Published online Aug 27, 2022. doi: 10.4240/wjgs.v14.i8.743
Peer-review started: April 3, 2022
First decision: June 2, 2022
Revised: June 24, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: August 27, 2022
Processing time: 143 Days and 7.8 Hours
Previous studies reported hypertension remission after gastrectomy for gastric cancer patients, and the remission rate was 11.1%-93.8%. We have reported the factors of hypertension remission previously, however, the follow-up time was six months. It is necessary to identify risk factors for hypertension for a relatively longer follow-up time.
To analyze the predictive factors for hypertension remission one year after gastrectomy of gastric cancer patients and to construct a risk model for hyper
We retrospectively collected the medical information of patients with concurrent gastric cancer and hypertension in a single clinical center from January 2013 to December 2020. Univariate and multivariate logistic regression of hypertension remission were conducted, and a nomogram model was established.
A total of 209 patients with concurrent gastric cancer and hypertension were included in the current study. There were 108 patients in the remission group and 101 patients in the non-remission group. The hypertension remission rate was 51.7% one year after gastrectomy. The remission group had younger aged patients (P = 0.001), larger weight loss (P = 0.001), lower portion of coronary heart disease (P = 0.017), higher portion of II-degree hypertension (P = 0.033) and higher portion of total gastrectomy (P = 0.008) than the non-remission group. Younger age (P = 0.011, odds ratio = 0.955, 95%CI: 0.922-0.990), higher weight loss (P = 0.019, odds ratio = 0.937, 95%CI: 0.887-0.989) and total gastrectomy (P = 0.039, odds ratio = 2.091, 95%CI: 1.037-4.216) were independent predictors for hypertension remission. The concordance index of the model was 0.769 and the calibration curve suggested great agreement. Furthermore, decision curve analysis showed that the model was clinically useful.
Younger age, higher weight loss and total gastrectomy were independent predictors for hyper
Core Tip: The purpose of the current study is to analyze the predictive factors for hypertension remission one year after gastrectomy of gastric cancer patients and to construct a risk model for hypertension remission. We found that younger age, higher weight loss and total gastrectomy were independent predictors for hypertension remission after gastrectomy for gastric cancer patients. The nomogram could visually display these results.
- Citation: Kang B, Liu XY, Cheng YX, Tao W, Peng D. Factors associated with hypertension remission after gastrectomy for gastric cancer patients. World J Gastrointest Surg 2022; 14(8): 743-753
- URL: https://www.wjgnet.com/1948-9366/full/v14/i8/743.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i8.743
Gastric cancer is the fifth most common cancer and the third most common cause of cancer-related death[1,2]. In China, gastric cancer patients account for about approximately 50% of the world’s population[3]. Despite improvements in treatment strategies, radical gastrectomy remains the cornerstone of gastric cancer treatment[4-6].
Hypertension is a major risk factor for cardiovascular disease and an important cause of morbidity and mortality[7,8]. It is estimated that, in 2025, hypertensive patients will account for nearly one-third of adults worldwide[9,10]. In China, the prevalence of hypertension has increased significantly because of urbanization, economic growth, and the aging population[11]. A total of 26.6%-33.6% of the general population is diagnosed with hypertension, resulting in an estimated 23 million deaths per year[12].
Obese patients could experience hypertension remission after bariatric surgery[13,14]. Previous studies reported hypertension remission after gastrectomy for gastric cancer patients, and the remission rate was 11.1%-93.8%[15-20]. We have reported the factors of hypertension remission previously, however, the follow-up time was six months[15].
It is necessary to identify risk factors for hypertension for a relatively longer follow-up time. Therefore, the purpose of the current study was to analyze the predictive factors for hypertension remission one year after gastrectomy in gastric cancer patients; moreover, we constructed a nomogram to visually display these associated factors.
We retrospectively collected the medical information of patients with concurrent gastric cancer and hypertension in a single clinical center from January 2013 to December 2020. This study was carried out in accordance with the World Medical Association Declaration of Helsinki. Ethical approval was obtained from the Institutional Ethics Committee of the local hospital (2022-133-2), and informed consent was obtained from all patients.
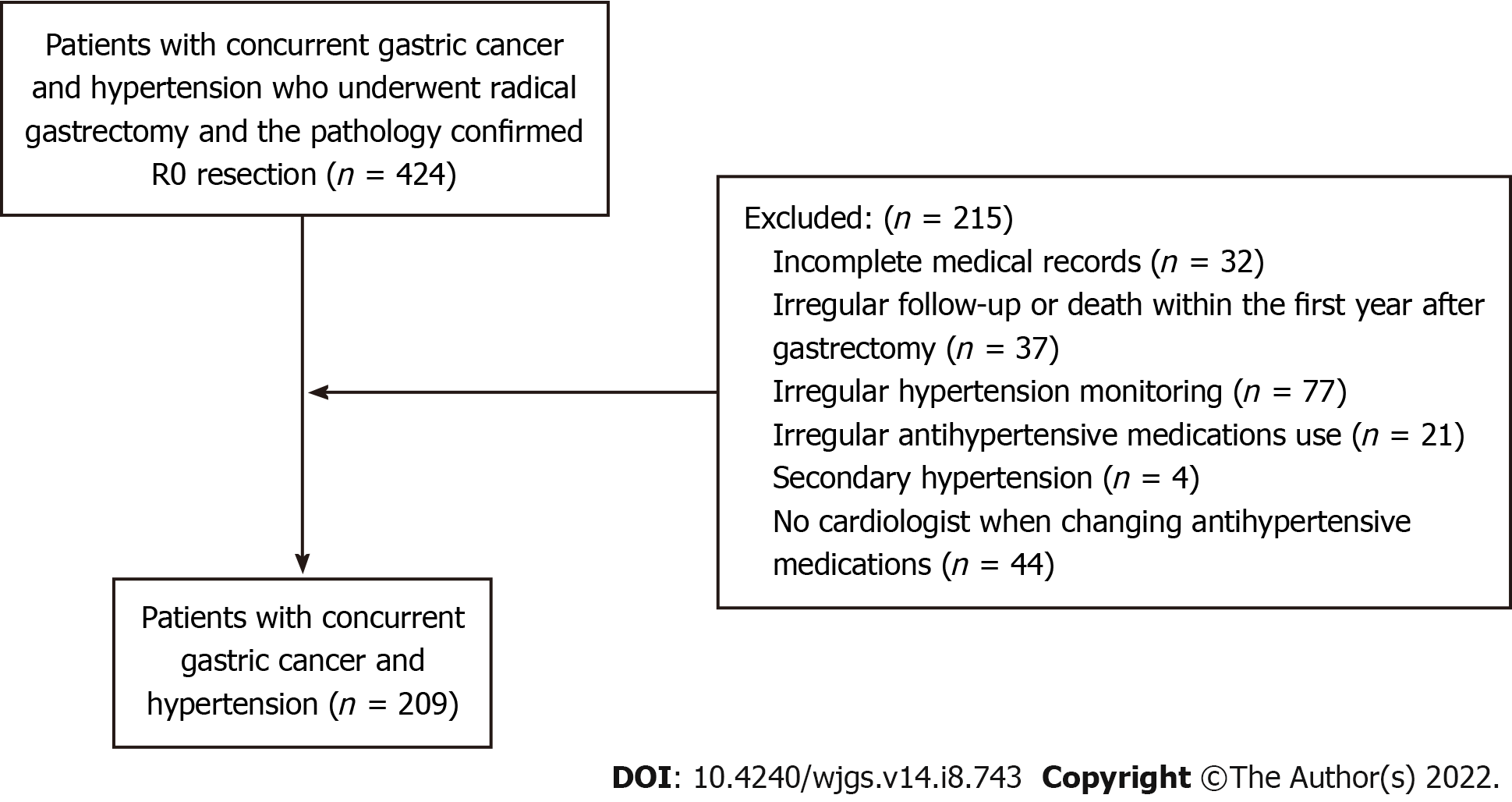
The analysis of this study was restricted to patients who: (1) Had concurrent gastric cancer and hypertension who underwent radical gastrectomy; and (2) had a pathology confirming R0 resection. On the other hand, those excluded had: (1) Incomplete medical records (n = 32); (2) Irregular follow-up or death within the first year after gastrectomy (n = 37); (3) Irregular hypertension monitoring (n = 77); (4) Irregular antihypertensive medications use (n = 21); (5) Secondary hypertension (n = 4); and (6) had no cardiologist when changing antihypertensive medications (n = 44). Finally, a total of 209 patients with concurrent gastric cancer and hypertension were included in this study, and the flow chart of patient selection is shown in Figure 1.
Hypertension (HTN) was defined as follows: the average systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg at least three times on different days. Hypertension was classified into I, II and III degrees. Degree I HTN was an average SBP was between 140 and159 mmHg or an average DBP between 90 and 99 mmHg; the degree II-HTN was as follows: the average SBP was between 160 and 179 mmHg or the average DBP was between 100 and 109 mmHg; and the degree III was as follows: the average SBP ≥ 180 mmHg or the average DBP ≥ 110 mmHg.
Hypertension remission was divided into two groups: the remission group and the non-remission group. The remission group was defined as follows: (1) SBP and/or DBP decreased with the same antihypertensive medications; (2) The antihypertensive medications were reduced or ceased. The non-remission group was defined as the antihypertensive medications that remained the same or increased. Weight loss was defined as: weight (one year after gastrectomy) minus preoperative weight.
Subtotal gastrectomy or total gastrectomy plus D2 Lymph node dissection was conducted according to the guidelines of the 2010 Japanese gastric cancer treatment guidelines (ver. 3)[21]. The gastrectomy type was based on the location and size of the tumor and the reconstruction methods included the Billroth I, Billroth II or Roux-en-Y methods. Patients were regularly followed up every three months for the first three years and every six months for the following two years.
Patients’ information was collected through the inpatient system, outpatient system and telephone interview. The collected information was as follows: age, sex, preoperative body mass index, preoperative weight, preoperative albumin, pre-operative hemoglobin, one-year postoperative weight, weight loss, smoking, drinking, type 2 diabetes mellitus (T2DM), coronary heart disease (CHD), hypertension classification, neoadjuvant chemotherapy, surgical techniques (subtotal gastrectomy or total gastrectomy), reconstruction methods, tumor stage, tumor size, hypertension duration and hypertension remission.
The continuous data are shown as the mean ± SD and the categorical data are shown as n (%). Chi-square tests, Fisher’s exact test or independent samples t tests were used to compare the difference between the remission group and the non-remission group.
Parameters were analyzed by univariate regression analysis for potential predictors of hypertension remission. Multivariate regression analysis was used to identify independent risk factors for hypertension remission. Then, a nomogram was generated. Bootstraps with 300 resamples were performed for internal validation. The predictive performance was assessed by Harrell’s concordance index (C-index). A calibration curve was plotted to evaluate the calibration of the nomogram. Decision curve analysis (DCA) was performed to evaluate the clinical usefulness of the nomogram.
Data were analyzed using SPSS (version 22.0) statistical software and R software (version 3.6.1). A bilateral P value of < 0.05 was considered statistically significant.
A total of 209 patients with concurrent gastric cancer and hypertension were included in the current study according to the inclusion and exclusion criteria (Figure 1). There were 108 patients in the remission group and 101 patients in the non-remission group. The hypertension remission rate was 51.7%.
We compared the baseline information and surgical information of the two groups. The remission group had younger patients (63.6 ± 8.7 years vs 67.4 ± 8.0 years, P = 0.001), larger weight loss (-8.2 ± 6.7 kg vs -5.6 ± 4.6 kg, P = 0.001), lower portion of CHD (8.3% vs 19.8%, P = 0.017), higher portion of II-degree hypertension (47.2% vs 31.7%, P = 0.033) and higher portion of total gastrectomy (31.5% vs 15.8%, P = 0.008) than the non-remission group. There was no significant difference in terms of other information (P > 0.05) (Table 1).
| Characteristics | Remission (n = 108) | Non-remission (n = 101) | P value |
| Age (yr) | 63.6 ± 8.7 | 67.4 ± 8.0 | 0.001b |
| Sex | 0.420 | ||
| Male | 70 (64.8) | 60 (59.4) | |
| Female | 38 (35.2) | 41 (40.6) | |
| Pre-operative BMI (kg/m2) | 23.4 ± 3.0 | 23.3 ± 32.9 | 0.770 |
| Pre-operative weight (kg) | 63.1 ± 10.0 | 61.9 ± 10.1 | 0.366 |
| Pre-operative albumin (g/L) | 39.5 ± 5.9 | 39.4 ± 5.3 | 0.902 |
| Pre-operative hemoglobin (g/L) | 117.9 ± 28.5 | 118.3 ± 24.4 | 0.922 |
| Weight loss (kg) | -8.2 ± 6.7 | -5.6 ± 4.6 | 0.001b |
| Smoking | 39 (36.1) | 41 (40.6) | 0.923 |
| Drinking | 44 (40.7) | 31 (30.7) | 0.130 |
| T2DM | 21 (19.4) | 19 (18.8) | 0.908 |
| CHD | 9 (8.3) | 20 (19.8) | 0.017a |
| Hypertension classification | 0.033a | ||
| I | 27 (25.0) | 25 (24.8) | |
| II | 51 (47.2) | 32 (31.7) | |
| III | 30 (27.8) | 44 (43.6) | |
| Neoadjuvant chemotherapy | 7 (6.5) | 7 (6.9) | 0.897 |
| Surgical techniques | 0.008b | ||
| Subtotal gastrectomy | 74 (68.5) | 85 (84.2) | |
| Total gastrectomy | 34 (31.5) | 16 (15.8) | |
| Reconstruction methods | 0.771 | ||
| B-I | 37 (34.3) | 36 (35.6) | |
| B-II | 15 (13.9) | 17 (16.8) | |
| R-Y | 56 (51.8) | 48 (47.6) | |
| Tumor stage | 0.174 | ||
| I | 37 (34.3) | 36 (35.6) | |
| II | 15 (13.9) | 17 (16.8) | |
| III | 56 (51.8) | 48 (47.6) | |
| Tumor size | 0.556 | ||
| < 5 cm | 92 (85.2) | 83 (82.2) | |
| ≥ 5 cm | 16 (14.8) | 18 (17.8) | |
| Hypertension duration | 0.346 | ||
| ≤ 5 yr | 53 (49.1) | 43 (42.6) | |
| > 5 yr | 55 (50.9) | 58 (57.4) |
Univariate analyses were conducted to identify potential risk factors for hypertension remission. In univariate logistic regression, younger age (P = 0.002, odds ratio = 0.947, 95%CI: 0.916-0.980) and higher weight loss (P = 0.002, odds ratio = 0.922, 95%CI: 0.875-0.971), CHD (P = 0.020, odds ratio = 0.368, 95%CI: 0.159-0.853) and total gastrectomy (P = 0.009, odds ratio = 2.441, 95%CI: 1.248-4.775) were statistically significant (Table 2).
| Risk factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 0.947 (0.916-0.980) | 0.002b | 0.955 (0.922-0.990) | 0.011a |
| Sex (male/female) | 0.794 (0.454-1.391) | 0.421 | ||
| Pre-operative BMI (kg/m2) | 1.014 (0.925-1.112) | 0.769 | ||
| Pre-operative weight (kg) | 1.013 (0.986-1.040) | 0.365 | ||
| Pre-operative albumin (g/L) | 1.003 (0.956-1.053) | 0.902 | ||
| Pre-operative hemoglobin (g/L) | 0.999 (0.989-1.010) | 0.922 | ||
| Weight loss (kg) | 0.922 (0.875-0.971) | 0.002b | 0.937 (0.887-0.989) | 0.019a |
| Smoking (yes/no) | 0.973 (0.557-1.700) | 0.923 | ||
| Drinking (yes/no) | 1.552 (0.877-2.748) | 0.131 | ||
| T2DM (yes/no) | 1.042 (0.523-2.077) | 0.908 | ||
| CHD (yes/no) | 0.368 (0.159-0.853) | 0.020a | 0.517 (0.212-1.265) | 0.148 |
| Hypertension classification (III/II/I) | 0.761 (0.533-1.087) | 0.133 | ||
| Neoadjuvant chemotherapy (yes/no) | 0.931 (0.315-2.753) | 0.897 | ||
| Surgical techniques (Total gastrectomy/subtotal gastrectomy) | 2.441 (1.248-4.775) | 0.009b | 2.091 (1.037-4.216) | 0.039a |
| Reconstruction methods (R-Y/B-II/B-I) | 1.318 (0.968-1.794) | 0.080 | ||
| Tumor stage (III/II/I) | 1.072 (0.795-1.445) | 0.650 | ||
| Tumor size (≥ 5 cm/< 5 cm) | 0.802 (0.384-1.674) | 0.557 | ||
| Hypertension duration (> 5 yr/≤ 5 yr) | 0.769 (0.446-1.328) | 0.346 | ||
Multivariate logistic regression was conducted to identify independent risk factors. In multivariate logistic regression, younger age (P = 0.011, odds ratio = 0.955, 95%CI: 0.922-0.990) and higher weight loss (P = 0.019, odds ratio = 0.937, 95%CI: 0.887-0.989) and total gastrectomy (P = 0.039, odds ratio = 2.091, 95%CI: 1.037-4.216) were independent predictors (Table 2).
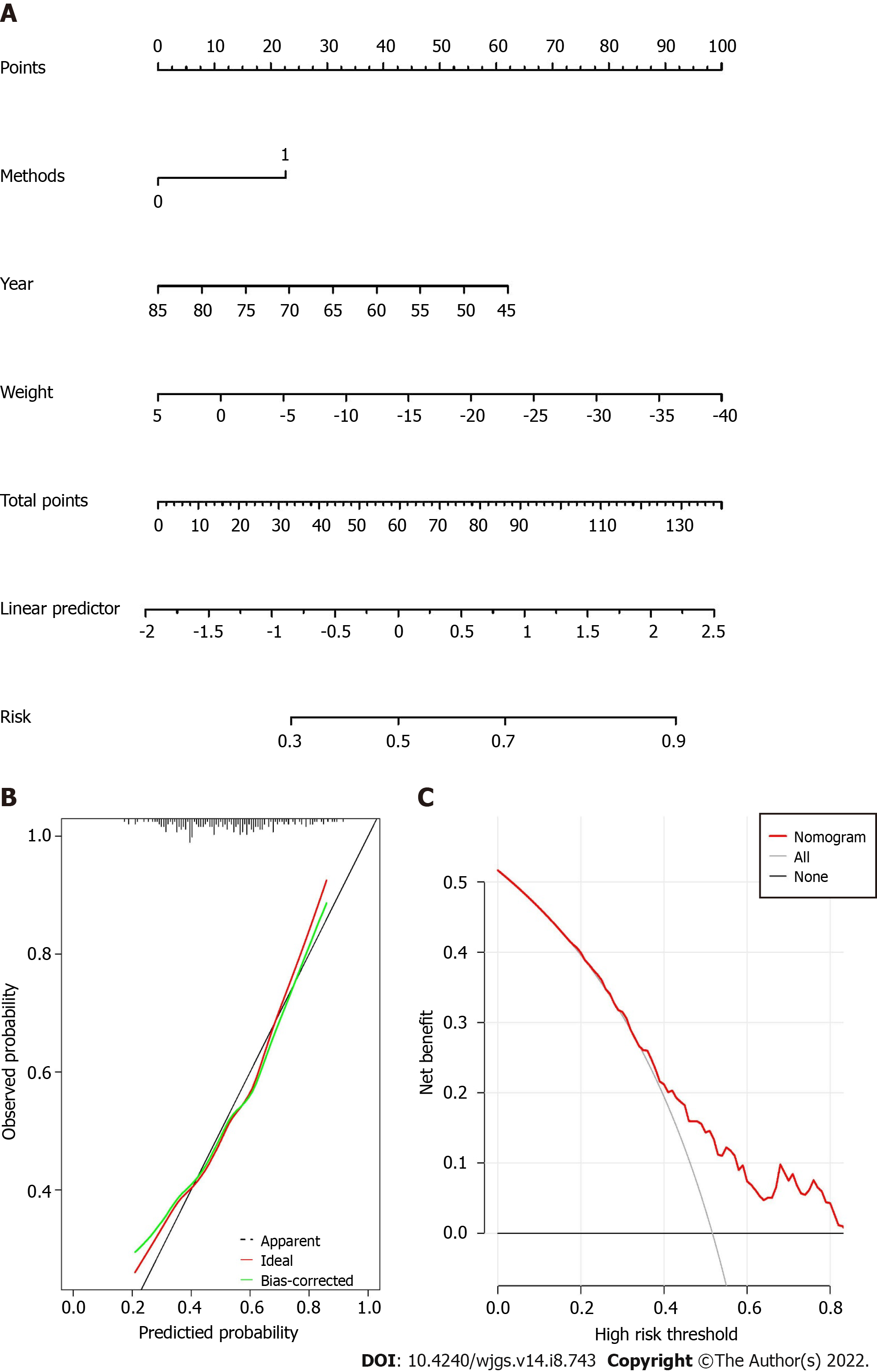
The nomogram was built as shown in Figure 2A. The score of each variable could be calculated by drawing vertical line upward to the point scale. The risk factors for hypertension remission could be calculated by summing the total points.
The C-index value of the nomogram was 0.769. The calibration curve of the nomogram suggested great agreement (Figure 2B).
The DCA for the nomogram is shown in Figure 2C, which indicated that when the threshold probability was larger than 0.33, the nomogram might add more benefit than the treat-all or treat-none strategies.
A total of 209 patients with concurrent gastric cancer and hypertension were included in the current study and the hypertension remission rate was 51.7% one year after gastrectomy. Younger age, higher weight loss and total gastrectomy were independent predictors for hypertension remission. The C-index of the model was 0.769 and the calibration curve suggested great agreement. Furthermore, decision curve analysis showed that the model was clinically useful.
Previous studies reported that patients with concurrent colorectal cancer and hypertension and/or T2DM could experience hypertension or T2DM remission[22,23]. In gastric cancer patients, remission of T2DM and hypertension was also observed after gastrectomy[20,24-28]. Onco-metabolic surgery was proposed because of the observation of hypertension and/or T2DM remission after gastrectomy for gastric cancer patients. Based on the current findings of hypertension and/or T2DM remission after gastric cancer and colorectal cancer surgery, we thought the onco-metabolic surgery might expand to gastrointestinal cancer surgery.
In terms of patients with concurrent gastric cancer and hypertension, the remission rate was 11.1%-93.8%[15-20]. We summarized these findings in Table 3. We previously reported that age and the surgical techniques used can predict the remission of hypertension six months after gastrectomy[15], however, the follow-up time was only 6 mo. Kim et al[16] reported that in early gastric cancer survivors with hypertension, gastrectomy resulted in better blood pressure control, which might be due to the gastrectomy itself, beyond weight loss. Therefore, it was necessary to identify exact risk factors for hypertension remission.
| Ref. | Year | Country | Sample size | Remission rate | Summary |
| Peng et al[15] | 2020 | China | 143 | 55.3% | Age and the surgical techniques used can predict the remission of hypertension 6 mo after gastrectomy. However, the follow-up time was only 6 mo |
| Kim et al[16] | 2019 | South Korea | 66 | 57.6% | In early gastric cancer survivors with hypertension, gastrectomy resulted in better blood pressure control, which may be due to the gastrectomy itself, beyond weight loss |
| Lee et al[17] | 2015 | South Korea | 351 | 11.1% | The results came from a nationwide cohort study with limited baseline information, no further information could be found in terms of risk factors for hypertension remission |
| Park et al[18] | 2020 | South Korea | 33 | 42.4% | The study focused on the comparison between the long-limb R-Y reconstruction between conventional R-Y reconstruction, the information for hypertension remission was limited |
| Wang et al[19] | 2020 | China | 16 | 93.8% | Elaborate parameters of endocrine hormone change, however, the sample size was too small |
The molecular mechanism of hypertension remission after gastrectomy for gastric cancer patients is unclear, but it might be related to bariatric surgery for obese patients[29,30]. There were many possible molecular mechanisms of hypertension remission for obese patients after bariatric surgery: elevated activation of the renin–angiotensin–aldosterone system in obese patients might normalize after surgery[31] and the improvement of gastrointestinal gut hormone levels and insulin resistance after surgery[32], a possible effect of these gut hormones on the sympathetic nervous system[33], adipokines and other inflammatory cytokines would lead to hypertension recovery[34]. Thus, similar to bariatric surgery, multiple factors might work together for hypertension remission after gastric cancer surgery[35-37]. Furthermore, it was reported that early hypertension remission might be related to endocrine hormones and late hypertension remission might be related to neurohumoral regulation[36,37].
For younger patients, vascular elasticity might contribute to the higher rate of hypertension remission[15]. Total gastrectomy had a wider extent than subtotal gastrectomy, and a larger volume of residual stomach in subtotal gastrectomy allowed more food than total gastrectomy, thus total gastrectomy might be associated with higher remission of hypertension[16]. The purpose of this study was different from previous studies reporting the remission of hypertension after gastrectomy for gastric cancer patients. Lee et al[17] found no risk factors for hypertension remission. Park et al[18] focused on the comparison between long-limb R-Y reconstruction and conventional R-Y reconstruction. The information for hypertension remission was limited. Another study from China focused on the elaborate parameters of endocrine hormone change, however, the sample size was too small[19]. In this study, we identified three independent predictive factors including younger age, total gastrectomy and higher weight loss, which led to hypertension remission after gastrectomy. Weight loss was an important factor for hypertension control, which was related to lifestyle changes that promoted hypertension remission[38-40].
Some limitations existed in this study. First, this was a retrospective single center study, which might cause selection bias and some detailed data were lost; Second, the follow-up time was relatively short; Third, we only established internal validation, and external validation is needed in the future; Fourth, some blood parameters including leptin, adiponectin, renin, angiotensin II and aldosterone are needed in the following experiments. Therefore, multi-center, large-sample studies with more parameters are needed in future studies to elaborately analyze the factors of hypertension remission.
In conclusion, younger age, higher weight loss and total gastrectomy were independent predictors for hypertension remission after gastrectomy for gastric cancer patients one year after surgery. The nomogram could visually display these results. Our study predicted that younger hypertension patients who underwent gastrectomy for gastric cancer might decrease anti-hypertensive medication and relieve hypertension-related comorbidities.
Previous studies reported hypertension remission after gastrectomy for gastric cancer patients, and the remission rate was 11.1%-93.8%. We have reported the factors of hypertension remission previously, however, the follow-up time was six months. It is necessary to identify risk factors for hypertension for a relatively longer follow-up time.
The purpose of the current study was to analyze the predictive factors for hypertension remission one year after gastrectomy in gastric cancer patients.
The purpose of the current study is to analyze the predictive factors for hypertension remission one year after gastrectomy of gastric cancer patients and to construct a risk model for hypertension remission.
Univariate and multivariate logistic regression of hypertension remission were conducted, and a nomogram model was established.
A total of 209 patients with concurrent gastric cancer and hypertension were included in the current study and the hypertension remission rate was 51.7% one year after gastrectomy. Younger age, higher weight loss and total gastrectomy were independent predictors for hypertension remission. The C-index of the model was 0.769 and the calibration curve suggested great agreement. Furthermore, decision curve analysis showed that the model was clinically useful.
Younger age, higher weight loss and total gastrectomy were independent predictors for hypertension remission after gastrectomy for gastric cancer patients. The nomogram could visually display these results.
Our study predicted that younger hypertension patients who underwent gastrectomy for gastric cancer might decrease anti-hypertensive medication and relieve hypertension-related comorbidities.
The authors are grateful for all the colleagues who helped in the preparation of this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Brisinda G, Italy; de Melo FF, Brazil; Sumi K, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64698] [Article Influence: 16174.5] [Reference Citation Analysis (177)] |
| 2. | Gao K, Wu J. National trend of gastric cancer mortality in China (2003-2015): a population-based study. Cancer Commun (Lond). 2019;39:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 4. | Matsui R, Inaki N, Tsuji T. Impact of diabetes mellitus on long-term prognosis after gastrectomy for advanced gastric cancer: a propensity score matching analysis. Surg Today. 2022;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Youn SI, Son SY, Lee K, Won Y, Min S, Park YS, Ahn SH, Kim HH. Quality of life after laparoscopic sentinel node navigation surgery in early gastric cancer: a single-center cohort study. Gastric Cancer. 2021;24:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 463] [Article Influence: 115.8] [Reference Citation Analysis (1)] |
| 7. | Carey RM, Whelton PK. New findings bearing on the prevention, detection and management of high blood pressure. Curr Opin Cardiol. 2021;36:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Filippini T, Malavolti M, Whelton PK, Vinceti M. Sodium Intake and Risk of Hypertension: A Systematic Review and Dose-Response Meta-analysis of Observational Cohort Studies. Curr Hypertens Rep. 2022;24:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2858] [Cited by in RCA: 3859] [Article Influence: 193.0] [Reference Citation Analysis (1)] |
| 10. | Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 2386] [Article Influence: 265.1] [Reference Citation Analysis (1)] |
| 11. | Wang J, Zhang L, Wang F, Liu L, Wang H; China National Survey of Chronic Kidney Disease Working Group. Prevalence, awareness, treatment, and control of hypertension in China: results from a national survey. Am J Hypertens. 2014;27:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 305] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 12. | Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Bärnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catalá-López F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mmHg, 1990-2015. JAMA. 2017;317:165-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1468] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 13. | Nudotor RD, Canner JK, Haut ER, Prokopowicz GP, Steele KE. Comparing remission and recurrence of hypertension after bariatric surgery: vertical sleeve gastrectomy versus Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2021;17:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Neff KJ, Baud G, Raverdy V, Caiazzo R, Verkindt H, Noel C, le Roux CW, Pattou F. Renal Function and Remission of Hypertension After Bariatric Surgery: a 5-Year Prospective Cohort Study. Obes Surg. 2017;27:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Peng D, Cheng YX, Tao W, Zou YY, Qian K, Zhang W. Onco-Metabolic Surgery: A Combined Approach to Gastric Cancer and Hypertension. Cancer Manag Res. 2020;12:7867-7873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Kim HJ, Cho EJ, Kwak MH, Eom BW, Yoon HM, Cho SJ, Lee JY, Kim CG, Ryu KW, Kim YW, Choi IJ. Effect of gastrectomy on blood pressure in early gastric cancer survivors with hypertension. Support Care Cancer. 2019;27:2237-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Lee EK, Kim SY, Lee YJ, Kwak MH, Kim HJ, Choi IJ, Cho SJ, Kim YW, Lee JY, Kim CG, Yoon HM, Eom BW, Kong SY, Yoo MK, Park JH, Ryu KW. Improvement of diabetes and hypertension after gastrectomy: a nationwide cohort study. World J Gastroenterol. 2015;21:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Park YS, Park DJ, Kim KH, Lee Y, Park KB, Min SH, Ahn SH, Kim HH. Nutritional safety of oncometabolic surgery for early gastric cancer patients: a prospective single-arm pilot study using a historical control group for comparison. Surg Endosc. 2020;34:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Yang W, Zhu Y, Jin N, Wu W, Zheng F. Decreased hypertension in non-obese non-diabetic gastric cancer patients after gastrectomy. Asian J Surg. 2020;43:926-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Cheng YX, Peng D, Tao W, Zhang W. Effect of oncometabolic surgery on gastric cancer: The remission of hypertension, type 2 diabetes mellitus, and beyond. World J Gastrointest Oncol. 2021;13:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 21. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 22. | Peng D, Liu XY, Cheng YX, Tao W, Cheng Y. Improvement of Diabetes Mellitus After Colorectal Cancer Surgery: A Retrospective Study of Predictive Factors For Type 2 Diabetes Mellitus Remission and Overall Survival. Front Oncol. 2021;11:694997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Cheng YX, Tao W, Liu XY, Yuan C, Zhang B, Wei ZQ, Peng D. Hypertension Remission after Colorectal Cancer Surgery: A Single-Center Retrospective Study. Nutr Cancer. 2022;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012;18:49-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | An JY, Kim YM, Yun MA, Jeon BH, Noh SH. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J Gastroenterol. 2013;19:9410-9417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Wang KC, Huang KH, Lan YT, Fang WL, Lo SS, Li AF, Wu CW. Outcome after curative surgery for gastric cancer patients with type 2 diabetes. World J Surg. 2014;38:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Kim JH, Huh YJ, Park S, Park YS, Park DJ, Kwon JW, Lee JH, Heo YS, Choi SH. Multicenter results of long-limb bypass reconstruction after gastrectomy in patients with gastric cancer and type II diabetes. Asian J Surg. 2020;43:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Shin YL, Park SH, Kwon Y, Lee CM, Park S. Restoration for the foregut surgery: bridging gaps between foregut surgery practice and academia. J Minim Invasive Surg. 2021;24:175-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Arias A, Rodríguez-Álvarez C, González-Dávila E, Acosta-Torrecilla A, Novo-Muñoz MM, Rodríguez-Novo N. Arterial Hypertension in Morbid Obesity after Bariatric Surgery: Five Years of Follow-Up, a Before-And-After Study. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Climent E, Oliveras A, Pedro-Botet J, Goday A, Benaiges D. Bariatric Surgery and Hypertension. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013;15:14-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 32. | le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 552] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 33. | Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939-2946. [PubMed] |
| 34. | Woelnerhanssen B, Peterli R, Steinert RE, Peters T, Borbély Y, Beglinger C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg Obes Relat Dis. 2011;7:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Schiavon CA, Drager LF, Bortolotto LA, Amodeo C, Ikeoka D, Berwanger O, Cohen RV. The Role of Metabolic Surgery on Blood Pressure Control. Curr Atheroscler Rep. 2016;18:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Ahmed AR, Rickards G, Coniglio D, Xia Y, Johnson J, Boss T, O'Malley W. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg. 2009;19:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 38. | Hall ME, Cohen JB, Ard JD, Egan BM, Hall JE, Lavie CJ, Ma J, Ndumele CE, Schauer PR, Shimbo D; American Heart Association Council on Hypertension; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; and Stroke Council. Weight-Loss Strategies for Prevention and Treatment of Hypertension: A Scientific Statement From the American Heart Association. Hypertension. 2021;78:e38-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 39. | Kwee LC, Ilkayeva O, Muehlbauer MJ, Bihlmeyer N, Wolfe B, Purnell JQ, Xavier Pi-Sunyer F, Chen H, Bahnson J, Newgard CB, Shah SH, Laferrère B. Metabolites and diabetes remission after weight loss. Nutr Diabetes. 2021;11:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Ortiz-Gomez C, Romero-Funes D, Gutierrez-Blanco D, Frieder JS, Fonseca-Mora M, Lo Menzo E, Szomstein S, Rosenthal RJ. Impact of rapid weight loss after bariatric surgery on the prevalence of arterial hypertension in severely obese patients with chronic kidney disease. Surg Endosc. 2020;34:3197-3203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |