Published online Jan 27, 2023. doi: 10.4240/wjgs.v15.i1.105
Peer-review started: November 1, 2022
First decision: November 21, 2022
Revised: November 30, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 27, 2023
Processing time: 78 Days and 3.2 Hours
Transarterial chemoembolization (TACE) is an effective treatment for primary hepatocellular carcinoma (PHC). Radioactive iodine therapy has been used in the treatment of advanced PHC, especially in patients with portal vein tumor thrombosis. However, data on the therapeutic effect of TACE combined with radioactive iodine therapy in PHC are scarce.
To investigate the clinical efficacy of TACE combined with radioactive iodine implantation therapy in advanced PHC via perfusion computed tomography (CT).
For this study, 98 advanced PHC patients were recruited and divided randomly into the study and control groups. Patients in the study group were treated with TACE combined radioactive iodine implantation therapy. Patients in the control group were treated with only TACE. The tumor lesion length, clinical effect, serum alpha-fetoprotein (AFP) and CT perfusion parameters were compared before and after therapy, and statistical analysis was performed.
There was no significant difference in tumor length and serum AFP between the study and control groups (P > 0.05) before treatment. However, the tumor length and serum AFP in the study group were lower than those in the control group 1 mo and 3 mo after therapy. After 3 mo of treatment, the complete and partial remission rate of the study group was 93.88%, which was significantly higher than the control group (77.55%) (P < 0.05). Before treatment, there were no significant differences between the two groups on the perfusion CT variables, including the lesion blood volume, permeability surface, blood flow, hepatic artery flow and mean transit time (P > 0.05). After 3 mo of treatment, all perfusion CT variables were lower in the study group compared to the control group (P < 0.05). The survival time of patients in the study group was 22 mo compared to 18 mo in the control group, which was significantly different [log rank (Mantel-Cox) = 4.318, P = 0.038].
TACE combined with radioactive iodine implantation in the treatment of advanced PHC can inhibit the formation of blood vessels in tumor tissue and reduce the perfusion level of tumor lesions, thereby improving the clinical efficacy and prolonging the survival time of patients.
Core Tip: This randomized controlled trial was designed to investigate the short-term clinical efficacy of transarterial chemoembolization (TACE) combined with radioactive iodine implantation in the treatment of patients with primary hepatocellular carcinoma (PHC). The results demonstrated that this treatment could inhibit the formation of blood vessels in tumor tissue and reduce the perfusion level of tumor lesions better than TACE alone. Therefore, TACE combined with radioactive ion implantation could improve the clinical efficacy and prolong the survival time of patients with PHC.
- Citation: Wang L, Huang K, Zhang Y, Wu YF, Yue ZD, Fan ZH, Liu FQ, Li YW, Dong J. Short-term efficacy assessment of transarterial chemoembolization combined with radioactive iodine therapy in primary hepatocellular carcinoma. World J Gastrointest Surg 2023; 15(1): 105-113
- URL: https://www.wjgnet.com/1948-9366/full/v15/i1/105.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i1.105
Primary hepatocellular carcinoma (PHC) is a malignant tumor with a high incidence in the Chinese population. It can develop in hepatocytes and intrahepatic bile duct cells and cause clinical symptoms[1-3]. Surgical resection is the primary treatment for early-stage hepatocellular carcinoma. However, due to an insidious onset and atypical early symptoms, more than 80% of hepatocellular carcinoma patients are diagnosed with metastasis and are ineligible for surgical treatment[1,4-6].
Transarterial chemoembolization (TACE) is the main treatment for patients with inoperable hepatocellular carcinoma. It can release chemotherapeutic drugs rapidly and maintain a high blood concentration in the organ to inhibit rapid local tumor growth. However, its long-term efficacy is inadequate[5-8]. Radioactive iodine (125I) implantation is a new means of radiotherapy with a high radiation dose and precise localization. It is also a potential treatment option for patients with PHC[8-11]. As such, this study investigated the short-term clinical efficacy of TACE combined with 125I implantation in the treatment of patients with PHC.
