Published online Jul 27, 2022. doi: 10.4240/wjgs.v14.i7.670
Peer-review started: January 30, 2022
First decision: March 12, 2022
Revised: March 28, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: July 27, 2022
Processing time: 178 Days and 5.2 Hours
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. However, the number of patients with chronic kidney disease (CKD) is on the rise because of the increase in lifestyle-related diseases.
To establish a tailored management strategy for HCC patients, we evaluated the impact of comorbid renal dysfunction (RD), as stratified by using the estimated glomerular filtration rate (EGFR), and assessed the oncologic validity of hepatectomy for HCC patients with RD.
We enrolled 800 HCC patients who underwent hepatectomy between 1997 and 2015 at our university hospital. We categorized patients into two (RD, EGFR < 60 mL/min/1.73 m2; non-RD, EGFR ≥ 60 mL/min/1.73 m2) and three groups (severe CKD, EGFR < 30 mL/min/1.73 m2; mild CKD, 30 ≤ EGFR < 60 mL/min/1.73 m2; control, EGFR ≥ 60 mL/min/1.73 m2) according to renal function as defined by the EGFR. Overall survival (OS) and recurrence-free survival (RFS) were compared among these groups with the log-rank test, and we also analyzed survival by using a propensity score matching (PSM) model to exclude the influence of patient characteristics. The mean postoperative observation period was 64.7 ± 53.0 mo.
The RD patients were significantly older and had lower serum total bilirubin, aspartate amino
Comorbid mild RD had a negligible impact on the prognosis of HCC patients who underwent curative hepatectomy with appropriate perioperative management, and close attention to severe CKD is necessary to prevent postoperative bleeding and surgical site infection.
Core Tip: This retrospective study revealed that comorbid renal dysfunction (RD) had a negligible impact on the prognosis of hepatocellular carcinoma patients who underwent curative hepatectomy with appropriate perioperative management, and close attention to severe chronic kidney disease is necessary to prevent postoperative bleeding and surgical site infection. Of particular interest is the finding that regardless of the degree of comorbid RD, the overall survival rate and recurrence-free survival rate were comparable, even when using a propensity model to exclude the influence of patient characteristics, liver function, and other causes of death. Moreover, no RD patient, even severe RD patients, received maintenance hemodialysis after hepatectomy.
- Citation: Sakamoto Y, Shimada S, Kamiyama T, Sugiyama K, Asahi Y, Nagatsu A, Orimo T, Kakisaka T, Kamachi H, Ito YM, Taketomi A. Impact of comorbid renal dysfunction in patients with hepatocellular carcinoma on long-term outcomes after curative resection. World J Gastrointest Surg 2022; 14(7): 670-684
- URL: https://www.wjgnet.com/1948-9366/full/v14/i7/670.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i7.670
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death in many parts of the world and is estimated to be the fourth most common cause of cancer-related death worldwide[1-3]. Hepatectomy for the treatment of HCC has the highest controllability among local treatments and results in a good survival rate[4,5]. However, chronic kidney disease (CKD) affects 8% to 16% of the population worldwide, especially in developed countries, and the number of patients with CKD is on the rise; additionally, CKD is most commonly attributed to diabetes and/or hypertension[6]. Several studies have shown that patients with CKD who undergo any major surgery are at risk because they have more comorbidities, including coagulopathy and systemic atherosclerosis[7-9]. Previous reports have shown a relationship between preoperative renal dysfunction (RD) and prognosis and posto
We enrolled 800 HCC patients who underwent hepatectomy between January 1997 and December 2015 at the Gastroenterological Surgery Unit of Hokkaido University Hospital in Sapporo, Japan. Baseline information, including the etiology of chronic liver disease, serum biochemistry, severity of cirrhosis, performance status, and cancer stage, was recorded when the diagnosis was established. This study was conducted with the approval of the Institutional Review Board of Hokkaido University Hospital (No. 016-0354) and was performed in accordance with the Helsinki Declaration guidelines. Informed consent was obtained in the opt-out form on the website of Hokkaido University Hospital.
The diagnosis of HCC, disease progression and resectability status were assessed via general status, physical findings, serological tests, and imaging studies, including contrast-enhanced computed tomography, magnetic resonance imaging, and ultrasonography. Liver function was assessed with a blood liver function test, the Child-Pugh grade, the estimated indocyanine green retention rate at 15 min[15,16], and the technetium-99 m-galactosyl human serum albumin scintigraphy index[17]. To evaluate the feasibility of hepatectomy in HCC patients with RD, the primary endpoint of the present study was long-term outcomes [median survival time (MST)] after hepatectomy. The secondary endpoint was postoperative complications.
Preoperative RD was defined by the preoperative EGFR. CKD stage 3a (45 ≤ EGFR < 60 mL/min/1.73 m2) or higher according to KDIGO CKD guideline is reportedly associated with an increase in the risk of various diseases and mortality[18-20], so the RD group comprised patients with an EGFR < 60 mL/min/1.73 m2, and the non-RD group comprised patients with an EGFR ≥ 60 mL/min/1.73 m2. Moreover, we also categorized patients into three groups according to the RD as defined by the EGFR (severe CKD, EGFR < 30 mL/min/1.73 m2; mild CKD, 30 ≤ EGFR < 60 mL/min/1.73 m2; control, EGFR ≥ 60 mL/min/1.73 m2) because patients with ESRD who were undergoing dialysis were likely to be at high risk of developing HCC[21].
The criteria for hepatectomy were decided regardless of renal function. Surgical procedures were determined according to the patient’s liver function and general status, including the extent of disease[22], and were classified as anatomical resection (subsegmentectomy, segmentectomy, bisegmen-tectomy, and trisegmentectomy) or nonanatomical resection (partial resection). Postoperative complications of class II or higher according to the Clavien-Dindo classification system were recorded[23]. Postoperative mortality was defined as death within 90 d after surgery.
All the patients were managed pre- and postoperatively according to previous reports[22]. In particular, the nephrology team was consulted on cases of severe CKD, and preparations for emergency hemodialysis were made prior to surgery. For six patients in the RD group on maintenance hemodialysis, hemodialysis was scheduled to be performed the day before surgery, one day postoperatively, and then three times per week thereafter.
Categorical data were compared with the χ2 test. Continuous data were compared between the RD and non-RD groups by the Mann-Whitney U test and among the three groups (severe CKD, mild CKD, and non-RD) by the Kruskal-Wallis U test. The EGFR values before and one month after hepatectomy in patients with severe CKD were compared by a paired t test. Overall survival (OS) and recurrence-free survival (RFS) curves were drawn using the Kaplan-Meier method with the generalized log-rank test for in all 800 patients, and 110 pairs of matched HCC patients were selected by using a propensity score matching (PSM) model. This PSM model was constructed with patients’ age, etiology, and laboratory data such as the levels of serum total bilirubin (T-bil), aspartate aminotransferase (AST), aspartate aminotransferase (ALT), and hemoglobin A1c (HbA1c). Univariate and multivariate analyses were performed using Cox proportional hazards regression models. A P value less than 0.05 was considered statistically significant. All statistical analyses were conducted with JMP 16 software (SAS Institute Inc., Cary, NC, United States) or GraphPad Prism 7 (GraphPad Software, Inc., La Jolla CA, United States).
The patients in the RD group (128 patients, 16.0%) were significantly older (P < 0.0001), had a lower prevalence of hepatitis B (P < 0.001), had lower serum T-bil, AST, ALT, alpha-fetoprotein (AFP), and AFP isoform, lectin affinity (AFP-L3) levels (P < 0.001, P < 0.05, P < 0.01, P < 0.01, and P < 0.05, respectively), had a higher prevalence of non-hepatitis B virus (HBV) and non-hepatitis C virus (HCV) (NBNC) (P < 0.001), and had higher serum HbA1c, blood urea nitrogen (BUN), and Cr levels (P < 0.05, respectively) than the patients in the non-RD group (Table 1). The preoperative characteristics of the severe CKD, mild CKD and non-RD patient groups are summarized in Table 2. Nineteen patients had severe CKD, including six patients who received routine preoperative hemodialysis, and 109 patients had mild CKD. Age (73.0, 69.0, and 63.0 years; P < 0.0001), female ratio (31.6%, 10.1%, and 18.3%; P < 0.05), BUN (38.0 mg/dL, 19.0 mg/dL, and 14.0 mg/dL; P < 0.0001), and Cr (2.4 mg/dL, 1.0 mg/dL, and 0.7 mg/dL; P < 0.0001) and AFP-L3 levels (21.7%, 0%, and 3.1%; P < 0.05) in the severe CKD patient group were significantly higher than those in the other patient groups. On the other hand, the serum albumin (3.8 g/dL, 4.1 g/dL, and 4.1 g/dL; P < 0.01), T-bil (0.4 mg/dL, 0.7 mg/dL, and 0.8 mg/dL; P < 0.001), ALT (21.0 IU/L, 34.0 IU/L, and 40.0 IU/L; P < 0.05), and cholinesterase levels (181.0 IU/L, 249.0 IU/L, and 245.0 IU/L; P < 0.01) in the severe CKD group were significantly lower than those in the other patient groups. The NBNC ratio (31.6%, 47.7%, and 28.5%; P < 0.001) and HbA1c level (5.5%, 5.9%, and 5.3%; P < 0.05) in the mild CKD patient group were higher and the HBV ratio (26.3%, 22.0%, and 39.1%; P < 0.01) in the severe and mild CKD groups was lower than those in the non-RD group. The mean follow-up time was 64.7 ± 53.0 mo after hepatectomy.
| RD (EGFR < 60), n = 128 | Non-RD (EGFR ≥ 60), n = 672 | P value | |
| Age (yr) | 69.5 ± 8.6 | 63.0 ± 10.4 | < 0.0001 |
| Sex | |||
| Male | 111 (86.7) | 549 (81.7) | 0.17 |
| Female | 17 (13.3) | 123 (18.3) | - |
| Etiology | |||
| HBV | 29 (22.7) | 263 (39.1) | < 0.001 |
| HCV | 41 (32.0) | 218 (32.4) | 0.93 |
| NBNC | 58 (45.3) | 191 (28.5) | < 0.001 |
| Child-Pugh grade | |||
| A | 124 (96.9) | 649 (96.6) | 0.86 |
| B | 4 (3.1) | 23 (3.4) | - |
| Laboratory data | |||
| Plt (×104/μL) | 16.2 ± 6.2 | 15.5 ± 7.3 | 0.26 |
| PT (%) | 94.9 ± 13.7 | 0.08 | |
| Alb (g/dL) | 4.0 ± 0.4 | 4.1 ± 0.4 | 0.32 |
| T-bil (mg/dL) | 0.7 ± 0.3 | 0.8 ± 0.4 | < 0.001 |
| AST (IU/L) | 35.5 ± 31.2 | 43.0 ± 43.4 | < 0.05 |
| ALT (IU/L) | 31.5 ± 30.0 | 40.0 ± 36.1 | < 0.01 |
| ChE (IU/L) | 238.0 ± 89.8 | 245.0 ± 81.3 | 0.92 |
| ICG15R (%) | 14.4 ± 7.3 | 13.6 ± 10.6 | 0.61 |
| HbA1c (%) | 5.7 ± 1.1 | 5.3 ± 1.1 | < 0.05 |
| BUN (mg/dL) | 20.0 ± 10.8 | 14.0 ± 4.0 | < 0.0001 |
| Cr (mg/dL) | 1.1 ± 1.6 | 0.7 ± 0.1 | < 0.0001 |
| AFP (ng/mL) | 10.3 (1.4-164321.4) | 19.9 (0-5986980) | < 0.01 |
| AFP-L3 (%) | 0.0 ± 23.8 | 3.1 ± 24.4 | < 0.05 |
| PIVKA-II (mAU/mL) | 11385.0 (0-436410) | 136.0 (0-664680) | 0.68 |
| CKD stage | P value | |||
| Severe (EGFR < 30), n = 19 | Mild (30 ≤ EGFR < 60), n = 109 | Non-RD (EGFR ≥ 60), n = 672 | ||
| Age (yr) | 73.0 ± 8.9 | 69.0 ± 8.6 | 63.0 ± 10.4 | < 0.0001 |
| Sex | ||||
| Male | 13 (68.4) | 98 (89.9) | 549 (81.7) | < 0.05 |
| Female | 6 (31.6) | 11 (10.1) | 123 (18.3) | - |
| Etiology | ||||
| HBV | 5 (26.3) | 24 (22.0) | 263 (39.1) | < 0.01 |
| HCV | 8 (42.1) | 33 (30.3) | 218 (32.4) | 0.59 |
| NBNC | 6 (31.6) | 52 (47.7) | 191 (28.5) | < 0.001 |
| Child-Pugh grade | ||||
| A | 19 (100.0) | 105 (96.3) | 649 (96.6) | 0.71 |
| B | 0 (0.0) | 4 (3.7) | 23 (3.4) | - |
| Laboratory data | ||||
| Plt (×104/μL) | 14.5 ± 5.2 | 16.3 ± 6.4 | 15.5 ± 7.3 | 0.76 |
| PT (%) | 94.9 ± 10.1 | 95.2 ± 14.3 | 91.7 ± 14.7 | 0.35 |
| Alb (g/dL) | 3.8 ± 0.3 | 4.1 ± 0.4 | 4.1 ± 0.4 | < 0.01 |
| T-bil (mg/dL) | 0.4 ± 0.2 | 0.7 ± 0.3 | 0.8 ± 0.4 | < 0.001 |
| AST (IU/L) | 27.0 ± 17.4 | 38.0 ± 32.5 | 43.0 ± 43.4 | 0.07 |
| ALT (IU/L) | 21.0 ± 19.0 | 34.0 ± 30.9 | 40.0 ± 36.1 | < 0.05 |
| ChE (IU/L) | 181.0 ± 68.1 | 249.0 ± 90.0 | 245.0 ± 81.3 | < 0.01 |
| ICG15R (%) | 10.5 ± 6.2 | 15.3 ± 7.3 | 13.6 ± 10.6 | 0.18 |
| HbA1c (%) | 5.5 ± 1.0 | 5.9 ± 1.1 | 5.3 ± 1.1 | < 0.05 |
| BUN (mg/dL) | 38.0 ± 15.8 | 19.0 ± 5.2 | 14.0 ± 4.0 | < 0.0001 |
| Cr (mg/dL) | 2.4 ± 3.2 | 1.0 ± 0.2 | 0.7 ± 0.1 | < 0.0001 |
| AFP (ng/mL) | 51.5 (2.1-164321.4) | 6.5 (1.4-37525.5) | 19.9 (0-5986980) | 0.61 |
| AFP-L3 (%) | 21.7 ± 30.6 | 0.0 ± 21.6 | 3.1 ± 24.4 | < 0.05 |
| PIVKA-II (mAU/mL) | 1309.0 (10-167600) | 105.0 (0-436410) | 136.0 (0-664680) | 0.93 |
As listed in Table 3, the proportion of curability A or B was significantly higher in the RD patients than in the non-RD patients (91.4% vs 83.8%; P < 0.05). Vascular invasion and advanced fibrosis (F stage 3 and 4) were significantly lower in the RD patients than in the non-RD patients (8.6% vs 21.6%; P < 0.001, 32.0% vs 53.2%; P < 0.0001, respectively). The intraoperative variables and other tumor characteristics of the severe, mild CKD and non-RD groups were almost comparable for all groups. In this analysis, the curability of the severe and mild CKD group patients was higher than that of the non-RD group patients (P < 0.05); on the other hand, the proportion of vascular invasion and advanced fibrosis in the patients with severe and mild CKD was significantly lower than that of the non-RD group patients (P < 0.01 and P < 0.001, respectively). The resected liver weight (365 g, 222 g, and 252 g, P = 0.24) in the severe CKD patient group tended to be higher than that in the other patient groups, although the difference was not statistically significant (Table 4).
| CKD stage | P value | ||
| RD (EGFR < 60), n = 128 | Non-RD (EGFR ≥ 60), n = 672 | ||
| Intraoperative variables | |||
| Operative time (min) | 323.0 ± 125.0 | 329.0 ± 108.0 | 0.70 |
| Blood loss (mL) | 380.0 ± 3230.1 | 425.0 ± 1577.3 | 0.42 |
| Procedure of resection | |||
| Anatomical resection | 99 (77.3) | 498 (74.1) | 0.44 |
| Nonanatomical resection | 29 (22.7) | 174 (25.9) | - |
| Resected liver weight (g) | 239.0 ± 459.3 | 252.0 ± 630.0 | 0.57 |
| Curability | |||
| A + B | 117 (91.4) | 563 (83.8) | < 0.05 |
| C | 11 (8.6) | 109 (16.2) | - |
| Tumor characteristics | |||
| Tumor size (cm) | 4.5 ± 3.9 | 4.4 ± 4.6 | 0.85 |
| Tumor number | 1.0 ± 1.7 | 1.0 ± 2.8 | 0.55 |
| PStage1 | |||
| I | 8 (6.3) | 53 (7.9) | 0.11 |
| II | 62 (48.4) | 272 (40.5) | - |
| III | 40 (31.3) | 207 (30.8) | - |
| IV | 18 (14.1) | 140 (20.8) | - |
| Pathological grade | |||
| Well | 24 (18.7) | 95 (14.1) | 0.29 |
| Mod-por | 104 (81.3) | 577 (85.9) | - |
| Vascular invasion1 | |||
| Yes | 11 (8.6) | 145 (21.6) | < 0.001 |
| No | 117 (91.4) | 527 (78.4) | - |
| Liver fibrosis score2 | |||
| 0-1 | 44 (34.4) | 143 (21.2) | < 0.0001 |
| 2 | 43 (33.6) | 172 (25.6) | - |
| 3 | 22 (17.2) | 149 (22.2) | - |
| 4 | 19 (14.8) | 208 (31.0) | - |
| CKD stage | P value | |||
| Severe (EGFR < 30), n = 19 | Mild (30 ≤ EGFR < 60), n = 109 | Non-RD (EGFR ≥ 60), n = 672 | ||
| Intraoperative variables | ||||
| Operative time (min) | 311.0 ± 112.0 | 331.0 ± 127.0 | 329.0 ± 108.0 | 0.52 |
| Blood loss (mL) | 389.0 ± 1254.1 | 380.0 ± 3464.9 | 425.0 ± 1577.3 | 0.64 |
| Procedure of resection | ||||
| Anatomical resection | 13 (68.4) | 86 (78.9) | 498 (74.1) | 0.46 |
| Nonanatomical resection | 6 (31.6) | 23 (21.1) | 174 (25.9) | - |
| Resected liver weight (g) | 365.0 ± 388.5 | 222.0 ± 471.3 | 252.0 ± 630.0 | 0.24 |
| Curability | ||||
| A + B | 19 (100.0) | 98 (89.9) | 563 (83.8) | < 0.05 |
| C | 0 (0.0) | 11 (10.1) | 109 (16.2) | - |
| Tumor characteristics | ||||
| Tumor size (cm) | 5.8 ± 4.0 | 4.5 ± 3.8 | 4.4 ± 4.6 | 0.41 |
| Tumor number | 1.0 ± 2.1 | 1.0 ± 1.6 | 1.0 ± 2.8 | 0.44 |
| pStage1 | ||||
| I | 1 (5.3) | 7 (6.4) | 53 (7.9) | 0.45 |
| II | 8 (42.1) | 54 (49.5) | 272 (40.5) | - |
| III | 7 (36.8) | 33 (30.3) | 207 (30.8) | - |
| IV | 3 (15.8) | 15 (13.8) | 140 (20.8) | - |
| Pathological grade | ||||
| Well | 2 (10.5) | 22 (20.2) | 95 (14.1) | 0.84 |
| Mod-por | 17 (89.5) | 87 (79.8) | 577 (85.9) | - |
| Vascular invasion1 | ||||
| Yes | 2 (10.5) | 9 (8.3) | 145 (21.6) | < 0.01 |
| No | 17 (89.5) | 100 (91.7) | 527 (78.4) | - |
| Liver fibrosis score2 | ||||
| 0-1 | 7 (36.8) | 37 (34.0) | 143 (21.2) | < 0.001 |
| 2 | 8 (42.1) | 35 (32.1) | 172 (25.6) | - |
| 3 | 3 (15.8) | 19 (17.4) | 149 (22.2) | - |
| 4 | 1 (5.3) | 18 (16.5) | 208 (31.0) | - |
Although the overall postoperative complication rates were similar between the RD and non-RD patients, the proportions of postoperative bleeding and surgical site infection were significantly higher in the RD patients (5.5% vs 1.8%; P < 0.05, 3.9% vs 1.8%; P < 0.05, respectively) (Table 5). In the comparison between the patients with severe CKD and those with mild CKD, there was no difference in postoperative complications. Postoperative complications were also not significantly different among the three groups, except for bleeding, which was higher than that in the severe CKD group (P < 0.05) (Table 6). Regarding these bleeding complications, three RD patients (2.3%) and eight non-RD patients (1.2%) required reoperation to control postoperative bleeding. There were no complications of ascites, pleural effusion, liver failure, or surgical site infection in six patients who required maintenance hemodialysis before surgery. The duration of postoperative hospital stay was not significantly different among the three groups (16.0, 16.0, and 16.0 d; P = 0.92). There was no mortality during hospitalization in the severe CKD group, but one patient each in the mild CKD and non-RD groups died during hospitalization. In the mild CKD group, one patient died due to postoperative gastrointestinal perforation and an intraabdominal abscess. In the non-RD group, one patient died due to postoperative liver failure.
| CKD stage | P value | ||
| RD (EGFR < 60), n = 128 | Non-RD (EGFR ≥ 60), n = 672 | ||
| All complications | 33 (25.8) | 169 (25.1) | 0.96 |
| Major complication (Grade ≥ 2) | 20 (15.6) | 112 (16.7) | 0.91 |
| Bile leakage | 12 (9.8) | 44 (6.5) | 0.33 |
| Ascites | 6 (4.7) | 27 (4.0) | 0.90 |
| Pleural effusion | 4 (3.1) | 37 (5.5) | 0.41 |
| Pneumonia | 6 (5.3) | 12 (1.8) | 0.70 |
| Bleeding | 7 (5.5) | 12 (1.8) | < 0.05 |
| Liver failure | 1 (0.8) | 9 (1.3) | 0.55 |
| Surgical site infection | 5 (3.9) | 12 (1.8) | < 0.05 |
| Duration of postoperative hospital stay (d) | 16.0 ± 14.5 | 16.0 ± 19.3 | 0.17 |
| Died during hospitalization | 11 (0.8) | 12 (0.1) | 0.96 |
| CKD stage | P value | |||
| Severe (EGFR < 30), n = 19 | Mild (30 ≤ EGFR < 60), n = 109 | Non-RD (EGFR ≥ 60), n = 672 | ||
| All complications | 5 (26.3) | 28 (25.7) | 169 (25.1) | 0.99 |
| Major complication (Grade ≥ 2) | 3 (15.8) | 17 (15.6) | 112 (16.7) | 0.98 |
| Bile leakage | 2 (10.5) | 10 (9.2) | 44 (6.5) | 0.40 |
| Ascites | 2 (10.5) | 4 (3.7) | 27 (4.0) | 0.45 |
| Pleural effusion | 0 (0.0) | 4 (3.7) | 37 (5.5) | 0.68 |
| Pneumonia | 1 (5.3) | 5 (4.6) | 12 (1.8) | 0.84 |
| Bleeding | 2 (10.5) | 5 (4.6) | 12 (1.8) | < 0.05 |
| Liver failure | 0 (0.0) | 1 (0.9) | 9 (1.3) | 0.55 |
| Surgical site infection | 0 (0.0) | 5 (4.6) | 12 (1.8) | 0.07 |
| Duration of postoperative hospital stay (d) | 16.0 ± 15.3 | 16.0 ± 14.4 | 16.0 ± 9.3 | 0.92 |
| Died during hospitalization | 0 (0.0) | 11 (0.9) | 12 (0.1) | 0.96 |
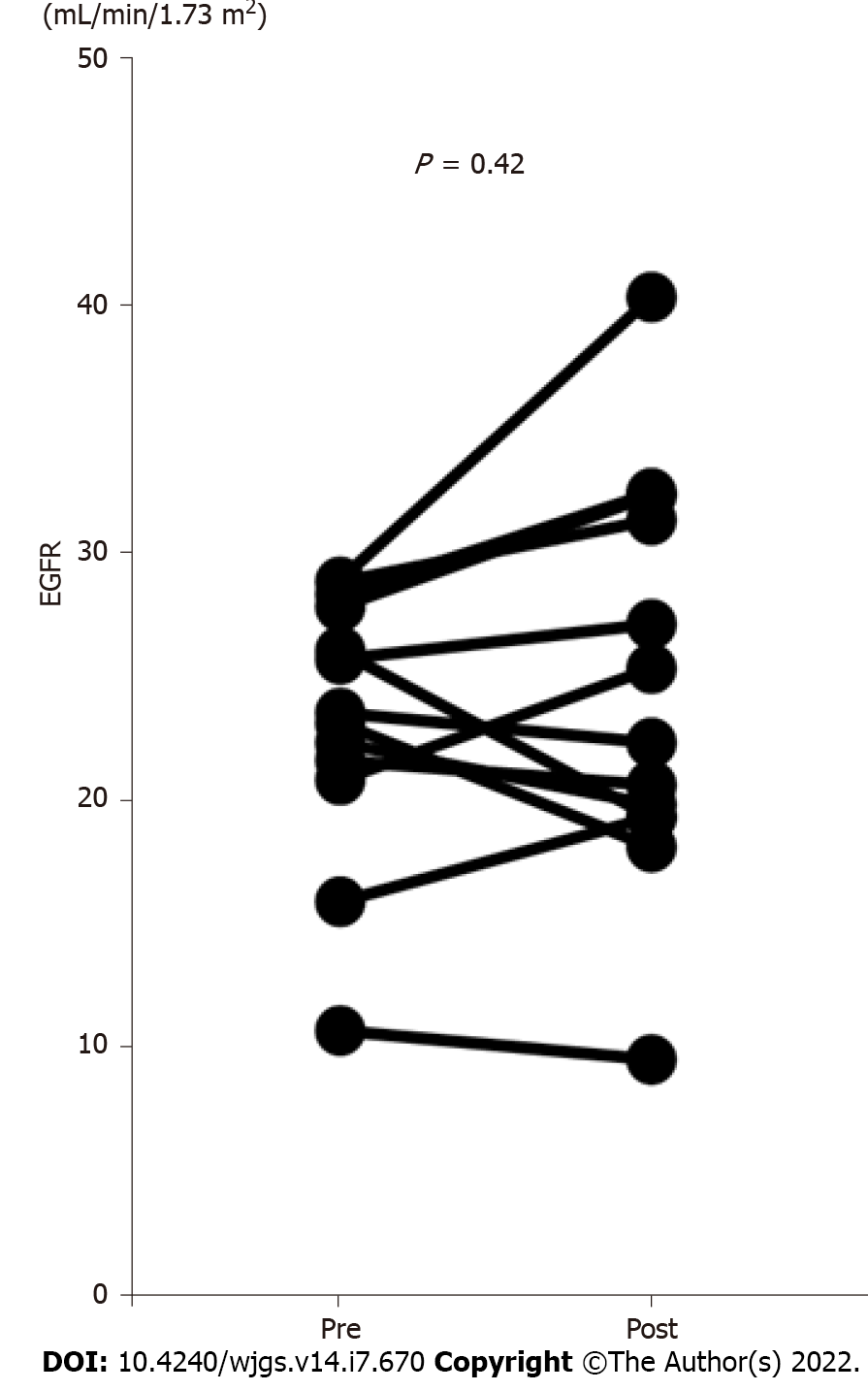
We compared the EGFR values before and one month after hepatectomy in the patients with CKD stage 4 or 5 according to the KDIGO CKD guidelines who did not receive maintenance hemodialysis (n = 13)[18] (Figure 1). The EGFR values did not decrease after the operation; furthermore, no patient received maintenance hemodialysis after hepatectomy.
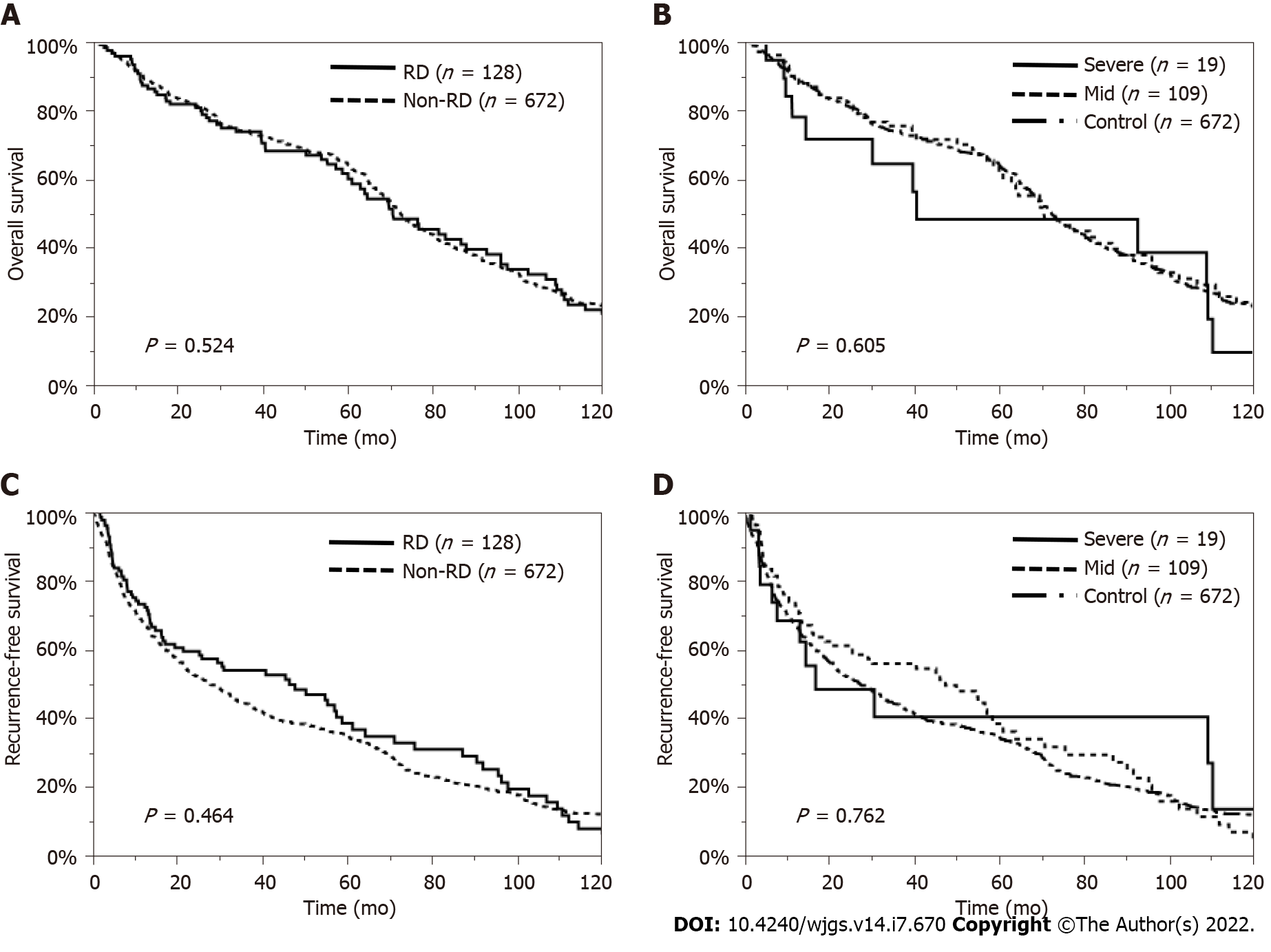
The MST was 70.6 mo in the RD patients and 72.4 mo in the non-RD patients (P = 0.524). The 1-, 3-, 5-, and 10-year OS rates were 87.3%, 74.0%, 60.2%, and 20.6% in the RD patients and 89.9%, 74.1%, 64.6%, and 23.1% in the non-RD patients, respectively (Figure 2A). Moreover, the MST was 40.8 mo in the severe CKD group, 70.9 mo in the mild CKD group and 72.4 mo in the non-RD group (P = 0.605). The 1-, 3-, 5-, and 10-year OS rates were 78.2%, 64.5%, 48.4%, and 9.7% in the severe CKD group, 89.0%, 75.5%, 62.2%, and 22.5% in the mild CKD group and 89.9%, 74.1%, 64.6%, and 23.1% in the non-RD group, respectively (Figure 2B). The median RFS time was 46.2 mo in the RD patients and 27.4 mo in the non-RD patients (P = 0.464) (Figure 2C). The median RFS time was 17.0 mo in the severe CKD group, 47.5 mo in the mild CKD group and 27.4 mo in the non-RD group (P = 0.762) (Figure 2D).
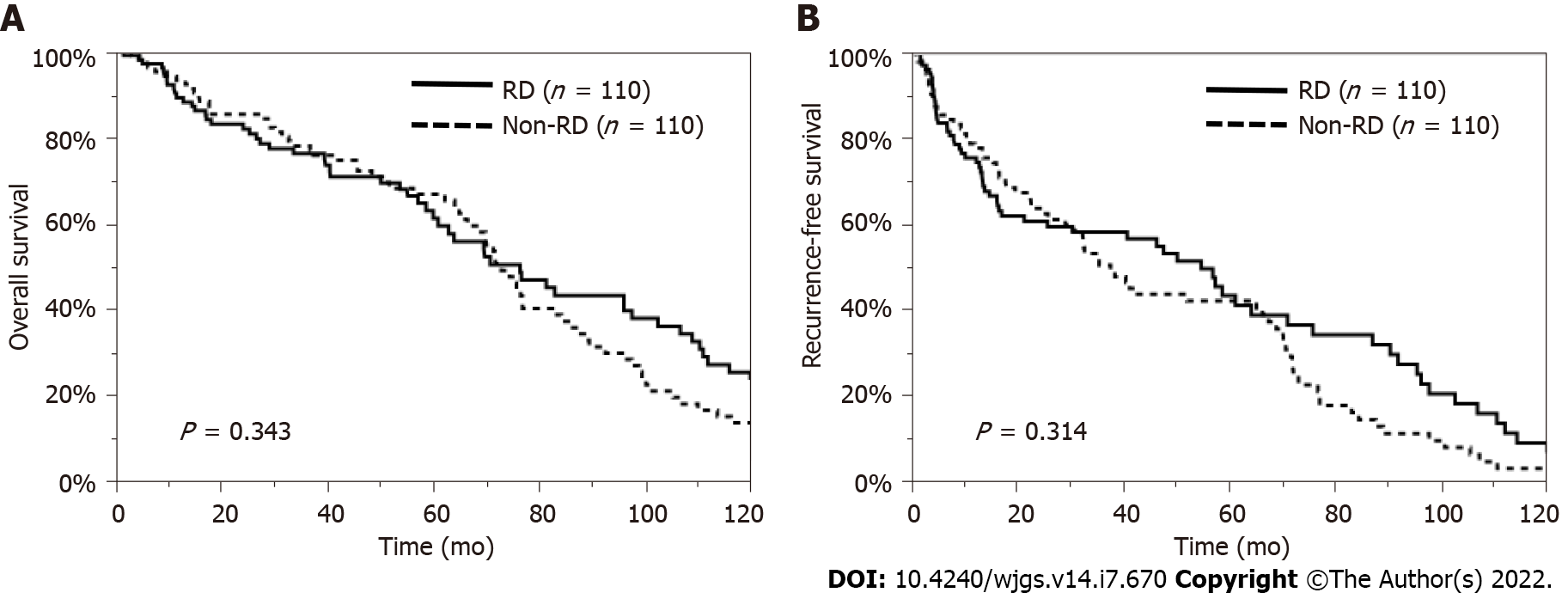
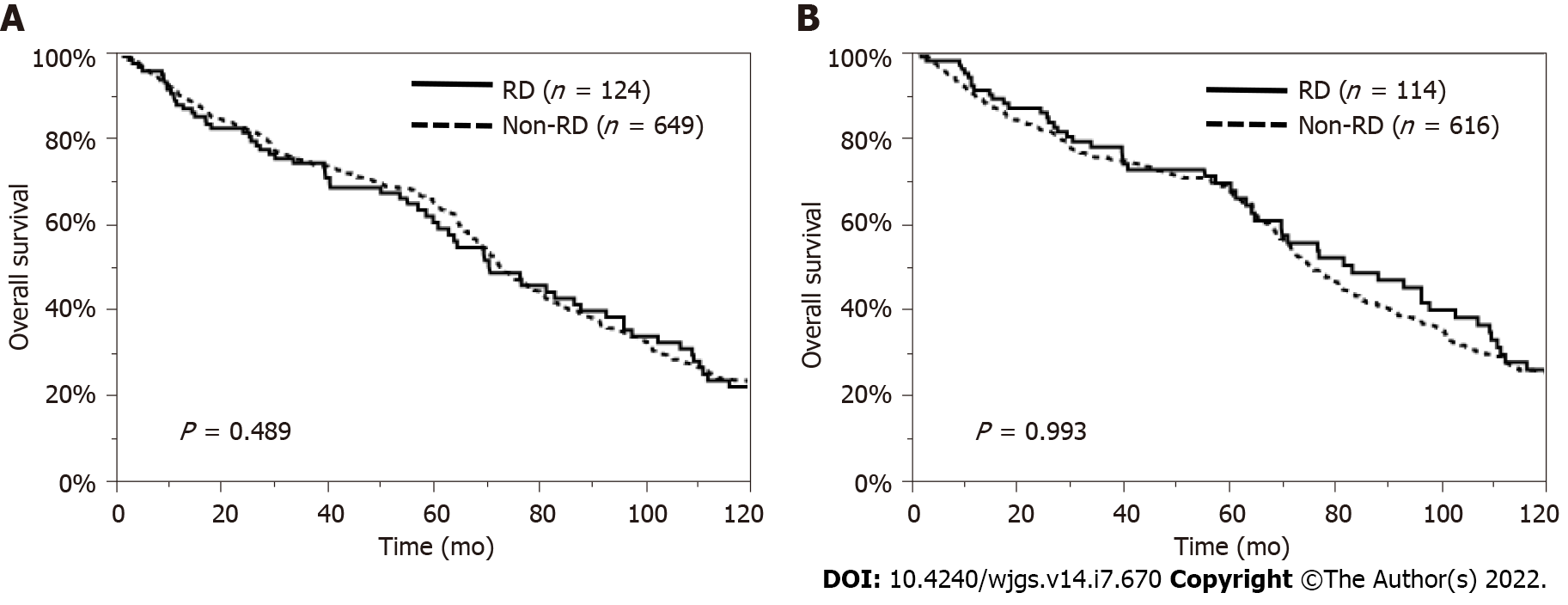
Regarding patient characteristics, the RD patients were significantly older, had a lower proportion of HBV and a higher proportion of NBNC, and had lower serum T-bil, AST, and ALT levels and higher serum HbA1c levels than the non-RD patients. Therefore, we examined the impact of preoperative RD on the OS and RFS rates, excluding the influence of these factors, by using a propensity model. This PSM model was constructed with patients’ age, etiology, and laboratory data, such as the levels of serum T-bil, AST, ALT, and HbA1c, so a total of 110 pairs of matched HCC patients undergoing hepatectomy were selected in this model (Supplementary Table 1). The comparison of the OS and RFS rates between the matched patients with and without RD showed no significant difference (P = 0.343, P = 0.314, respectively) (Figure 3). In addition, considering the influence of liver function or other causes of death, we also analyzed survival in patients with Child-Pugh grade A disease and in those who died from cancer-related causes. The OS rate was similar between the RD and non-RD patients with Child-Pugh grade A disease (P = 0.489, Figure 4A) and in those who died from cancer-related causes (P = 0.993, Figure 4B).
Table 7 shows the prognostic factors for OS and RFS in the HCC patients with RD in this cohort. In the RD patients, the multivariate analysis showed that the presence of multiple tumors was an independent factor for both OS and RFS [OS: hazard ratio (HR) = 2.44, 95% confidence interval (CI): 1.04-5.75, P = 0.040, RFS: HR = 3.77, 95%CI: 1.61-8.97, P = 0.002].
| Variable (RD patients) | Univariate analysis (OS) | Multivariate analysis (OS) | Univariate analysis (RFS) | Multivariate analysis (RFS) | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age > 60 yr | 2.33 (1.14-5.63) | < 0.05 | 3.85 (0.81-22.53) | 0.092 | 2.08 (1.01-5.01) | < 0.05 | 0.98 (0.26-4.76) | 0.978 |
| Male | 1.37 (0.73-2.82) | 0.371 | 1.66 (0.88-3.48) | 0.122 | ||||
| HBV+ | 1.00 (0.56-1.67) | 0.995 | 1.02 (0.57-1.73) | 0.947 | ||||
| HCV+ | 0.97 (0.60-1.52) | 0.899 | 1.03 (0.64-1.63) | 0.889 | ||||
| NBNC | 1.03 (0.66-1.60) | 0.898 | 0.96 (0.61-1.50) | 0.849 | ||||
| Child-Pugh grade B | 2.16 (0.12-10.17) | 0.44 | 0.90 (0.05-4.13) | 0.919 | ||||
| Plt < 13.8 | 0.88 (0.54-1.40) | 0.598 | 0.76 (0.46-1.22) | 0.257 | ||||
| PT < 80 | 0.89 (0.47-1.57) | 0.706 | 1.05 (0.57-1.81) | 0.858 | ||||
| Alb < 4.0 | 1.25 (0.80-1.95) | 0.321 | 1.23 (0.78-1.92) | 0.376 | ||||
| T-bil > 1.2 | 0.87 (0.30-2.01) | 0.772 | 1.03 (0.40-2.18) | 0.95 | ||||
| AST > 38 | 1.15 (0.74-1.79) | 0.534 | 1.31 (0.85-2.05) | 0.223 | ||||
| ALT > 44 | 0.71 (0.43-1.14) | 0.162 | 1.21 (0.76-1.90) | 0.421 | ||||
| ChE < 168 | 2.40 (1.22-4.32) | < 0.01 | 1.06 (0.31-3.15) | 0.921 | 3.15 (1.59-5.79) | < 0.01 | 2.21 (0.17-1.35) | 0.147 |
| ICGR15 > 15 | 0.94 (0.60-1.48) | 0.800 | 1.36 (0.87-2.14) | 0.176 | ||||
| HbA1c > 5.6 | 1.49 (0.87-2.55) | 0.145 | 0.94 (0.57-1.56) | 0.823 | ||||
| AFP > 10 | 1.51 (0.97-2.39) | 0.068 | 2.07 (1.32-3.28) | < 0.01 | 0.79 (0.29-2.03) | 0.634 | ||
| AFP-L3 > 10 | 2.97 (1.74-5.01) | < 0.0001 | 2.57 (0.99-6.70) | 0.051 | 2.21 (1.33-3.59) | < 0.01 | 2.22 (0.87-5.98) | 0.095 |
| PIVKA-II > 40 | 1.85 (1.17-3.00) | < 0.01 | 2.57 (0.64-11.50) | 0.186 | 1.53 (0.97-2.46) | 0.067 | ||
| Operative time > Ave | 0.96 (0.62-1.50) | 0.868 | 0.98 (0.62-1.53) | 0.916 | ||||
| Blood loss > Ave | 1.31 (0.78-2.13) | 0.282 | 1.17 (0.70-1.89) | 0.533 | ||||
| Anatomical resection | 1.06 (0.64-1.85) | 0.833 | 0.79 (0.48-1.37) | 0.391 | ||||
| Resected liver weight > Ave | 2.05 (1.18-3.44) | < 0.01 | 0.99 (0.37-2.66) | 0.978 | 1.53 (0.87-2.59) | 0.137 | ||
| Tumor size > Ave | 1.94 (1.18-3.10) | < 0.01 | 1.06 (0.33-3.30) | 0.918 | 1.88 (1.14-3.04) | < 0.05 | 1.86 (0.63-5.40) | 0.258 |
| Tumor number > 1 | 2.13 (1.30-3.45) | < 0.01 | 2.44 (1.04-5.75) | < 0.05 | 3.46 (2.04-5.85) | < 0.0001 | 3.77 (1.61-8.97) | < 0.01 |
| Pathological grade (mod-por) | 1.23 (0.70-2.34) | 0.505 | 1.32 (0.76-2.47) | 0.337 | ||||
| Vascular invasion (Vp+, Vv+) | 4.92 (2.21-9.84) | < 0.0001 | 1.88 (0.61-5.14) | 0.26 | 4.08 (1.86-8.00) | < 0.01 | 1.89 (0.70-4.60) | 0.198 |
| Liver fibrosis score 3, 4 | 1.29 (0.80-2.03) | 0.278 | 1.37 (0.86-2.16) | 0.186 | ||||
We revealed here that the prognoses for survival and recurrence in HCC patients with and without RD who underwent curative hepatectomy were similar, even if patients had severe CKD. This finding indicated that comorbid RD had a negligible impact on the prognosis of HCC patients who underwent curative hepatectomy. However, preoperative RD affected some kinds of postoperative complications, such as postoperative bleeding and surgical site infection. It has been reported that progressive CKD is associated with adverse clinical outcomes, including ESRD, cardiovascular disease, and increased mortality[24,25]. The prognosis of HCC patients with RD might be affected by these comorbidities. In addition, Toyoda et al[21] reported that the survival rate of patients who required dialysis was significantly lower than that of nondialysis controls. On the other hand, Shirata et al[14] mentioned that liver resection for Child-Pugh A patients with RD is safe and has comparable oncological outcomes compared to those for non-RD patients, but the selection of liver resection candidates among Child-Pugh B patients with RD should be stricter. In our study, there was no significant difference in either OS or RFS between the patients with and without RD, even if the patients had severe CKD. Moreover, because there were some differences in patient characteristics, such as age, etiology, liver function, and HbA1c levels, between patients with and without RD, we also performed PSM. The OS and RFS rates were comparable between the patients with and without RD after PSM. These results indicated that curative hepatectomy might be effective for the long-term prognosis of HCC patients, regardless of the presence of concomitant RD.
RD has been reported to be a risk factor for the development of massive ascites, pleural effusion, respiratory failure, and acute renal failure in patients after hepatectomy[11,12]. Our study showed that the proportion of patients who experienced these complications was similar between those with and without RD. The following reasons might explain these results. First, there were low frequencies of ascites and pleural effusion. Second, we might perform hepatectomy in RD patients whose liver function was better because serum T-bil, AST, and ALT levels were lower in the RD patients than in the non-RD patients. Regarding acute renal failure, the EGFR values did not decrease after liver resection; furthermore, no patient with stage 4 or 5 disease who was not on hemodialysis was treated after hepatectomy; instead, they were given appropriate perioperative care. Some reports have also shown that blood loss is higher in RD patients than in non-RD patients[11], but the amounts of blood loss were similar between the RD and non-RD patients in our study. On the other hand, the rate of postoperative bleeding was significantly higher in the RD patients. Regarding the higher proportion of postoperative bleeding in the RD patients, especially in those with severe CKD, some degree of coagulopathy and tissue weakness in patients with CKD might influence this complication[26]. Surgical site infection might also be related to the immune dysfunction of CKD patients[27]. Therefore, we should ensure blood stanching before closing the abdomen.
In the present study, the proportion of postoperative surgical site infections was also higher in the RD patients than in the non-RD patients, so more careful postoperative management is needed for RD patients. In addition to curative liver resection, hepatectomy requires careful follow-up of patients. As demonstrated in the univariate and multivariate analyses, the RD patients with multiple tumors tended to have a poor prognosis. We might have to carefully monitor and perform additional treatments for patients with multiple tumors. Moreover, from an oncological point of view, some reports have shown an increased risk of various cancers in patients with severe CKD, especially those on dialysis[28-30]. The incidences of various cancers, including kidney, bladder, and thyroid cancers, other endocrine tumors, and multiple myeloma, are higher in ESRD patients than in non-ESRD patients[31,32]. Patients who require dialysis are likely to be at risk of developing HCC, and patients with ESRD may be at high risk of developing HCC[21].
There are some limitations to this study. First, the liver function of the RD patients was better than that of the non-RD patients because physicians might exclude RD patients with severe liver function. Second, the number of HCC patients with RD, especially those with severe CKD who underwent hepatectomy, was rather small; therefore, we could not investigate rehepatectomy for patients with RD who experienced HCC recurrence, and we could not entirely conclude that severe RD has a negligible impact on the prognosis of HCC patients. Third, this study was a retrospective study. Additional studies on larger cohorts of HCC patients with RD are required to reveal the pathogenesis of HCC and RD.
We revealed that comorbid mild RD has a negligible impact on the prognosis of HCC patients who undergo curative hepatectomy with appropriate perioperative management, and close attention to severe CKD is necessary to prevent postoperative bleeding and surgical site infection.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, on the other hand, the number of patients with chronic kidney disease (CKD) is on the rise because of the increase in lifestyle-related diseases.
To establish a tailored management strategy for HCC patients with CKD.
To evaluate the impact of comorbid renal dysfunction (RD), as stratified by using the estimated glomerular filtration rate (EGFR), and assessed the oncologic validity of hepatectomy for HCC patients with RD.
We enrolled 800 HCC patients who underwent hepatectomy between 1997 and 2015 at our university hospital. We categorized patients into two and three groups according to renal function as defined by the EGFR. Overall survival (OS) and recurrence-free survival (RFS) were compared among these groups and we also analyzed survival by using a propensity score matching (PSM) model to exclude the influence of patient characteristics.
The RD patients were significantly older and had lower serum total bilirubin, aspartate aminotransferase, and aspartate aminotransferase levels than the non-RD patients, and no patient received maintenance hemodialysis after surgery. Although the overall postoperative complication rates were similar between the RD and non-RD patients, the proportions of postoperative bleeding and surgical site infection were significantly higher in the RD patients, and postoperative bleeding was the highest in the severe CKD group. Regardless of the degree of comorbid RD, OS and RFS were comparable, even after PSM between the RD and non-RD groups to exclude the influence of patient characteristics, liver function, and other causes of death.
Comorbid mild RD had a negligible impact on the prognosis of HCC patients who underwent curative hepatectomy with appropriate perioperative management, and close attention to severe CKD is necessary to prevent postoperative bleeding and surgical site infection.
The present study will be useful for management of HCC patients with CKD in future.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Giacomelli L, Italy; Qiu X, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1501] [Article Influence: 187.6] [Reference Citation Analysis (0)] |
| 2. | Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, Zhao Y. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10:2993-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2909] [Article Influence: 484.8] [Reference Citation Analysis (17)] |
| 4. | Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systematic Review and Meta-analysis. Ann Surg. 2021;273:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 5. | Orcutt ST, Anaya DA. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control. 2018;25:1073274817744621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 6. | Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 2019;322:1294-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 1016] [Article Influence: 169.3] [Reference Citation Analysis (0)] |
| 7. | Matsumoto S, Takayama T, Wakatsuki K, Tanaka T, Migita K, Nakajima Y. Short-term and long-term outcomes after gastrectomy for gastric cancer in patients with chronic kidney disease. World J Surg. 2014;38:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Drolet S, Maclean AR, Myers RP, Shaheen AA, Dixon E, Donald Buie W. Morbidity and mortality following colorectal surgery in patients with end-stage renal failure: a population-based study. Dis Colon Rectum. 2010;53:1508-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Nathan DP, Tang GL. The impact of chronic renal insufficiency on vascular surgery patient outcomes. Semin Vasc Surg. 2014;27:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kaibori M, Matsui Y, Kwon AH, Tokoro T, Kamiyama Y. Prognosis of hepatocellular carcinoma after hepatectomy in patients with renal dysfunction. World J Surg. 2005;29:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Orii T, Takayama T, Haga I, Fukumori T, Amada N. Efficacy of a liver resection for hepatocellular carcinoma in patients with chronic renal failure. Surg Today. 2008;38:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Toshima T, Shirabe K, Yoshiya S, Muto J, Ikegami T, Yoshizumi T, Maehara Y. Outcome of hepatectomy for hepatocellular carcinoma in patients with renal dysfunction. HPB (Oxford). 2012;14:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Mian AN, Schwartz GJ. Measurement and Estimation of Glomerular Filtration Rate in Children. Adv Chronic Kidney Dis. 2017;24:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 14. | Shirata C, Hasegawa K, Kokudo T, Yamashita S, Yamamoto S, Arita J, Akamatsu N, Kaneko J, Sakamoto Y, Kokudo N. Liver Resection for Hepatocellular Carcinoma in Patients with Renal Dysfunction. World J Surg. 2018;42:4054-4062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Shimizu T, Aoki T, Park KH, Matsumoto T, Shiraki T, Sakuraoka Y, Mori S, Iso Y, Ishizuka M, Kubota K. Volumetric assessment and clinical predictors of cirrhosis in patients undergoing hepatectomy for hepatocellular carcinoma with presumed normal liver function. Hepatol Int. 2021;15:1258-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Harada K, Mizuguchi T, Katagiri Y, Kawamoto M, Nakamura Y, Meguro M, Ota S, Sasaki S, Miyanishi K, Sonoda T, Mori M, Shinomura Y, Kato J, Hirata K. Area between the hepatic and heart curves of (99m)Tc-galactosyl-human serum albumin scintigraphy represents liver function and disease progression for preoperative evaluation in hepatocellular carcinoma patients. J Hepatobiliary Pancreat Sci. 2012;19:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Adeera L, Paul ES, Rudy WB, Josef C, Angel LM De Francisco, Paul E De Jong, Kathryn EG, Brenda RH, Kunitoshi I, Edmund JL, Andrew SL, Miguel CR, Michael GS, Haiyan W, Colin TW, Christopher GW. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1438] [Cited by in RCA: 1667] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 19. | Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073-2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3261] [Cited by in RCA: 3078] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 20. | Huang YJ, Hsu YL, Chuang YH, Lin HY, Chen YH, Chan TC. Association between renal function and cardiovascular mortality: a retrospective cohort study of elderly from health check-up. BMJ Open. 2021;11:e049307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Toyoda H, Hiraoka A, Tada T, Michitaka K, Takaguchi K, Tsuji K, Itobayashi E, Takizawa D, Hirooka M, Koizumi Y, Ochi H, Joko K, Kisaka Y, Shimizu Y, Tajiri K, Tani J, Taniguchi T, Toshimori A, Fujioka S, Kumada T. Characteristics and Prognosis of Hepatocellular Carcinoma in Japanese Patients Undergoing Dialysis. Ther Apher Dial. 2017;21:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24855] [Article Influence: 1183.6] [Reference Citation Analysis (0)] |
| 24. | Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schöttker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Ärnlöv J; CKD Prognosis Consortium. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 462] [Cited by in RCA: 644] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 25. | Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 1083] [Article Influence: 270.8] [Reference Citation Analysis (2)] |
| 26. | Nunns GR, Moore EE, Chapman MP, Moore HB, Stettler GR, Peltz E, Burlew CC, Silliman CC, Banerjee A, Sauaia A. The hypercoagulability paradox of chronic kidney disease: The role of fibrinogen. Am J Surg. 2017;214:1215-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Syed-Ahmed M, Narayanan M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv Chronic Kidney Dis. 2019;26:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 28. | Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823-2831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 840] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 29. | Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol. 2010;23:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ, Fang TC. Increased risk of cancer in chronic dialysis patients: a population-based cohort study in Taiwan. Nephrol Dial Transplant. 2012;27:1585-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1654] [Cited by in RCA: 2427] [Article Influence: 303.4] [Reference Citation Analysis (0)] |
| 32. | Tsuzuki T, Iwata H, Murase Y, Takahara T, Ohashi A. Renal tumors in end-stage renal disease: A comprehensive review. Int J Urol. 2018;25:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |