Published online Jun 27, 2022. doi: 10.4240/wjgs.v14.i6.611
Peer-review started: September 27, 2021
First decision: January 12, 2022
Revised: January 26, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 27, 2022
Processing time: 273 Days and 2.5 Hours
Extramedullary plasmacytoma (EMP) of the gastrointestinal tract is an extremely rare disease. Clinical manifestations of EMPs are varied and depend on the location and progression of the tumor.
Here, we firstly report a case of intestinal perforation with abdominal abscess caused by EMP of the small intestine in a 55-year-old female patient. The patient received emergency surgery immediately after the necessary preoperative procedures. During the operation, EMP was found to have caused the perforation of the small intestine and the formation of multiple abscesses in the abdominal cavity. Partial resection of the small intestine with peritoneal irrigation and drainage was performed. EMP was finally confirmed by postoperative histo
EMP of the small intestine may have spontaneous perforation, which requires emergency surgery. Surgical resection can obtain good therapeutic effects.
Core Tip: Extramedullary plasmacytoma (EMP) of the gastrointestinal tract is an extremely rare disease, accounting for only 7% of all EMPs. Clinical manifestations of EMPs are varied and depend on the location and progression of the tumor. Here, we firstly report a case of intestinal perforation with abdo
- Citation: Wang KW, Xiao N. Intestinal perforation with abdominal abscess caused by extramedullary plasmacytoma of small intestine: A case report and literature review. World J Gastrointest Surg 2022; 14(6): 611-620
- URL: https://www.wjgnet.com/1948-9366/full/v14/i6/611.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i6.611
Plasmacytoma is a malignant tumor that originates from bone marrow hematopoietic tissue. It is characterized by an imbalance in the monoclonal proliferation of plasma cells. Extramedullary plasmacytoma (EMP) refers to a localized monoclonal plasma cell proliferation that occurs in soft tissues without bone marrow involvement. It is a rare type of malignant monoclonal plasma cell lesion, accounting for approximately 2%-3% of all plasmacytomas[1,2]. Plasmacytoma primarily occurs in the upper respi
A 55-year-old female was admitted to the Department of Emergency of our hospital with sudden abdominal pain and abdominal distension.
The patient’s symptoms started 3 d prior and were accompanied by nausea and vomiting without gas or defecation. Since onset, the patient had a loss of appetite, limited diet, poor sleep and decreased urination. No significant change in body weight was noted.
The patient’s previous medical history was not remarkable. She and her family had no history of multiple myeloma (MM) or other gastrointestinal diseases.
The patient has no personal and family history.
During physical examination, the patient had a normal heart rate and mild hypotension. The patient’s abdomen was slightly distended, and the abdominal tenderness was more severe in the left upper abdomen accompanied by rebound pain and muscle tension.
Laboratory tests showed the following: White blood cells 10.5 × 10-9/L, neutrocyte (NE) 9.63 × 10-9/L, NE% 91.7%, hemoglobin 108 g/L, and platelet 330 × 10-9/L. Liver and kidney function were normal.
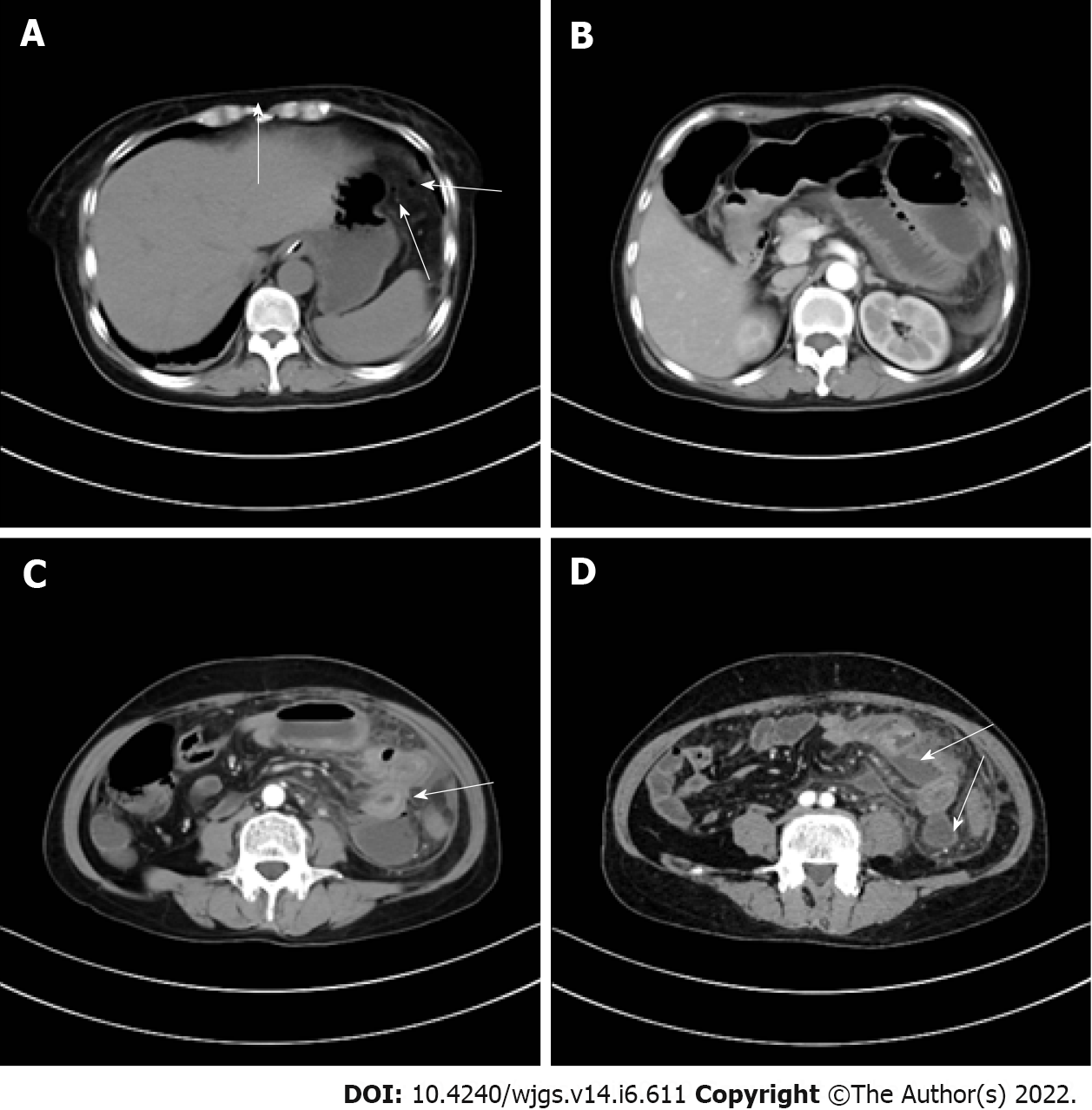
Enhanced computed tomography (CT) showed that the small intestinal lumen in the upper left abdomen was dilated with gas and fluid accumulation, and showed multiple fluid-gas level changes were noted. The intestinal wall was edematous and thickened, and the density of the surrounding fat interspace had increased. Small air bubbles were scattered under the left diaphragm, and multiple encapsulated effusions were observed between the small intestines. These imaging findings suggested local perforation and multiple abscesses in the abdominal cavity (Figure 1).
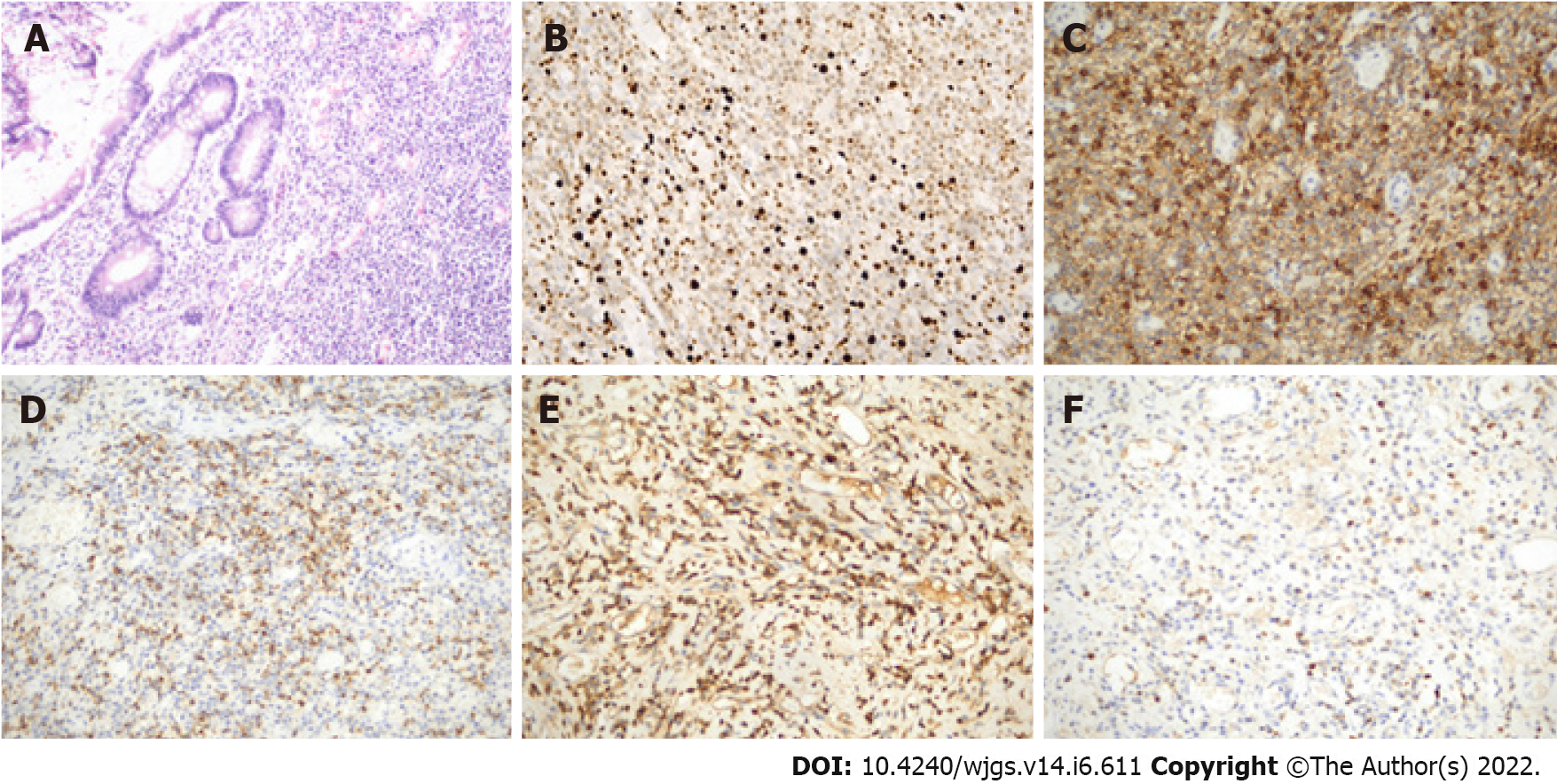
Microscopic analysis showed that the pathological specimen displayed a large number of neoplastic plasma cells with inflammatory cell infiltration (Figure 2A). These plasma cells were positive for CD38 (+), CD138 (+), kappa (+), lambda (week+), CD79a (week+), and MUM1 (+) and negative for creatine kinase (-), CD117 (-), Dog-1 (-), S-100 (-), B cell lymphoma-2 (-), beta-catenin (-), CD56 (-), immunoglobulin G4 (-) and Pax-5 (-) with a Ki-67 proliferative index of 10% (Figures 2B-F). The final pathological specimens were highly suspicious of plasmacytoma. Postoperative laboratory tests showed that the bone marrow cytology was normal and no abnormal monoclonal plasma cells were detected in the flow cytometric analysis. Urine free light chain and serum immunofixation electrophoresis were also normal. Lytic lesions were not found on X-rays. Therefore, the final diagnosis of this patient was primary EMP of the small intestine.
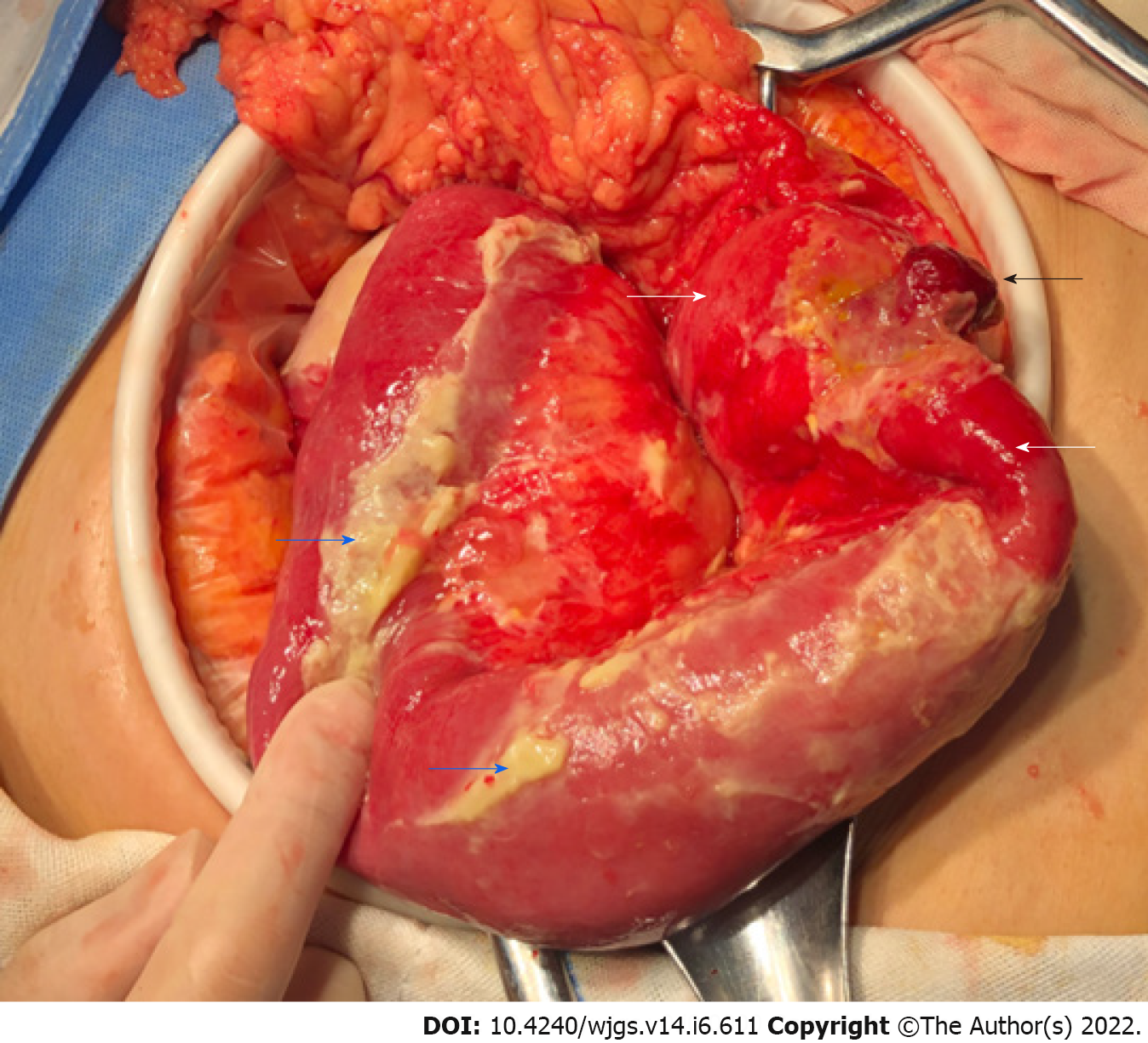
Considering that the patient may have a perforation of the digestive tract, we performed emergency surgery. During the operation, we found that the small intestinal serosa 100 cm away from the Treitz ligament had a dark-red polyp-like protrusion with a perforation approximately 0.5 cm in diameter at the top. The local intestinal wall was hyperemic, edematous and thickened, and the surface of the surrounding small intestine and lateral peritoneum was covered with many purulent masses (Figure 3). Several abscesses were observed between the left paracolic groove and small intestine and filled with a yellow, turbid fluid. After the abscesses were removed, the abdominal cavity was flushed with a large amount of warm normal saline. Then, a segment of the jejunum 33 cm in length was resected, and a primary side-to-end anastomosis of the small intestine was performed. The lumen of the intestinal tube 6 cm from the nearest end resection margin was narrow with a diameter of approximately 1.5 cm. The serosal surface was similar to a polypoid with a size of approximately 2 cm × 1 cm × 1 cm.
The patient had a good postoperative recovery with no complications, and she was discharged smoothly from the hospital one week after her surgery. As of August 1, 2021, she has been regularly followed up for 2 years at an outpatient clinic, and there have been no signs of recurrence or metastasis.
Primary plasmacytoma of the small intestine is rare in clinical practice. Here, we firstly report a case of intestinal perforation with abdominal abscess caused by EMP of the small intestine in a 55-year-old female. The diagnosis is based on a pathologically confirmed small intestinal mass with clonal growth of plasma cells, normal bone marrow histological examination, and normal serum monoclonal immunoglobulin levels[14]. EMP can be divided into two types: Primary and secondary. EMP can also present as a secondary tumor of another plasma cell neoplasm, such as MM[15]. MM must be excluded before the diagnosis of primary EMP[16]. The case we reported had no positive laboratory or imaging findings of MM, which met the diagnostic criteria of primary EMP. In this paper, we performed a review of the well-documented primary gastrointestinal EMP cases in the last 20 years and presented these results in table form[4-7,11,17-45] (Table 1). These results show that gastrointestinal EMP is common in patients over the age of 50 years, and the incidence rate is higher in men compared with women (2:1). The clinical manifestations of gastrointestinal EMPs vary with the location of the tumor and lack specificity. In the early stage, this disease is often asymptomatic, and patients often seek medical treatment because of pain or discomfort caused by local tumor compression. Other clinical manifestations include gastrointestinal bleeding or obstruction, changes in bowel habits, etc. In our case, the patient presented with sudden abdominal pain and abdominal distension, which may have been caused by intestinal perforation. CT images usually show an infiltrating mass with clear boundaries. When the mass is large, a liquefied necrotic area may appear in the center. However, until now, there has been no description of the specific imaging characteristics of EMP[46]. Therefore, the role of imaging examinations in differentiating gastrointestinal EMP from other neoplastic diseases is limited. EMP may be occasionally misdiagnosed as cancer[47], stromal tumors or inflammatory bowel disease[41]. Hence, the accurate diagnosis of gastrointestinal EMP still depends on histopathological results. For gastrointestinal EMP, endoscopic biopsy is a convenient and practical diagnostic method.
| Ref. | Age | Gender | Location | Presentation | Operative | Non-operative | Outcome |
| Katodritou et al[17], 2008 | 68 | Male | Stomach | Upper-gastrointestinal bleeding | None | Bortezomib, dexamethasone | No recurrence 13 mo after diagnosis |
| Park et al[18], 2009 | 50 | Female | Stomach | None | Endoscopic submucosal dissection | None | No recurrence during 12 mo follow-up |
| Krishnamoorthy et al[19], 2010 | 57 | Male | Stomach | Upper-gastrointestinal bleeding | Gastrectomy | None | N/A |
| Park et al[20], 2014 | 70 | Male | Stomach | Indigestion | Endoscopic submucosal resection | Oral thalidomide therapy | No recurrence during 24 mo follow-up |
| Zhao et al[21], 2014 | 79 | Male | Stomach | Epigastric pain | Surgical resection | None | No recurrence during 8 mo follow-up |
| Fukuhara et al[22], 2016 | 36 | Male | Stomach | Dyspnoea, fatigue | Total gastrectomy, lymphadenectomy | Chemotherapy and autologous peripheral blood stem-cell transplantation | No recurrence during 18 mo follow-up |
| Kang et al[23], 2016 | 78 | Female | Stomach | Epigastric pain | Refused | High-dose dexamethasone | Completely regressed and remission was maintained for over 1 yr |
| Takahashi et al[24], 2016 | 64 | Female | Stomach | Loss of appetite and reduced body weight | Surgical resection | None | No recurrence during 36 mo follow-up |
| Oliveira et al[25], 2017 | 61 | Male | Stomach | Upper gastrointestinal bleeding | Endoscopic polypectomy | None | No recurrence during 6 yr follow-up |
| Ding et al[6], 2019 | 65 | Male | Stomach | Epigastric discomfort and mass | Distal gastrectomy | None | No recurrence during 3 mo follow-up |
| Weidenbaum et al[26], 2022 | 83 | Female | Stomach | None | None | Radiation therapy, chemotherapy | N/A |
| Carneiro et al[27], 2009 | 72 | Male | Duodenum | Epigastric pain, vomiting and weight loss | Resection of the fourth part of the duodenum and proximal segment of jejunum | None | No recurrence after 12 mo follow-up |
| Ammar et al[28], 2010 | 69 | Female | Duodenum | Fatigue, melaena | Percutaneous transhepatic biliary drainage | Extra-corporeal radiotherapy | N/A |
| Yoshida et al[29], 2004 | 70 | Female | Ileum | High fever, bowel obstruction | Combined resection of the terminal ileum and ascending colon | Chemotherapy | Died of cachexia 4 mo after surgery |
| Moriyama et al[30], 2006 | 73 | Female | Ileum | Abdominal pain | Local resection of the tumor | None | No recurrence after 28 mo follow-up |
| Gabriel et al[31], 2014 | 62 | Male | Ileocecum | Melena | Right hemicolectomy | None | N/A |
| Zhang et al[32], 2017 | 63 | Female | Ileocecum | Episodic pain around the umbilicus | Right hemicolectomy surgery | None | N/A |
| Hanawa et al[7], 2019 | 63 | Male | Ileocecum | Abdominal distention and weight loss | Surgically removed stenotic lesion of small intestine | Anti-Crohn’s disease | No recurrence during 36 mo follow-up |
| Evans et al[5], 2020 | 35 | Male | Appendix | Upper abdominal pain | Appendectomy | None | Alive without evidence of disease |
| Doki et al[33], 2008 | 64 | Male | Ascending colon | Aggravated pain in the right lower abdomen | Surgical resection | Chemotherapy (recurrence) | Recurrence 4 mo after surgery. Dead after 12 mo |
| Zhu et al[11], 2017 | 67 | Female | Ascending colon | Abdominal pain, and reduced gas and stool passage | Refused | Chemotherapy | Died of agranulocytosis and sepsis |
| Han et al[34], 2014 | 49 | Male | Transverse colon | Periumbilical abdominal pain | Extended laparoscopic left hemicolectomy | None | No recurrence during 36 mo follow-up |
| Lee et al[35], 2013 | 45 | Male | Descending colon | Lower abdominal pain, diarrhoea, weight loss | Laparoscopic extended left hemicolectomy with lymph node dissection | None | No recurrence during 36 mo follow-up |
| Zihni et al[36], 2014 | 54 | Male | Descending colon | Abdominal pain | Left hemicolectomy, small bowel resection | None | Died on the thirty-fifth post-operative day due to sepsis |
| Lattuneddu et al[37], 2004 | 86 | Male | Sigmoid colon | Abdominal pain, rectal bleeding and asthenia | Segmental resection of the left colon, with a complementary colecystectomy | None | No recurrence during 6 mo follow-up |
| Jones et al[38], 2008 | 65 | Male | Sigmoid colon | Dysuria, constant left lower quadrant abdominal pain | Sigmoid colon resection | None | N/A |
| 57 | Male | Sigmoid colon | Fatigue, hematochezia | Hartmann resection of the sigmoid colon | None | Died on day 19 after surgery | |
| Mjoli et al[39], 2016 | 42 | Male | Sigmoid colon | Rectal bleeding | Sigmoid colectomy | None | No recurrence during 3 mo follow-up |
| Kitamura et al[40], 2018 | 77 | Female | Sigmoid colon | Lower abdominal pain, nausea | Resection of the sigmoid colon, artificial anus | None | No recurrence during 14 mo follow-up |
| Gupta et al[41], 2007 | 42 | Male | Colon (multiple sites) | Diarrhea, progressive weight loss and malaise | Subtotal colectomy | Adjuvant chemotherapy (melphalan, prednisolone) | No recurrence during 17 mo follow-up |
| Nakagawa et al[42], 2011 | 84 | Female | Cecum, rectum | None | Endoscopic mucosal resection | None | N/A |
| Gohil et al[43], 2015 | 55 | Male | Rectum | Perianal pain, altered bowel habits | Surgical resection | Adjuvant radiotherapy | No recurrence during 17 mo follow-up |
| Bhangoo et al[44], 2021 | 82 | Male | Rectosigmoid colon | Rectal bleeding and obstruction | Open sigmoid low anterior resection | Radiotherapy | N/A |
| Lin et al[4], 2021 | 80 | Male | Rectum | Change of his bowel habit and inhibited defecation | Radical resection of the mass by laparoscope | None | N/A |
| Antunes et al[45], 2010 | 61 | Male | Anal canal | Abdominal discomfort, tenesmus, perineal pain | None | Radiotherapy | No recurrence during 24 mo follow-up |
Given the rarity of gastrointestinal EMP, unified treatment guidelines for this disease are not available. At present, complete surgical resection is a good choice for the treatment of gastrointestinal EMP. Several studies have reported that patients with gastrointestinal EMP can be completely cured after surgical resection of tumors[21,24,34,40]. Most of the patients underwent routine surgery. However, the EMP patient we reported with perforation of the small intestine required emergency surgery. In addition to perforation of small intestinal EMPs, perforation of colon EMPs can also occur. Kitamura et al[40] reported one case of EMP in the sigmoid colon with perforation. The patient underwent emergency surgery without postoperative adjuvant chemotherapy with no recurrence after 14 mo of regular follow-up. In recent years, endoscopic treatments, such as endoscopic mucosal resection or endoscopic submucosal dissection, have become increasingly popular in gastrointestinal EMP surgery and have obtained a good therapeutic effect[18,20,25]. Due to the high sensitivity of primary EMP to radiotherapy, local radiotherapy is also an effective treatment method[45,48]. At present, many hospitals use radiotherapy as an adjuvant treatment for patients with gastrointestinal EMP after surgery to prevent local recurrence or metastasis. Moreover, radiotherapy can also represent an additional therapeutic option for cases with incomplete resection, lymph node involvement or recurrence. There are also some results suggesting that EMP is well controlled with a dose of 40 Gy or more[49]. In cases that are small, well-defined, or postexcision with positive margins, 40 Gy is acceptable[50]. Currently, most studies in this area are retrospective, and more prospective randomized controlled studies are needed to verify these results.
EMP is a low malignancy tumor with a good prognosis. Local recurrence or recurrence at other sites occurred in 7.5% and 10% of patients, respectively, and the 15-year survival rate was 78%[51]. Given that EMP may recur or progress to MM in some patients, regular long-term follow-up is recommended and necessary. Detailed medical records, physical examination, laboratory tests, including complete blood cell count, beta-2 microglobulin and immunoglobulin levels, renal function, and imaging examination of the abdomen are required for patients during follow-up[52].
In conclusion, EMP of the small intestine is extremely rare and lacks specific clinical and imaging manifestations. EMP may be associated with spontaneous perforation, which requires emergency surgery. We firstly report a case of intestinal perforation caused by EMP of the small intestine. The diagnosis of EMP still depends on the histopathological results. Surgical resection and radiotherapy can obtain good therapeutic effects. The cooperation of a multidisciplinary team, including pathologists, hematologists, radiologists and surgeons, is needed to develop the best diagnostic and therapeutic plan for gastrointestinal EMP.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Majerović M, Croatia; Perse M, Slovenia; Vij M, India A-Editor: Yao QG, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Knowling MA, Harwood AR, Bergsagel DE. Comparison of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol. 1983;1:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 253] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Clinical course of solitary extramedullary plasmacytoma. Radiother Oncol. 1999;52:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144:86-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Lin QT, Cai XR. Extramedullary plasmacytoma involving rectum: A case report and literature review. Radiol Case Rep. 2021;16:785-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Evans MG, Zhao X, Lin F, Wang BY. First Reported Case of Extramedullary Plasmacytoma of the Appendix. Gastroenterology Res. 2020;13:85-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Ding W, Tan Y, Qian Y, Xue W, Wang Y, Xi C, Gu K, Xu Y, Xu X. Primary plasmablastic plasmacytoma in the stomach of an immunocompetent adult: A case report. Medicine (Baltimore). 2019;98:e14235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Hanawa Y, Higashiyama M, Horiuchi K, Ayaki K, Ito S, Mizoguchi A, Nishii S, Wada A, Inaba K, Sugihara N, Furuhashi H, Takajo T, Shirakabe K, Watanabe C, Tomita K, Komoto S, Nagao S, Miura S, Shimazaki H, Takeuchi K, Ueno H, Hokari R. Crohn's Disease Accompanied with Small Intestinal Extramedullary Plasmacytoma. Intern Med. 2019;58:2019-2023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Glasbey JC, Arshad F, Almond LM, Vydianath B, Desai A, Gourevitch D, Ford SJ. Gastrointestinal manifestations of extramedullary plasmacytoma: a narrative review and illustrative case reports. Ann R Coll Surg Engl. 2018;100:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Comba IY, Torres Luna NE, Cooper C, W Crespo M, Carilli A. A Rare Case of Extramedullary Plasmacytoma Presenting as Massive Upper Gastrointestinal Bleeding. Cureus. 2019;11:e3993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Iosif E, Rees C, Beeslaar S, Shamali A, Lauro R, Kyriakides C. Gastrointestinal bleeding as initial presentation of extramedullary plasma cell neoplasms: A case report and review of the literature. World J Gastrointest Endosc. 2019;11:308-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Zhu Z, Cao H, Chen L. Incomplete Colonic Obstruction Caused by Extramedullary Plasmacytoma. Clin Gastroenterol Hepatol. 2017;15:e69-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Alnimer L, Zakaria A, Alshare B, Samhouri Y, Raphael M. A Rare Case of Small Bowel Extramedullary Plasmacytomas Presenting With Intestinal Obstruction. Cureus. 2021;13:e15704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Ignjatović M, Bezmarević M, Cerović S. Solitary extramedullary plasmacytoma of the duodenum and pancreas: A case report and review of the literature. Vojnosanit Pregl. 2016;73:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, Samson D; Working Group of the UK Myeloma Forum; British Committee for Standards in Haematology; British Society for Haematology. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin Oncol (R Coll Radiol). 2004;16:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Fagkrezos D, Manes K, Paraskeva K, Lenos M, Triantopoulou C, Apessou D, Maniatis P. Secondary extramedullary plasmacytoma of sigmoid colon in a patient with multiple myeloma: a case report. J Med Case Rep. 2018;12:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wallace E, Stewart Z, Theriot D, Shaffer W, Guillory S, Hanemann M, Danrad R, Spieler B. Atypical Presentation of Extramedullary Plasmacytoma. Ochsner J. 2018;18:101-103. [PubMed] |
| 17. | Katodritou E, Kartsios C, Gastari V, Verrou E, Mihou D, Banti A, Lazaraki G, Lazaridou A, Kaloutsi V, Zervas K. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32:339-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Park CH, Lee SM, Kim TO, Kim DU, Jung WJ, Kim GH, Song GA. Treatment of solitary extramedullary plasmacytoma of the stomach with endoscopic submucosal dissection. Gut Liver. 2009;3:334-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Krishnamoorthy N, Bal MM, Ramadwar M, Deodhar K, Mohandas KM. A rare case of primary gastric plasmacytoma: an unforeseen surprise. J Cancer Res Ther. 2010;6:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Park SY, Moon HS, Seong JK, Jeong HY, Yoon BY, Hwang SW, Song KS. Successful treatment of a gastric plasmacytoma using a combination of endoscopic submucosal dissection and oral thalidomide. Clin Endosc. 2014;47:564-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Zhao ZH, Yang JF, Wang JD, Wei JG, Liu F, Wang BY. Imaging findings of primary gastric plasmacytoma: a case report. World J Gastroenterol. 2014;20:10202-10207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Fukuhara S, Tazawa H, Okanobu H, Kida M, Kido M, Takafuta T, Nishida T, Ohdan H, Sakimoto H. Successful treatment of primary advanced gastric plasmacytoma using a combination of surgical resection and chemotherapy with bortezomib: A case report. Int J Surg Case Rep. 2016;27:133-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Kang DY, Kim GB, Choi BS, Seo JW, Lim HJ, Hong R, Park SG. Successful treatment of a primary gastric plasmacytoma mimicking intractable gastric ulcer by using high-dose dexamethasone therapy: a case report. J Med Case Rep. 2016;10:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Takahashi T, Morita T, Matsuno Y. Primary Extramedullary Plasmacytoma in the Gastroduodenal Canal Associated With Epstein-Barr Virus-Associated Adenocarcinoma of the Stomach: A Case Report. Int J Surg Pathol. 2016;24:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Oliveira RC, Amaro P, Julião MJ, Cipriano MA. Primary gastric plasmacytoma: a rare entity. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Weidenbaum C. Case report: gastric plasmacytoma resistant to radiation therapy. Postgrad Med. 2022;134:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Carneiro FP, Sobreira MN, Maia LB, Sartorelli AC, Franceschi LE, Brandão MB, Calaça BW, Lustosa FS, Lopes JV. Extramedullary plasmocytoma associated with a massive deposit of amyloid in the duodenum. World J Gastroenterol. 2009;15:3565-3568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Ammar T, Kreisel F, Ciorba MA. Primary antral duodenal extramedullary plasmacytoma presenting with melena. Clin Gastroenterol Hepatol. 2010;8:A32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Yoshida T, Soda K, Yamada S, Nakahara M, Nishida J, Kametaka M, Konishi F. Biclonal extramedullary plasmacytoma arising in the peritoneal cavity: report of a case. Surg Today. 2004;34:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Moriyama H, Kawahara K, Noguchi T, Kikuchi R, Wada S, Takeno S, Kashima K. Primary extramedullary plasmacytoma of the small intestine. A case report and review of the literature. J Exp Clin Cancer Res. 2006;25:129-134. [PubMed] |
| 31. | Gabriel EM, Savu M. Discovery of a rare ileocecal plasmacytoma. J Surg Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Zhang D, Cao D, Shen D, Mulmi Shrestha S, Yin Y. Extramedullary plasmacytoma occuring in ileocecum: A case report and literature review. Medicine (Baltimore). 2017;96:e9313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Doki T, Takeuchi O, Kaiho T, Tsuchiya S, Matsuzaki O, Miyazaki M. Primary isolated extramedullary plasmacytoma of the colon. Int J Colorectal Dis. 2008;23:719-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Han YJ, Park SJ, Park MI, Moon W, Kim SE, Ku KH, Ock SY. Solitary extramedullary plasmacytoma in the gastrointestinal tract: report of two cases and review of literature. Korean J Gastroenterol. 2014;63:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Lee SH, Ahn BK, Baek SU, Chang HK. Primary Isolated Extramedullary Plasmacytoma in the Colon. Gastroenterology Res. 2013;6:152-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Zihni İ, Dinç R, Canpolat S, Cengiz F, Uslu A. Extramedullary plasmacytoma of the colon: a case report. Ulus Cerrahi Derg. 2014;30:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Lattuneddu A, Farneti F, Lucci E, Garcea D, Ronconi S, Saragoni L. A case of primary extramedullary plasmacytoma of the colon. Int J Colorectal Dis. 2004;19:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Jones JE, Brand MI, Saclarides TJ, Jakate S. Primary extramedullary plasmacytomas of the colon. Am Surg. 2008;74:873-874. [PubMed] |
| 39. | Mjoli M, Vorajee N, Naidoo Y, Madiba T. Solitary extramedullary plasmacytoma of the colon, rectum and anus. S Afr J Surg. 2016;54:45-47. [PubMed] |
| 40. | Kitamura F, Doi K, Ishiodori H, Ohchi T, Baba H. Primary extramedullary plasmacytoma of the sigmoid colon with perforation: a case report. Surg Case Rep. 2018;4:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Gupta V, Nahak B, Sakhuja P, Agarwal AK, Kumar N, Mishra PK. Primary isolated extramedullary plasmacytoma of colon. World J Surg Oncol. 2007;5:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Nakagawa Y, Nagai T, Okawara H, Nakashima H, Hisamatsu A, Syutou M, Yamauchi M, Kai S, Nakayama T, Yokoyama S, Murakami K, Fujioka T. Minute primary extramedullary plasmacytomas of the large intestine. Endoscopy. 2011;43 Suppl 2 UCTN:E105-E106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Gohil MH, Bhavsar DC, Suryanarayana U, Jetly DH. Plasmacytoma rectum extending to para-rectal region. J Cancer Res Ther. 2015;11:662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Bhangoo RS, McCullough AE, Yang M. Obstructive rectosigmoid colon solitary extramedullary plasmacytoma. Dig Liver Dis. 2021;53:496-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Antunes MI, Bujor L, Grillo IM. Anal canal plasmacytoma-An uncommon presentation site. Rep Pract Oncol Radiother. 2010;16:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Ryu SW, Cohen-Hallaleh V. Imaging features of extramedullary plasmacytoma. J Med Imaging Radiat Oncol. 2020;64:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Parnell K, Ahmed M, Smalligan RD, Nadesan S. Extramedullary plasmacytoma mimicking colon carcinoma: an unusual presentation and review of the literature. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Barzenje DA, Kolstad A, Ghanima W, Holte H. Long-term outcome of patients with solitary plasmacytoma treated with radiotherapy: A population-based, single-center study with median follow-up of 13.7 years. Hematol Oncol. 2018;36:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Alghisi A, Borghetti P, Maddalo M, Roccaro AM, Tucci A, Mazzola R, Magrini SM, Lo Casto A, Bonù ML, Tomasini D, Pasinetti N, Peretto G, Bertagna F, Tomasi C, Buglione M, Triggiani L. Radiotherapy for the treatment of solitary plasmacytoma: 7-year outcomes by a mono-institutional experience. J Cancer Res Clin Oncol. 2021;147:1773-1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Tsang RW, Campbell BA, Goda JS, Kelsey CR, Kirova YM, Parikh RR, Ng AK, Ricardi U, Suh CO, Mauch PM, Specht L, Yahalom J. Radiation Therapy for Solitary Plasmacytoma and Multiple Myeloma: Guidelines From the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;101:794-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 51. | Galieni P, Cavo M, Pulsoni A, Avvisati G, Bigazzi C, Neri S, Caliceti U, Benni M, Ronconi S, Lauria F. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85:47-51. [PubMed] |
| 52. | Lopes da Silva R. Extramedullary plasmacytoma of the small intestine: clinical features, diagnosis and treatment. J Dig Dis. 2012;13:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |