Published online Aug 27, 2022. doi: 10.4240/wjgs.v14.i8.731
Peer-review started: April 9, 2022
First decision: May 12, 2022
Revised: May 23, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: August 27, 2022
Processing time: 136 Days and 23.5 Hours
Approximately 10%-20% of the cases of acute pancreatitis have acute necrotizing pancreatitis. The infection of pancreatic necrosis is typically associated with a prolonged course and poor prognosis. The multidisciplinary, minimally invasive “step-up” approach is the cornerstone of the management of infected pancreatic necrosis (IPN). Endosonography-guided transmural drainage and debridement is the preferred and minimally invasive technique for those with IPN. However, it is technically not feasible in patients with early pancreatic/peripancreatic fluid collections (PFC) (< 2-4 wk) where the wall has not formed; in PFC in paracolic gutters/pelvis; or in walled off pancreatic necrosis (WOPN) distant from the stomach/duodenum. Percutaneous drainage of these infected PFC or WOPN provides rapid infection control and patient stabilization. In a subset of patients where sepsis persists and necrosectomy is needed, the sinus drain tract between WOPN and skin-established after percutaneous drainage or surgical necro
Core Tip: In expert hands, percutaneous direct endoscopic necrosectomy through the sinus drainage tract, established after percutaneous drainage or surgical necrosectomy drain, plays a vital role as a minimally invasive, safe, and effective adjunct in the management of infected pancreatic necrosis.
- Citation: Vyawahare MA, Gulghane S, Titarmare R, Bawankar T, Mudaliar P, Naikwade R, Timane JM. Percutaneous direct endoscopic pancreatic necrosectomy. World J Gastrointest Surg 2022; 14(8): 731-742
- URL: https://www.wjgnet.com/1948-9366/full/v14/i8/731.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i8.731
Acute necrotizing pancreatitis may be seen in about 10%-20% of the cases of acute pancreatitis and is frequently associated with a protracted course. The infection of pancreatic necrosis is a serious com
The minimally invasive and preferred endosonography-guided transmural drainage and debridement approach may be technically impossible in early pancreatic/peripancreatic fluid colle