From January 2016 to June 2018, 98 patients with PHC, who were scheduled for treatment with interventional embolization chemotherapy, were selected as study subjects. They were randomly divided into the study group (n = 49) and the control group (n = 49). The inclusion criteria included: (1) Diagnosis of PHC according to the criteria in the Guidelines for Diagnosis and Treatment of Primary Hepatocellular Carcinoma[10,12-15]; (2) PHC confirmed by computed tomography (CT), magnetic resonance imaging and liver puncture biopsy; (3) Patients aged 19-79 years; (3) PHC patients with preoperative liver function grade A or B according to the Child-Pugh classification; (4) Stage C and D lesions according to the Barcelona Clinic Liver Cancer (BCLC) staging system[10,12-15]; (5) Preoperative assessment of survival time > 3 mo; and (6) PHC patients with survival status score 0-2 based on the Eastern Cooperative Oncology Group Performance Status[13]. The exclusion criteria included: (1) Metastatic hepatocellular carcinoma; (2) Biliary obstruction due to tumor infiltration of the bile duct; (3) Hepatic artery-portal vein fistula formation; (4) Mental illness and intellectual disability; (5) Severe renal dysfunction; and (6) Other contraindications to treatment. Study protocols were reviewed by ethics experts and implemented with presurgical informed consent from patients and families.
Treatment method: The control group was treated with TACE, which included the following chemotherapy drugs: 0.75-1.25 g of 5-fluorouracil; 80-120 mg of cisplatin; 20 mg of oxaliplatin; 80-140 mg of epirubicin; and super-liquidated iodine oil as an embolic agent. The doses of chemotherapy drugs and iodine oil were adjusted according to the tumor size and blood supply.
The study group was treated with TACE combined with 125I implantation. After 1 wk of TACE, a CT scan was performed to confirm the location, structure and specific size of the tumor and its surrounding tissues, and CT navigation and localization were performed. 125I particles were placed in the patients after CT determined that the needle tip reached the target area, and the distribution was recorded. The puncture needle was withdrawn after successful placement was confirmed by CT scan, and the site was sterilized and bandaged.
Evaluation indices: After being admitted and treated, 3 mL of venous blood was drawn to measure serum alpha-fetoprotein (AFP) by enzyme-linked immunoassay using an enzyme-labeled instrument (BD Biosciences, Franklin Lakes, NJ, United States). The CT perfusion parameters measured were blood volume (BV), permeability surface (PS), blood flow (BF), hepatic artery flow (HAF), and mean transit time (MTT).
Lesions were classified as complete remission (CR), partial remission (PR), stable disease and progressive disease according to the changes in the lesions before and after treatment. CR was defined as solid tumors, other than nodal disease, where the target lesion completely disappeared or all target nodes had shrunk to normal size for 4 wk or more. PR was defined as the sum of long diameters selected for target lesions and short diameters selected for target nodes reduced by ≥ 30% when compared to baseline for 4 wk or more. Progressive disease was defined by the sum of the target lesion diameters exceeding the reference value (smallest sum of the measured target lesion diameters) by 20% or more and the absolute value increased by ≥ 5 mm or ≥ 1 new lesions having appeared and not completely/partially in remission before the lesions grew in size or increased in number. Stable disease was defined when the volume and number of lesions were between PR and progressive disease.
SPSS 21.0 software (IBM Corp., Armonk, NY, United States) was used for statistical comparative analysis of the data. The measurement data, such as tumor length and AFP level, were expressed by mean ± SD, and the t test was adopted for comparison between groups. χ2 test was adopted for comparative analysis between groups (clinical efficacy and other count data). The Kaplan-Meier method was used to model the survival analysis. P < 0.05 was considered a statistically significant difference.
In the study group, the patients ranged from 43-years-old to 76-years-old (56.3 ± 7.2 years) and included 28 males and 21 females. Thirty patients were BCLC stage C and 19 patients were BCLC stage D. The maximum diameter of the tumor lesion was 6.31 ± 2.00 cm. There were 32 cases of Child-Pugh grade A and 17 cases of Child-Pugh grade B PHC. In the control group, the patients ranged from 40-years-old to 75-years-old (55.5 ± 6.8 years) and included 31 males and 18 females. There were 34 cases of BCLC stage C and 15 cases of BCLC stage D. The maximum diameter of tumor lesion was 6.14 ± 1.89 cm. There were 30 cases of Child-Pugh grade A and 19 cases of Child-Pugh grade B PHC. There were no statistically significant differences between these baseline characteristics of the two groups (P > 0.05).
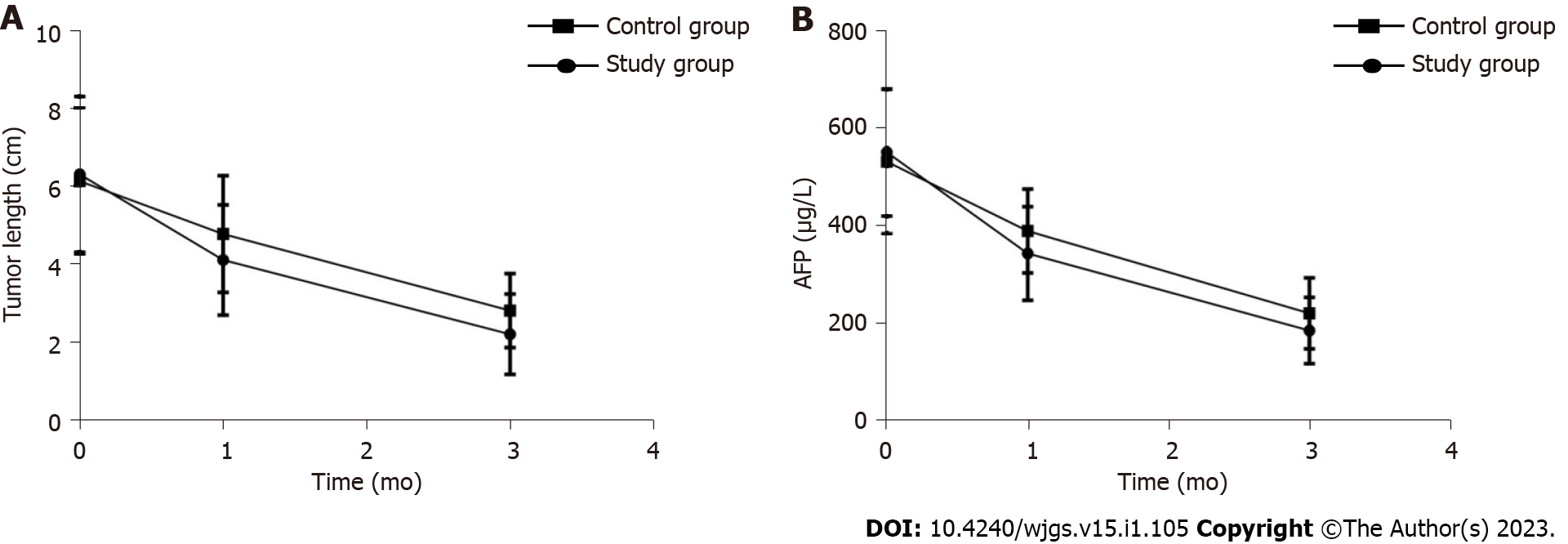
Before treatment, there was no statistically significant difference in tumor lengths between the study group and the control group (P > 0.05). The tumor lengths of the study group were significantly lower than those of the control group after 1 mo and 3 mo of treatment (P < 0.05) (Table 1 and Figure 1A).
| Group | n | Before treatment | After 1 mo of treatment | After 3 mo of treatment |
| Study group | 49 | 6.31 ± 2.00 | 4.11 ± 1.42 | 2.20 ± 1.04 |
| Control group | 49 | 6.14 ± 1.89 | 4.78 ± 1.50 | 2.81 ± 0.95 |
| t value | 0.432 | -2.271 | -3.031 | |
| P value | 0.666 | 0.025 | 0.003 |
Before treatment, the difference in serum AFP between the study group and the control group were not statistically significant (P > 0.05). After 1 mo and 3 mo of treatment, the serum AFP of the study group was lower than that of the control group (P < 0.05) (Table 2 and Figure 1B).
| Group | n | Before treatment | After 1 mo of treatment | After 3 mo of treatment |
| Study group | 49 | 549.8 ± 130.7 | 342.0 ± 96.5 | 184.3 ± 67.8 |
| Control group | 49 | 530.6 ± 148.0 | 388.5 ± 86.0 | 219.5 ± 73.0 |
| t value | 0.681 | -2.518 | -2.473 | |
| P value | 0.498 | 0.013 | 0.015 |
After 3 mo of treatment, the CR + PR rate in the study group was 93.88%, which was higher than in the control group (77.55%, P < 0.05) (Table 3).
| Group | n | CR | PR | SD | PD | CR + PR |
| Study group | 49 | 15 | 29 | 3 | 0 | 46 (93.88) |
| Control group | 49 | 9 | 29 | 11 | 0 | 38 (77.55) |
| χ2 value | 5.333 | |||||
| P value | 0.021 |
Before treatment, there was no statistically significant difference between the BV, PS, BF, HAF and MTT measurements of the lesions in the study group and the control group (P > 0.05). After 3 mo, the BV, PS, BF, HAF and MTT measurements in the study group were lower than those in the control group (P < 0.05) (Table 4).
| Perfusion parameters | Study group, n = 49 | Control group, n = 49 | Z value | P value |
| BV, mL/(100 g/min) | ||||
| Before treatment | 23.16 ± 3.29 | 22.57 ± 4.02 | 0.795 | 0.429 |
| After 3 mo of treatment | 8.40 ± 2.20 | 10.01 ± 2.54 | -3.354 | 0.001 |
| PS, mL/(100 g/min) | ||||
| Before treatment | 27.17 ± 5.48 | 26.20 ± 5.81 | 0.850 | 0.397 |
| After 3 mo of treatment | 12.64 ± 2.60 | 14.20 ± 3.13 | -2.684 | 0.009 |
| BF, mL/(100 g/min) | ||||
| Before treatment | 254.8 ± 58.1 | 247.6 ± 63.4 | 0.586 | 0.559 |
| After 3 mo of treatment | 83.0 ± 24.7 | 100.2 ± 32.5 | -2.949 | 0.004 |
| HAF, % | ||||
| Before treatment | 0.67 ± 0.17 | 0.64 ± 0.17 | 0.873 | 0.385 |
| After 3 mo of treatment | 0.24 ± 0.08 | 0.31 ± 0.10 | -3.826 | 0.000 |
| MTT, s | ||||
| Before treatment | 7.60 ± 1.63 | 7.80 ± 1.55 | -0.622 | 0.535 |
| After 3 mo of treatment | 5.20 ± 0.81 | 5.83 ± 0.96 | -3.511 | 0.001 |
The patients in both groups were followed up and observed. There was no statistically significant difference between the 3-year survival rate of patients in the study group and the control group (P > 0.05). However, the survival time of patients in the study group was 22 mo, which was significantly longer than 18 mo in the control group [log rank (Mantel-Cox) = 4.318, P = 0.038] (Table 5 and Figure 2).
| Group | n | 1 yr | 2 yr | 3 yr |
| Study group | 49 | 46 (93.88) | 34 (69.39) | 21 (42.86) |
| Control group | 49 | 44 (89.8) | 30 (61.22) | 13 (26.53) |
| χ2 value | 0.544 | 0.721 | 2.882 | |
| P value | 0.461 | 0.396 | 0.090 |
Epidemiological studies suggest that the incidence rate of liver cancer in China has reached 29/100000, with a mortality rate of 26.04/100000[3,10,12,16]. PHC is caused by various factors including hepatitis B virus infection, aflatoxin, toxic substances, alcohol, nitrite, environmental pollution, etc[7,11,15,17]. Surgery is the most effective treatment for PHC. However, due to insidious early symptoms, the time for surgical treatment is often missed[10,17-19].
TACE is the first choice of treatment for inoperable liver cancer surgery[7,20,21]. It directly delivers embolic agents, iodinated oil and chemotherapeutic drugs, which can cause tumor ischemia and hypoxia, and are injected into the hepatic artery through a catheter. This catheter also blocks the blood supply, which inhibits tumor growth and metastasis[1,7,11,18,21]. Unfortunately, as the clinical utilization of TACE increased, several disadvantages were found, including need for multiple treatments, incomplete embolizations, and increased chance of recurrence and metastasis due to vascular endothelial growth factor release. A single TACE treatment typically has a dissatisfactory long-term treatment effect.
125I implantation is a new minimally invasive interventional technique that is effective in treating lung cancer, liver cancer, and kidney cancer[8-11]. Radioactive particles, like 125I, are encased in a very small silver rod or titanium alloy and form a very small particle that contains a very strong radioactive isotope[8,9,11]. 125I particles are a type of brachytherapy. Due to the shorter range (1 cm action radius), lower capacity and weak penetration ability of brachytherapy, there is less impact on normal cells while still effectively killing tumor cells. After the radioactive particles are implanted inside the tumor, rays are continuously emitted to kill tumor cells for a certain period of time.
The results of this study showed that after 1 mo and 3 mo of treatment, the tumor lengths in the study group were lower than those in the control group, and the CR + PR rate of the study group was significantly higher than that of the control group, suggesting that TACE combined with 125I implantation has a better anti-tumor effect than TACE alone and can significantly inhibit tumor growth[8-11]. The survival time of the patients in the study group was 22 mo, which was significantly longer than 18 mo in the control group. This result suggests that TACE combined with 125I implantation can prolong the survival time of patients with PHC, and TACE and radionuclide therapy are an effective combination.
AFP is a broad-spectrum tumor marker with high sensitivity and specificity in monitoring disease changes and diagnosing PHC[1,2,10,22]. This study found that the serum AFP in the study group was lower than that in the control group after 1 mo and 3 mo of treatment, indicating that TACE combined with 125I implantation can reduce the level of AFP. This likely occurred due to the ability of 125I particles to ionize water molecules and cause direct damage to DNA. This affects the DNA repair mechanisms and can reduce AFP levels.
The CT perfusion imaging technique can effectively evaluate the hemodynamic changes of hepatocellular carcinoma tumors. This provides feedback on the micro-angiogenesis of tumor tissues and the surrounding tissues, which will direct the treatment of the cancer[12,16,23]. This study showed that after 3 mo of treatment the BV, PS, BF, HAF, and MTT measurements in the study group were lower than those in the control group, indicating that TACE combined with 125I implantation can effectively reduce perfusion levels of tumor lesions, which improves clinical efficacy. 125I particles implanted into tumor tissue release low-energy γ-rays, which exert direct killing effects, induce an inflammatory response, promote antigen-presenting cells such as macrophages to process and take up antigenic information, and promote B cells and T cells to participate in the tumor immune process[9,11]. In addition, the mammalian target of rapamycin pathway may form radiotherapy-specific proteins after several hours of irradiation. This activates lymphocytes, and the cytokine network regulatory mechanism is stimulated through the secretion of large amounts of cytokines, which activates tumor-specific immune processes to kill tumor cells. Related studies suggested that lower doses of γ-rays are more beneficial because they increase the responsiveness of lymphocytes, promote the production of antibodies, enhance the toxic effect on tumor cells, and improve the treatment effect[8,12].
This study confirmed that TACE combined with 125I implantation for the treatment of patients with advanced PHC could better inhibit the formation of blood vessels in tumor tissues and reduce the perfusion level of tumor lesions compared to TACE alone. Therefore, with the development of technology, the combined multidisciplinary treatment improves the anti-tumor effect and plays a synergistic role in prolonging the survival time of patients, which is worthy of further clinical research.
Primary hepatocellular carcinoma (PHC) is a malignant tumor with a high incidence in the Chinese population. Transarterial chemoembolization (TACE) is an effective treatment for PHC. Radioactive iodine (125I) therapy has been used in the treatment of advanced PHC, especially in patients with portal vein tumor thrombosis.
Due to insidious onset and atypical early symptoms of PHC, more than 80% of hepatocellular carcinoma patients are diagnosed with metastasis and are ineligible for surgical treatment. Therefore, it is crucial to develop effective treatment methods, such as TACE and 125I therapy. However, the data on the therapeutic effect of TACE combined with 125I therapy in PHC is scarce.
To investigate the short-term efficacy of TACE combined with 125I in patients with PHC.
Ninety-eight patients with PHC were recruited and randomly divided into the study group (n = 49, treatment with TACE and 125I therapy) and the control group (n = 49, treatment with TACE alone). The tumor length, alpha-fetoprotein (AFP) level, and computed tomography (CT) perfusion were recorded. Complete remission, partial remission (PR), stable disease and progressive disease were evaluated for all patients. Then, the efficacy was compared between the control group and the study group.
The tumor length and serum AFP level were lower in the study group compared to those in the control group after 1 mo and 3 mo of therapy. After 3 mo of treatment, the complete and PR rate in the study group was higher than in the control group (93.88% vs 77.55%, P < 0.05). Furthermore, CT perfusion parameters, including blood volume, permeability surface, blood flow, hepatic artery flow, and mean transit time, were all lower in the study group than in the control group (P < 0.05). The survival time of patients in the study group was 22 mo, which was significantly longer than 18 mo in the control group [log rank (Mantel-Cox) = 4.318, P = 0.038].
For advanced PHC patients, TACE combined with 125I implantation better inhibits the formation of blood vessels in tumor tissues and further reduces the perfusion level of tumor lesions compared to TACE alone. The combination of TACE and 125I therapy improves clinical efficacy and plays a synergistic role in prolonging the survival time of patients.
TACE combined with 125I implantation or other therapeutic methods, such as radiofrequency ablation, programmed cell death ligand 1 therapy, and immune therapy, should be investigated in advanced PHC patients in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abe K, Japan; Martins VH, Italy; Noverati N, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Chan SL, Yeo W, Mo F, Chan AWH, Koh J, Li L, Hui EP, Chong CCN, Lai PBS, Mok TSK, Yu SCH. A phase 2 study of the efficacy and biomarker on the combination of transarterial chemoembolization and axitinib in the treatment of inoperable hepatocellular carcinoma. Cancer. 2017;123:3977-3985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Ruan JY, Lin JT, Xiong Y, Chen ZZ, Chen JH, Yu HJ. Clinical Characteristics of Transarterial Chemoembolization in Treatment of Primary Hepatocellular Carcinoma Complicated With Respiratory Distress Syndrome. Technol Cancer Res Treat. 2020;19:1533033820970673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Chen PD, Chen LJ, Chang YJ. Long-Term Survival of Combined Hepatocellular-Cholangiocarcinoma: A Nationwide Study. Oncologist. 2021;26:e1774-e1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, Gasbarrini A, Pech M, Peck-Radosavljevic M, Popovič P, Rosmorduc O, Schott E, Seidensticker M, Verslype C, Sangro B, Malfertheiner P. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:1164-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 5. | Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, Liu X, Zheng L, Chen J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer. 2021;127:3782-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 6. | Ikeda M, Kudo M, Aikata H, Nagamatsu H, Ishii H, Yokosuka O, Torimura T, Morimoto M, Ikeda K, Kumada H, Sato T, Kawai I, Yamashita T, Horio H, Okusaka T; Miriplatin TACE Study Group. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: a phase III randomized trial. J Gastroenterol. 2018;53:281-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 542] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 8. | Chen L, Sun T, Kan X, Chen S, Ren Y, Cao Y, Yan L, Liang B, Xiong B, Zheng C. Transarterial chemoembolization combined with iodine-125 seed implantation for patients with hepatocellular carcinoma: a retrospective controlled study. J Int Med Res. 2020;48:300060520944309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Sun H, Zhang M, Liu R, Liu Y, Hou Y, Wu C. Endovascular implantation of (125)I seed combined with transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma. Future Oncol. 2018;14:1165-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Li S, Guo JH, Lu J, Wang C, Wu H, Wang H, Zha J, Fan R. I(125) irradiation stent for treatment of hepatocellular carcinoma with portal vein thrombosis: A meta-analysis. Cancer Radiother. 2021;25:340-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Peng S, Yang QX, Zhang T, Lu MJ, Yang G, Liu ZY, Zhang R, Zhang FJ. Lobaplatin-TACE combined with radioactive 125I seed implantation for treatment of primary hepatocellular carcinoma. Asian Pac J Cancer Prev. 2014;15:5155-5160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Yuan D, Gao Z, Zhao J, Zhang H, Wang J. (125)I seed implantation for hepatocellular carcinoma with portal vein tumor thrombus: A systematic review and meta-analysis. Brachytherapy. 2019;18:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Sahai V, Griffith KA, Beg MS, Shaib WL, Mahalingam D, Zhen DB, Deming DA, Zalupski MM. A randomized phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: BilT-01. Cancer. 2022;128:3523-3530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 14. | Fessas P, Naeem M, Pinter M, Marron TU, Szafron D, Balcar L, Saeed A, Jun T, Dharmapuri S, Gampa A, Wang Y, Khan U, Muzaffar M, Navaid M, Lee PC, Bulumulle A, Yu B, Paul S, Nimkar N, Bettinger D, Hildebrand H, Abugabal YI, Pressiani T, Personeni N, Nishida N, Kudo M, Kaseb A, Huang YH, Ang C, Pillai A, Rimassa L, Naqash AR, Sharon E, Cortellini A, Pinato DJ. Early Antibiotic Exposure Is Not Detrimental to Therapeutic Effect from Immunotherapy in Hepatocellular Carcinoma. Liver Cancer. 2021;10:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Borde T, Nezami N, Laage Gaupp F, Savic LJ, Taddei T, Jaffe A, Strazzabosco M, Lin M, Duran R, Georgiades C, Hong K, Chapiro J. Optimization of the BCLC Staging System for Locoregional Therapy for Hepatocellular Carcinoma by Using Quantitative Tumor Burden Imaging Biomarkers at MRI. Radiology. 2022;304:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Okushin K, Tateishi R, Takahashi A, Uchino K, Nakagomi R, Nakatsuka T, Minami T, Sato M, Fujishiro M, Hasegawa K, Eguchi Y, Kanto T, Kubo S, Yoshiji H, Miyata H, Izumi N, Kudo M, Koike K. Current status of primary liver cancer and decompensated cirrhosis in Japan: launch of a nationwide registry for advanced liver diseases (REAL). J Gastroenterol. 2022;57:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, Chen MS, Shi M. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol. 2022;40:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 18. | Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 19. | Chen H, Nan G, Wei D, Zhai RY, Huang M, Yang WW, Xing BC, Zhu X, Xu HF, Wang XD, Zhang XY, Zhu BR, Liu P, Cao G, Gao S, Hao CY, Yang RJ, Guo JH, Zhang X, Gao K, Wang K, Wang JF, Li ZY, Zhu LZ, Ding R, Li J, Zhao L, Shao YJ, Liu HC, Xia JL, Wang L, Kong LM, Chen ZN, Bian H. Hepatic Artery Injection of (131)I-Metuximab Combined with Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma: A Prospective Nonrandomized, Multicenter Clinical Trial. J Nucl Med. 2022;63:556-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kim D, Lee JH, Moon H, Seo M, Han H, Yoo H, Seo H, Lee J, Hong S, Kim P, Lee HJ, Chung JW, Kim H. Development and evaluation of an ultrasound-triggered microbubble combined transarterial chemoembolization (TACE) formulation on rabbit VX2 liver cancer model. Theranostics. 2021;11:79-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 497] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 22. | Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, Yang X, Huang A, Zhang X, Zhou S, Sun H, Wang Y, Ge N, Xu X, Tang Z, Lau W, Fan J, Wang J, Zhou J. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res. 2018;24:2074-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 23. | Mikhail AS, Pritchard WF, Negussie AH, Inkiyad G, Long DJ, Mauda-Havakuk M, Wakim PG, van der Sterren W, Levy EB, Lewis AL, Karanian JW, Wood BJ. Cone-Beam Computed Tomography-Based Spatial Prediction of Drug Dose After Transarterial Chemoembolization Using Radiopaque Drug-Eluting Beads in Woodchuck Hepatocellular Carcinoma. Invest Radiol. 2022;57:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |