Published online Jun 27, 2022. doi: 10.4240/wjgs.v14.i6.567
Peer-review started: December 1, 2021
First decision: April 16, 2022
Revised: April 21, 2022
Accepted: May 21, 2022
Article in press: May 21, 2022
Published online: June 27, 2022
Processing time: 207 Days and 22.6 Hours
Patients with hepatocellular carcinoma complicated with main portal vein tumor thrombosis (mPVTT) and cirrhotic portal hypertension (CPH) have an extremely poor prognosis, and there is a lack of a clinically effective treatment paradigm.
To evaluate the efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPS) combined with radioactive seed strand for the treatment of mPVTT patients with CPH.
The clinical data of 83 consecutive patients who underwent TIPS combined with 125I seed strand placement for mPVTT and CPH from January 2015 to December 2018 were retrospectively reviewed. Procedure-related data (success rate, relief of portal vein pressure and CPH symptoms, and adverse events), PVTT response, and patient survival were assessed through a 2-year follow-up.
The success rate was 100.0% without perioperative death or procedure-related severe adverse events. The mean portal vein pressure was significantly decreased after the procedure (22.25 ± 7.33 mmHg vs 35.12 ± 7.94 mmHg,
TIPS combined with radioactive seed strand might be effective and safe in treating mPVTT patients with CPH.
Core Tip: We adequately evaluated whether transjugular intrahepatic portosystemic shunt combined with radioactive seed strand placement was safe in adverse events and effective in portal vein tumor thrombosis response and prolonging survival time for the treatment of patients with main portal vein tumor thrombosis and cirrhotic portal hypertension through a retrospective cohort study with 2 years of follow-up.
- Citation: Yan XH, Yue ZD, Zhao HW, Wang L, Fan ZH, Wu YF, Meng MM, Zhang K, Jiang L, Ding HG, Zhang YN, Yang YP, Liu FQ. Transjugular intrahepatic portosystemic shunt with radioactive seed strand for main portal vein tumor thrombosis with cirrhotic portal hypertension. World J Gastrointest Surg 2022; 14(6): 567-579
- URL: https://www.wjgnet.com/1948-9366/full/v14/i6/567.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i6.567
Portal vein tumor thrombosis (PVTT) is common in patients with hepatocellular carcinoma (HCC), with an incidence of 44.0%-62.2%[1]. Main PVTT (mPVTT) is defined as PVTT invading the main trunk of the portal vein, accounting for approximately 19.5%-35.2% of PVTT[2-4]. The prognosis of patients with PVTT is poor and the median overall survival is only 2.7-4.0 mo without treatment[5].
HCC is mostly based on cirrhosis, and is usually complicated with cirrhotic portal hypertension (CPH). The decompensated stage of CPH is often accompanied by high-mortality events, e.g., esophagogastric variceal bleeding (EGVB) and refractory ascites/hydrothorax. EGVB is associated with a mortality of 10%-20% at 6 wk[6], and refractory ascites is associated with a reduction in the survival rate to 50% at 6 mo[7]. Once PVTT is combined with cirrhosis-related decompensated events, it would worsen the disease and accelerate the death of patients.
The treatment strategies for PVTT include palliative surgical resection, transarterial chemoembolization (TACE), external radiotherapy, chemotherapy, and targeted therapy[2,8,9], but these treatments are usually infeasible and unsatisfactory in patients with decompensated CPH. Transjugular intrahepatic portosystemic shunt (TIPS) is an effective treatment for CPH[10,11] and eliminates pylemphraxis with the covered stent, but the stent has no substantial therapeutic effect on mPVTT and results in PVTT progression and stent stenosis.
In recent years, the application of radioactive seed placement, such as the low-energy radionuclide 125I[12-14], has attracted attention and achieved promising efficacy when combined with portal vein stents. Radioactive seed strand placement is one method of endovascular brachytherapy. The purpose of this study was to retrospectively analyze the clinical efficacy of TIPS combined with radioactive seed strand placement for mPVTT patients with CPH from January 2015 to December 2018.
The study was approved by the Ethics Committee and Institutional Review Board of Peking University Ninth School of Clinical Medicine. A consecutive cohort of 83 patients with HCC who underwent TIPS combined with 125I seed strand placement for mPVTT and CPH from January 2015 to December 2018 was retrospectively reviewed. Patients with incomplete clinical data or loss to follow-up were excluded from the analysis. Among 81 patients, 70 (84.3%) were males and 13 (15.7%) were females, aged 35-79 years (mean 56.46 years). There were 62 (74.7%) cases of EGVB, 14 (16.9%) cases of refractory ascites/hydrothorax, and 7 (8.4%) cases of both. Child–Pugh grading included 23 (27.7%) cases with grade A, 52 (62.7%) cases with grade B, and 8 (9.6%) cases with grade C. According to cTNM staging, 55 (66.3%) cases were stage IIIB, 19 (22.9%) cases were stage IVA, and 9 (10.8%) cases were stage IVB. The baseline characteristics of the patients are presented in Table 1.
| Characteristics | n (%)/mean ± SD/M (P25-P75) |
| Gender (male/female) | 70/13 (84.3/15.7) |
| Age (yr) | 56.46 ± 8.97 |
| BMI | 22.83 ± 2.99 |
| Etiology of cirrhosis (HBV/HCV/alcoholic/other) | 66/8/4/5 (79.5/9.6/4.8/6.0) |
| Cirrhosis-related decompensated events (EGVB/Refractory ascites or hydrothorax/Both) | 62/14/7 (74.7/16.9/8.4) |
| EGV degree (mild/moderate/severe) | 7/36/40 (8.4/43.4/48.2) |
| Ascites degree (no/mild/moderate-severe) | 8/24/51 (9.6/28.9/61.4) |
| Preoperative HVPG (mmHg) | 19.96 ± 9.01 |
| Child–Pugh grade (A/B/C) | 23/52/8 (27.7/62.7/9.6) |
| Intrahepatic HCC morphology (unifocal/multifocal) | 47/36 (56.6/43.4) |
| Sum of longest viable tumor diameters (cm) | 6.62 ± 2.77 |
| ≤ 5/5-8/> 8 | 23/44/16 (27.7/53.0/19.3) |
| BCLC stage (C/D) | 75/8 (90.4/9.6) |
| cTNM stage (IIIB/IVA/IVB) | 55/19/9 (66.3/22.9/10.8) |
| PLT (109/L) | 108.24 ± 86.09 |
| PT (s) | 14.89 ± 3.89 |
| ALT (U/L) | 31.40 ± 29.29 |
| AST (U/L) | 49.63 ± 45.00 |
| TBil (μmol/L) | 31.74 ± 17.68 |
| Albumin (g/L) | 35.08 ± 4.85 |
| AFP (ng/mL)1 | 769.49 (16.69-2345.11) |
| Log10(AFP) | 2.40 ± 1.26 |
| Combined TACE/RFA/targeted therapy | 83/52/41 (100/62.7/49.4) |
Procedure-related data [success rate, relief of portal vein pressure (PVP) and CPH symptoms, and adverse events], mPVTT response, and patient survival were assessed through a 2-year follow-up. The success rate was defined by the planned stent and seed successfully placed. PVTT response was determined according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST)[15] by experienced radiologists: (1) Complete response (CR) was defined as disappearance of PVTT; (2) Partial response (PR) was a ≥ 30% reduction of the PVTT lesion compared with baseline; (3) Progressive disease (PD) was defined as ≥ 20% enlargement of the PVTT lesion than baseline; (4) Stable disease (SD) referred to the PVTT lesion that did not reach the standard of PR and PD. The objective response rate (ORR) of PVTT was the sum of CR and PR. Patient survival was defined as the period from the day of operation to patient death from any cause or to the last follow-up time point.
Adverse events were classified as shunt-related adverse events and radiation-related adverse events. Shunt-related adverse events consisted of post-TIPS hepatic encephalopathy (HE), the recurrence of CPH, shunt stenosis, and shunt-induced potential distant metastasis. The recurrence of CPH was determined as recurrent EGVB or hepatic ascites/hydrothorax, which principally resulted from shunt or intra-stent stenosis. Shunt stenosis was indicated by the recurrence of CPH events and confirmed by imaging [e.g., enhanced computed tomography (CT) or portal venography]. Shunt-induced potential distant metastasis was defined as new-onset hematogenous metastasis after shunt opening of TIPS, which was diagnosed by systemic imaging or pathology. Radiation-related adverse events included radiation injury and seed strand or 125I seed translocation.
All patients were fully evaluated before the procedure: (1) The severity of esophagogastric varices (EGV) was graded by gastroscopy; (2) The degree of ascites was graded by ultrasound examination[16]; (3) Child-Pugh was used for evaluation of liver function; (4) Tumors were staged according to both the international Barcelona Clinic Liver Cancer (BCLC) staging system[17] and cTNM staging system[18]; and (5) intrahepatic tumor size was determined as the sum of the longest viable tumor diameters of typical intrahepatic target lesions according to mRECIST[15], measured by experienced radiologists.
The indications for the procedure were as follows: (1) mPVTT secondary to HCC, as confirmed by percutaneous biopsy or enhanced CT/magnetic resonance imaging /positron emission tomography imaging; (2) Intrahepatic CPH confirmed by imaging examinations and hepatic venous pressure gradient (HVPG) measurement; (3) Failure of prior conservative treatment for cirrhosis-related decompensated events such as EGVB or refractory ascites/hydrothorax; and (4) Life expectancy > 2 mo. The contraindications were any one of the following: (1) Uncomplicated prehepatic portal hypertension; (2) Severe cardiac, cerebral, respiratory, renal insufficiency or other systemic malignancy; (3) Rapid progression in hepatic insufficiency; (4) Intrahepatic tumor hampering the procedure; (5) Allergy to contrast agent; and (6) Pregnancy or lactation. The operation was performed by interventional physicians with more than 15 years of experience. The benefits and potential risks of the procedure were explained thoroughly to all patients and their families, and then, written informed consent was signed.
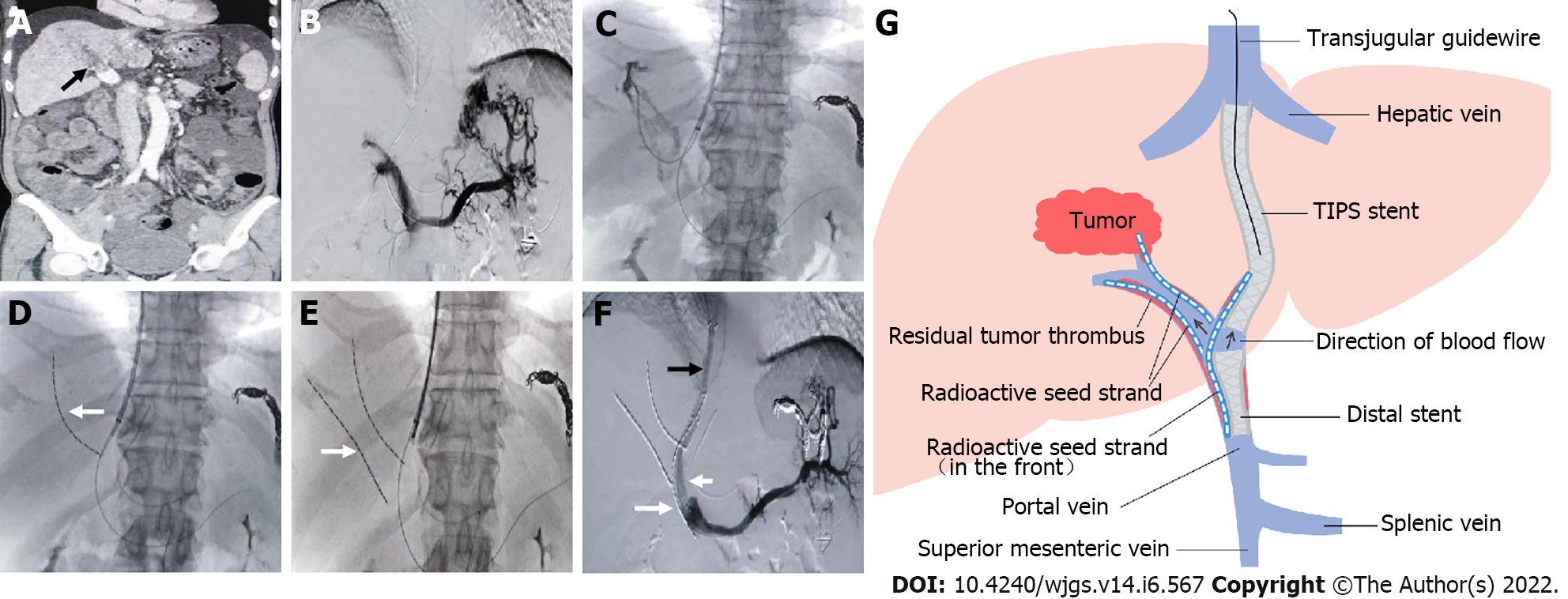
During the procedure, the right internal jugular vein was punctured routinely under local anesthesia. After intubation to the inferior vena cava and hepatic vein, HVPG was measured, and then RUPS-100 (Cook Inc., United States) was inserted. According to preoperative imaging and angiography, the appropriate position and angle were determined to puncture the intrahepatic portal vein from the hepatic vein or inferior vena cava of the hepatic segment. After successful puncture, an angiographic catheter was inserted for portal venography, and the puncture set was placed into the intrahepatic portal vein.
Before shunting, PVP was measured, and then PVTT was grabbed and aspirated as much as possible. Two ultrasmooth guidewires were inserted through the outer sheath of RUPS-100, one of which was retained in the splenic vein, and the other introduced a 4-5F single-bend or cobra catheter that was selected to the distal end of branch PVTT. Then, a 6F guiding catheter was replaced, and a radioactive seed strand was implanted via the guiding catheter. Next, a 6-8 mm balloon was introduced through the outer sheath to dilate the shunt, and then a 7-8 mm Fluency covered stent (Bard Inc., United States) was placed. According to the extent of mPVTT, a distal 10-12 mm covered stent was placed for the entire coverage of mPVTT.
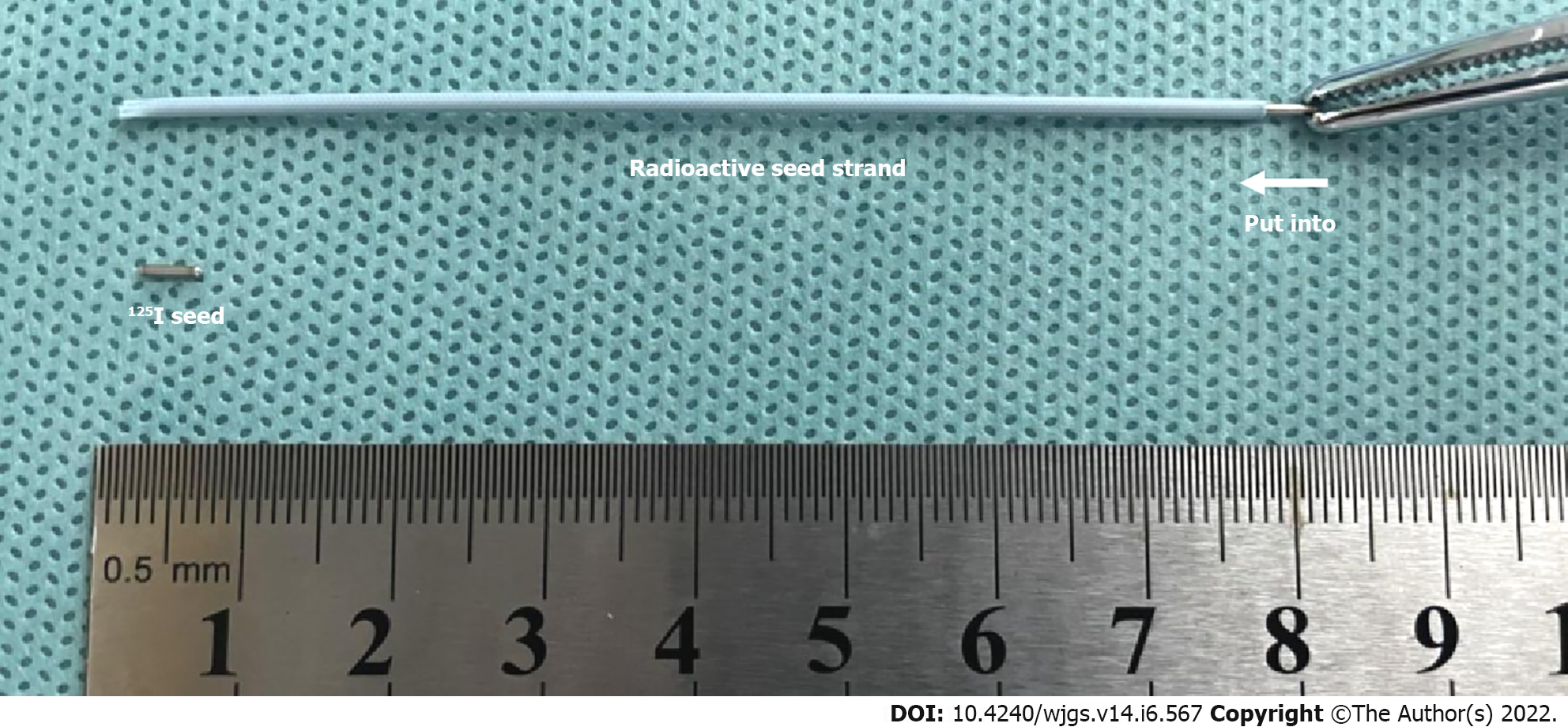
The radioactive seed (Isotope & Radiation Corp., China) was fully loaded into a 4F catheter in vitro, creating the radioactive seed strand (Figure 1). Then, the radioactive seed strand was placed outside the stents via a 6F guiding catheter (by the guidewire retained in the splenic vein). The radioactive seed strand was compressed and fixed to the portal vein by the stents. The length of the radioactive seed strand was usually more than 10 mm at both ends of the PVTT. Finally, PVP after shunting was measured, and portal venography was performed again (Figure 2).
TACE was used for intrahepatic tumors and PVTT lesions every 1-3 mo by using an embolic agent (lipiodol 3-30 mL) and chemotherapy drugs (epirubicin 10-20 mg and hydroxycamptothecine 5-15 mg). TACE was performed in all patients (ranging from 1-12 times per patient and an average of 4.2 times).
Radiofrequency ablation (RFA) was also carried out for intrahepatic tumors in patients with good coagulation function and platelet count and the inability to sequentially undergo TACE due to arterial occlusion after repeated arterial intervention. The RFA equipment was WHK-IB, Beijing Welfare Electronics Co., China. 52 of 83 patients underwent RFA (ranging from 1-3 times per patient and an average of 1.6 times).
According to patients' specific conditions and wishes, 41 patients received targeted therapy such as sorafenib or lenvatinib.
All patients were followed up by telephone at a 4-6-wk interval postoperatively until death or their last follow-up. At 3, 6, 12, and 24 mo after the operation, patients were required to undergo a hospital revisit to assess PVTT response and adverse events. Sequential TACE or RFA was performed on the intrahepatic primary lesions. In addition, positive and timely management was given for adverse events such as post-TIPS HE, shunt stenosis, and recurrence of CPH.
Continuous variables conforming to a normal distribution are presented as the mean ± SD and median (interquartile range) [M (P25-P75)] for those with a nonnormal distribution. Categorical variables are presented as percentages (%). The mean values of two related samples were compared by using the paired samples t test. In survival analysis, the Kaplan-Meier curve was performed for description, the log-rank test was utilized for comparison, and Cox regression was carried out for correlated factor analysis. Variables satisfying the proportional hazards assumption were included in the multivariate analysis using Cox regression. P < 0.05 was considered a statistically significant difference. IBM SPSS software version 26.0 was used for statistical analysis.
The success rate of the procedure was 100.0% (83/83), without perioperative death or procedure-related serious adverse events. The number of implanted seeds ranged from 29 to 95, with an average of 47 per patient. The mean PVP was significantly decreased after the procedure (22.25 ± 7.33 mmHg vs 35.12 ± 7.94 mmHg,
The mean follow-up period was 14.5 ± 9.4 mo (range 1-37 mo). HE developed in a total of 16 patients (19.3%) after the procedure, most of whom had mild HE in clinical stages 1-2. The cumulative recurrence rates of CPH at 6, 12, and 24 mo were 9.6% (8/83), 22.9% (19/83), and 33.7% (28/83), respectively. The cumulative rates of shunt stenosis at 6, 12, and 24 mo were 13.3% (11/83), 28.9% (24/83), and 38.6% (32/83), respectively (Table 2). During follow-up, no seed strand shift or 125I seed fall-off and translocation occurred, and no radiation injury (such as radiation-induced liver disease or gastrointestinal ulceration) was observed.
| Items | 6 mo | 12 mo | 24 mo |
| Cumulative survival rate (%) | 83.1 | 49.7 | 21.8 |
| Cumulative rate of shunt stenosis (%) | 13.3 | 28.9 | 38.6 |
| Cumulative recurrence rate of CPH (%) | 9.6 | 22.9 | 33.7 |
Four patients failed to be assessed on account of death within 2 mo. The ORR of PVTT was 67.5% (Table 3). Among patients who presented PD, all 6 cases related to PVTT exceeded the distal portal system, e.g., the mesenteric vein or splenic vein.
| PVTT response | CR | PR | SD | PD | Response (ORR) |
| Number (%) | 15 (18.1) | 41 (49.4) | 17 (20.5) | 6 (7.2) | 56 (67.5) |
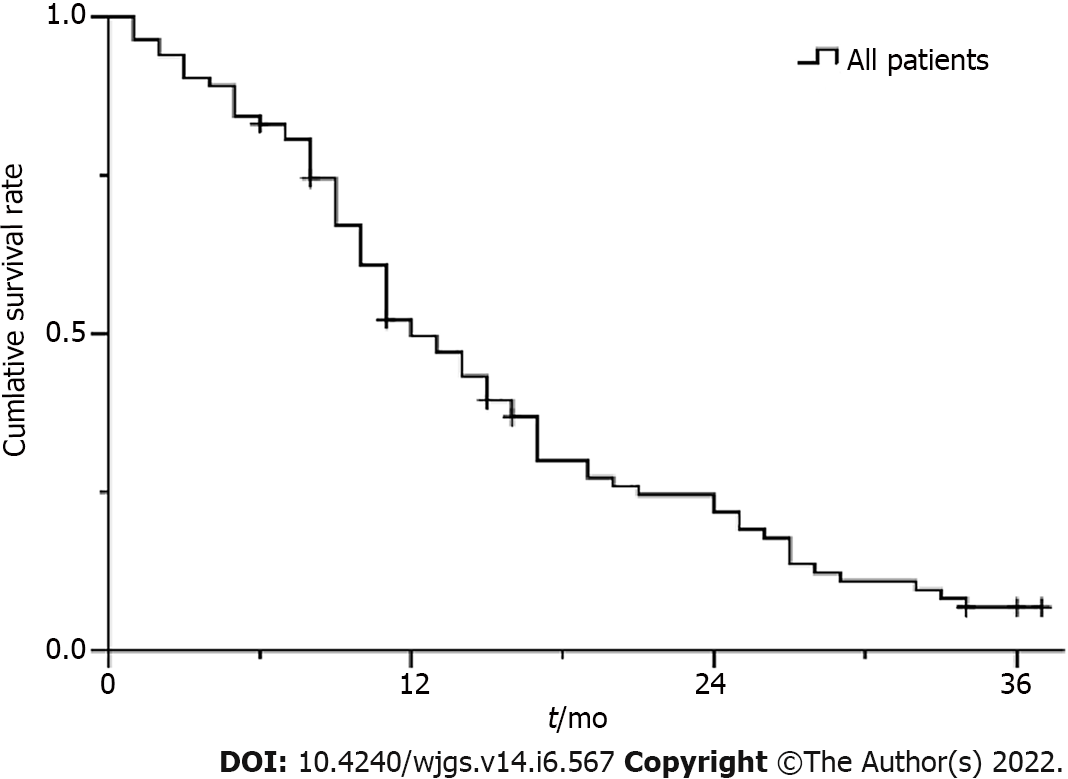
The Kaplan-Meier survival curve is shown in Figure 3. The median survival time was 12.0 ± 1.3 mo [95% confidence interval (CI): 9.5-14.5]. The cumulative survival rates at 6, 12, and 24 mo were 83.1%, 49.7%, and 21.8%, respectively.
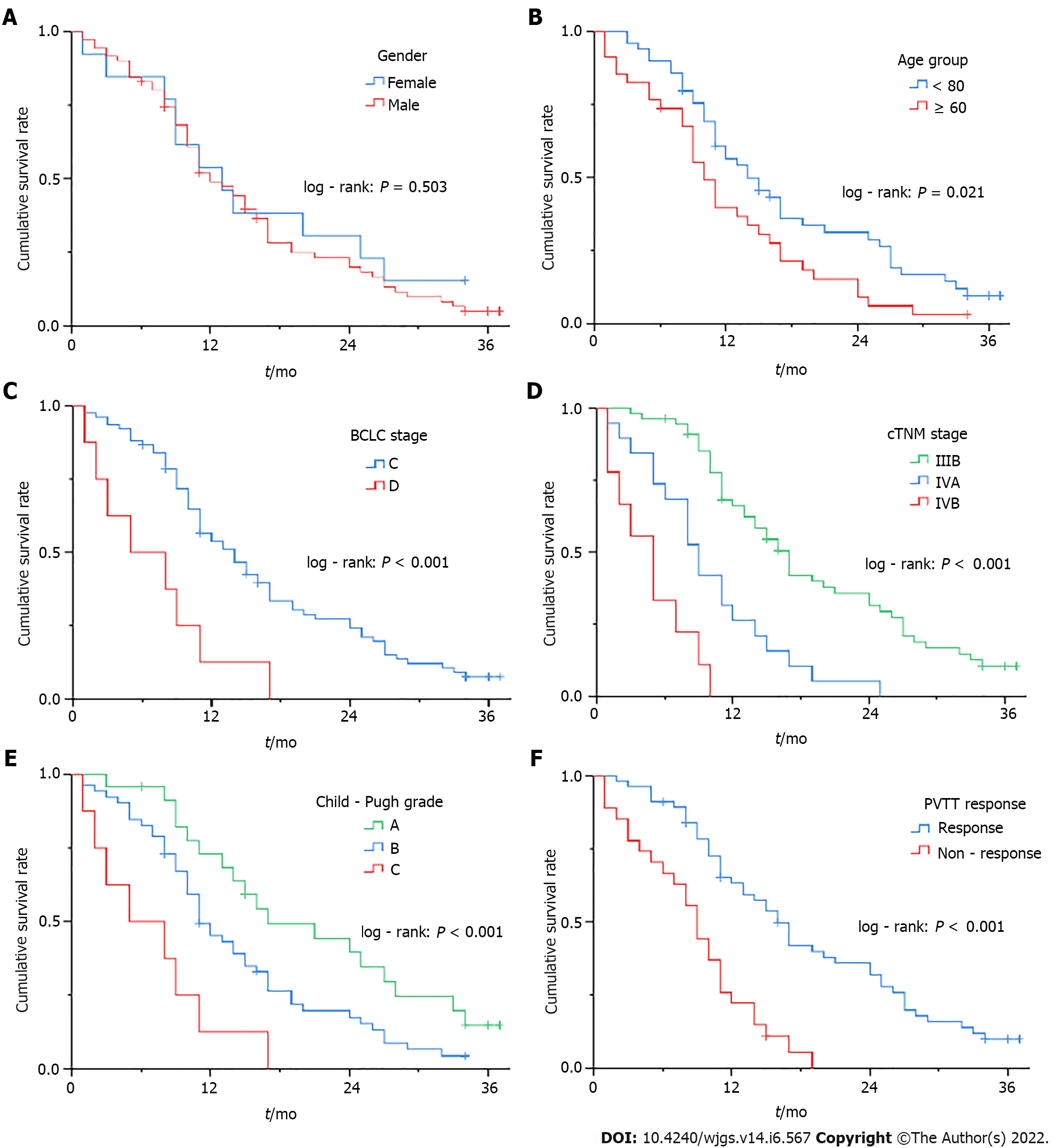
In the stratification analysis using the survival curves and log-rank test, patients with age < 60, Child-Pugh grade A or B, BCLC stage C, cTNM stage IIIB or IVA, and PVTT response had significant survival benefits (P < 0.05) in the comparison of their respective groups (Figure 4 and Table 4). Notably, cTNM staging showed a more detailed stratification capability than BCLC staging.
| Stratification indicator | Log-rank | P value |
| Gender | 0.448 | 0.503 |
| Age group | 5.311 | 0.021 |
| EGV degree | 0.448 | 0.600 |
| Ascites degree | 1.308 | 0.520 |
| Child-Pugh grade | 15.810 | < 0.001 |
| Intrahepatic HCC morphology | 0.174 | 0.677 |
| Group of tumor diameters | 1.685 | 0.431 |
| BCLC stage | 10.883 | < 0.001 |
| cTNM stage | 51.774 | < 0.001 |
| Combined with RFA | 0.275 | 0.600 |
| Combined with targeted therapy | 0.001 | 0.978 |
| PVTT response | 22.617 | < 0.001 |
| Post-TIPS HE | 0.255 | 0.613 |
| Shunt stenosis | 0.027 | 0.868 |
| Recurrence of CPH | 0.235 | 0.628 |
In Cox regression analysis, the relevant parameters including body mass index (BMI), Child-Pugh grade, cTNM stage, and PVTT response, were independent prognostic factors as indicated in the multivariate Cox regression model (Table 5).
| Variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender (female/male) | 1.237 | 0.650-2.355 | 0.518 | |||
| Age (years) | 1.039 | 1.011-1.068 | 0.006 | |||
| BMI | 0.781 | 0.701-0.871 | < 0.001 | 0.861 | 0.768-0.965 | 0.010 |
| EGV degree (mild/moderate/severe) | 1.130 | 0.796-1.605 | 0.493 | |||
| Ascites degree (no/mild/moderate-severe) | 1.055 | 0.760-1.464 | 0.748 | |||
| Preoperative HVPG (mmHg) | 1.006 | 0.979-1.034 | 0.668 | |||
| Child-Pugh grade | < 0.001 | |||||
| A/B | 1.856 | 1.068-3.225 | 0.028 | 2.243 | 1.270-3.961 | 0.005 |
| A/C | 4.999 | 2.099-11.907 | < 0.001 | 7.308 | 2.898-18.425 | < 0.001 |
| Intrahepatic HCC morphology (unifocal/multifocal) | 0.909 | 0.570-1.447 | 0.687 | |||
| Sum of longest viable tumor diameters (cm) | 1.070 | 0.988-1.158 | 0.097 | |||
| BCLC stage (C/D) | 3.216 | 1.509-6.851 | 0.002 | |||
| cTNM stage (IIIB/IVA/IVB) | 3.269 | 2.228-4.795 | < 0.001 | 2.745 | 1.726-4.366 | < 0.001 |
| PLT (109/L) | 1.000 | 0.997-1.003 | 0.917 | |||
| PT (s) | 1.006 | 0.959-1.056 | 0.802 | |||
| ALT (U/L) | 1.004 | 0.994-1.013 | 0.465 | |||
| AST (U/L) | 1.003 | 0.998-1.008 | 0.173 | |||
| TBil (μmol/L) | 1.022 | 1.008-1.035 | 0.001 | |||
| Albumin (g/L) | 0.929 | 0.886-0.974 | 0.002 | |||
| Log10(AFP) (ng/mL) | 1.341 | 1.097-1.639 | 0.004 | |||
| Combined RFA (no/yes) | 0.885 | 0.552-1.419 | 0.612 | |||
| Combined targeted therapy (no/yes) | 0.994 | 0.627-1.574 | 0.978 | |||
| Reduction of PVP (mmHg) | 1.025 | 0.983-1.069 | 0.247 | |||
| PVTT response (nonresponse/response) | 0.302 | 0.176-0.516 | < 0.001 | 0.472 | 0.259-0.859 | 0.014 |
| Post-TIPS HE (no/yes) | 0.864 | 0.482-1.551 | 0.625 | |||
| Shunt stenosis (no/yes) | 1.039 | 0.650-1.662 | 0.873 | |||
| Recurrence of CPH (no/yes) | 1.122 | 0.694-1.814 | 0.639 | |||
With the development of multidisciplinary teamwork, HCC complicated with PVTT has attracted increasing interest and research. Owing to the biological characteristics of HCC and anatomical features of the liver, HCC cells tend to invade the intrahepatic vasculature, especially the portal venous system[19]. In the past few years, the application of 125I seeds[12-14] has provided a new therapy for advanced HCC. In our study, the ORR of PVTT reached 67.5% after 125I seed strand placement. In multivariate survival analysis, PVTT response had a significant effect on patient survival, which could reduce the risk of death [hazard ratio (HR) = 0.472]. Additionally, no radiation injury was observed during postoperative follow-up. In short, radioactive seed strand placement may be an effective approach for the local treatment of PVTT.
It is a biological effect of ionizing radiation that 125I relies on by continuously releasing low-energy γ rays to kill tumor cells and then achieve the purpose of treatment. With a half-value layer of only 17 mm in equivalent tissue, 125I rarely involves adjacent tissues or organs. Thus, radioactive seed strand placement has the advantages of a high local dose to the tumor thrombus and less damage to normal tissues.
In addition, radioactive seed strands also have the following advantages: first, the length of the seed strand can be determined according to the length of the tumor thrombus, and the seeds in the catheter are arranged neatly; second, the seed strand implanted in the portal vein branch does not shift, nor does the seed strand that is fixed in the main portal vein by stents; and finally, radioactive seed have antitumor and anti-intimal hyperplasia effects, which can prevent stent stenosis. However, as a drawback of this approach, when the diameter of the tumor thrombus is large, the effective radiation dose may not be achieved.
In clinical practice, the management of HCC patients with PVTT often neglects the effective diagnosis and treatment of CPH. PVTT patients complicated with CPH usually have an extremely poor prognosis. TIPS is an established treatment for CPH and its decompensated events by establishing a shunt between the intrahepatic portal vein and the hepatic vein or inferior vena cava. In our study, PVP was signi
In addition, TIPS still has the following effects: first, it can improve liver functional reserve by improving portal blood supply to normal liver tissue and then prevent fatal liver failure caused by PVTT and provide favorable conditions for the subsequent treatment of intrahepatic primary lesions; next, the covered stent of TIPS plays a part in covering and compressing PVTT; and last, TIPS is able to resolve portal hypertension not only caused by cirrhosis but also due to the combination of intrahepatic cirrhosis and prehepatic PVTT[20,21].
TIPS combined with radioactive seed strand placement and sequential TACE/RFA for mPVTT with CPH may reduce the mortality risk from decompensated events of CPH (i.e., nonneoplastic mortality risk) as well as reduce neoplastic mortality risk by controlling PVTT and primary lesions, prolonging survival. In our study, the median survival time of patients was 12.0 ± 1.3 mo (95%CI: 9.5-14.5), and the cumulative survival rates at 6, 12 and 24 mo were 83.1%, 49.7% and 21.8%, respectively. In a systematic review[13] of 6 retrospective studies involving mPVTT patients whose CPH was unclear, after percutaneous transhepatic 125I seed strand with stent placement combined with TACE, the median survival time was 10.3 mo (range 4.9-12.5 mo), and the cumulative survival rates at 6, 12 and 24 mo were 74.5% (range 61.8%-88.9%), 48.7% (range 32.4%-54.5%) and 20.1% (range 14.1%-26.1%), respectively. Huo et al[22] reported that in mPVTT patients partly mixed with CPH, the 2-year cumulative survival rate after palliative resection was 17.1%. Our results were similar to theirs. Despite similar survival results, it is necessary to differentiate and treat CPH in the management of PVTT or mPVTT patients.
In regard to postoperative long-term complications, our results showed that the cumulative rates of shunt stenosis at 6, 12 and 24 mo were 13.3%, 28.9% and 38.6%, respectively. Luo et al[23] and Yu et al[24] reported that after 125I seed strand with stent placement combined with TACE, the cumulative stent patency rates were 43.2% and 46.5% at 12 mo and 26.1% and 25.7% at 24 mo, respectively. Our results were clearly superior to theirs, which might be related to the following reasons: TIPS dredging the blood flow of the portal vein, full use of covered stents, and our postoperative anticoagulation treatment.
Furthermore, by survival analysis, shunt-related adverse events, including post-TIPS HE, shunt stenosis and recurrence of CPH, had no significant influence on survival, which might be related to the timely management of these complications, such as removal of HE inducements, balloon dilatation and/or stent reimplantation for shunt stenosis.
Regarding shunt-induced potential distant metastasis, 5 new cases of pulmonary metastasis and 1 new case of adrenal metastasis were observed. This small number of cases observed might be related to the censoring of death and the nonadherence of patients to the revisit and systematic examination. Further study is needed to expand the sample. However, it cannot be ignored that distant metastasis may be reduced to some extent by PVTT grab and aspiration before shunting, the entire coverage of mPVTT using covered stents, the PVTT response obtained by radioactive seed strand, and active intervention for intrahepatic lesions.
Among other factors that affected survival, cTNM staging showed a more detailed stratification capability than BCLC staging and showed an independent significant association with survival, with an increased risk of death for each increase in cTNM stage (HR = 2.745). Child-Pugh grade was an important factor affecting survival throughout, and the mortality risk in patients with grade C (HR = 7.308) and grade B (HR = 2.243) was much higher than those with grade A. Combining the Child-Pugh liver function grade and the cTNM tumor stage may be of great significance for the assessment of prognosis and survival.
Concerning other tumor-related factors, intrahepatic HCC morphology had no significant effect on survival, and the sum of longest viable tumor diameters approached significance, which might be related to active interventional treatment for intrahepatic primary lesions. Combined RFA was not significant, which might be related to RFA as an additional therapy after TACE for intrahepatic lesions. Combined targeted therapy was also not significant, and some high-quality studies[25,26] showed that targeted therapy did not achieve satisfactory outcomes in the treatment of HCC with PVTT.
BMI exerted a significant influence on survival (HR = 0.861). Patients with advanced HCC and decompensated cirrhosis often present malnutrition, so attention should be given to improving nutrition.
In addition, radioembolizaton was not used in combination therapy because it was not approved during the time of the study, but it could be considered for treatment in the future.
This single-arm retrospective cohort study has inherent limitations. Further relevant studies are warranted to follow and expand on the findings.
In conclusion, the key points of this initial study may be summarized as follows: (1) TIPS combined with radioactive seed strand placement might be effective and safe in treating mPVTT with CPH, which could effectively alleviate symptoms of portal hypertension and prolong patient survival time; (2) In the management of PVTT or mPVTT patients, it is necessary to differentiate and effectively treat CPH; (3) Combining Child-Pugh liver function grade and cTNM tumor stage may be of guiding significance for the assessment of prognosis and survival.
Main portal vein tumor thrombosis (mPVTT) is common in patients with hepatocellular carcinoma (HCC). Mostly based on cirrhosis, HCC is usually complicated with cirrhotic portal hypertension (CPH), which is often accompanied by high-mortality decompensated events such as esophagogastric variceal bleeding and refractory ascites/hydrothorax.
HCC patients with PVTT have a poor prognosis with median survival of only 2.7-4.0 mo. Once mPVTT is combined with cirrhotic decompensated events, it would deteriorate the disease and accelerate the death of patients. However, there is a lack of a clinical treatment paradigm for mPVTT patients with CPH.
This cohort study is to evaluate the efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPS) combined with radioactive seed strand for the treatment of mPVTT complicated with CPH. It might contribute new perspectives into clinical treatment management.
The clinical data of 83 consecutive patients who underwent TIPS combined with 125I seed strand placement for mPVTT and CPH from January 2015 to December 2018 were retrospectively reviewed, and the efficacy and safety were adequately evaluated by a 2-year follow-up.
There was universal improvement in CPH and apparent relief of its decompensated complications after operation. The majority of patients had at least a decrease in the extent of PVTT and the objective response rate of PVTT was 67.5%. The cumulative rate of shunt stenosis and recurrence rate of CPH were low within the first year. The median survival time was 12.0 ± 1.3 mo (95% confidence interval: 9.5-14.5).
TIPS combined with radioactive seed strand might be effective and safe in the treatment of mPVTT with CPH, which could effectively alleviate symptoms of portal hypertension and prolong patient survival time.
In the management of HCC patients with PVTT or mPVTT, it is necessary to differentiate and effectively treat CPH. The treatment of mPVTT with CPH is still a clinical difficulty and requires multidisciplinary teamwork. Future studies may require randomized controlled trials to verify our results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lidofsky S, United States; Maslennikov R, Russia A-Editor: Yao QG, China S-Editor: Gao CC L-Editor: A P-Editor: Cai YX
| 1. | Zhang ZM, Lai EC, Zhang C, Yu HW, Liu Z, Wan BJ, Liu LM, Tian ZH, Deng H, Sun QH, Chen XP. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surg. 2015;20:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J Gastroenterol. 2016;22:7289-7300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Wang K, Guo WX, Chen MS, Mao YL, Sun BC, Shi J, Zhang YJ, Meng Y, Yang YF, Cong WM, Wu MC, Lau WY, Cheng SQ. Multimodality Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Large-Scale, Multicenter, Propensity Mathching Score Analysis. Medicine (Baltimore). 2016;95:e3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Kaneko S, Tsuchiya K, Yasui Y, Inada K, Kirino S, Yamashita K, Osawa L, Hayakawa Y, Sekiguchi S, Higuchi M, Takaura K, Maeyashiki C, Tamaki N, Takeguchi T, Takeguchi Y, Nagano T, Nakanishi H, Itakura J, Takahashi Y, Himeno Y, Hoshi A, Kurosaki M, Izumi N. Strategy for advanced hepatocellular carcinoma based on liver function and portal vein tumor thrombosis. Hepatol Res. 2020;50:1375-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2290] [Article Influence: 229.0] [Reference Citation Analysis (3)] |
| 7. | Adebayo D, Neong SF, Wong F. Refractory Ascites in Liver Cirrhosis. Am J Gastroenterol. 2019;114:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Liu PH, Huo TI, Miksad RA. Hepatocellular Carcinoma with Portal Vein Tumor Involvement: Best Management Strategies. Semin Liver Dis. 2018;38:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J Gastroenterol. 2019;25:4360-4382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 10. | Sankar K, Moore CM. Transjugular Intrahepatic Portosystemic Shunts. JAMA. 2017;317:880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 12. | Yuan D, Gao Z, Zhao J, Zhang H, Wang J. 125I seed implantation for hepatocellular carcinoma with portal vein tumor thrombus: A systematic review and meta-analysis. Brachytherapy. 2019;18:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Zhang L, Hu B, Li W, Huang P, Zhang S, Zhong BY, Ni CF. 125I Irradiation Stent for Hepatocellular Carcinoma with Main Portal Vein Tumor Thrombosis: A Systematic Review. Cardiovasc Intervent Radiol. 2020;43:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Li S, Guo JH, Lu J, Wang C, Wu H, Wang H, Zha J, Fan R. I125 irradiation stent for treatment of hepatocellular carcinoma with portal vein thrombosis: A meta-analysis. Cancer Radiother. 2021;25:340-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3295] [Article Influence: 219.7] [Reference Citation Analysis (36)] |
| 16. | Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 611] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 17. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6039] [Article Influence: 862.7] [Reference Citation Analysis (3)] |
| 18. | Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 544] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 19. | Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X, Lau WY, Wu M. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition). Liver Cancer. 2020;9:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 20. | Liu L, Zhao Y, Qi X, Cai G, He C, Guo W, Yin Z, Chen H, Chen X, Fan D, Han G. Transjugular intrahepatic portosystemic shunt for symptomatic portal hypertension in hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol Res. 2014;44:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Qiu B, Li K, Dong X, Liu FQ. Transjugular Intrahepatic Portosystemic Shunt for Portal Hypertension in Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Cardiovasc Intervent Radiol. 2017;40:1372-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Huo L, Wei W, Yan Z, Lei Z, Xie Y, Gong R, Huang S, Jia N, Xia Y. Short-term and long-term outcomes of liver resection for HCC patients with portal vein tumor thrombus. Cell Biosci. 2019;9:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Luo JJ, Zhang ZH, Liu QX, Zhang W, Wang JH, Yan ZP. Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int. 2016;10:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Yu TZ, Zhang W, Liu QX, Li WH, Ma JQ, Zhang ZH, Yang MJ, Wang JH, Chen B, Zeng SC, Luo JJ, Liu LX, Yan ZP. Endovascular brachytherapy combined with portal vein stenting and transarterial chemoembolization improves overall survival of hepatocellular carcinoma patients with main portal vein tumor thrombus. Oncotarget. 2017;8:12108-12119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4648] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 26. | Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, Cai M, Shan H. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology. 2014;272:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |