d before and 5 d after surgery. Liver tissue samples and bone marrow mesenchymal stem cells were collected from euthanized experimental and control mice 5 d after surgery.
Assessed indicators: Changes in liver function [ALT, AST, and alkaline phosphatase (ALP) levels] were assessed by biochemical assays at different time points. Changes in the level of leukocytes, erythrocytes, platelets, etc. were detected by routine blood tests at different time points. Pathological liver changes were observed in the tissue sections at different time points. Serum TPO and c-Mpl levels were determined using enzyme-linked immunosorbent assay (ELISA). The expression of Jak/signal transducers and activators of transcription (STAT), in extracted mouse bone marrow mesenchymal stem cells, was detected with Western blotting.
Groups: Mice with a silenced TPO receptor gene and C57BL/6J mice were used to establish the model of cirrhosis with hypersplenism. These mice then all underwent OLT. The experimental group received rhTPO treatment for 3 consecutive days before surgery and 5 consecutive days after surgery, while the control group received saline instead at the same dose and frequency.
Sample collection: Same as described above.
Assessed indicators: Same as described above.
SPSS, version 22.0 (IBM Corp., Armonk, NY, United States) was used for analysis. Normally distributed quantitative data were expressed as mean ± SD, and a t-test was used for comparison between the groups. The data with skewed distributions were expressed as M (range), and a Mann-Whitney U test was used for comparison between the groups. Count data were expressed as absolute numbers or percentages, and a χ² test or a Fisher’s exact test was used for comparisons between the groups. Data with repeated measurements were analyzed using repeated-measures analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
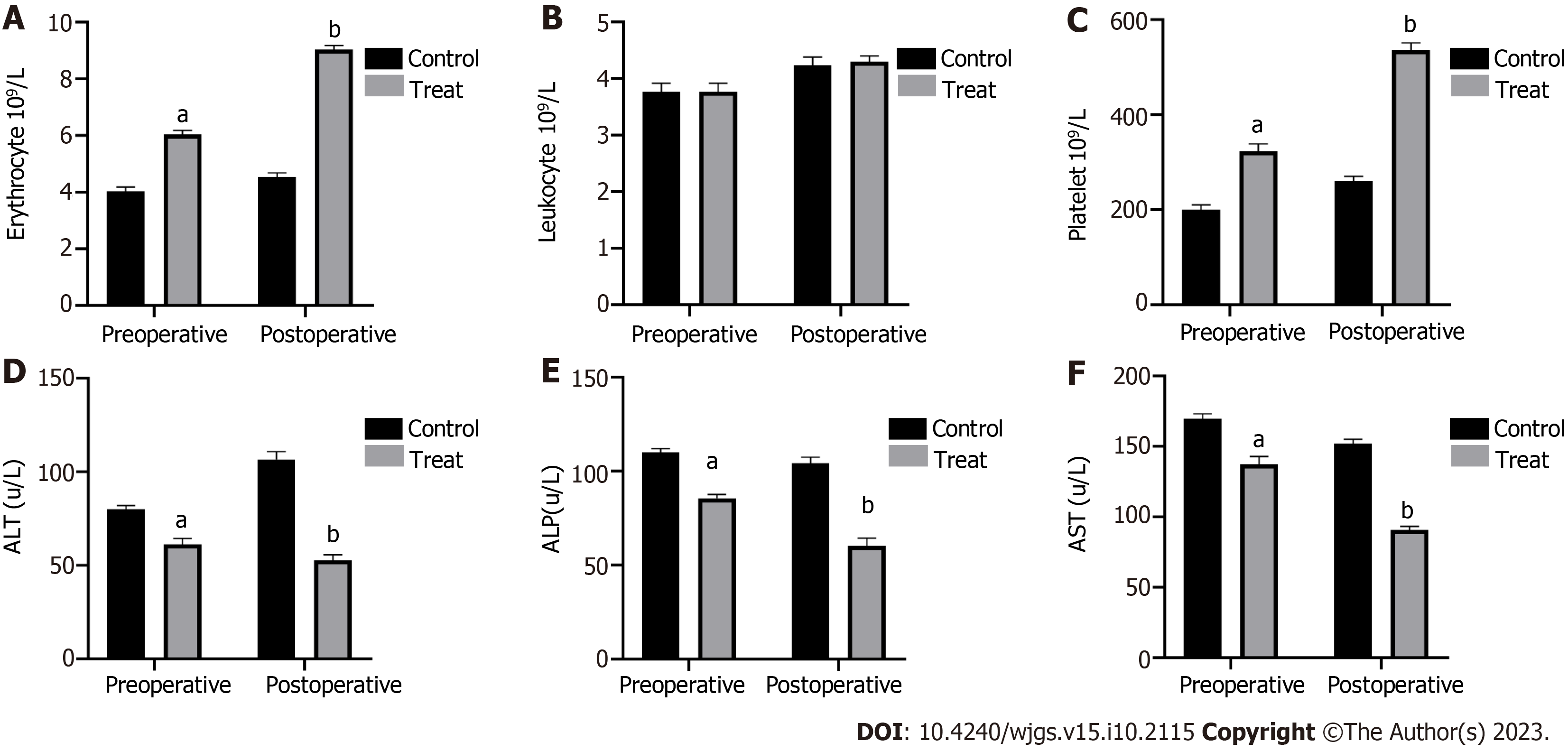
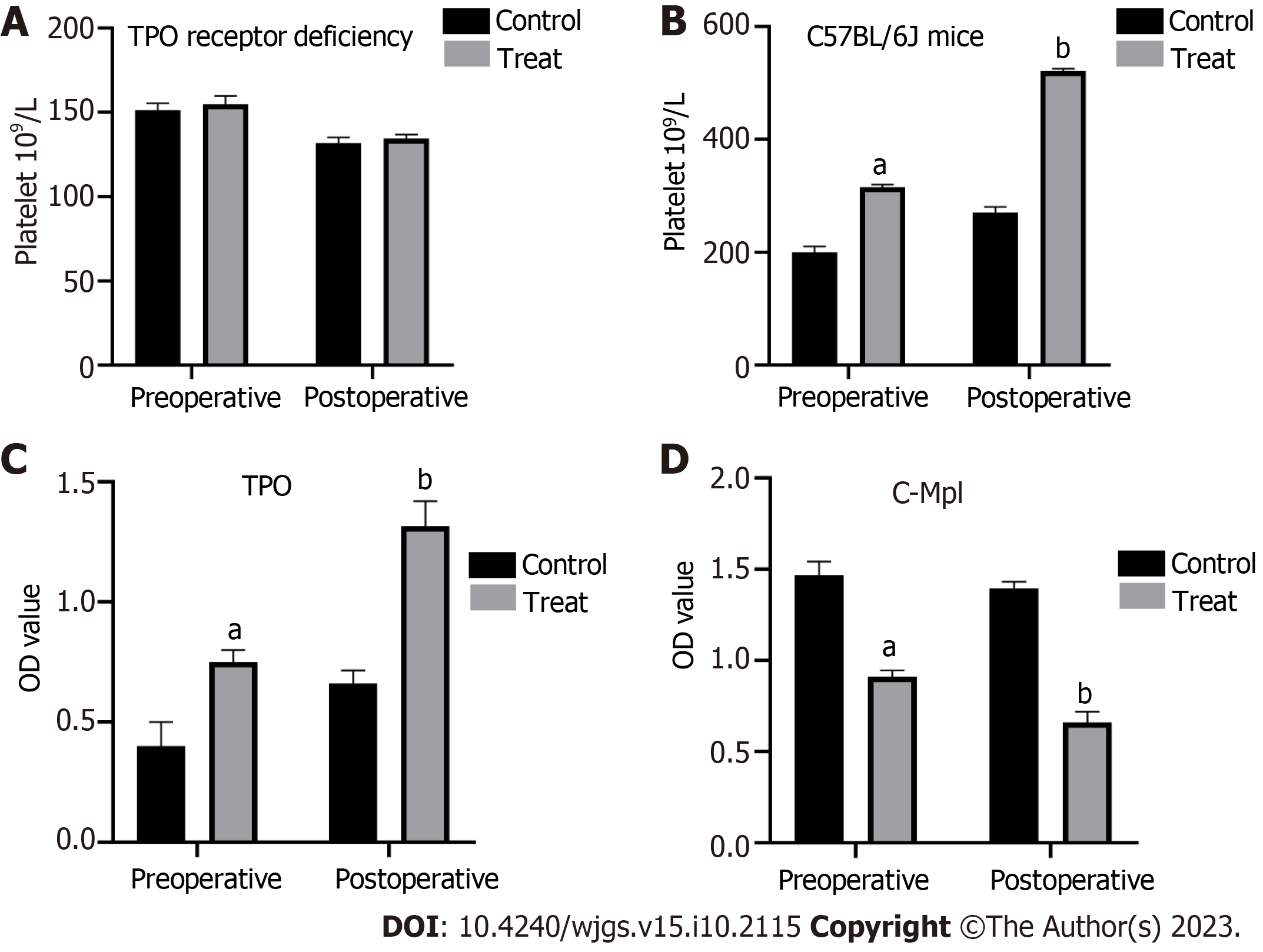
C57BL/6J mice were treated with CCl4 to establish a model of cirrhosis with hypersplenism. Subsequently, these mice underwent OLT. In the experimental group, rhTPO was administrated for 3 consecutive days before and 5 consecutive days after surgery, while in the control group, an equal amount of saline was administrated at the same frequency instead. Routine blood tests and liver function assessments of the experimental and control mice were performed 1 d before and 5 d after surgery to monitor the changes. The results showed that TPO pre-treatment significantly ameliorated erythropenia and thrombocytopenia during the perioperative period of liver transplantation in the experimental C57BL/6 mice compared to the control mice (Figure 1A and C). However, the leukocyte count was not affected by TPO pre-treatment (Figure 1B). Importantly, TPO pre-treatment significantly improved the perioperative liver function of these mice (Figure 1D-F). In contrast, TPO receptor-deficient mice responded poorly to TPO pre-treatment compared to C57BL/6J mice. As such, TPO pre-treatment failed to elevate the perioperative platelet counts in TPO receptor-deficient mice (Figure 2A and B).
ELISA was used to determine the differences in TPO and c-Mpl levels between the experimental and control groups, 1 d before and 5 d after surgery. Compared to the control group, the results from the experimental group showed that the TPO treatment significantly increased serum TPO after liver transplantation, which in turn, led to a decrease in the c-Mpl level (Figure 2C and D).
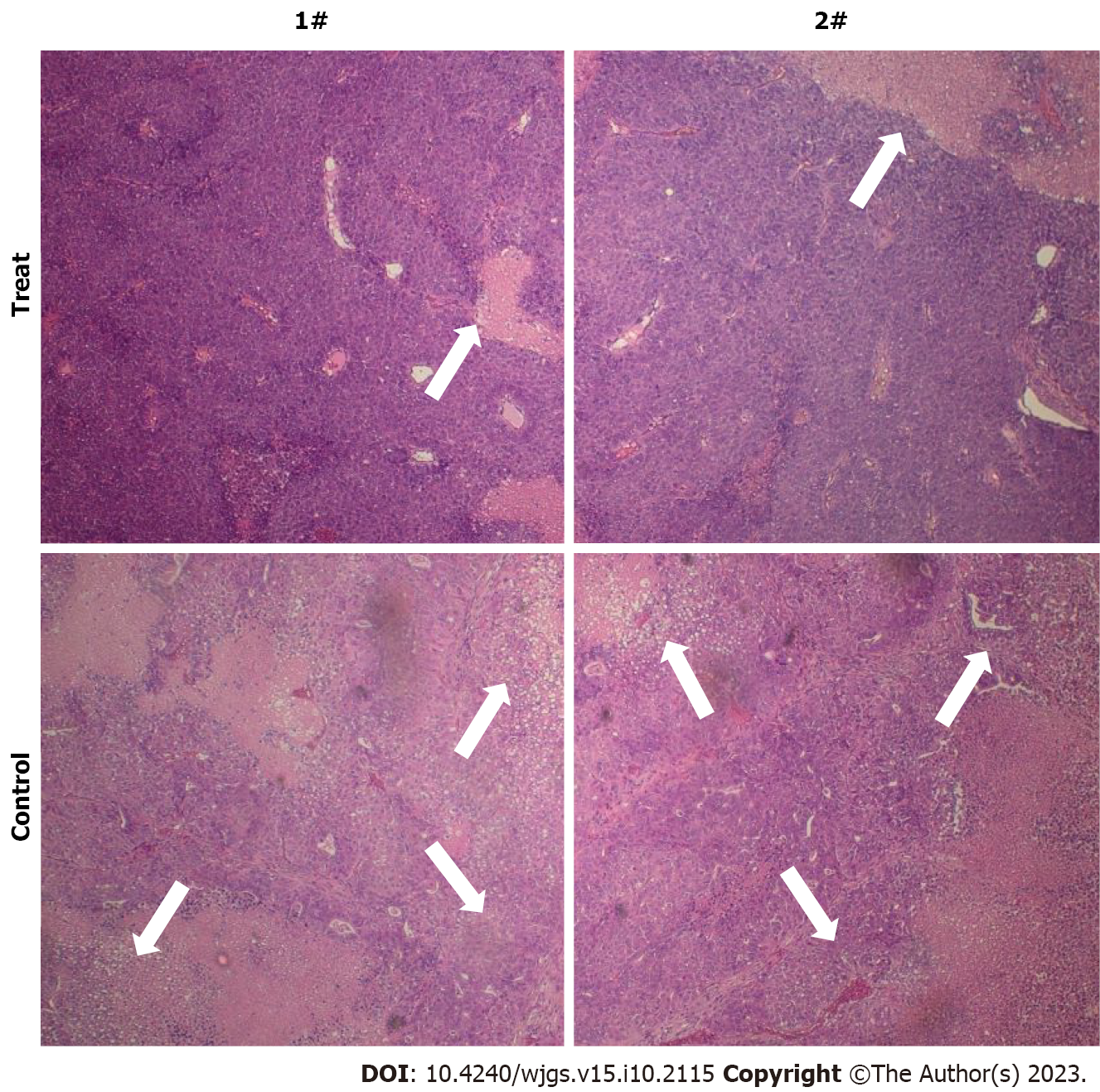
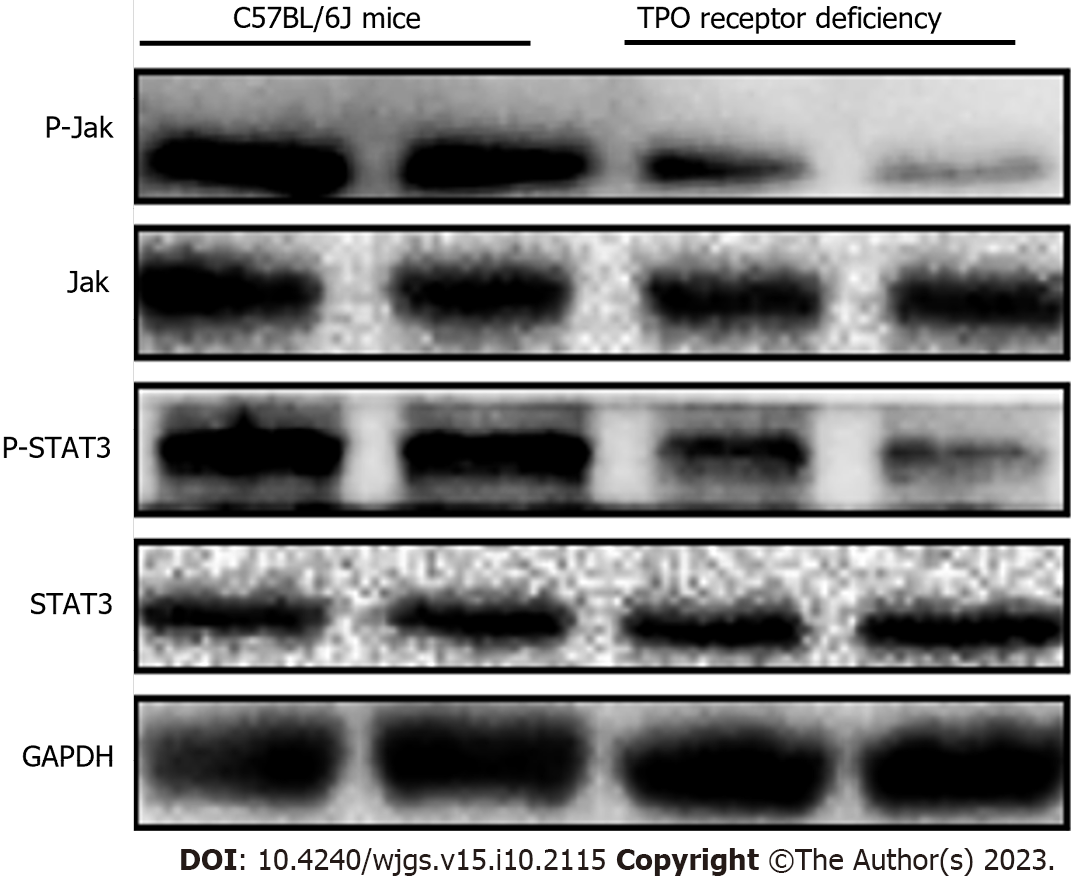
HE staining showed that the mice in the experimental group which received TPO pre-treatment had significantly reduced inflammatory responses in the liver tissues compared to those in the control group (Figure 3). Considering that the Jak/STAT pathway plays an important role in the differentiation and maturation of megakaryocytes and the promotion of platelet production, we examined the differential expression of the Jak/STAT pathway proteins between the TPO receptor-deficient mice and the C57BL/6J mice. TPO pre-treatment significantly enhanced the expression of the Jak/STAT pathway proteins in the bone marrow stem cells of C57BL/6J mice compared to those of the TPO receptor-deficient mice (Figure 4).
TPO is a glycoprotein with 332 amino acids produced mainly by hepatocytes and is an endogenous cytokine that stimulates the growth and differentiation of megakaryocytes. It has stimulative effects on megakaryopoiesis at all stages, including the proliferation of precursor cells and the development and maturation of polyploid megakaryocytes. Once TPO binds to its receptor, c-Mpl, it induces the homodimerization of c-Mpl, activating the family of JAK in signaling pathways, including Jak/STAT, P13K/Akt, Ras/MAPK, etc., and the secretion of a series of signaling molecules to induce megakaryocyte differentiation and maturation and promote platelet production. The secretion of TPO is mainly influenced by the number of peripheral blood platelets, and TPO, in turn, acts to maintain a stable number of peripheral blood platelets[7-10]. rhTPO is a purified full-length glycosylated TPO produced by Chinese hamster ovary cells that are modified using recombinant gene technology. It has the same amino acid sequence as TPO and is fully glycosylated with similar platelet-elevating pharmacological effects as endogenous TPO[11-13]. Most patients with cirrhosis and hypersplenism who require OLT are in a decompensated stage of cirrhosis, with comorbidities, such as hepatitis or a long history of excessive alcohol consumption. As such, thrombocytopenia tends to be more severe, and postoperative recovery is usually slower in these patients than those with cirrhosis and hypersplenism alone. However, the therapeutic effects of rhTPO in these patients have not yet been elucidated.
Several studies have employed in-situ hybridization techniques to demonstrate that the liver is the primary organ expressing mRNA of TPO, despite the expression of TPO mRNA in other organs[14-16]. It is now confirmed that the main site of TPO production in the body is the liver. Under physiological conditions, TPO is cleared from the blood by binding to its receptors on the platelet surface. As the platelet count increases, TPO clearance also increases, resulting in a decreasing blood TPO level. Conversely, blood TPO level increases when the platelet count decreases[17,18]. There are two major contributors to thrombocytopenia in cirrhosis: Abnormal platelet distribution and a decreased production of hepatic TPO. Abnormal platelet distribution is related to the circuiting platelets being trapped in the spleen, and the degree of entrapment is positively associated with the size of the spleen. The decreased production of hepatic TPO is related to TPO production in organs, specifically 70% of TPO originates from the liver and only 30% originates from the kidneys and other organs. The indications for rhTPO during the per-OLT period in patients with cirrhosis and hypersplenism are as follows: (1) Patients with hypersplenism who have reduced circulating platelets before liver trans
The present study found that preoperative administration of rhTPO significantly improved peri-liver transplantation thrombocytopenia in mice with cirrhosis and hypersplenism. Blocking the expression of TPO receptors exacerbated peri-OLT thrombocytopenia. The concentration of TPO decreased, while the concentration of c-Mpl increased in compensation, in the mouse model of cirrhosis with hypersplenism. TPO pre-treatment significantly increased the postoperative TPO concentration in mice, which in turn, led to a decrease in the c-Mpl concentration. TPO pre-treatment significantly enhanced the expressions of proteins involved in the Jak/STAT pathway in the bone marrow stem cells of C57BL/6J mice, which is consistent with the results from other studies. Additionally, two animal studies have shown that TPO can promote liver regeneration and ameliorate liver fibrosis by promoting platelet production[19,20]. A clinical study has also shown that platelet transfusion can improve liver function in patients with chronic liver disease and cirrhosis. In the present study, we demonstrated that TPO can regulate the levels of the biochemical indicators, such as ALT, ALP, and AST in C57BL/6J mice, regardless of the timing of its administration (before or after surgery). Our results also validated that TPO can improve liver function in mice by enhancing the effects of platelets. However, there are still some limitations and deficiencies in this study. First, the study was limited to a mouse model, and the clinical effects of TPO on perioperative liver transplant patients need to be further explored in the future. In addition, the study found that TPO exerts pharmacological effects by activating the Jak/Stat3 pathway, and the specific molecular mechanisms in this pathway still need to be further demonstrated by basic experiments.
In conclusion, we found that preoperative prophylactic use of TPO has a therapeutic effect on perioperative thrombocytopenia in cirrhotic hyper splenic mice undergoing liver transplantation. In addition, TPO pretreatment can significantly improve the liver function of perioperative mice. TPO pretreatment also improved postoperative liver inflammation and reduced liver cell necrosis in mice.
During cirrhosis, the liver undergoes significant impairment, leading to various complications, including thrombocytopenia and bleeding tendency. Thrombopoietin (TPO) is a hormone produced by the liver that plays a crucial role in regulating platelet production and clearance. However, in cirrhotic patients, the liver’s ability to synthesize and clear TPO is compromised. The impaired liver function in cirrhosis results in reduced TPO synthesis. TPO is primarily produced in the liver sinusoidal endothelial cells, and when the liver is damaged, the production of TPO is significantly decreased. This reduction in TPO levels leads to a decrease in the production of new platelets in the bone marrow, contributing to thrombocytopenia.
It is important to manage thrombocytopenia and bleeding tendency in cirrhotic patients. Treatment options may include platelet transfusions, medications that stimulate platelet production (such as TPO receptor agonists), and interventions to address the underlying liver dysfunction. Close monitoring and collaboration with a healthcare provider are crucial in managing these complications in cirrhotic patients.
To evaluate the clinical effectiveness of recombinant human TPO (rhTPO) in managing perioperative thrombocytopenia during liver transplantation in cirrhotic mice with hypersplenism. We aimed to assess whether rhTPO administration could effectively increase platelet count and reduce bleeding complications in this specific population.
To achieve this objective, we conducted a controlled experiment using a cirrhotic mouse model with hypersplenism. The mice were divided into two groups: A treatment group receiving rhTPO and a control group receiving a placebo or standard care. We monitored the platelet counts of the mice before and after liver transplantation, as well as during the perioperative period.
The results of our study demonstrated that preoperative administration of rhTPO effectively improved perioperative thrombocytopenia in mice with cirrhosis and hypersplenism undergoing liver transplantation (OLT). This finding suggests that rhTPO may have potential clinical efficacy in managing thrombocytopenia in cirrhotic patients undergoing liver transplantation. Furthermore, we found that blocking the expression of TPO receptors exacerbated peri-OLT thrombocytopenia, indicating the importance of the TPO/c-Mpl pathway in platelet regulation during liver transplantation in cirrhotic mice with hypersplenism. In our study, we observed a decrease in TPO concentration in the mouse model of cirrhosis with hypersplenism, while the concentration of c-Mpl increased in compensation. However, pre-treatment with TPO significantly increased the postoperative TPO concentration in mice, leading to a decrease in the c-Mpl concentration. This suggests that TPO administration can regulate the TPO/c-Mpl pathway and potentially improve platelet production and function. Additionally, TPO pre-treatment significantly enhanced the protein ex
Pre-treatment with TPO not only exhibited therapeutic effects on perioperative thrombocytopenia in the mice with cirrhosis and hypersplenism, who underwent liver transplantation but also significantly enhanced the perioperative liver function.
Overall, our study provides evidence supporting the clinical efficacy of rhTPO in managing perioperative thrombocytopenia during liver transplantation in cirrhotic mice with hypersplenism. These findings may have implications for the development of potential therapeutic strategies for managing thrombocytopenia in cirrhotic patients undergoing liver transplantation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Frager SZ, United States; Jeong SH, South Korea S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 852] [Article Influence: 213.0] [Reference Citation Analysis (1)] |
| 2. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 3. | Dai JF, Xia RX. [Effect of rhTPO and rhIL-11 on Thrombocytopenia after Chemotherapy in Leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30:711-717. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Zhu Q, Yang S, Zeng W, Li M, Guan Z, Zhou L, Wang H, Liu Y, Gao Y, Qiu S, Chen C, Li H, Zheng S, Yuan Y, Zhang H, Pan X. A Real-World Observation of Eltrombopag and Recombinant Human Thrombopoietin (rhTPO) in Lymphoma Patients With Chemotherapy Induced Thrombocytopenia. Front Oncol. 2021;11:701539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | McArthur K, Chappaz S, Kile BT. Apoptosis in megakaryocytes and platelets: the life and death of a lineage. Blood. 2018;131:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Gollomp K, Lambert MP, Poncz M. Current status of blood 'pharming': megakaryoctye transfusions as a source of platelets. Curr Opin Hematol. 2017;24:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Kuter DJ. Milestones in understanding platelet production: a historical overview. Br J Haematol. 2014;165:248-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Wang B, Zheng J. Platelet generation in vivo and in vitro. Springerplus. 2016;5:787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. Br J Haematol. 2014;165:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Chuai Y, Nie W, Wang A, Dai G. Thrombopoietin receptor agonists for prevention and treatment of chemotherapy-induced thrombocytopenia in patients with solid tumours. Cochrane Database Syst Rev. 2017;11:CD012035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Miao J, Leblebjian H, Scullion B, Parnes A. A single center experience with romiplostim for the management of chemotherapy-induced thrombocytopenia. Am J Hematol. 2018;93:E86-E88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Al-Samkari H, Marshall AL, Goodarzi K, Kuter DJ. The use of romiplostim in treating chemotherapy-induced thrombocytopenia in patients with solid tumors. Haematologica. 2018;103:e169-e172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Cantoni S, Carpenedo M, Mazzucconi MG, De Stefano V, Carrai V, Ruggeri M, Specchia G, Vianelli N, Pane F, Consoli U, Artoni A, Zaja F, D'adda M, Visentin A, Ferrara F, Barcellini W, Caramazza D, Baldacci E, Rossi E, Ricco A, Ciminello A, Rodeghiero F, Nichelatti M, Cairoli R. Alternate use of thrombopoietin receptor agonists in adult primary immune thrombocytopenia patients: A retrospective collaborative survey from Italian hematology centers. Am J Hematol. 2018;93:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Santoro C, Volpicelli P, Baldacci E, Ferrara G, Di Rocco A, Ferretti A, Porrazzo M, Mazzucconi MG. Repeated successful use of eltrombopag in chronic primary immune thrombocytopenia: description of an intriguing case. Clin Case Rep. 2017;5:1385-1388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Mittelman M, Platzbecker U, Afanasyev B, Grosicki S, Wong RSM, Anagnostopoulos A, Brenner B, Denzlinger C, Rossi G, Nagler A, Garcia-Delgado R, Portella MSO, Zhu Z, Selleslag D. Eltrombopag for advanced myelodysplastic syndromes or acute myeloid leukaemia and severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled, phase 2 trial. Lancet Haematol. 2018;5:e34-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Kanno M, Onoda T, Meguro T, Sato H, Mitsui T. Eltrombopag with i.v. immunoglobulin for safe splenectomy in refractory immune thrombocytopenia. Pediatr Int. 2018;60:191-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Kaushansky K. Thrombopoiesis. Semin Hematol. 2015;52:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Chan S, Chan GC, Ye J, Lian Q, Chen J, Yang M. Thrombopoietin Protects Cardiomyocytes from Iron-Overload Induced Oxidative Stress and Mitochondrial Injury. Cell Physiol Biochem. 2015;36:2063-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg. 2008;248:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Czajka P, Przybyłkowski A, Nowak A, Postula M, Wolska M, Mirowska-Guzel D, Czlonkowska A, Eyileten C. Antiplatelet drugs and liver fibrosis. Platelets. 2022;33:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |