Published online Jul 27, 2022. doi: 10.4240/wjgs.v14.i7.696
Peer-review started: April 15, 2022
First decision: May 12, 2022
Revised: May 26, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: July 27, 2022
Processing time: 102 Days and 14.9 Hours
Colorectal adenocarcinoma is the third most common cancer worldwide. It accounts for almost 10% of all cancer-related deaths. Skeletal muscle is a very unusual site for metastasis from colorectal cancers and is associated with a poor prognosis and high mortality.
To review the literature for cases of skeletal muscle metastasis (SMM) from colorectal adenocarcinoma.
A systematic literature search using a validated search strategy was carried out to identify the incidence of SMM associated with colorectal adenocarcinoma. The studies identified were tabulated in a PRISMA, and data was extracted in a tabulated form.
Twenty-nine studies were included in this literature review. SMM was most commonly detected in the thigh muscles. Most of the tumours had originated from the rectum or the right colon. The histopathology of the primary tumour was generally advanced. The mean time interval between the primary tumour and onset of SMM was 22 mo. In 3 cases, asymptomatic SMM had been picked up by advanced imaging systems, like fluorodeoxyglucose-positron emission tomography scan.
SMM from colorectal adenocarcinomas is a rare complication. However, it is possible that the low incidence could be due to under-reporting. Early use of advanced imaging techniques and a high index of clinical suspicion might increase the reporting of SMM from colorectal adenocarcinoma.
Core Tip: Skeletal muscle metastasis (SMM) from a colorectal adenocarcinoma is a rare complication. Presentation usually occurs at a late stage, and prognosis remains poor. However, with a high index of suspicion and early use of advanced investigative modalities, like fluorodeoxyglucose-positron emission tomography scan, SMM can be detected and treated at an earlier stage. Further research is required to better understand the prognosis and pathophysiology of SMM.
- Citation: Kulkarni N, Khalil A, Bodapati S. Skeletal muscle metastasis from colorectal adenocarcinoma: A literature review. World J Gastrointest Surg 2022; 14(7): 696-705
- URL: https://www.wjgnet.com/1948-9366/full/v14/i7/696.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i7.696
Colorectal cancer is the third most common cancer worldwide, with at least 1.8 million new cases reported across the globe in 2018, and accounting for almost 10% of all cancer-related deaths worldwide[1,2]. Fortunately, there have been significant improvements in the life expectancy and survival rates after colorectal cancer. In particular, over the last 40 years, 5-year survival rates after a diagnosis of colorectal cancer have increased from 22% to 57%[2]. The improvement in survival has been attributed to a plethora of reasons, including screening and surveillance programmes, advanced endoscopic diagnostic and therapeutic techniques, use of minimally invasive surgical approaches (like laparoscopic and robotic techniques), and refined adjuvant and neoadjuvant chemotherapy and radiotherapy options.
Metastasis of colorectal cancer occurs via lymphatic, hematogenous and direct-spread routes, with the most common secondary sites being the liver, lungs, peritoneum, lymph nodes, and bones[3]. Intriguingly, although skeletal muscles constitute almost 50% of the total body mass, the incidence of metastasis to skeletal muscles from all forms of cancers is extremely low[4]. Many studies have commented on the possible reasons for the relatively low incidence of metastases to skeletal muscles. Hypotheses include variable blood flow to skeletal muscles, rare incidence of microvasculature damage due to cancer cells in skeletal muscles, and production of a low molecular weight non-protein factor that may inhibit tumour cell proliferation[5].
The aim of this study was to review the literature for cases of skeletal muscle metastasis (SMM) from colorectal adenocarcinoma.
A systematic literature search was carried out in December 2021, using a validated search strategy as described below.
The search was performed using Reference Citation Analysis, PubMed, Medline, Embase, Cochrane Library and Google Scholar databases. Journals, as well as society websites, were also searched using the search terms “skeletal muscle metastasis”, “colorectal cancer”, “case reports”, and “review.” The search strategy was standardized using the PRISMA guidelines. Two researchers (Khalil A, Bodapati S) reviewed the summary and abstracts of the articles. A full-text review was then performed by all three authors.
Articles that were not available in English language were excluded from the study. Only studies with full texts available that included data for pathological evidence of SMM from colorectal origin were considered. Studies with pathology data other than adenocarcinoma were excluded. No other exclusion criteria were used. The data were extracted by the three researchers and included patient characteristics, year of publication, site of primary tumour, presenting symptom, type of surgery performed for the primary lesion, site of skeletal and non-skeletal metastasis, time interval for onset of skeletal metastasis, and final outcome.
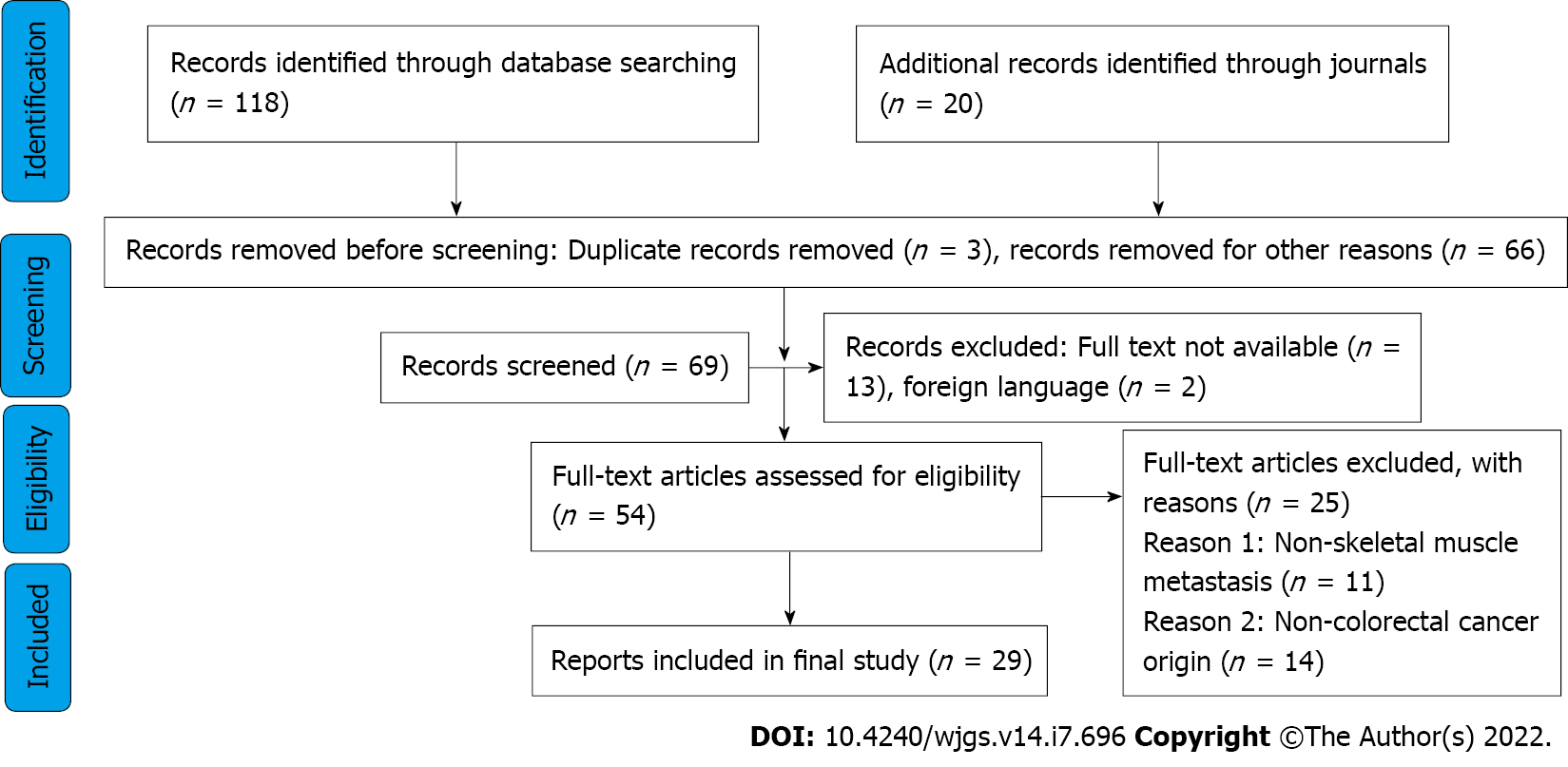
Information about the number of relevant citations, number and reasons of studies excluded after full assessment, as well as number of studies included in the systematic review fit in a well-designed PRISMA diagram, as presented in Figure 1.
The initial search yielded 138 eligible studies, of which 29 ultimately fit our inclusion criteria for the review (all case reports). These studies covered a total of 30 patients. Detailed characteristics of the studies are shown in Table 1.
| Case | Ref. | Age/sex | Site of primary tumour | Presenting symptom | Surgery of primary tumour | Histology of primary | Site of skeletal metastasis and treatment | Non-skeletal metastases | Time interval in mo | Follow-up/outcome |
| 1 | Hasegawa et al[14], 2000 | 60/M | Transverse colon | Not described | Transverse colon resection and lymph node dissection + FOLFOX | Adenocarcinoma | Right extensor carpi ulnaris muscle; a major part of the right extensor carpi ulnaris and the extensor digiti minimi muscle were resected, warranting a sufficient margin of 5 cm of normal tissue from the tumour | Multiple hepatic metastases detected 14 mo after primary resection and was resected | 24 | Alive |
| 2 | Buemi et al[3], 2019 | 69/F | Right colon | Pain when mobilizing left leg + elevated CEA of 7.7 ng/mL | Right hemicolectomy | pT3N0M0 (0/44 lymph nodes) | Left gluteus muscle; lesion was resected en bloc | 7 | Alive; 6 yr after colectomy and 65 mo after resection of the muscular metastasis she was tumour free with normal CEA level | |
| 3 | Yi et al[17], 2015 | 67/M | Caecum | Swelling and pain | Right hemicolectomy and subsequent chemotherapy with a regimen containing oxaliplatin | Poorly differentiated | Right thenar muscles | Liver, right kidney, right abdominal wall, left axillary and right subclavicular lymph nodes, skin of right thigh; treatment was given with palliative systemic chemotherapy (FOLFIRI) | Synchronous | Dead (9 mo after diagnosis) |
| 4 | Araki et al[18], 1994 | 66/M | Ascending colon | Painful lump | Right hemicolectomy | Right teres major; excision of the mass was performed | 6 | Dead (31 mo after surgery) | ||
| 5 | Manafi-Farid et al[19], 2019 | 23/M | Rectum | Incidentally detected in FDG-PET studies | Proctocolectomy preceded by neoadjuvant chemotherapy and followed by adjuvant chemotherapy, including the FOLFOX regimen | pT3N1 | Multiple: Deltoid, external oblique, biceps, tongue; excisional biopsy of the deltoid muscle lesion proved to be metastatic adenocarcinoma; commenced chemotherapy (FOLFIRI) | Lung/adrenal gland/scalp | 24 | Alive |
| 6 | Torosian et al[20], 1987 | 69/M | Transverse colon | Extended right colectomy | Left thigh; en bloc resection was performed | 60 | Not specified | |||
| 7 | Okada et al[21], 2009 | 70/M | Rectum | Painful lump | Rectal resection | Right thigh; resection and chemotherapy were given | Lung | 12 | Alive; the resection of SMM made a positive contribution to his quality of life | |
| 8 | Chang et al[22], 1994 | 62/M | Descending colon | Painful lump | Left tibialis anterior; excision of the mass was performed | Synchronous | Not specified | |||
| 9 | Yoshikawa et al[23], 1999 | 54/M | Sigmoid colon | Severe buttocks pain | Partial sigmoid colectomy | Right buttocks; en bloc resection performed | Multiple metastases | 24 | Died after 8 mo from multiple metastases | |
| 10 | Guo et al[16], 2021 | 43/M | Ascending colon | Right thigh mass 4 cm × 4 cm with intolerable pain | Laparoscopic extended right hemicolectomy and four cycles of chemotherapy with CapeOX | PT4N2bM0; poorly differentiated adenocarcinoma | Right thigh; a complete resection was suggested but was refused by the patient; unresponsive to FOLFIRI; switched to bevacizumab, irinotecan, and capecitabine | Bony metastasis and multiple lymph node metastases around the abdominal aorta | 5 | Deteriorated and died 9 mo after primary resection |
| 11 | Tatsuta et al[24], 2022 | 83/M | Ascending colon | Pain in the back of his neck | Curative resection | Adenocarcinoma | Cervical (neck muscle); he was prescribed palliative radiation therapy because of his poor performance status | None | 11 | Died 2 mo after diagnosis of muscle metastasis |
| 12 | Iusco et al[25], 2005 | 73/F | Ascending colon | Painful lump | Right hemicolectomy | Dukes C | Left calf; the mass was excised and received adjuvant radiotherapy | None | 24 | Alive; no sign of recurrence at a 2-yr follow-up |
| 13 | Landriscina et al[9], 2013 | 71/F | Right colon | Detected on PET/CT scan | Right hemicolectomy with subsequent systemic neoadjuvant chemotherapy for liver metastasis followed by radical hepatectomy | Poorly differentiated adenocarcinoma | Deltoid, sternocleidomastoid and other multiple sites; chemotherapy with FOLFOX was administered for 3 cycles but discontinued due to traumatic femur fracture | Liver/lung | 23 | Disease progression and death |
| 14 | Hattori et al[26], 2008 | 64/F | Rectum | Asymptomatic; increased CEA; discovered by FDG-PET | Abdominoperineal rectal resection | Moderately differentiated adenocarcinoma | Right thoracic paraspinal muscles; en bloc excision was performed including the paraspinal muscles | Solitary lung metastasis, which was resected 3 yr previously by lobectomy with subsequent immunochemotherapy | 96 | Alive |
| 15 | Choi et al[27], 2008 | 83/F | Rectum | Painful lump | Low anterior resection and right liver lobectomy | T2N1M1 | Semimembranous muscle of right thigh | Solitary pulmonary nodule in left lobe | 48 | Died of heart failure on second postoperative day |
| 16 | Doroudinia et al[28], 2019 | 48/M | Rectum | Subcutaneous lump | Abdominoperineal rectal resection followed by adjuvant radiotherapy and chemotherapy | High grade mucinous adenocarcinoma | Right proximal thigh; the patient became a candidate for tumour excision (metastasectomy) followed by additional course of chemotherapy. | None | 38 | Not specified |
| 17 | Tunio et al[29], 2013 | 28/M | Transverse colon | Abdominal pain and hard nodule at anterior abdominal wall | Extended right hemicolectomy; radiotherapy; FOLFOX4 | Mucinous moderately differentiated adenocarcinoma T4N2bM0 | Rectus abdominis muscle and right gluteus maximus; underwent palliative radiotherapy followed by systemic chemotherapy | None | 11 | Alive at time of publication with progressive disease |
| 18 | Simeunovic et al[30], 2014 | 55/F | Rectum | Lower back pain and left hip pain as first manifestation of the primary tumour | Radiotherapy; chemotherapy with FOLFOX | Poorly differentiated adenocarcinoma | Left adductor muscle | None | Synchronous | Not specified |
| 19 | Prabhu et al[31], 2017 | 69/M | Rectum | Severe low back ache | Neoadjuvant; abdominoperineal resection; capecitabine | Adenocarcinoma with signet ring cell features T3N2; Dukes C1 | Multiple skeletal muscles: left sartorious, left vastus lateralis, left infraspinatus, left levator scapulae, left tenth Intercostal muscle, right subscapularis muscle | None | 4 | Not specified |
| 20 | Tai et al[32], 2014 | 81/M | Caecum | Severe right shoulder pain | Palliative chemotherapy; palliative right hemicolectomy | Poorly differentiated adenocarcinoma | Right supraspinatus muscle | Right lobe of lung | Synchronous | Patient transitioned to hospice |
| 21 | Farraj et al[33], 2021 | 52/F | Rectum | Noted with preoperative staging | Low anterior resection; adjuvant combination of oxaliplatin, capecitabine, and pelvic external beam radiation therapy | Moderately differentiated adenocarcinoma T2N1a | Left psoas muscle | None | Synchronous | Patient is currently maintained on platinum doublet chemotherapy with control of metastatic disease |
| 22 | Salar et al[34], 2012 | 67/F | Rectum | Deep pelvic and left buttock pain | EUA; submucosal polypectomy | Tubullovillous adenomatous polyp with high grade dysplasia | Left piriformis muscle | None | 18 | Patient began cycles of chemoradiotherapy with plans for further surgical resection |
| 23 | Homan et al[35], 2000 | 72/F | Descending colon | Surgical resection; FOLFOX | Thigh | NA | ||||
| 24 | Takada et al[36], 2011 | 71/M | Sigmoid colon | Radiotherapy; FOLFOX; resection “Hartmann” | Stage III adenocarcinoma | Left iliopsoas muscle; received radiotherapy and 15 courses of FOLFOX + bevacizumab for decreasing large and unresectable tumour; then resection was performed | GI metastasis | 60 | 5 mo after resection of muscle metastasis, there was no recurrence | |
| 25 | Naik et al[37], 2005 | 56/M | Ascending colon | A lump | Resection; chemotherapy FOLFOX; radiotherapy | Mucin secreting adenocarcinoma | Rectus abdominis muscle; resection was performed | NA | 60 | Not specified |
| 26 | Burgueño Montañés and López Roger[38], 2002 | 60/M | Rectosigmoid | Exophthalmos | Radiotherapy; FOLFOX | Lateral rectus muscle | Not specified | |||
| 27 | García-Fernández et al[39], 2012 | 32/M | Colon | Palpebral oedema, conjunctival chemosis, severe exophthalmos, complete ptosis in left eye and limitation in eye movement mainly in abduction and supraversion | Resistant to chemotherapy | Stage IV | Superior rectus elevator muscle of upper eyelid complex and external rectus muscle | Due to the patient generally feeling unwell, radiotherapy was not considered, and an intravenous bolus of corticoids was given, without response, resulting in the death of the patient | ||
| 28 | Lampenfeld et al[40], 1990 | 75/F | Rectum | Progressive growth of left buttock mass | Excision of mass | Adenocarcinoma | Left gluteus maximus and medius | 24 | ||
| 29 | Laurence and Murray[41], 1970; Case 1 | 70/F | Caecum | Painful mass in posteroexternal aspect of right calf and leg oedema | Right hemicolectomy | Ulcerated villous adenocarcinoma | Right calf; en bloc resection was performed | Generalized metastasis | 24 | Died due to generalized metastasis |
| 30 | Laurence and Murray[41], 1970; Case 2 | 51/M | Transverse colon | Right colectomy | Right forearm; en bloc resection was performed | Generalized metastasis | Synchronous | Died due to generalized metastasis |
The median age of the patients was 67 years (range: 23-83 years), with 19 male patients and 11 female patients. The primary tumour was present in the right colon in 10 patients, transverse colon in 4, left colon in 5, and rectum in 11. The presenting symptoms were pain (6 patients), palpable lump (4 patients), painful lump (9 patients), and ocular symptoms (2 patients). Three of the patients had the SMM incidentally diagnosed by imaging. Only 3 of the reported cases mention an early primary lesion (tubulovillous adenoma with high-grade dysplasia or T2 stage tumours). Six cases reported indicated that the primary lesion was of an advanced nature (T3 or T4). The tumours were either moderately or poorly differentiated in 6 cases. Four of the reported cases indicated that the primary lesion was either a mucin-secreting tumour or signet ring cell tumour.
The mean time interval between the diagnosis of the primary tumour and presentation of SMM was 22 mo. Six cases were diagnosed synchronously with the metastasis. There were a wide range of skeletal muscles that were involved in the metastasis, as follows: Upper limb (extensor carpi ulnaris, thenar, deltoid, biceps); lower limb (thigh, tibialis anterior, semimembranous, adductor, sartorius, vastus lateralis); trunk (teres major, glutei, external oblique, neck muscles, paraspinal, rectus abdominus, intercostal, psoas, piriformis); and, extraocular muscles (lateral rectus, superior rectus). However, the most common site of metastasis was the thigh muscle. In 8 cases, the skeletal muscles were the only site of metastasis.
There was no detailed information about the duration of follow-up and final outcome of the disease; however, 10 case reports mentioned that the patients did not survive the disease.
Colorectal cancers account for 10.7% of all new cancers reported worldwide[2]. Our literature review has shown that since 1970, only 30 cases of SMM due to colorectal adenocarcinomas have been reported. This highlights the extremely low incidence of skeletal muscle as a metastatic site due to colorectal adenocarcinoma.
The primary pathology in the majority of the patients was in the rectum (11 patients) and the right colon (10 patients). Left-sided colonic tumours accounted for 5 of the cases and transverse colon for 4. A large meta-analysis carried out by Prasanna et al[6] highlighted the different metastatic patterns of colorectal cancers, depending on the site of the primary tumour. This study showed that right colonic tumours were more frequently associated with peritoneal seeding, and rectal tumours were more frequently associated with lung, brain and bone metastases compared to left colonic tumours. Though SMMs were not mentioned in this meta-analysis, the general pattern of higher metastases in right colonic and rectal tumours was also seen in our review. Only 8 patients had no documented simultaneous metastasis in non-skeletal muscles. The other patients had metastases in non-skeletal muscle sites.
The most common presenting symptom of the SMM was a painful lump (9 patients). Six patients had a palpable lump with no description of pain, and 6 patients had pain as the presenting symptom. Three patients had the SMM diagnosed incidentally by imaging. The importance of advanced imaging techniques, especially fluorodeoxyglucose-positron emission tomography (FDG-PET) scanning, for diagnosis of SMM has been highlighted by Emmering et al[7]. Lesions that cannot be detected by routine contrast computed tomography or magnetic resonance imaging can be observed by FDG-PET scans. FDG-PET had a significant impact on early diagnosis and patient management in 51% of cases with muscle metastasis. Hence, if there is a suspicion of SMM, the early use of FDG-PET should be encouraged for diagnosis.
Our review showed that most of the primary tumours were of an advanced nature (either T3 or T4 with positive lymph node status and poor differentiation). Three patients had mucinous features, and 1 patient had signet ring cell features. This raises the possibility that colorectal cancers with advanced aggressive features on the primary pathology have a higher incidence of SMM. Studies have shown that colorectal cancers with advanced pathological features have worse outcomes than early cancers[8]. It has been proposed that the presence of other coexisting pathologies could increase the chances of getting SMM due to colorectal adenocarcinomas. Landriscina et al[9] commented that dermatomyositis and other paraneoplastic syndromes could increase the chances of getting SMM. Kanani et al[10] also documented a case of multiple SMM associated with colorectal adenocarcinoma and non-Hodgkin’s lymphoma with ulcerative colitis. However, no other studies in our literature review commented on any other coexisting pathologies.
The use of minimally invasive approaches has revolutionized the surgical treatment of colorectal cancers. Colorectal resections are now routinely undertaken with the laparoscopic and robotic approaches. Patients have smaller incisions, shorter hospital stays and equal oncological outcomes[11]. The use of laparoscopic surgery for colorectal procedures started in 1990 but became more widespread only in the 21st century. Our case reports were from a lengthy time period, beginning in 1970. Only two case reports specifically mention the use of a laparoscopic approach for the resection. Previous studies have shown that the incidence of distant metastasis and peritoneal seeding is not different between laparoscopic and open approaches[12]. In our search. we did not find any studies that observed that the laparoscopic approach led to fewer distant metastases. However, due to the advantage of decreased environmental exposure due to operating in closed cavities and smaller incisions, the possibility always remains that peritoneal seeding and subsequent metastasis incidence could be lower in minimally invasive approaches.
The incidence of SMM was detected in up to 5.6% of patients in a post-mortem series of cancer patients[13]. However, the incidence of SMM due to colorectal cancers is still extremely low and has been reported to be about 0.028%[14]. The outcome from SMM is generally poor. A large study investigating soft tissue metastases postulated that the survival time from diagnosis to death is 5.4 mo[15]. The studies included in our review were all case reports, and the duration of follow-up was not documented in most of these studies. Hence, it is not possible to comment on the exact mortality of SMM from our study. However, the presence of SMM generally indicates disseminated disease, which would indicate a very poor prognosis.
There have been previous studies that have studied the incidence of SMM due to colorectal cancer[16]. However, we found SMM has been documented in 30 patients in the literature. We believe that this is the maximum number of cases of SMM due to colorectal cancers that have been reported in the literature. All the studies identified were case reports, and very few of these had long-term follow-up. Hence, it is not possible to definitely comment on the treatment strategies and long-term outcomes for these patients. This study again highlights that there is a paucity of literature on SMM due to colorectal adenocarcinoma. This is certainly a field that needs more research in the future.
Our review showed that SMM from colorectal adenocarcinomas is a rare complication. However, it is possible that the low incidence could be due to under-reporting. Early use of advanced imaging techniques like FDG-PET and a high index of clinical suspicion might increase the reporting of SMM from colorectal adenocarcinoma.
Skeletal muscle metastasis (SMM) is a rare complication of colorectal adenocarcinomas. The study was conducted to explore, in more detail, the present literature of this unusual finding.
The study encompassed a thorough review of the present literature on SMM due to colorectal adenocarcinoma. Our goal was to highlight the significance of this type of metastasis and increase awareness for early diagnosis and detection.
The aim of this study was to review the literature for cases of SMM from colorectal adenocarcinoma.
A systematic literature search was carried out in December 2021. The search strategy was standardized using the PRISMA guidelines.
SMM were most commonly detected in the thigh muscles. Most of the tumours originated from the rectum or the right colon. The mean time interval between the primary lesion and onset of SMM was 22 mo.
Our review showed that SMM from colorectal adenocarcinomas is a rare complication. However, it is possible that the low incidence could be due to under-reporting. Early use of advanced imaging techniques, like fluorodeoxyglucose-positron emission tomography, and a high index of clinical suspicion might increase the reporting of SMM from colorectal adenocarcinoma.
This study again highlights that there is a paucity of literature on SMM after colorectal adenocarcinoma. This is certainly a field that needs more research in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Tang D, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | World Cancer Research Fund. Worldwide cancer data. [cited 10 March 2022]. Available from: https://www.wcrf.org/dietandcancer/worldwide-cancer-data/. |
| 2. | Cancer Research UK. Bowel cancer statistics. [cited 10 March 2022]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer. |
| 3. | Buemi A, Aunak S, Mansvelt B. The Gluteus Muscle an Unusual Localization of a Colon cancer metastasis: Case report and review of literature. Clin Sur. 2019;4:2575. |
| 4. | Damron TA, Heiner J. Distant soft tissue metastases: a series of 30 new patients and 91 cases from the literature. Ann Surg Oncol. 2000;7:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Djaldetti M, Sredni B, Zigelman R, Verber M, Fishman P. Muscle cells produce a low molecular weight factor with anti-cancer activity. Clin Exp Metastasis. 1996;14:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 6. | Prasanna T, Karapetis CS, Roder D, Tie J, Padbury R, Price T, Wong R, Shapiro J, Nott L, Lee M, Chua YJ, Craft P, Piantadosi C, Sorich M, Gibbs P, Yip D. The survival outcome of patients with metastatic colorectal cancer based on the site of metastases and the impact of molecular markers and site of primary cancer on metastatic pattern. Acta Oncol. 2018;57:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Emmering J, Vogel WV, Stokkel MP. Intramuscular metastases on FDG PET-CT: a review of the literature. Nucl Med Commun. 2012;33:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Pozos-Ochoa LI, Lino-Silva LS, León-Takahashi AM, Salcedo-Hernández RA. Prognosis of Signet Ring Cell Carcinoma of the Colon and Rectum and their Distinction of Mucinous Adenocarcinoma with Signet Ring Cells. A Comparative Study. Pathol Oncol Res. 2018;24:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Landriscina M, Gerardi AM, Fersini A, Modoni S, Stoppino LP, Macarini L, Sanguedolce F, Bufo P, Neri V. Multiple skeletal muscle metastases from colon carcinoma preceded by paraneoplastic dermatomyositis. Case Rep Med. 2013;2013:392609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kanani H, Gandhi N, Sheth A, Kulkarni N. Multiple primary malignant neoplasms: a case of colonic adenocarcinoma and non-Hodgkin's lymphoma on a background of ulcerative colitis. Ann R Coll Surg Engl. 2022;104:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;CD003145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 431] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 12. | Zanghì A, Cavallaro A, Piccolo G, Fisichella R, Di Vita M, Spartà D, Zanghì G, Berretta S, Palermo F, Cappellani A. Dissemination metastasis after laparoscopic colorectal surgery vs conventional open surgery for colorectal cancer: a metanalysis. Eur Rev Med Pharmacol Sci. 2013;17:1174-1184. [PubMed] |
| 13. | Nocuń A, Chrapko B. Multiple and solitary skeletal muscle metastases on 18F-FDG PET/CT imaging. Nucl Med Commun. 2015;36:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 14. | Hasegawa S, Sakurai Y, Imazu H, Matsubara T, Ochiai M, Funabiki T, Suzuki K, Mizoguchi Y, Kuroda M, Kasahara M. Metastasis to the forearm skeletal muscle from an adenocarcinoma of the colon: report of a case. Surg Today. 2000;30:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Plaza JA, Perez-Montiel D, Mayerson J, Morrison C, Suster S. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer. 2008;112:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Guo Y, Wang S, Zhao ZY, Li JN, Shang A, Li DL, Wang M. Skeletal muscle metastasis with bone metaplasia from colon cancer: A case report and review of the literature. World J Clin Cases. 2021;9:9285-9294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Yi C, Liu Z, Chu Y, Li S, Liu L, Li J, Yu X. Ileocecal adenocarcinoma with overexpression of P53 protein metastasized to the thenar muscle: report of a rare case and review of literature. Int J Clin Exp Pathol. 2015;8:13546-13551. [PubMed] |
| 18. | Araki K, Kobayashi M, Ogata T, Takuma K. Colorectal carcinoma metastatic to skeletal muscle. Hepatogastroenterology. 1994;41:405-408. [PubMed] |
| 19. | Manafi-Farid R, Ayati N, Eftekhari M, Fallahi B, Masoumi F. A Rare Presentation of Colorectal Cancer with Unusual Progressive Intramuscular and Subcutaneous Metastatic Spread. Asia Ocean J Nucl Med Biol. 2019;7:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Torosian MH, Botet JF, Paglia M. Colon carcinoma metastatic to the thigh--an unusual site of metastasis. Report of a case. Dis Colon Rectum. 1987;30:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Okada T, Nishimura T, Nakamura M, Sakata K, Setoguchi M, Ihara K. [Skeletal muscle metastasis of rectal carcinoma--a case report]. Gan To Kagaku Ryoho. 2009;36:2248-2250. [PubMed] |
| 22. | Chang PC, Low HC, Mitra AK. Colonic carcinoma with metastases to the tibialis anterior muscle--a case report. Ann Acad Med Singap. 1994;23:115-116. [PubMed] |
| 23. | Yoshikawa H, Kameyama M, Ueda T, Kudawara I, Nakanishi K. Ossifying intramuscular metastasis from colon cancer: report of a case. Dis Colon Rectum. 1999;42:1225-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (2)] |
| 24. | Tatsuta K, Harada T, Nishiwaki Y. Cervical Skeletal Muscle Metastasis of Colorectal Cancer. Intern Med. 2022;61:263-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Iusco D, Sarli L, Mazzeo A, Donadei E, Roncoroni L. Calf metastasis of colorectal cancer: report of a case. Chir Ital. 2005;57:783-787. [PubMed] |
| 26. | Hattori H, Nishimura H, Matsuoka H, Yamamoto K. FDG-PET demonstration of asymptomatic skeletal muscle metastasis from colorectal carcinoma. J Orthop Sci. 2008;13:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Choi PW, Kim CN, Kim HS, Lee JM, Heo TG, Park JH, Lee MS, Chang SH. Skeletal Muscle Metastasis from Colorectal Cancer: Report of a Case. J Korean Soc Coloproctol. 2008;24. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Doroudinia A, Mehrian P, Dorudinia A, Kaghazchi F. Rectal adenocarcinoma presenting with thigh muscle metastasis as the only metastatic site. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 29. | Tunio MA, Al Asiri M, Riaz K, Al Shakwer W, Alarifi A. Skeletal Muscle Metastasis Secondary to Adenocarcinoma of Colon: A Case report and review of literature. J Gastroint Dig Syst. 2013;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Simeunovic K, Sharma D, Jain V, Botic J, Ramasamy V, Anklesaria A, Korsten M, Ahmed A. A Rare Case of a Skeletal Muscle Mass as an Initial Presentation of Metastatic Colon Carcinoma. Official J Am College Gas. 2014;109:S412. |
| 31. | Prabhu M, Raju SHVN, Sachani H. Rectal Carcinoma Metastases to Multiple Skeletal Muscles-Role of F-18 FDG PET/CT. Indian J Nucl Med. 2017;32:214-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Tai CH, Park C, Bhardwaj R, Jean RD, Harshan M, Ilyas N, Swaminath A. Skeletal Muscle (SM) Metastasis: A Rare Initial Presentation of Metastatic Colon Cancer. Official J Am College Gas. 2014;114. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Farraj K, Im J, Gonzalez LF, Lu A, Portnoy R 3rd, Podrumar A. Solitary Cystic Psoas Muscle Metastasis From Rectosigmoid Adenocarcinoma. J Investig Med High Impact Case Rep. 2021;9:23247096211024067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 34. | Salar O, Flockton H, Singh R, Reynolds J. Piriformis muscle metastasis from a rectal polyp. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 35. | Homan HH, Mühlberger T, Kuhnen C, Steinau HU. [Intramuscular extremity metastasis of adenocarcinoma of the colon]. Chirurg. 2000;71:1392-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Takada J, Watanabe K, Kuraya D, Kina M, Hayashi S, Hamada H, Katsuk Y. [An example of metastasis to the iliopsoas muscle from sigmoid colon cancer]. Gan To Kagaku Ryoho. 2011;38:2294-2297. [PubMed] |
| 37. | Naik VR, Jaafar H, Mutum SS. Heterotopic ossification in skeletal muscle metastasis from colonic adenocarcinoma--a case report. Malays J Pathol. 2005;27:119-121. [PubMed] |
| 38. | Burgueño Montañés C, López Roger R. [Exophthalmos secondary to a lateral rectus muscle metastasis]. Arch Soc Esp Oftalmol. 2002;77:507-510. [PubMed] |
| 39. | García-Fernández M, Castro-Navarro J, Saiz-Ayala A, Álvarez-Fernández C. [Orbital metastases in colorectal cancer: a case report]. Arch Soc Esp Oftalmol. 2012;87:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Lampenfeld ME, Reiley MA, Fein MA, Zaloudek CJ. Metastasis to skeletal muscle from colorectal carcinoma. A case report. Clin Orthop Relat Res. 1990;193-196. [PubMed] |
| 41. | Laurence AE, Murray AJ. Metastasis in skeletal muscle secondary to carcinoma of the colon--presentation of two cases. Br J Surg. 1970;57:529-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 0.7] [Reference Citation Analysis (1)] |