Published online Jul 27, 2022. doi: 10.4240/wjgs.v14.i7.714
Peer-review started: March 22, 2022
First decision: June 11, 2022
Revised: June 14, 2022
Accepted: July 5, 2022
Article in press: July 5, 2022
Published online: July 27, 2022
Processing time: 126 Days and 17.4 Hours
Splenic artery aneurysm (SAA) is a rare vascular lesion conventionally treated by resection or interventional therapy. The surgical procedure usually involves splenectomy, and interventional therapy may cause post-embolization syndro
We report a patient with an asymptomatic SAA (3.5 cm in diameter), which was en-bloc resected laparoscopically using indocyanine green (ICG) fluorescence imaging to preserve the spleen and its function.
ICG fluorescence imaging for spleen preservation in laparoscopic SAA resection is safe and may be beneficial in avoiding splenectomy and maintaining splenic function.
Core Tip: Currently, there are three main treatment methods for splenic artery aneurysm (SAA): Endovascular treatment, open surgery, and laparoscopic surgery. Laparoscopic SAA resection is inevitably concomitant with splenectomy due to end-organ ischemia at times. We here present a case of SAA treated by laparoscopic resection using indocyanine green fluorescence imaging for preserving spleen and its function. This is the first case successfully treated by this method reported in the literature.
- Citation: Cheng J, Sun LY, Liu J, Zhang CW. Indocyanine green fluorescence imaging for spleen preservation in laparoscopic splenic artery aneurysm resection: A case report. World J Gastrointest Surg 2022; 14(7): 714-719
- URL: https://www.wjgnet.com/1948-9366/full/v14/i7/714.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i7.714
With further understanding of spleen function, and occurrence of complications such as overwhelming post-splenectomy infection, thrombocytosis, and portal vein thrombosis after splenectomy, surgeons have realized the importance of splenic preservation[1]. Protecting normal splenic artery blood flow is the key to maintain spleen function[2]. Preserving the spleen and its function is an important issue in the management of splenic artery aneurysm (SAA). We here report the application of indocyanine green (ICG)-enhanced fluorescence for spleen preservation in a patient during laparoscopic SAA resection. We also review the relevant literature.
A 50-year-old man was admitted to hospital due to an asymptomatic SAA found on medical examination.
Abdominal ultrasound showed a posterior pancreatic mass, which was diagnosed as an SAA 3.5 cm in diameter three days ago without any symptoms.
The patient denied a history of surgery or abdominal trauma, and had a free previous medical history.
His personal history and family history were unremarkable. He denied history of consuming alcohol, tobacco, and psychoactive drugs.
No positive signs were found on abdominal examination and other physical examinations.
Blood tests, blood biochemistry, coagulation function, urine and routine stool tests were all normal.
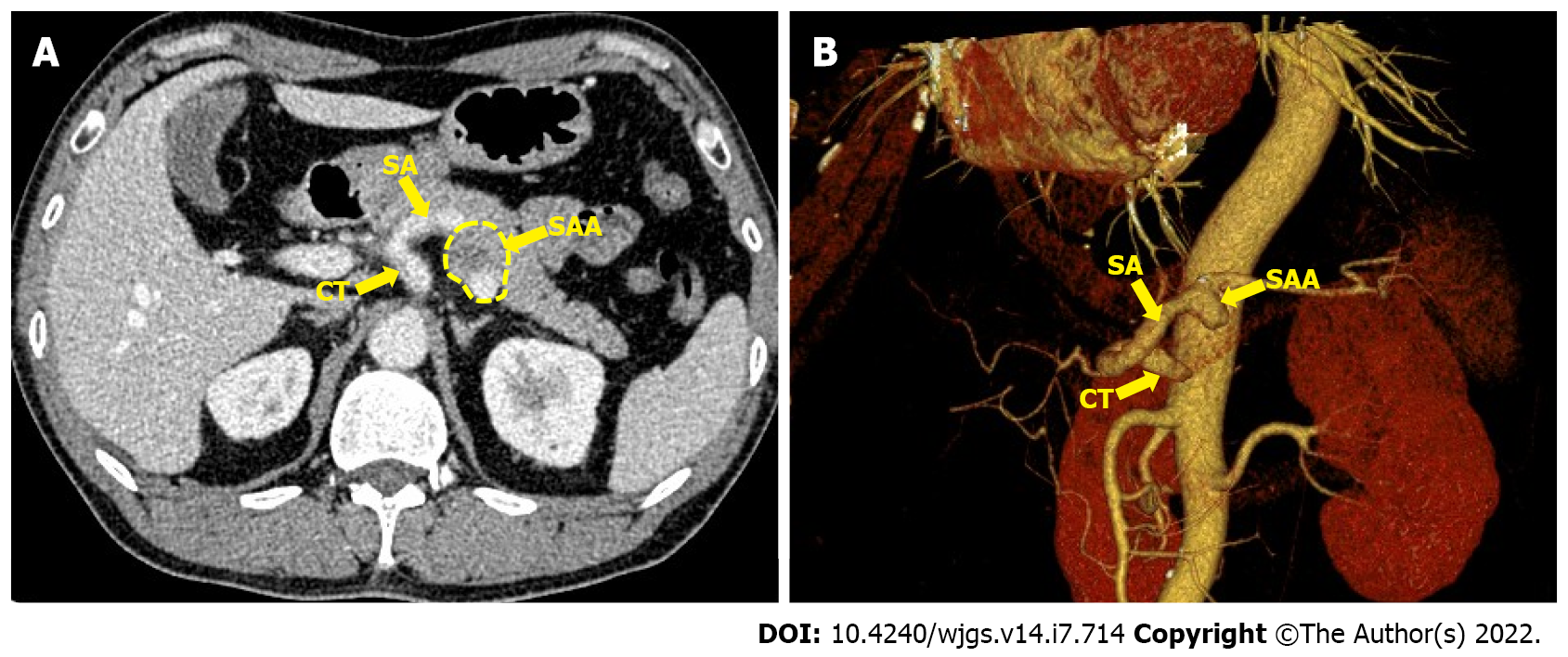
Ultrasound showed a posterior pancreatic mass and an SAA was considered. A contrast-enhanced celiac trunk (CT) scan revealed an SAA 3.5 cm in diameter with thrombosis located in the posterior pancreas. 3D virtual imaging revealed a 3.5 cm SAA located at approximately 3 cm from the CT (Figure 1).
The final diagnosis of the presented case is an asymptomatic SAA (3.5 cm in diameter).
Surgical treatment was selected based on the anatomic location of the aneurysm, possible rupture of the SAA and the patient’s choice. Endovascular treatment was not proposed as endovascular repair may increase the risk of subsequent complications and re-interventions during long-term follow-up[3]. Thus, laparoscopic SAA resection with ICG fluorescence imaging was performed.
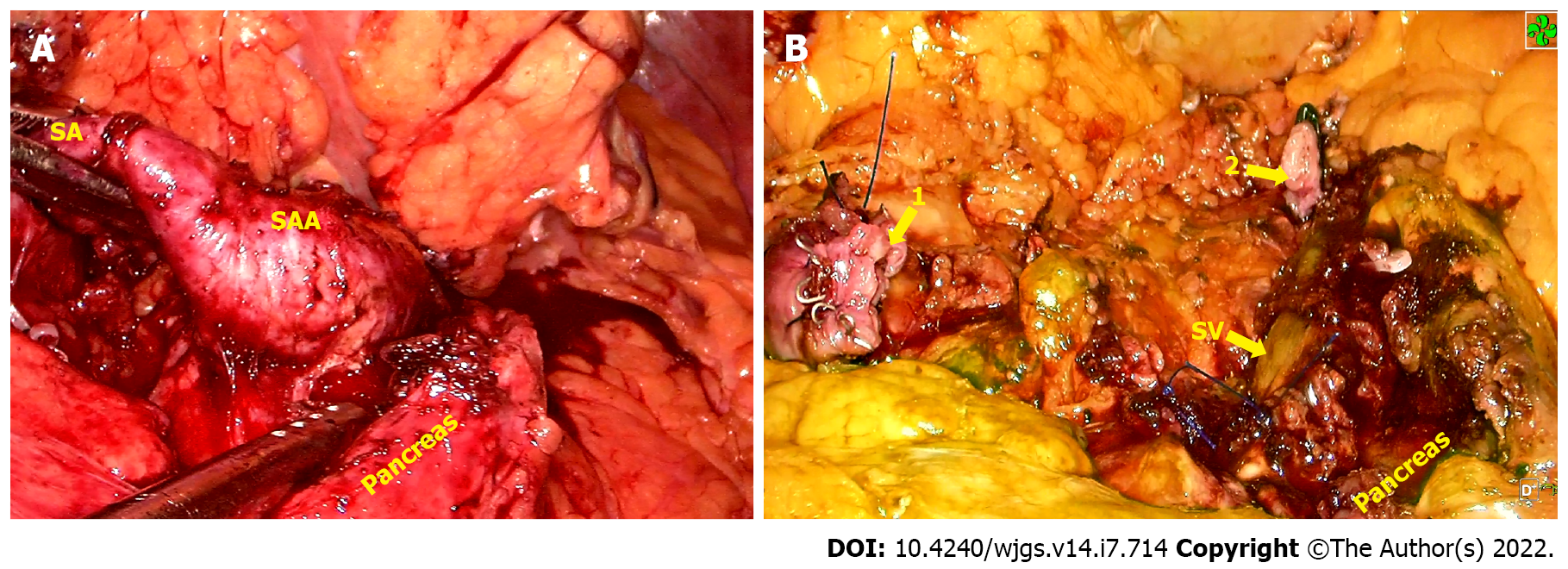
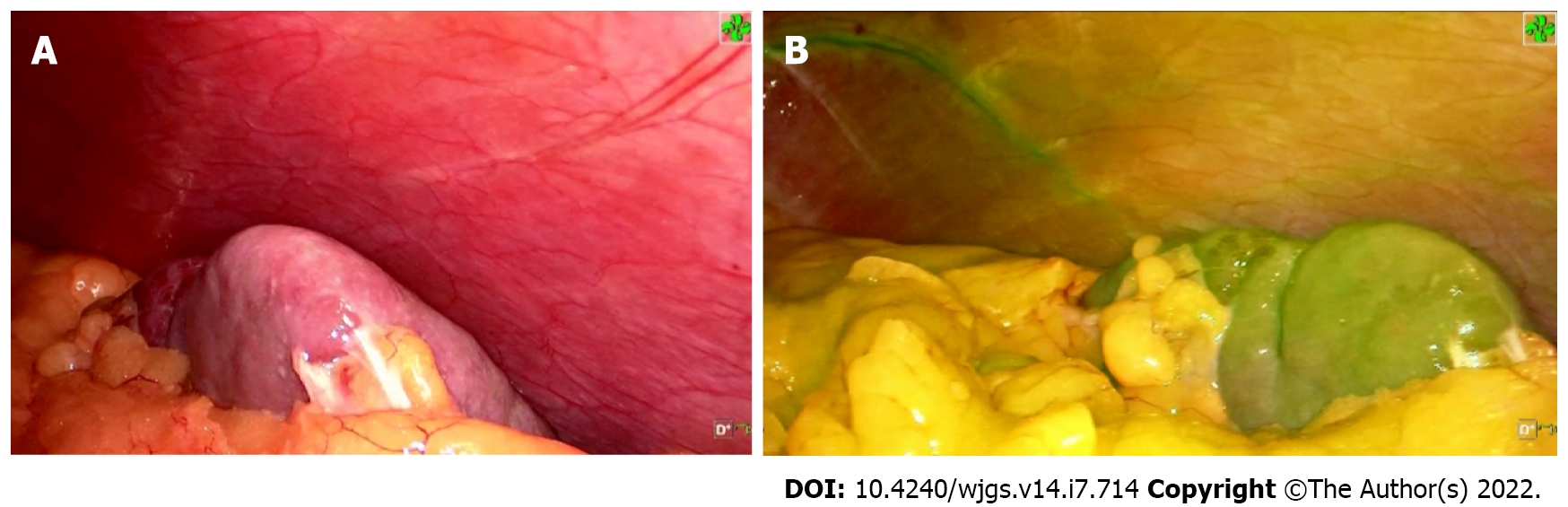
Five ports were inserted in the abdomen at a 15 mmHg pressure pneumoperitoneum. After that, the patient was placed in the reverse trendelenburg position. First, the gastrocolic ligament was divided to expose the pancreatic edge, identify splenic artery and aneurysm, then the proximal and distal parts were separated and ligated, respectively. An aneurysm, about 3.5 cm × 3.0 cm in size, was located approximately 3 cm from the CT, it had grown into the retroperitoneal pancreas parenchyma and was densely adhered to the splenic vein (Figure 2). It was partially ruptured with a 0.5 cm cleft, and protruded into the pancreatic parenchyma with thrombogenesis. The collateral vessels of the aneurysm were completely dissected, thus the aneurysm was en-bloc resected following separation of the surrounding tissues using an ultrasonic knife (Figure 2). At the end of the procedure, 2.5 mg ICG was injected into the peripheral vein, the whole spleen was stained green 6 min 50 s later, and the color faded completely 12 min 20 s after ICG injection, respectively (Figure 3). We irrigated the surgical field with normal saline and a tube was placed to drain the fluid. The operative time was approximately 140 min and blood loss was 50 mL.
Three days later, contrast-enhanced CT showed no splenic ischemia, localized fluid collections or splenic vein thrombosis, and the abdominal drainage tube was removed. The patient was discharged on postoperative day 8 after well recovery without any complications. Histopathology confirmed an aneurysm of the splenic artery. During the follow-up period, the blood platelet count was normal, and no abdominal pain, pancreatic insufficiency or recurrence of the aneurysm as well as no splenic infarction were observed.
SAAs are the most common visceral aneurysms accounting for 60%-70% of all cases, with an estimated prevalence of 1% in the population[4]. Early recognition and treatment of an SAA are essential, as 2%-10% present with rupture, resulting in a mortality rate of 25%-70% depending on the underlying pathology[5]. The management of an asymptomatic SAA is still controversial. SAAs with high-risk characteristics for rupture such as lesions > 2 cm in size, pregnancy and portal hypertension should be treated[6]. The mean diameter of non-ruptured SAAs was 2.2 cm, while that of ruptured SAAs was 3.1 cm according to one of the largest series published[7]. Investigators have been inclined to raise the standard to 2.5 cm due to the very low rupture risk in aneurysms below the standard, which is supported by retrospective studies[8].
Aneurysmectomy and endovascular repair are usually performed to treat SAAs. However, the splenectomy rate is approximately 76% during surgical treatment regardless of the size of the aneurysm[9]. Moreover, distal pancreatectomy or aneurysmectomy with vascular reconstruction have occasionally been performed concomitantly[10]. Even with spleen preservation, end-organ ischemia risk can occur after surgery and after interventional therapy. The most common ischemic incidents were post-embolization syndromes presenting as fever, abdominal pain, elevated leukocyte level and multiple splenic abscesses at the high rate of 31.8%[2]. Moreover, recanalization, coil migration and splenic infarction with abscess formation may occur. Laparoscopic ligation of a SAA in the proximal splenic artery is another method of preventing potential rupture of the SAA; however, there is still a risk of deficient residual blood flow to the spleen, thus leading to splenic infarction and possible evolution into a splenic abscess[11]. In the present case, the SAA was 3.5 cm in diameter, located approximately 3 cm from the CT, and it ruptured and eroded into the pancreatic parenchyma, indicating that it required immediate treatment. We chose SAA resection instead of ligation or other procedures for the following reasons: First, the SAA protruded into the pancreatic parenchyma with thrombogenesis and could potentially cause an abdominal infection; second, SAA may recur if the collateral circulation of the SAA was not blocked completely; third, the SAA’ anatomical position nearby the CT, leading to a high risk of recanalization and coil migration with interventional therapy. It was crucial to find a way of assessing the blood supply to the spleen after surgery and to determine the optimal surgical strategy during preoperative evaluation. Preoperative 3D virtual reconstruction and intraoperative ultrasound are usually used to confirm the residual blood flow in the spleen[11]. However, collateral vessels of the splenic hilum are difficult to confirm due to abundant blood vessels in the posterior wall of the stomach and the pancreatic tail, surrounding the splenic hilum. In the present case, the collateral vessels of the spleen were too abundant and small to be seen clearly on the 3D images. ICG is widely used in general surgery for staining liver segments, locating hepatic carcinoma, visualizing bile ducts and evaluating anastomotic blood supply due to its special attribute of fluorescence imaging[12-14]. The price of ICG is affordable for most patients at $18.8 United States dollars. Based on the characteristics of ICG and experience of fluorescence imaging-guided laparoscopic hepatectomy, ICG fluorescence imaging can detect segmental blood supply to spleen theoretically. However, it is rarely reported in splenic surgery. A recent study showed that ICG could visualize the spleen to assess the splenic blood supply, facilitating laparoscopic partial splenectomy[15]. Based on the characteristics of ICG visualization, we injected 2.5 mg ICG into a peripheral vein at the end of surgery, the whole spleen was stained green 6 min 50 s later, which indicated that fluorescence staining was complete and the splenic blood supply was satisfactory. The staining faded completely 12 min 20 s after ICG injection, which indicated that the splenic vein reflux was normal with a low risk of congestive splenomegaly. During the follow-up period, the blood platelet count was normal at all time points after surgery, and no abdominal pain, pancreatic insufficiency or recurrence of the aneurysm as well as no splenic infarction were observed. ICG fluorescence imaging is an effective and easy way to assess residual blood supply to the spleen and determine whether to preserve the spleen after surgical treatment of SAA.
ICG fluorescence imaging for spleen preservation in laparoscopic SAA resection is safe and may be beneficial in avoiding splenectomy and maintaining splenic function.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giordano A, Italy; Sultan AAEA, Egypt S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Rottenstreich A, Kleinstern G, Spectre G, Da'as N, Ziv E, Kalish Y. Thromboembolic Events Following Splenectomy: Risk Factors, Prevention, Management and Outcomes. World J Surg. 2018;42:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Lakin RO, Bena JF, Sarac TP, Shah S, Krajewski LP, Srivastava SD, Clair DG, Kashyap VS. The contemporary management of splenic artery aneurysms. J Vasc Surg. 2011;53:958-64; discussion 965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Zhu C, Zhao J, Yuan D, Huang B, Yang Y, Ma Y, Xiong F. Endovascular and Surgical Management of Intact Splenic Artery Aneurysm. Ann Vasc Surg. 2019;57:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Marone EM, Peri A, Argenti F, Pugliese L, Rinaldi LF, Pietrabissa A. Robotic Treatment of Complex Splenic Artery Aneurysms with Deep Hilar Location: Technical Insights and Midterm Results. Ann Vasc Surg. 2020;68:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Carr SC, Mahvi DM, Hoch JR, Archer CW, Turnipseed WD. Visceral artery aneurysm rupture. J Vasc Surg. 2001;33:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Varnavas G, Dolapsakis C. A giant splenic artery aneurysm. CMAJ. 2020;192:E608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Abbas MA, Stone WM, Fowl RJ, Gloviczki P, Oldenburg WA, Pairolero PC, Hallett JW, Bower TC, Panneton JM, Cherry KJ. Splenic artery aneurysms: two decades experience at Mayo clinic. Ann Vasc Surg. 2002;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Shukla AJ, Eid R, Fish L, Avgerinos E, Marone L, Makaroun M, Chaer RA. Contemporary outcomes of intact and ruptured visceral artery aneurysms. J Vasc Surg. 2015;61:1442-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Hamid HKS, Suliman AEA, Piffaretti G, Spiliopoulos S, Tetreau R, Tozzi M, Pulli R. A systematic review on clinical features and management of true giant splenic artery aneurysms. J Vasc Surg. 2020;71:1036-1045.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Mulpuri VB, Samanta J, Gupta P, Gupta V. En bloc resection in giant bilobed splenic artery aneurysm. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Wei YH, Xu JW, Shen HP, Zhang GL, Ajoodhea H, Zhang RC, Mou YP. Laparoscopic ligation of proximal splenic artery aneurysm with splenic function preservation. World J Gastroenterol. 2014;20:4835-4838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | van den Bos J, Al-Taher M, Schols RM, van Kuijk S, Bouvy ND, Stassen LPS. Near-Infrared Fluorescence Imaging for Real-Time Intraoperative Guidance in Anastomotic Colorectal Surgery: A Systematic Review of Literature. J Laparoendosc Adv Surg Tech A. 2018;28:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Hong D, Cheng J, Wu W, Liu X, Zheng X. How to Perform Total Laparoscopic Duodenum-Preserving Pancreatic Head Resection Safely and Efficiently with Innovative Techniques. Ann Surg Oncol. 2021;28:3209-3216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Nishino H, Seo S, Hatano E, Nitta T, Morino K, Toda R, Fukumitsu K, Ishii T, Taura K, Uemoto S. What is a precise anatomic resection of the liver? J Hepatobiliary Pancreat Sci. 2021;28:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Zhang T, Han W, Liu ZJ, Feng ZD, Niu X, Sun HT, Qiu F, Yang TC, JI Y. The role of indocyanine green fluorescence imaging in laparoscopic partial splenectomy. Zhonghua Gandan Waike Zazhi. 2021;27:367-370. [DOI] [Full Text] |