Acute necrotizing pancreatitis may be seen in about 10%-20% of the cases of acute pancreatitis and is frequently associated with a complex and prolonged course. Infection is a serious complication of pancreatic necrotic collection, with a mortality rate of 20%-30%[1]. The drainage and/or debridement of necrotic material are indicated for symptomatic necrotic collections, either for infection (the commonest indication) or if sterile, then for persistent pain, gastrointestinal luminal obstruction, biliary obstruction, fistulas, or persistent systemic inflammatory response syndrome[1].
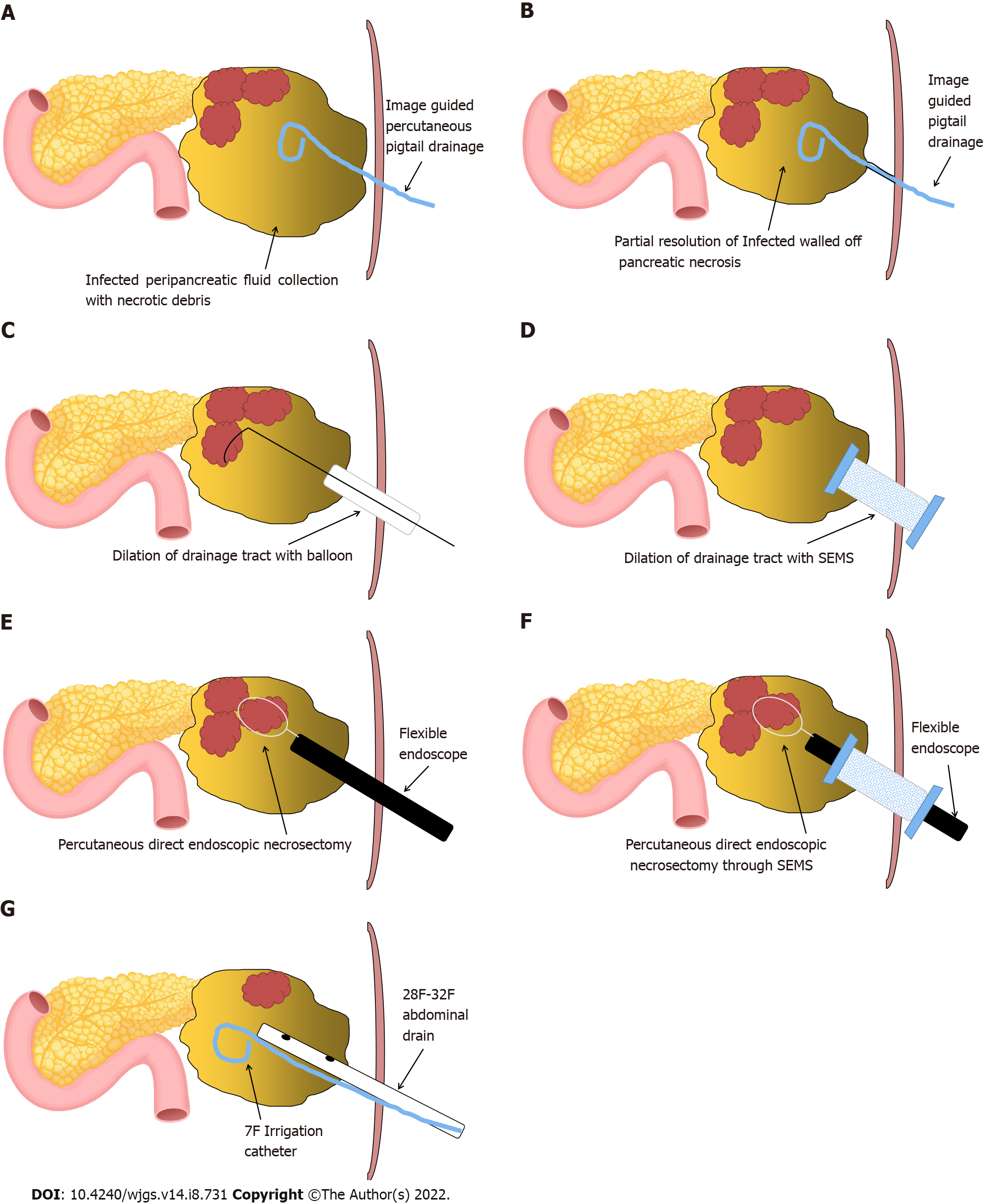
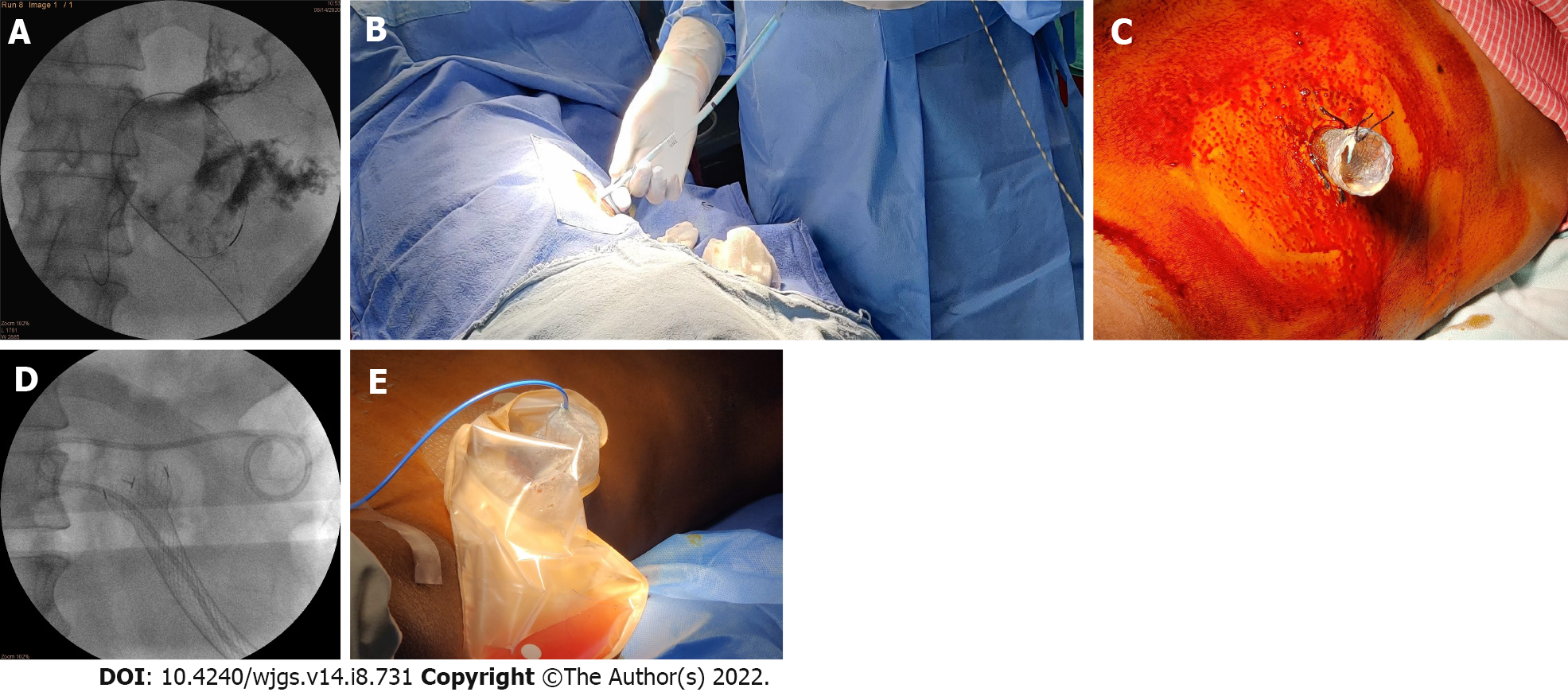
The preferred modality for the drainage of infected WOPN is endoscopic ultrasonography-guided transmural drainage (transgastric/transduodenal) with a lumen-apposing metal stent or plastic stents along with direct endoscopic necrosectomy, depending upon the symptoms and quantity of the solid component in the WOPN cavity[6,7]. Endoscopic transmural drainage is not technically feasible if: (1) Infection occurs during the early stage (< 2-4 wk) of acute necrotizing pancreatitis where pancreatic necrosis is not walled off; (2) WOPN is far away (> 10 mm) from the stomach/duodenum; (3) necrosis extends into paracolic gutters or pelvis; (4) the patient is very sick and unfit for the procedure; and (5) local expertise is not available. Image-guided percutaneous drainage of a symptomatic pancreatic necrotic collection is crucial in the treatment of these individuals. Percutaneous drainage of an infected PFC typically allows pancreatic necrosis to wall off and stabilize a sick patient while also controlling the infection source. Percutaneous drainage catheters are available in sizes ranging from 8 F to 32 F. It can be placed under imaging guidance by an interventional radiologist (Figure 1A). The drain size is usually gradually increased to around 28 F-32 F at regular intervals before PDEN. Percutaneous drainage with an esophageal fully covered self-expandable metal stent (SEMS) insertion may obviate the need for these multiple procedures[8]. Exclusive percutaneous drainage is effective in 35%-51% of symptomatic WOPN patients[2,9,10]. As a result, in the remaining subset of patients, debridement of infected necrotic debris is necessary. A matured sinus tract after percutaneous drainage or a surgically-placed drain after necrosectomy can be utilized for PDEN if there is an incomplete clinical improvement following percutaneous drainage.
PDEN, also known as sinus tract endoscopy, is a minimally invasive technique that involves passing a flexible endoscope through the matured tract connecting WOPN and skin, the drainage tract established following surgical necrosectomy drain or percutaneous drainage-to debride infected necrotic material. If percutaneous or surgically-placed drain alone does not result in a complete clinical response, PDEN can be used to debride the infected necrotic material. In the literature, PDEN has been the subject of various case series and case reports[3,5,8,11-27] (Table 1). Although the retroperitoneal route is the preferred safe route for PDEN because there is no risk of peritoneal contamination, a transperitoneal route has been reported. A fully covered SEMS, when used for drainage tract dilatation, may help to prevent infectious material from escaping into the peritoneal cavity, thereby preventing peritonitis. The main indications of PDEN are summarized in Table 2.
| Ref. | Number of patients | Initial intervention | PDEN/stent assisted PDEN | Anaesthesia | Median PDEN sessions | Additional intervention-number of patients | Clinical success rate (%) | Procedure related complications-number of patients | Mortality (%) |
| Carter et al[11], 2000 | 14 | ON-4, PD-10 | PDEN | GA | 2 | Surgery-1 | 85.7 | Bleeding-1 | 14.3 |
| Mui et al[12], 2005 | 13 | ON-4, PD-10 | PDEN | TIVA | 3 | ERCP-9, Surgery-1 | 76.9 | Colonic perforation-1; catheter dislodgement-1 | 7.7 |
| Dhingra et al[14], 2015 | 15 | PD-15 | PDEN | TIVA | 4 | Surgery-1 | 93.3 | Bleeding-1; pancreatico-cutaneous Fistula-1 | 6.7 |
| Mathers et al[15], 2016 | 10 | PD-10 | PDEN | TIVA; GA if clinically warranted | 1.5 | None | 100 | Pancreatico-cutaneous Fistula-1 | 0 |
| Goenka et al[18], 2018 | 10 | PD-10 | PDEN | TIVA | 2.3 | Transmural, DEN-2, Surgery-1 | 90 | Pneumo-peritoneum-2 | 0 |
| Saumoy et al[19], 2018 | 9 | PD-9 | Stent-assisted PDEN | GA | 3 | None | 88.9 | None | 11.1 |
| Thorsen et al[20], 2018 | 5 | PD-3; transmural; DEN-2 | Stent-assisted PDEN | TIVA or GA | 6 | Transmural DEN-1 | 80 | Abdominal Pain-5; pancreatico-cutaneous fistula-2 | 20 |
| Tringali et al[21], 2018 | 3 | PD-3 | Stent-assisted PDEN | TIVA | 3 | 0 | 100 | None | 0 |
| Jain et al[5], 2020 | 53 | PD-53 | PDEN | TIVA | 4 | Surgery-8 | 79.2 | Pancreatico-cutaneous fistula-4; bleeding-1; aspiration pneumonia-2; peritonitis-2; paralytic ileus-1; subcutaneous emphysema-1 | 20.8 |
| Ke et al[25], 2021 | 37 | PD-37 | Stent-assisted PDEN | NA | 4 | Surgery-8 | 86.5 | Bleeding-6; pancreatico-cutanoeus fistula-7; colonic fistula-4; gastro-duodenal fistula-4 | 13.5 |
| Indications |
| < 2-4 wk-Infected acute pancreatic/peripancreatic collection in which percutaneous drainage is required early and infection persists even after percutaneous drainage alone |
| > 2-4 wk-Infected walled off pancreatic necrosis unsuitable for transmural drainage: (1) Location (Paracolic/pelvic extension); (2) Distance > 1 cm; (3) Coagulopathy; (4) Multiple collaterals-Endosonography guided can be done |
Although PDEN has been performed under general anaesthesia in a few case series[11,19], it has mostly been done under conscious sedation or total intravenous anaesthesia without endotracheal intubation (TIVA)[14,18,21,27]. A deep plane of anaesthesia can be achieved with TIVA. Propofol is used for induction and maintenance, while ketamine is used to provide analgesia during spontaneous ventilation with an oxygen mask[28]. When compared to general, regional, and combined anaesthesia, TIVA is significantly associated with a reduction in inflammatory markers, particularly C-reactive protein, potentially reducing the post-procedure systemic inflammatory response and complications[29]. However, elderly patients or those with the American Society of Anaesthesiologists’ poor physical status should be treated with extreme caution.
After the sinus tract between the skin and WOPN has matured (usually 7-10 d after percutaneous drainage) (Figure 1B), it can be dilated with a wire-guided controlled radial expansion balloon or Amplatz dilators, depending on the length of the sinus tract, to facilitate an easy passage of the flexible endoscope into WOPN (Figure 1C). As Amplatz dilators have a smaller nose compared to Savary Gillard dilators, they can be used to dilate longer sinus tract more easily and safely. As the diameter of the upper gastrointestinal endoscope ranges from 9 to 10 mm, the sinus tract dilation is typically planned up to 10 to 12 mm. Another method for sinus tract dilatation is to gradually increase the drain size to around 28-32 F at regular intervals. If the drainage tract is longer and a patent tract is required for a longer period of time, an esophageal fully covered SEMS placement across the tract should be preferred to minimize repeated dilatation of the sinus tract (Figure 1D). Because of its wide diameter, the fully covered SEMS keeps the sinus tract patent and enables easy and several passes of the flexible endoscope during PDEN. Percutaneous drainage and tract dilatation with a fully covered SEMS placement followed by necrosectomy may be done in a single step, eliminating the multiple steps involved in PDEN[8].
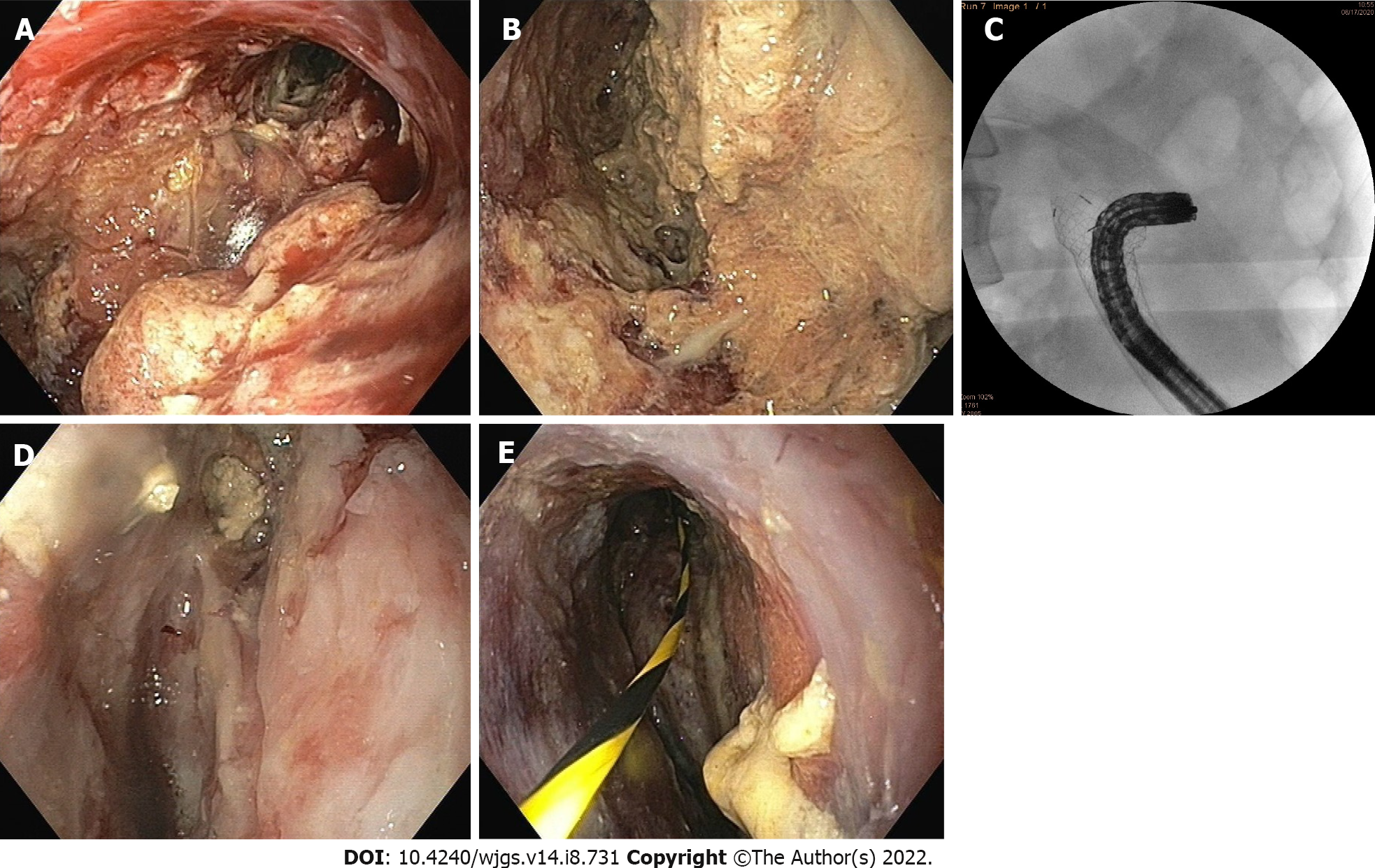
PDEN is carried out using carbon dioxide insufflation. The most crucial step for PDEN is to irrigate the cavity with sterile normal saline for the early evacuation of pus and liquefied necrotic debris. A rat-tooth forceps, a polyp retrieval basket, a snare, a dormia basket, or an automated rotor resection device can be used to remove necrotic debris (Figure 1E and F). The most important precaution to take during PDEN is to only remove loose debris with a gentle traction. Forceful traction will lead to intracavitary bleeding or perforation of the WOPN wall. After the necrosectomy session, it is preferable to keep a 30-32 F drain and a 7-8 F irrigation catheter in place to keep the tract dilated for easy passage of the scope during the subsequent necrosectomy and irrigation of the cavity with normal saline, respectively (Figure 1G). The necrosectomy sessions may vary depending on the infected solid component of WOPN. The key end objectives of PDEN are: (1) Symptom control with near-complete removal of the infected necrotic debris; and (2) visualization of healthy granulation tissue along the cavity wall[18]. The drainage catheter can be gradually changed with smaller diameter catheters every week after the PDEN sessions are completed and the patient’s symptoms have improved, for an early sinus tract closure.
PDEN can be carried out in a critically ill patient at bedside as it can be done under deep sedation. The main advantage of PDEN is an easier access to various extensions deep within the abdomen with a flexible endoscope as compared to a rigid laparoscope or nephroscope. Like a lumen-apposing metal stent, a fully covered SEMS used in PDEN reduces the need for frequent dilations while also eliminating peritoneal contamination in a transperitoneal approach. The significant adverse event of PDEN is pancreatico-cutaneous fistula, which can occur in up to 7% of the patients[5]. However, dual-percutaneous and transluminal drainage can help to minimize this complication[30]. Table 3 summarizes the advantages and disadvantages of PDEN.
| No. | Advantages | Disadvantages |
| 1 | It can be done in critically ill patients where laparoscopy access is not possible- bed side | More invasive (compared to transmural necrosectomy) (Multiple interventions-percutaneous drainage followed by multiple tract dilation/drainage catheter exchanges, if not stent-assisted percutaneous direct endoscopic necrosectomy) |
| 2 | Subsequent liquefied necrosis drained by gravity | Small endoscopic accessories for necrosectomy-hence, time-consuming and labour-intensive procedure (compared to VARD/surgical necrosectomy) |
| 3 | No intraperitoneal transmission (retroperitoneal approach); a fully covered self-expandable metal stent may help to prevent intraperitoneal transmission in transperitoneal approach | The need for repeated procedures for effective drainage (compared to VARD/surgical necrosectomy) |
| 4 | Access various extensions deep within the abdomen using the flexible endoscope’s angulation and versatility (Figures 3C and 6C) | Pancreatico-cutaneous fistula (compared to transmural necrosectomy) |
| 5 | Usually carried out under deep sedation; general anaesthesia avoided | - |
To better perceive the PDEN case situation, a study of two IPN cases with contrasting clinical settings is provided. The PDEN was carried out using distinct procedures and approaches in both the situations. One case had image-guided percutaneous drainage done in the early phase of acute pancreatitis due to a poor general condition, while the other case had a surgically-placed drain after open-necrosectomy. PDEN was carried out under TIVA.
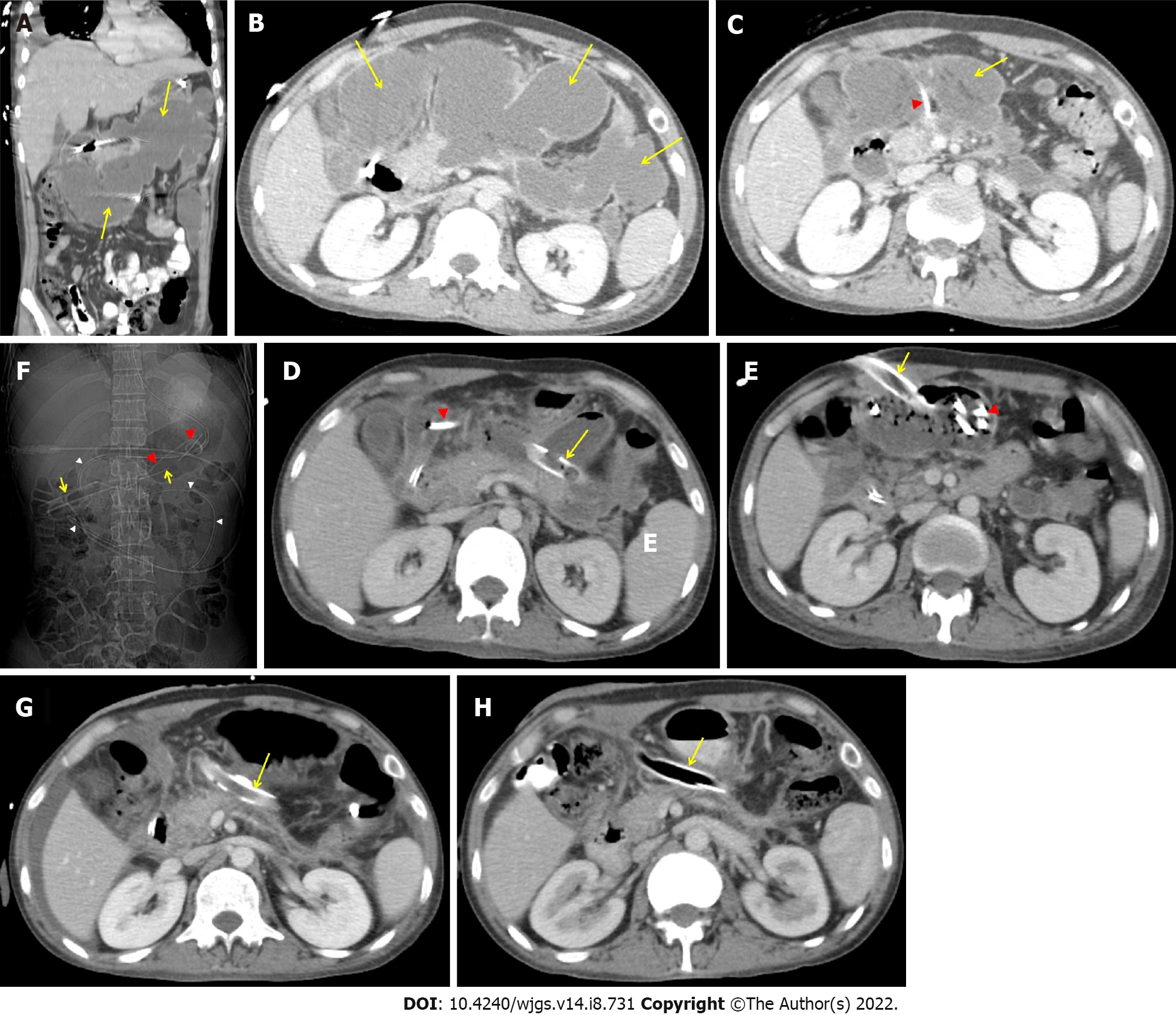
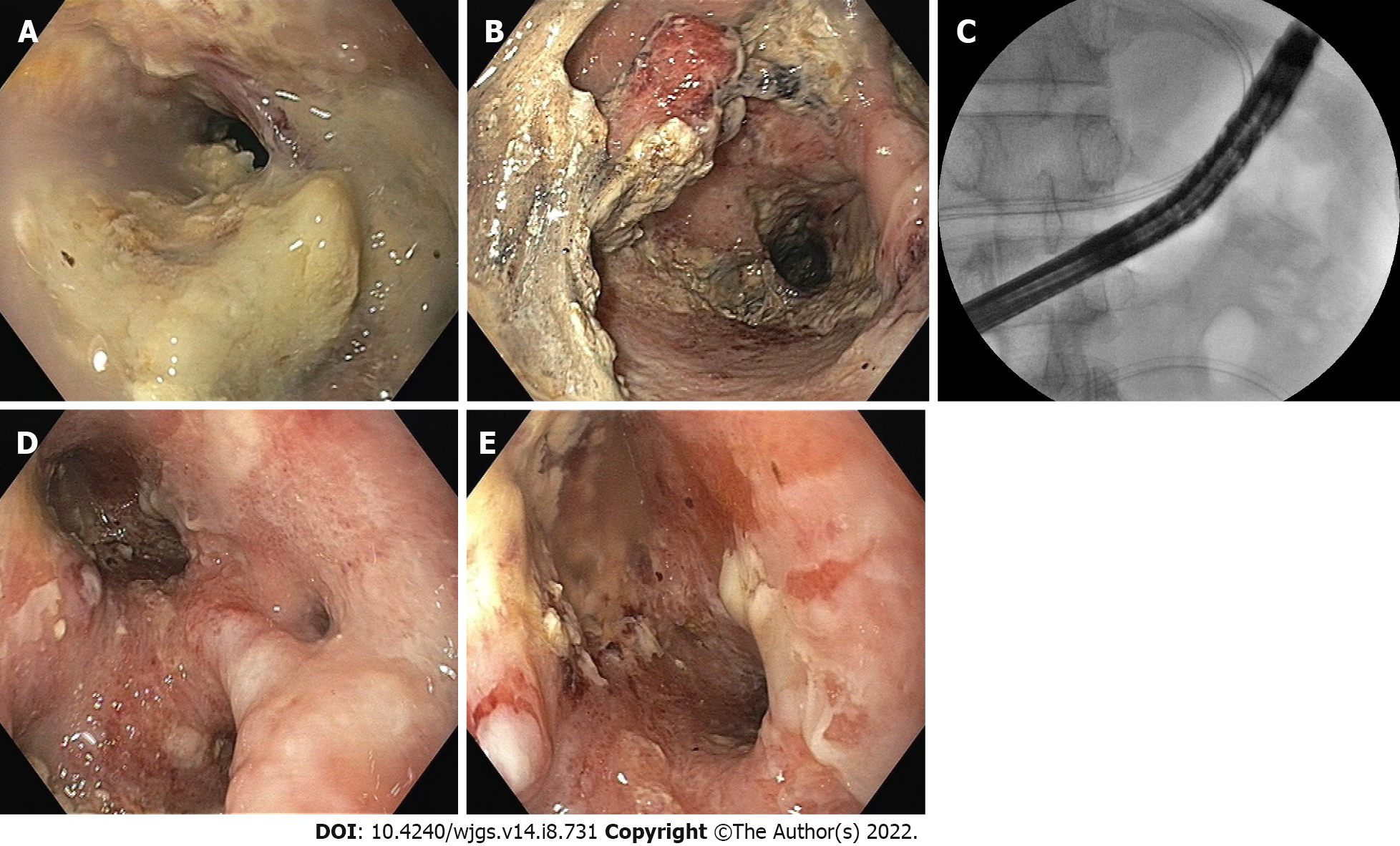
A 35-year-old male was treated for 2 wk for ethanol-induced moderately severe acute pancreatitis. On the 17th day of his illness, he was sent to our center with a persistent fever and loss of appetite. An abdominal contrast enhanced computed tomography (CECT) scan revealed a large irregular PFC in the upper abdomen (Figure 2A and B). Due to his poor health status and early PFC, an image-guided 14 F pigtail was inserted to drain the infected necrotic collection. Klebsiella pneumoniae was found in his pus culture, and it was sensitive to Carbepenams and Quinolones. The fever and leucocytosis continued even after the PFC was significantly reduced in size (Figure 2C). In order to irrigate the cavity, a 26 F drain and a 7 F irrigation catheter were inserted into the PFC following dilatation of the tract with a controlled radial expansion balloon over the guide-wire under fluoroscopy guidance (week 4) (Figure 2D). His health steadily improved, with fewer fever spikes and a lower leucocyte count. He did, however, continue to suffer from low-grade fever and systemic inflammatory response syndrome. As a result, following the dilatation of the tract with a controlled radial expansion balloon up to 12 mm, he underwent PDEN with a flexible upper gastrointestinal endoscope at week 5. A snare and rat-tooth forceps were used to remove the infected necrotic debris (Figure 3). A 7 F irrigation catheter and a 32 F drain were inserted for irrigation and for the subsequent necrosectomy sessions, respectively (Figure 2E and F). He had a second session of PDEN after 2 d. His general condition began to improve subsequently with the resolution of WOPN (Figure 2G and H). The drain was gradually reduced in size over a period of 4 wk, and it was eventually removed after 5 wk of PDEN treatment. At the 12-mo follow-up, he remained asymptomatic.
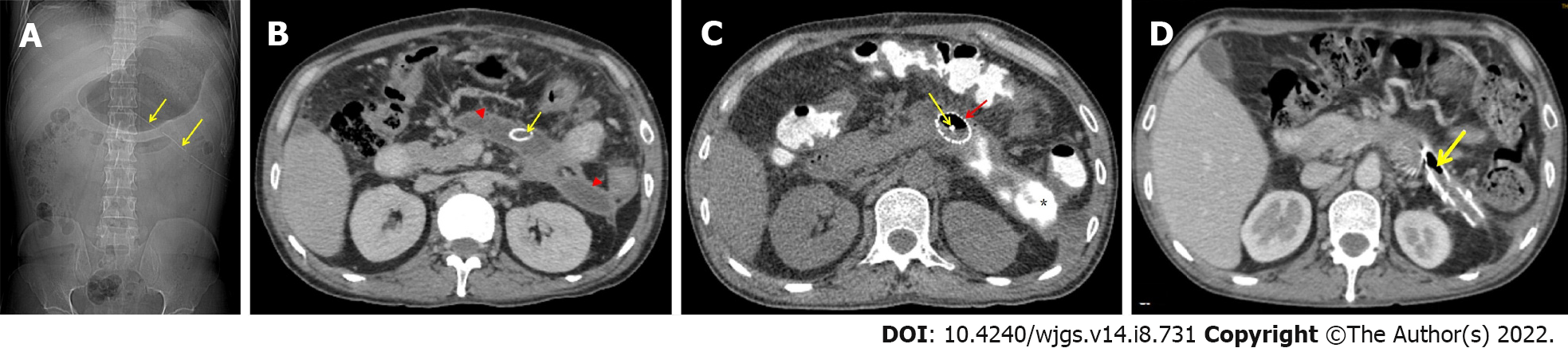
A 47-year-old male was managed for 4 wk for ethanol-induced moderately severe acute pancreatitis. At week 5, he had an exploratory laparotomy with WOPN drainage and necrosectomy for large symptomatic WOPN (not suited for transluminal drainage) with a 24 F drain in situ. He was admitted to our centre a week later with a fever, chills, and leucocytosis. The abdominal drain output was minimal with a residual WOPN on the CECT scan (Figure 4A and B). The sinus tract measured 9 to 10 cm in length. Hence, he was scheduled for stent-assisted PDEN. The drain was exchanged over the guide-wire with the catheter. The contrast was injected into the WOPN to delineate the cavity (Figure 5A). A 12-cm long esophageal fully covered SEMS with a 16 mm diameter was inserted across the tract after dilatation to 24 F using Amplatz dilators (Figure 4C; Figure 5B and C). The stent was secured to the skin with sutures (Figure 5C). The WOPN cavity was irrigated with a 7 F irrigation catheter, and a stoma bag was put over the SEMS to collect normal saline after the cavity was irrigated (Figure 4C; Figure 5D and E). He had PDEN through the fully covered SEMS 2 d later. He underwent three sessions of PDEN at 2-d intervals to remove the infected debris using a snare and rat tooth forceps (Figure 6). The fully covered SEMS was removed and replaced with a 32 F drain and a 7 F irrigation catheter after the clinical and haematological improvements. The irrigated normal saline was collected using the stoma bag. An abdominal CECT scan revealed complete resolution of WOPN (Figure 4D) after 1 wk. The drain size gradually decreased and the catheter was removed after 2 mo following stent removal, when the drain output was nil for a week. One month later, he again presented with abdominal pain with WOPN at the previous site on the CECT scan. The previously closed sinus tract spontaneously reopened with a discharge of clear liquid, indicating a pancreatico-cutaneous fistula. At the 10-mo follow-up, he remained asymptomatic with a pancreatico-cutaneous fistula.
To date, several case series and case reports on PDEN have been published[3,5,8,11-27] (Table 1). The largest observational study series of PDEN was reported by Garg et al[5], in which 53 patients with IPN underwent PDEN. 42 (79.2%) patients were successfully treated, with 34 patients recovering after PDEN alone and 8 patients recovering after the additional surgery. Eleven patients (7 after PDEN and 4 after surgery) died due to organ failure. The adverse events seen during PDEN included aspiration pneumonia, peritonitis, paralytic ileus, subcutaneous emphysema, and self-limiting haemorrhage. Four (7%) patients had pancreatico-cutaneous fistulas following the PDEN. Early organ failure and necrosis of more than 50% were found to be independent predictors of mortality. PDEN proved to be an effective therapy for IPN in the study[5].
Another observational study from the same group found that 14 of the 15 patients with IPN who received PDEN showed improvement. The adverse events were a pancreatico-cutaneous fistula and self-limiting haemorrhage. One patient required surgery but died as a result of organ failure. According to the authors, PDEN is a safe and effective minimally invasive technique for necrosectomy in IPN[14].
Carter et al[11] used PDEN in 4 and 10 patients with IPN along the drainage tract following previous open necrosectomy and percutaneous drainage, respectively. The procedure success rate was 78.6%, with a 14.3% mortality rate. The authors demonstrated a significant reduction in the postoperative organ dysfunction after PDEN[11]. A similar study was conducted by Mui et al[12] where PDEN was carried out in 4 and 9 patients with IPN via the drain tract following open necrosectomy and percutaneous drainage, respectively. Nine of the thirteen patients needed endoscopic retrograde cholangio-pancreaticography. The overall success rate and mortality rate of PDEN in the study were 76.9% and 7.7%, respectively. The authors concluded that PDEN and endoscopic retrograde cholangio-pancreaticography are useful adjuncts in the management of IPN[12].
A series by Goenka et al[18] of 10 patients with symptomatic, laterally-placed WOPN who underwent PDEN showed clinical success in 9 patients. Two patients developed pneumoperitoneum, which was managed conservatively. There was no mortality, cutaneous fistula, or recurrence during the follow-up. The authors concluded that PDEN can successfully manage laterally-placed WOPN[18].
In a recently published retrospective, historically-controlled cohort study by Ke et al[25], 37 patients with IPN who received stent-assisted PDEN were compared to 73 historically-control patients. While stent-assisted PDEN reduced hospital stay (38 d vs 48 d, P = 0.035) and new-onset sepsis (35% vs 56%, P = 0.037), and allowed for faster necrosectomy, it did not reduce the incidence of major complications and/or mortality (35% vs 52%, P = 0.095)[25].
All the studies in this regard have shown a comprehensive success rate with a minimal complication rate. Due to its minimally invasive nature, PDEN has been proven to significantly minimize the post-procedure organ dysfunction and new-onset sepsis, therefore improving outcomes in IPN patients. PDEN has been shown to treat laterally positioned WOPN that cannot be treated with transmural drainage. The stent-assisted PDEN has been shown to allow easy and multiple passes of the flexible endoscope, resulting in faster necrosectomy. Additionally, a fully covered SEMS prevents peritoneal contamination. The only unfavourable outcome of PDEN is pancreatico-cutaneous fistula. The major limitations of most of the above case series are: (1) The observational nature of the studies; (2) small sample size; (3) lack of uniformity in the procedural steps; and (4) biased case selection. However, large-scale studies may be challenging to conduct because IPN is a heterogeneous disease with substantial diversity in disease course and extent[4].
IPN is typically associated with a prolonged course and carries a poor prognosis with high mortality. The multidisciplinary, minimally invasive “step-up” approach is more favoured for the management of infected pancreatic necrotic collections. In a subset of patients in whom necrosectomy is essential, PDEN has emerged as a safe, effective, and minimally invasive adjunct in the armamentarium of IPN management. It may particularly be considered when a conventional drain is in situ by virtue of the previous percutaneous or surgical intervention.
We express our sincere thanks to Dr. Manish Gaikwad, Dr. (Mrs.) Vaishali Gaikwad, and Dr. (Mrs.) Nikita Vyawahare for helping us in writing and editing the manuscript. Also, we are especially thankful to our endoscopy technician team-Mr. Shailesh Chaware, Ms. Priyanka Tambe, and Mr. Satyaprakash-for their support during the procedure.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garret C, France; Jing D, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Wu RR
| 1. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of pancreatic necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 418] [Article Influence: 83.6] [Reference Citation Analysis (2)] |
| 2. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1037] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 3. | Logue JA, Carter CR. Minimally invasive necrosectomy techniques insevere acute pancreatitis: role of percutaneous necrosectomy and video-assisted retroperitoneal debridement. Gastroenterol Res Pract. 2015;2015:693040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Garg PK, Zyromski NJ, Freeman ML. Infected necrotizing pancreatitis: evolving interventional strategies from minimally invasive surgery to endoscopic therapy-evidence mounts, but one size does not fit all. Gastroenterology. 2019;156:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Jain S, Padhan R, Bopanna S, Jain SK, Dhingra R, Dash NR, Madhusudan KS, Gamanagatti SR, Sahni P, Garg PK. Percutaneous endoscopic step-up therapy is an effective minimally invasive approach for infected necrotizing pancreatitis. Dig Dis Sci. 2020;65:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 6. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1041] [Article Influence: 86.8] [Reference Citation Analysis (6)] |
| 7. | Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 8. | Binda C, Sbrancia M, La Marca M, Colussi D, Vizzuso A, Tomasoni M, Agnoletti V, Giampalma E, Ansaloni L, Fabbri C. EUS-guided drainage using lumen apposing metal stent and percutaneous endoscopic necrosectomy as dual approach for the management of complex walled-off necrosis: a case report and a review of the literature. World J Emerg Surg. 2021;16:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG; Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 466] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 10. | van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P; Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 474] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 11. | Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Mui LM, Wong SK, Ng EK, Chan AC, Chung SC. Combined sinus tract endoscopy and endoscopic retrograde cholangiopancreatography in management of pancreatic necrosis and abscess. Surg Endosc. 2005;19:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Navarrete C, Castillo C, Caracci M, Vargas P, Gobelet J, Robles I. Wide percutaneous access to pancreatic necrosis with self-expandable stent: new application (with video). Gastrointest Endosc. 2011;73:609-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Dhingra R, Srivastava S, Behra S, Vadiraj PK, Venuthurimilli A, Shalimar, Dash NR, Madhusudhan KS, Gamanagatti SR, Garg PK. Single or multiport percutaneous endoscopic necrosectomy performed with the patient under conscious sedation is a safe and effective treatment for infected pancreatic necrosis (with video). Gastrointest Endosc. 2015;81:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Mathers B, Moyer M, Mathew A, Dye C, Levenick J, Gusani N, Dougherty-Hamod B, McGarrity T. Percutaneous debridement and washout of walled-off abdominal abscess and necrosis using flexible endoscopy: a large single-center experience. Endosc Int Open. 2016;4:E102-E106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Sato S, Takahashi H, Sato M, Yokoyama M, Itoi T, Kawano Y, Kawashima H. A case of walled-off necrosis with systemic lupus erythematosus: Successful treatment with endoscopic necrosectomy. Semin Arthritis Rheum. 2016;46:e13-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Jürgensen C, Brückner S, Reichel S, Kilian M, Pannach S, Distler M, Weitz J, Neser F, Hampe J, Will U. Flexible percutaneous endoscopic retroperitoneal necrosectomy as rescue therapy for pancreatic necroses beyond the reach of endoscopic ultrasonography: A case series. Dig Endosc. 2017;29:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Goenka MK, Goenka U, Mujoo MY, Tiwary IK, Mahawar S, Rai VK. Pancreatic necrosectomy through sinus tract endoscopy. Clin Endosc. 2018;51:279-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Saumoy M, Kumta NA, Tyberg A, Brown E, Lieberman MD, Eachempati SR, Winokur RS, Gaidhane M, Sharaiha RZ, Kahaleh M. Transcutaneous endoscopic necrosectomy for walled-off pancreatic necrosis in the paracolic gutter. J Clin Gastroenterol. 2018;52:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Thorsen A, Borch AM, Novovic S, Schmidt PN, Gluud LL. Endoscopic necrosectomy through percutaneous self-expanding metal stents may be a promising additive in treatment of necrotizing pancreatitis. Dig Dis Sci. 2018;63:2456-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Tringali A, Vadalà di Prampero SF, Bove V, Perri V, La Greca A, Pepe G, Cozza V, Costamagna G. Endoscopic necrosectomy of walled-off pancreatic necrosis by large-bore percutaneus metal stent: a new opportunity? Endosc Int Open. 2018;6:E274-E278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Bader GA, Zibert K, Miller CB. A case of direct necrosectomy via sinus tract endoscopy in the retroperitoneum: a unique approach to a difficult problem. Am J Gastroenterol. 2019;114:S700-S701. |
| 23. | Nguyen AK, Song AJ, Swopes T, Ko A, Lim BS. Percutaneous endoscopic necrosectomy of complex walled-off lateral necrosis of the pancreas with the aid of laparoscopic Babcock forceps: A case report of an endoscopic and radiologic team approach. Perm J. 2019;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Pérez-Cuadrado-Robles E, Berger A, Perrod G, Ragot E, Cuenod CA, Rahmi G, Cellier C. Endoscopic treatment of walled-off pancreatic necrosis by simultaneous transgastric and retroperitoneal approaches. Endoscopy. 2020;52:E88-E89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Ke L, Li G, Wang P, Mao W, Lin J, Gao L, Ye B, Zhou J, Tong Z, Li W, Windsor J. The efficacy and efficiency of stent-assisted percutaneous endoscopic necrosectomy for infected pancreatic necrosis: a pilot clinical study using historical controls. Eur J Gastroenterol Hepatol. 2021;33:e435-e441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Zeuner S, Finkelmeier F, Waidmann O, Bojunga J, Zeuzem S, Friedrich-Rust M, Knabe M. Percutaneous endoscopic necrosectomy using an automated rotor resection device in severe necrotizing pancreatitis. Endoscopy. 2022;54:E362-E363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Patil G, Maydeo A, Dalal A, Iyer A, More R, Thakare S. Endoscopic retroperitoneal necrosectomy for infected pancreatic necrosis using a self-expandable metal stent. GE Port J Gastroenterol. 2021;28:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Nimmo AF, Absalom AR, Bagshaw O, Biswas A, Cook TM, Costello A, Grimes S, Mulvey D, Shinde S, Whitehouse T, Wiles MD. Guidelines for the safe practice of total intravenous anaesthesia (TIVA): Joint guidelines from the Association of Anaesthetists and the Society for Intravenous Anaesthesia. Anaesthesia. 2019;74:211-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 29. | Alhayyan A, McSorley S, Roxburgh C, Kearns R, Horgan P, McMillan D. The effect of anesthesia on the postoperative systemic inflammatory response in patients undergoing surgery: A systematic review and meta-analysis. Surg Open Sci. 2020;2:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Ross AS, Irani S, Gan SI, Rocha F, Siegal J, Fotoohi M, Hauptmann E, Robinson D, Crane R, Kozarek R, Gluck M. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: long-term clinical outcomes. Gastrointest Endosc. 2014;79:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |