Published online Aug 27, 2022. doi: 10.4240/wjgs.v14.i8.799
Peer-review started: February 14, 2022
First decision: April 5, 2022
Revised: May 8, 2022
Accepted: July 31, 2022
Article in press: July 31, 2022
Published online: August 27, 2022
Processing time: 191 Days and 0 Hours
Endoscopic treatment of pancreatic necrosis can be challenging and time-consuming because sticky necrotic debris is sometimes difficult to remove. The over-the-scope-grasper, a new tool that has recently become available for this purpose, might also be useful for other indications. However, clinical data on the efficacy and safety of this new device are lacking.
To evaluate the technical success and safety of the device in a multicenter setting.
The over-the-scope-grasper was used in nine selected endoscopic centers between November 2020 and October 2021 for appropriate indications. Overall, 56 procedures were included in the study. We retrospectively evaluated procedural parameters of all endoscopic interventions using a predefined questionnaire, with special respect to technical success, indications, duration of intervention, type of sedation, and complications. In the case of pancreatic necrosectomy, the access route, stent type, number of necrosis pieces removed, and clinical handling were also recorded.
A total of 56 procedures were performed, with an overall technical success rate of 98%. Most of the procedures were endoscopic pancreatic necrosectomies (33 transgastric, 4 transduodenal). In 70% of the procedures, access to the necrotic cavity was established with a lumen apposing metal stent. The technical success of pancreatic necrosectomy was 97%, with a mean of 8 pieces (range, 2-25 pieces) of necrosis removed in a mean procedure time of 59 min (range, 15-120 min). In addition, the device has been used to remove blood clots (n = 6), to clear insufficiency cavities before endoluminal vacuum therapy (n = 5), and to remove foreign bodies from the upper gastro
These first multicenter data demonstrate that the over-the-scope-grasper is a promising device for endoscopic pancreatic necrosectomy, which is also appropriate for removing foreign bodies and blood clots, or cleaning insufficiency cavities prior to endoluminal vacuum therapy.
Core Tip: The objective of our retrospective multicenter study was to evaluate the efficacy and safety of the over-the-scope-grasper, a new endoscopic grasping tool, originally designed for endoscopic pancreatic necrosectomy. A total of 56 procedures were evaluated, including 37 pancreatic necrosectomies with a technical success of 97%. In the other indications - removal of foreign bodies and blood clots or cleaning of insufficiency cavities before endoluminal vacuum therapy - the technical success rate was 100%. These first multicenter data show the over-the-scope-grasper as a promising tool for endoscopic pancreatic necrosectomy and beyond.
- Citation: Brand M, Bachmann J, Schlag C, Huegle U, Rahman I, Wedi E, Walter B, Möschler O, Sturm L, Meining A. Over-the-scope-grasper: A new tool for pancreatic necrosectomy and beyond - first multicenter experience. World J Gastrointest Surg 2022; 14(8): 799-808
- URL: https://www.wjgnet.com/1948-9366/full/v14/i8/799.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i8.799
Interventional endoscopy continues to evolve with new techniques, which allows minimally invasive treatment of gastroenterological diseases. The development and improvement of these methods have always been accompanied by the development of new, optimized equipment and tools[1-4].
In the case of endoscopic pancreatic necrosectomy, some new tools for endoscopic ultrasound (EUS) guided access to the necrotic cavity have been developed, such as lumen apposing metal stents (LAMS)[5]. Dedicated instruments for necrosectomy are scarce, although a new motorized device (EndoRotorTM) has been tested for this indication, providing encouraging data[6]. Therefore, in addition to suction and irrigation, various snares, baskets, or forceps are usually used to remove the tough and sticky necrotic tissue from the retroperitoneal cavity. Since these instruments are less suitable for this purpose, they often slip off from the necrotic tissue and necrosectomy is cumbersome and time consuming. Inci
The over-the-scope-grasper, an extra-large grasper attached to the tip of the endoscope, is a new tool developed to overcome the mentioned limitations, especially to facilitate pancreatic necrosectomy[7]. The aim of this retrospective study was to evaluate the efficacy and safety of the new device in a multicenter setting.
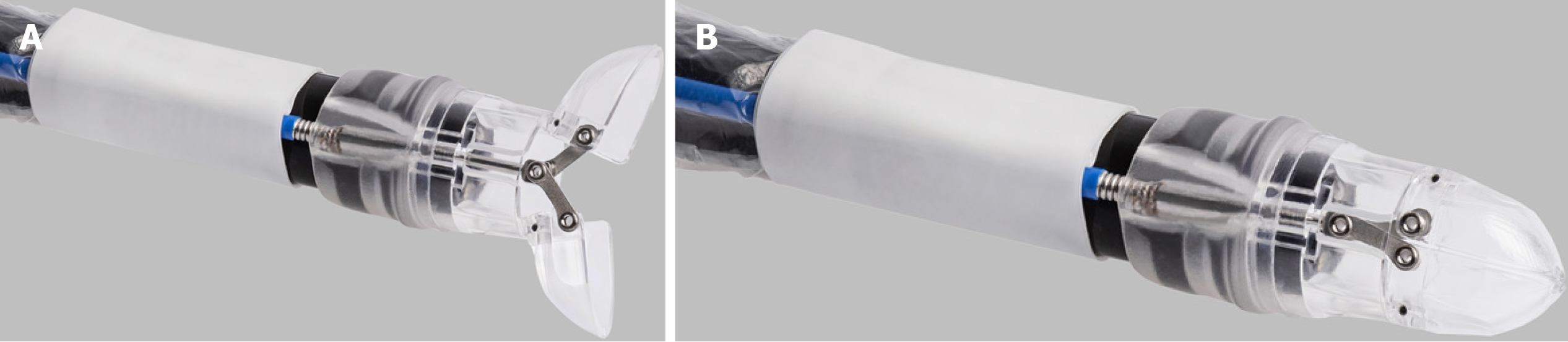
The over-the-scope-grasper (OTSG XcavatorTM - Ovesco Endoscopy AG, Tübingen, Germany) is an approved single use extra-large grasper attached to the tip of the endoscope. The device is made of transparent plastic to restrict the endoscopic view as little as possible. With a diameter of 14.7 mm (forceps closed), the grasping tool can be well inserted through large caliber LAMS. The diameter of open forceps (28.4 mm) allows grasping larger pieces of tissue or necrotic debris. The volume inside the closed grasper is just over 1 cm3. A central 1.1 mm opening at the tip of the device allows additional guidance and stiffening of the endoscope by a guidewire, if necessary. The instrument is connected to a semi-rigid spout that is fixed onto the endoscope’s tip (Figure 1). The 1650 mm flexible shaft of the instrument is fixed to the ring and connected proximally to a standard handgrip for opening and closing the grasping tool. To prevent the mucosa from becoming trapped between the endoscope and the cable, both (system and endoscope) are covered with a transparent plastic sheath.
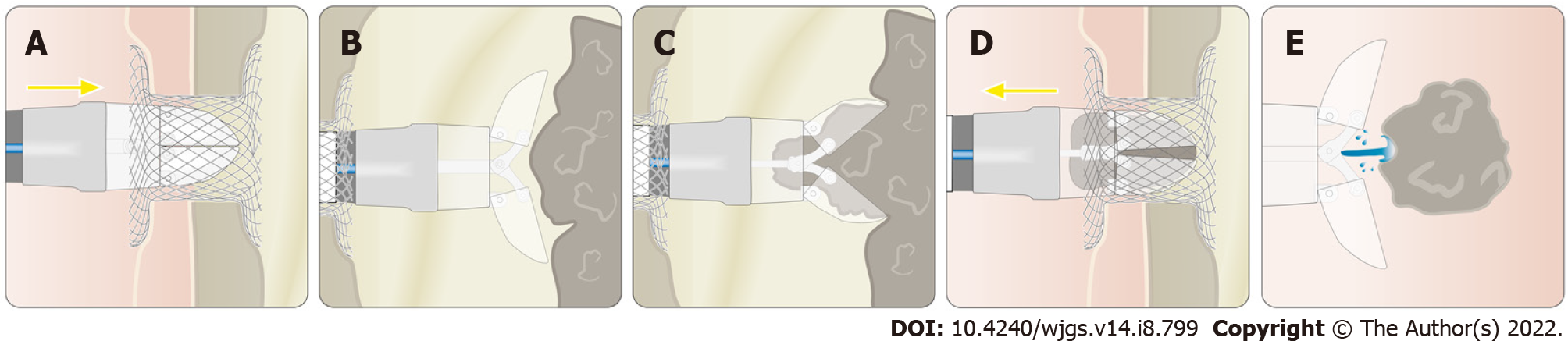
The device was applied as follows: The endoscope with the attached grasping tool was inserted into the necrosis cavity. Inside the cavity, the necrotic tissue was grasped by opening the tool and advancing the endoscope while the tissue was sucked into the grasper. After closing the device, the endoscope was withdrawn into the stomach, the grasper was opened, and the tissue was pushed out of the grasper by irrigation through the working channel (Figures 2 and 3, Video).
In this multicentric retrospective study, the over-the-scope-grasper was used in selected centers in the early phase of its market launch and 5 mo beyond (from November 2020 to October 2021). After a dedicated introduction into the system, the device was applied by experienced endoscopists for appropriate indications. Preparation and application of the system took place as previously described[7].
The main study objective was to evaluate the technical success of the device application, defined as the smooth advancement of the grasper into the target region, capturing and removing the foreign body/necrotic tissue.
Other outcome parameters were indications, duration of intervention, type of sedation, and complications. In the case of necrosectomy, the access route, stent type, number of necrosis pieces removed, and clinical handling (cleaning, additional instruments, etc.) were also considered. Complications were classified according to the American Society for Gastrointestinal Endoscopy Lexicon[8]. The overall procedure time was calculated from the first insertion to the last removal of the endoscope, while the “grasper on time" corresponds to the time period during which the grasper was attached to the endoscope.
To evaluate procedural parameters in a standardized manner, for each procedure a predefined questionnaire was retrospectively completed by the endoscopist. Data were extracted from the clinical database at each center and submitted in an anonymous form to the coordinating center, where all data were collected centrally and in an anonymized form. A complete case analysis was performed for all 56 procedures. Experience of at least four procedures was mandatory to have patients included in our prospective registry.
Data analyses were performed using Microsoft Excel (version 16.54). Due to the non-interventional study design, no between-group significance tests were performed, and only descriptive statistics were used (mean and range). Before each endoscopic procedure, the patients gave their written consent to the procedure. Retrospective analysis of clinical data was approved by the local ethics committee without requiring separate written informed consent from each patient for data analysis (Ethics Committee of the University of Würzburg).
In nine centers, the over-the-scope-grasper was used in 56 procedures (in 50 patients) performed between November 2020 and October 2021. All procedures were on-label uses. Details about the number of patients from each center are shown in the supplementary data (Supplementary Table 1).
The overall technical success of the device application was 98% (55 of 56 procedures). In one case (pancreatic necrosectomy with transduodenal access), the device could not be inserted into the necrosis cavity due to an unfavorable angle of entry.
Most of the procedures (66%, n = 37) were pancreatic necrosectomies, with preferred transgastric approach (33 transgastric vs 4 transduodenal). EUS-guided access to the necrosis cavity was achieved via LAMS (70%, n = 26) or via double pigtail stents (30%, n = 11). Three different types of SEMS were used. Almost all LAMS (25/26) had a small diameter (15 or 16 mm). The first necrosectomy session was performed in a mean of 35.7 (14 – 90) d after the beginning of the pancreatitis (Table 1).
| Number of cases | 37 |
| Number of patients | 31 |
| Sedation | 34× NAPS |
| 3× anesthesia | |
| Mean time to first necrosectomy | 35.7 d (14-90 d) |
| Mean dimension of won | 10.1 cm × 6.5 cm × 4.8 cm |
| Estimated percentage of necrosis within each collection | 57% (20%-90%) |
| Mean number of DEN session for WON resolution | 4.5 (1-13) |
| Access route/mean duration | Total (n = 37/59 min) |
| 33× transgastric (58 min) | |
| 4× transduodenal (65 min) | |
| LAMS (type, diameter) | 26× LAMS |
| 15× PlumberTM (16 mm) | |
| 8× hot AxiosTM (15 mm) | |
| 1× hot AxiosTM (20 mm) | |
| 2× SpaxusTM (16 mm) | |
| 11× double pigtail stents | |
| Additional tool | 37× irrigation pump |
| 8× snare | |
| Handling | 19× endoscope removed for cleaning |
| 18× removal of endoscope not necessary |
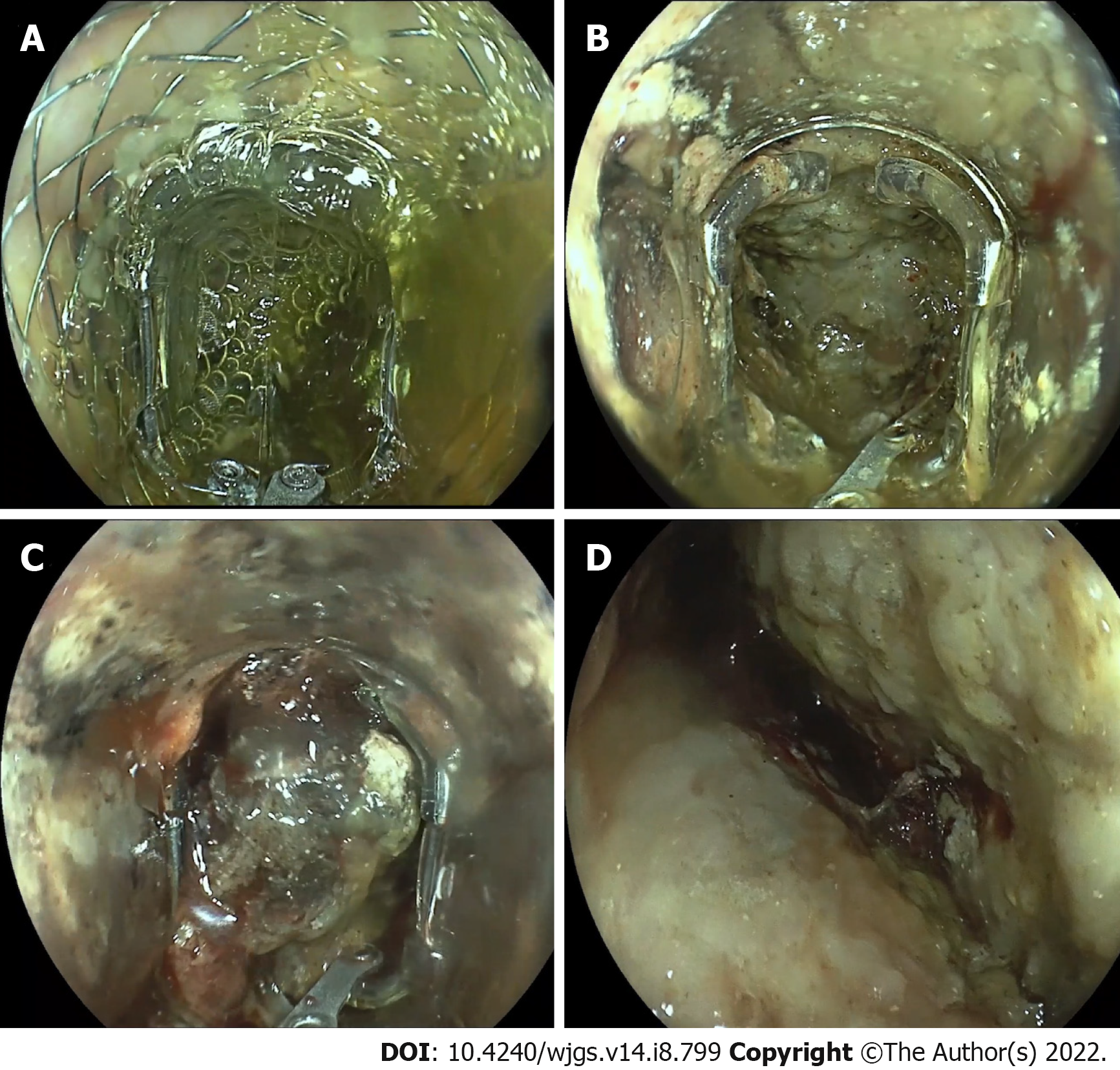
The technical success of necrosectomy was 97%, with a mean of 8 pieces (2-25 pieces) of necrosis removed. The mean overall procedure time was 59 min (range, 15-120 min), of which the grasper was used for a mean of 32 min (range, 10-70 min). In eight cases, an additional snare was used to pull the tissue into the grasping tool. In all cases, an irrigation pump was used to push the necrotic tissue out of the grasper. However, in 51%, removal of the endoscope was necessary to clean the device outside the patient. Almost all necrosectomies were performed under sedation. In three patients, the procedure was performed under general anesthesia because prolonged ventilation was required due to the severity of the pancreatitis.
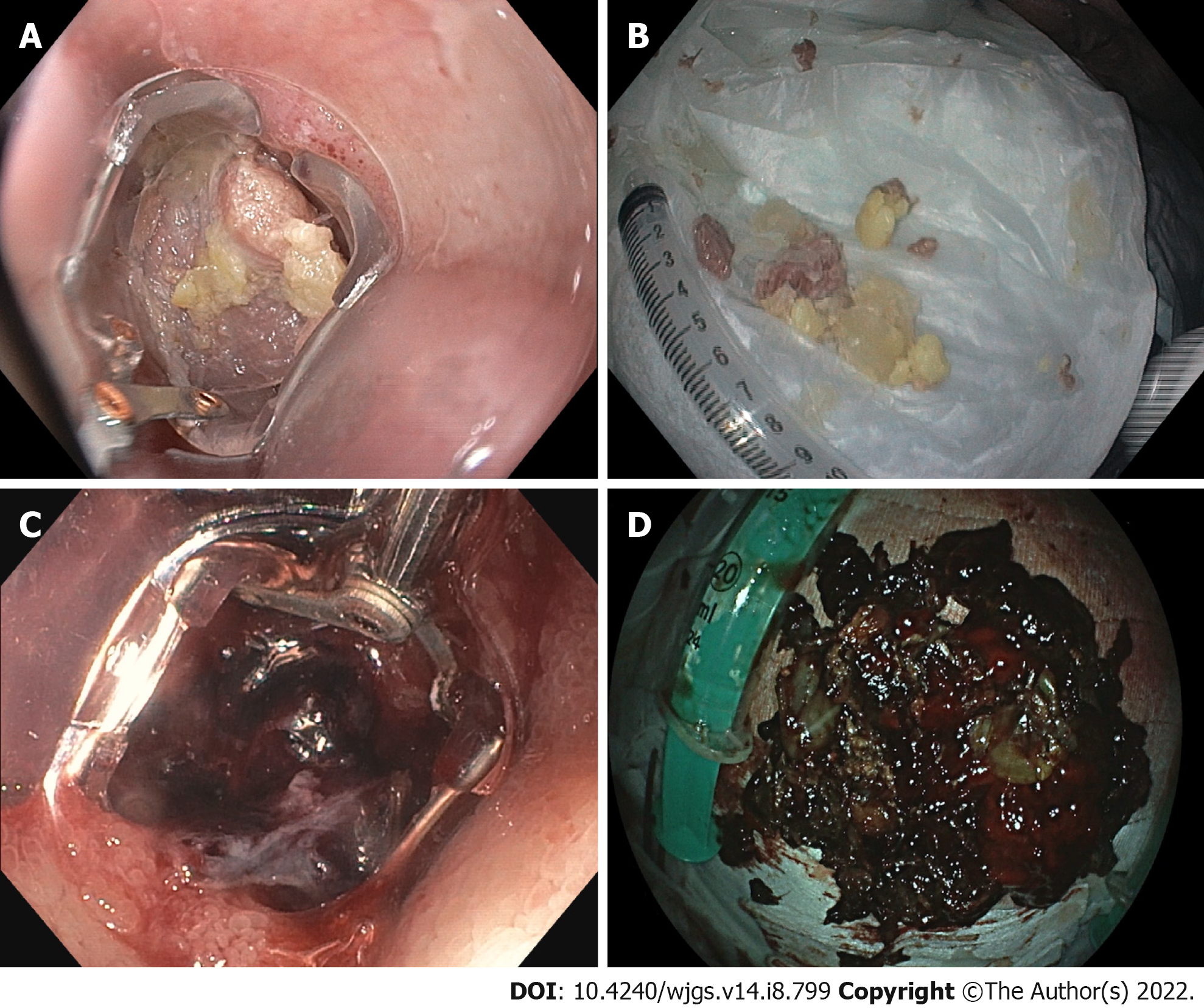
In addition to endoscopic necrosectomy, the device has been used for other appropriate indications (19 cases, Table 2). In eight patients, the tool was used to remove foreign bodies from the upper gastrointestinal tract (Figure 4). In each case, complete removal of the foreign body was achieved. In six cases, the device was used to remove large blood clots in case of upper gastrointestinal bleeding. In addition to pancreatic necrosectomy, the device was also used to clear insufficiency cavities prior to endoluminal vacuum therapy (n = 5). In all these cases, the technical success rate was 100%.
| Foreign bodies | |
| Number of cases | 8 |
| Number of patients | 8 |
| Sedation | 7× NAPS |
| 1× anesthesia | |
| Mean duration | 31.5 min (15-60 min) |
| Location | 5× esophagus |
| 3× stomach | |
| Type of foreign body | 5× meat bolus |
| 2× tablets (intoxication) | |
| 1× button cell batteries | |
| Additional tool | 1× forceps |
| 1× net | |
| Blood clots/bleeding: | |
| Number of cases | 6 |
| Number of patients | 6 |
| Sedation | 5× NAPS |
| 1× anesthesia | |
| Mean duration | 52.2 min (20-100 min) |
| Location | 4× stomach |
| 2× duodenum | |
| Additional treatment | 3× OTSC |
| 1× TTS clip | |
| 2× no treatment required | |
| Prior to endoluminal vacuum therapy: | |
| Number of cases | 5 |
| Number of patients | 5 |
| Sedation | 5× NAPS |
| Mean duration | 22 min (20-30 min) |
| Location | 5× rectum |
| Additional tool | 4× irrigation pump |
| 1× snare | |
Overall, five mild complications occurred. In three cases, dislocation of the LAMS occurred during endoscopic necrosectomy. None of these cases resulted in further problems (bleeding, etc.). In all three cases, pigtail stents were inserted instead to keep access to the necrosis open.
In one case, superficial laceration of the upper esophageal sphincter occurred during insertion of the device. In another case, minor bleeding occurred during necrosectomy, which could be treated endoscopically (no transfusion required). No moderate or severe/fatal complications were reported in any of the 56 procedures.
Direct endoscopic necrosectomy (DEN) of pancreatic necrosis is an important development in interventional endoscopy and has significantly improved the prognosis of these patients[9]. The method is well established and has been further developed in recent years, especially with new, specially shaped LAMS that facilitate EUS-guided access to the necrosis cavity[5]. To our knowledge, new devices designed for necrosectomy have not yet been developed[10-12]. Therefore, DEN is often performed by a combination of sucking debris through the working channel, removing necrotic material with a removal device, and applying irrigation. This method is often time consuming, as effective suction needs a free working channel, therefore used devices (snares, etc.) have to be introduced and removed frequently. The devices used so far also have disadvantages in necrosectomy. Frequently, snares or baskets cannot be fully opened in the narrow retroperitoneal necrosis cavity, thus grabbing of tissue can be difficult. In addition, snares often cut through the soft necrotic tissue rather than capturing it. Therefore, other systems for necrosectomy have been tested recently, such as the EndoRotorTM (Interscope Inc., Northbridge, Massachusetts, United States), a technically complex device originally developed for polypectomy and available only in a few centers[6,13,14].
The over-the-scope-grasper is a simple tool developed that can overcome several of the problems mentioned above. Since the grasper is mounted on the tip of the endoscope, the working channel remains free, allowing the necrotic tissue to be captured and aspirated simultaneously. The new device also cuts through the soft tissue, but the captured material remains in the grasper and can be removed. Furthermore, the grasping tool is easy to open even in tight space and can be even used in half-opened position. However, in foxhole-like branched necrotic cavities, the device is less applicable due to its size. Since the system can be attached to a standard gastroscope, it is quickly and easily ready for use and does not require any special additional equipment.
In our study, the new device was used in nine centers after a dedicated introduction into the system. No moderate or severe/fatal complications were reported in a total of 56 cases, underlining the ease of use and safety of the system.
Insertion of the device through the pharynx and esophagus but also entry into the necrosis cavity was usually straightforward. However, the transgastric approach to necrosis appears to be more favorable because the device significantly extends the tip of the endoscope, which may hinder manipulation within the duodenum. This should already be considered when creating the EUS access, as an unfavorable access angle (e.g., in the duodenum) can make insertion of the grasping tool impossible.
Removal of necrotic material with new device works well, even in small LAMS diameters (15 to 16 mm). However, there is little a risk of stent dislocation, especially if the grasper has captured much tissue. LAMS with a larger diameter (20 mm) may be advantageous in this situation. For effective use, a therapeutic gastroscope with a large working channel is recommended. To improve the suction performance, we recommend using a combined suction-irrigation attachment directly at the upper end of the working channel. Irrigation with a pump is also helpful to flush the necrotic pieces out of the grasper. Cleaning the grasper outside the patient is time consuming and frequent passage through the upper esophageal sphincter is an additional burden to the patient. Therefore, we recommend wetting the surface of the device with an Anti-Fog solution, to reduce the necrotic material sticking at the grasper and to improve the visibility through the transparent plastic cover.
Insufficiency cavities after gastrointestinal surgery are often treated by endoluminal vacuum therapy[15]. To achieve rapid healing of the insufficiency, the cavity is previously cleansed of pus and necrotic tissue. For this purpose, the new grasping tool can be used in the same way as for pancreatic necrosectomy if the access to the insufficiency cavity is large enough.
With respect to endoscopic removal of foreign bodies from the gastrointestinal tract, examiners experience that in case of extra-large or hard foreign bodies, the grasper may slip off the foreign body. Here, additional use of a snare might be helpful to pull the foreign body firmly into the grasper[7]. In case of small foreign bodies, the grasping tool completely encloses the foreign body, preventing it from being lost in the pharynx and eliminating the risk of aspiration. Therefore, the system is particularly suitable for removing button cell batteries and small magnets.
Last but not least, the new device appeared to be a helpful tool in the management of upper gastrointestinal bleeding. In addition to quick removal of large blood clots, the transparent plastic scoops of the grasper can be used to compress the bleeding vessel. Thus, after removal of the blood clot, the bleeding source can be compressed while an instrument (clip, injection needle, etc.) is inserted through the free working channel. After opening the device, the source of bleeding can then be treated directly, making hemostasis potentially easier and faster.
In summary, our data highlight the usefulness of this new device in several indications, but the study has several limitations. Due to the retrospective design, the study may be affected by selection bias in favor of the device. The multicenter study design with heterogeneous patient populations and operator experience may also lead to bias (e.g., referral bias). Since this is a retrospective study, a standardized approach to the necrosectomy was not possible. Therefore, only descriptive statistical methods are used and any benefit from the device cannot be quantified or statistically proven.
These first multicenter data demonstrate that the over-the-scope-grasper is a promising device for endoscopic pancreatic necrosectomy. Other appropriate indications seem to be cleaning insufficiency cavities prior to endoluminal vacuum therapy and removal of foreign bodies. In the management of upper gastrointestinal bleeding, the grasping tool has been reported to be a useful device beyond the removal of blood clots. However, prospective studies including more patients should be conducted to demonstrate the efficacy and clinical utility of the device and to gather even more information on the safety of the device.
Endoscopic treatment of pancreatic necrosis can be challenging and time consuming because sticky necrotic debris is sometimes difficult to remove. The over-the-scope-grasper, a new tool that has recently become available for this purpose, might also be useful for other indications.
To evaluate the technical success and safety of the new over-the-scope-grasper in a multicenter setting.
We retrospectively evaluated the use of the over-the-scope-grasper in nine selected endoscopic centers and aimed to investigate the technical success and safety of device use.
We retrospectively evaluated 56 procedures performed between November 2020 and October 2021. In addition to technical success and complications, we evaluated procedural parameters such as the indications, duration of the procedure, type of sedation, and, in the case of pancreatic necrosectomy, the access route, stent type, and number of pieces of necrosis removed.
The overall technical success rate was 98%. The technical success of pancreatic necrosectomy (37 cases) was 97%, with a mean of eight pieces of necrosis removed in a mean of 59 min. In addition, the device has been used to remove blood clots (n = 6) to clear insufficiency cavities before endoluminal vacuum therapy (n = 5), and to remove foreign bodies from the upper gastrointestinal tract (n = 8). In these cases, the technical success rate was 100%. No moderate or severe/fatal complications were reported.
The over-the-scope-grasper is a promising device for endoscopic pancreatic necrosectomy, which is also appropriate for removing foreign bodies and blood clots, or cleaning insufficiency cavities prior to endoluminal vacuum therapy.
Prospective studies including more patients should be conducted to demonstrate the efficacy and clinical utility of the device.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Binda C, Italy; Gunay S, Turkey; Maydeo A, India S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Yoshida N, Toyonaga T, Murakami T, Hirose R, Ogiso K, Inada Y, Rani RA, Naito Y, Kishimoto M, Ohara Y, Azuma T, Itoh Y. Efficacy of a Novel Narrow Knife with Water Jet Function for Colorectal Endoscopic Submucosal Dissection. Gastroenterol Res Pract. 2017;2017:5897369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Wedi E, Koehler P, Hochberger J, Maiss J, Milenovic S, Gromski M, Ho N, Gabor C, Baulain U, Ellenrieder V, Jung C. Endoscopic submucosal dissection with a novel high viscosity injection solution (LiftUp) in an ex vivo model: a prospective randomized study. Endosc Int Open. 2019;7:E641-E646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Knoop RF, Wedi E, Petzold G, Bremer SCB, Amanzada A, Ellenrieder V, Neesse A, Kunsch S. Endoscopic submucosal dissection with an additional working channel (ESD+): a novel technique to improve procedure time and safety of ESD. Surg Endosc. 2021;35:3506-3512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Fan C, Xu K, Huang Y, Liu S, Wang T, Wang W, Hu W, Liu L, Xing M, Yang S. Viscosity and degradation controlled injectable hydrogel for esophageal endoscopic submucosal dissection. Bioact Mater. 2021;6:1150-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, Yan L, Laique SN, Kowalski T, Loren DE, Taylor LJ, Munigala S, Bhat YM. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc. 2016;83:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 6. | Stassen PMC, de Jonge PJF, Bruno MJ, Koch AD, Trindade AJ, Benias PC, Sejpal DV, Siddiqui UD, Chapman CG, Villa E, Tharian B, Inamdar S, Hwang JH, Barakat MT, Andalib I, Gaidhane M, Sarkar A, Shahid H, Tyberg A, Binmoeller K, Watson RR, Nett A, Schlag C, Abdelhafez M, Friedrich-Rust M, Schlachterman A, Chiang AL, Loren D, Kowalski T, Kahaleh M. Safety and efficacy of a novel resection system for direct endoscopic necrosectomy of walled-off pancreas necrosis: a prospective, international, multicenter trial. Gastrointest Endosc. 2022;95:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Brand M, Hofmann N, Ho CN, Meining A. The over-the-scope grasper (OTSG). Endoscopy. 2021;53:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1852] [Article Influence: 123.5] [Reference Citation Analysis (1)] |
| 9. | Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 10. | Rimbaș M, Rizzati G, Gasbarrini A, Costamagna G, Larghi A. Endoscopic necrosectomy through a lumen-apposing metal stent resulting in perforation: is it time to develop dedicated accessories? Endoscopy. 2018;50:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Jha AK, Goenka MK, Kumar R, Suchismita A. Endotherapy for pancreatic necrosis: An update. JGH Open. 2019;3:80-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Bezmarević M, van Dijk SM, Voermans RP, van Santvoort HC, Besselink MG. Management of (Peri)Pancreatic Collections in Acute Pancreatitis. Visc Med. 2019;35:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Rizzatti G, Rimbas M, Impagnatiello M, Gasbarrini A, Costamagna G, Larghi A. Endorotor-Based Endoscopic Necrosectomy as a Rescue or Primary Treatment of Complicated Walled-off Pancreatic Necrosis. A Case Series. J Gastrointestin Liver Dis. 2020;29:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Kaul V, Diehl D, Enslin S, Infantolino A, Tofani C, Bittner K, Tariq R, Aslam R, Ayub K. Safety and efficacy of a novel powered endoscopic debridement tissue resection device for management of difficult colon and foregut lesions: first multicenter U.S. experience. Gastrointest Endosc. 2021;93:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Loske G. Endoscopic negative pressure therapy of the upper gastrointestinal tract. Chirurg. 2019;90:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |