Published online Apr 27, 2022. doi: 10.4240/wjgs.v14.i4.315
Peer-review started: November 11, 2021
First decision: January 9, 2022
Revised: January 20, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: April 27, 2022
Processing time: 164 Days and 4.7 Hours
Intraoperative methylene blue testing (IMBT), air leak testing, or endoscopy is used to assess the anastomotic integrity of esophagojejunostomy during open total gastrectomy for gastric cancer. Totally laparoscopic radical gastrectomy has been widely used to treat gastric cancer in the last few decades. However, reports on testing anastomotic integrity in totally laparoscopic radical gastrectomy are limited.
To explore the effects of IMBT on the incidence of postoperative anastomotic leaks (PALs) and identify the risk factors for PALs in totally laparoscopic radical gastrectomy.
From January 2017 to December 2019, patients who underwent totally laparoscopic radical gastrectomy at the Shaanxi Provincial People's Hospital were retrospectively analyzed. According to whether or not they experienced an IMBT, the patients were divided into an IMBT group and a control group. If the IMBT was positive, an intraoperative suture was required to reinforce the anastomosis. The difference in the incidence of PALs was compared, and the risk factors were investigated.
This study consisted of 513 patients, 211 in the IMBT group and 302 in the control group. Positive IMBT was shown in seven patients (3.3%) in the IMBT group, and no PAL occurred in these patients after suture reinforcement. Multivariate analysis showed that risk factors for predicting positive IMBT were body mass index (BMI) > 25 kg/m2 (hazard ratio [HR] = 8.357, P = 0.009), operation time > 4 h (HR = 55.881, P = 0.002), and insufficient surgical experience (HR = 15.286, P = 0.010). Moreover, 15 patients (2.9%) developed PALs in 513 patients, and the rates of PALs were significantly lower in the IMBT group than in the control group [2 of 211 patients (0.9%) vs 13 of 302 patients (4.3%), P = 0.0026]. Further analysis demonstrated that preoperative complications (HR = 13.128, P = 0.017), totally laparoscopic total gastrectomy (HR = 9.075, P = 0.043), and neoadjuvant chemotherapy (HR = 7.150, P = 0.008) were independent risk factors for PALs.
IMBT is an effective method to evaluate the integrity of anastomosis during totally laparoscopic radical gastrectomy, thus preventing technical defect-related anastomotic leaks. Preoperative complications, totally laparoscopic total gastrectomy, and neoadjuvant chemotherapy are independent risk factors for PALs.
Core Tip: We reviewed the outcomes of 513 consecutive patients with gastric cancer who underwent totally laparoscopic radical gastrectomy with and without intraoperative methylene blue testing at Shaanxi Provincial People's Hospital from January 2017 to December 2019. We found that intraoperative methylene blue testing is an effective method to evaluate the integrity of anastomosis during totally laparoscopic radical gastrectomy and could reduce the incidence of postoperative anastomotic leaks. Preoperative complications, totally laparoscopic total gastrectomy, and neoadjuvant chemotherapy are independent risk factors for postoperative anastomotic leaks.
- Citation: Deng C, Liu Y, Zhang ZY, Qi HD, Guo Z, Zhao X, Li XJ. How to examine anastomotic integrity intraoperatively in totally laparoscopic radical gastrectomy? Methylene blue testing prevents technical defect-related anastomotic leaks . World J Gastrointest Surg 2022; 14(4): 315-328
- URL: https://www.wjgnet.com/1948-9366/full/v14/i4/315.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i4.315
Gastric cancer is one of the most common cancers worldwide, ranking fifth in incidence and third in mortality[1]. Totally laparoscopic radical gastrectomy has been widely used to treat gastric cancer[2-4]. Postoperative anastomotic leak (PAL) is a severe complication, and occurs in 1.7%-5.7% of patients with gastric cancer[5-7]. These complications could prolong hospital stay, increase medical expenses, cause poor quality of life, and subsequently worsen the long-term survival of patients[8-10].
It is well known that the defects of intraoperative anastomotic techniques are closely related to PALs[11-13]. Therefore, some PALs might be avoided if insufficiently integral anastomoses were immediately reinforced. Intraoperative methylene blue testing (IMBT), intraoperative air leak test, or intraoperative endoscopy has been used to assess the anastomotic integrity of esophagojejunostomy during open total gastrectomy for gastric cancer[6,14-15]. However, to the best of our knowledge, no study has assessed the integrity of anastomosis during totally laparoscopic radical gastrectomy. Compared with open surgery, totally laparoscopic radical gastrectomy has the disadvantages of two-dimensional images, poor hand-eye coordination, limited operating space, fulcrum effect, and lack of haptic feedback[16-17]. Furthermore, according to the ERAS guidelines, abdominal drains should not routinely be placed after gastrectomy, which requires high-quality anastomosis[18-19]. Thus, a reliable anastomosis leak test is vital during totally laparoscopic radical gastrectomy.
In this study, we used IMBT to check the anastomotic integrity of esophagojejunostomy or gastrojejunostomy during totally laparoscopic radical gastrectomy. This is the first study to assess the anastomotic integrity during totally laparoscopic radical gastrectomy. We aimed to explore the effects of IMBT on the incidence and risk factors for PALs.
We performed a retrospective review of patients who underwent totally laparoscopic radical gastrectomy from January 2017 to December 2019. In our department, some surgeons think that IMBT is useful, while others are skeptical regarding its effects. Thus, two groups were formed: An IMBT group and a control group. Staging of the tumor was performed following the eighth edition of the AJCC Guidelines for gastric cancer[20]. This study was approved by the Ethics Committee of Shaanxi Provincial People's Hospital.
The inclusion criteria were: (1) Patients who underwent totally laparoscopic radical gastrectomy for gastric cancer and adenocarcinoma of the gastroesophageal junction from January 2017 to December 2019; (2) Gastric cancer or adenocarcinoma of the gastroesophageal junction diagnosed via endoscopy and pathological identification; and (3) Patients whose surgical and demographic data were complete and reliable. The exclusion criteria were: (1) Patients who underwent totally laparoscopic distal gastrectomy that used Billroth-I anastomosis; (2) Those who were converted to open surgery; (3) Those who were found to have distant metastases intraoperatively; (4) Those who did not undergo radical resection; and (5) Those who gave up treatment or were transferred to another hospital.
All surgeries were performed laparoscopically. Totally laparoscopic total gastrectomy was reconstructed via an overlap anastomosis[21], and totally laparoscopic distal gastrectomy was reconstructed via a Billroth-II anastomosis[22]. Lymph node dissection was performed according to the Japanese Gastric Cancer Treatment Guidelines 2014 (ver. 4)[23]. This study used a 45-mm linear stapler (Johnson Company, United States) for the overlap anastomosis and a 60-mm linear stapler (Johnson Company, United States) for the Billroth-II anastomosis. In our department, we preferred the Billroth II anastomosis and Roux-en-Y esophagojejunostomy rather than the Billroth I anastomosis. A Billroth I anastomosis needs to preserve a large residual stomach, leading to insufficient tumor margins and significant anastomotic tension when the tumor location is relatively high and the diameter is large. In China, most gastric cancer cases are found in advanced stages, and the diameter of the tumor is often large compared to Japan and Korea[24-26]. In addition, Billroth I anastomosis has a greater risk of remnant gastritis and reflux esophagitis[27-28].
Postoperative management was conducted according to the Japanese Gastric Cancer Treatment Guidelines (ver.4)[23]: The nasogastric tube was removed on postoperative day 1, and the abdominal drainage tube removed on postoperative day 5 without symptoms or inflammatory reactions. Abdominal CT, gastrointestinal tract angiography, or endoscopy was performed when an anastomotic leak was suspected.
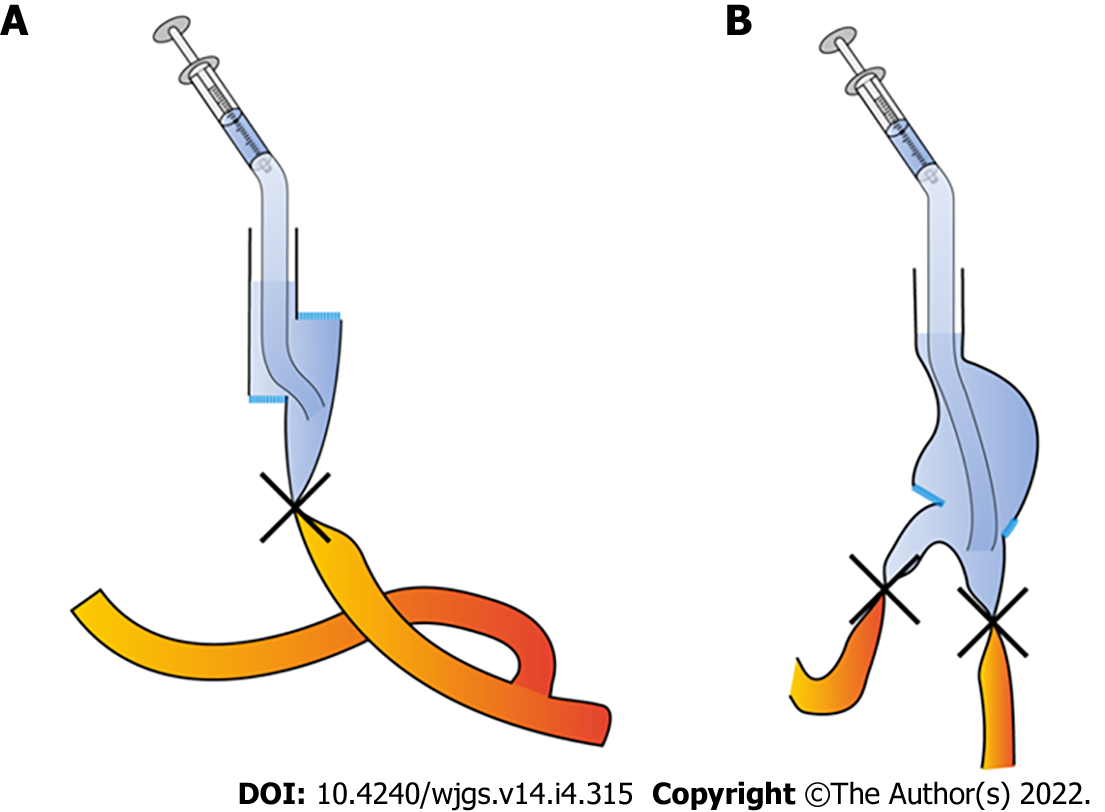
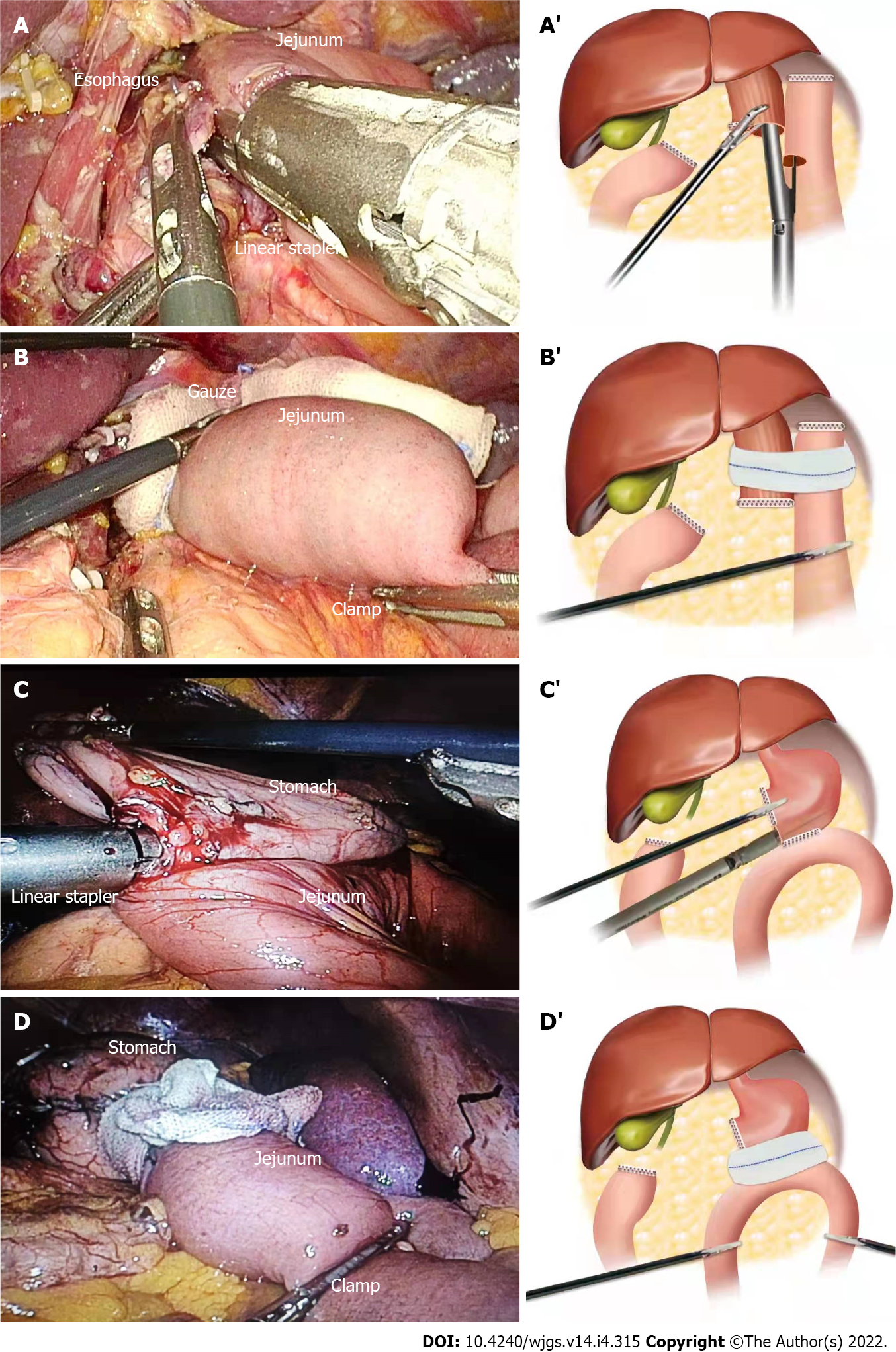
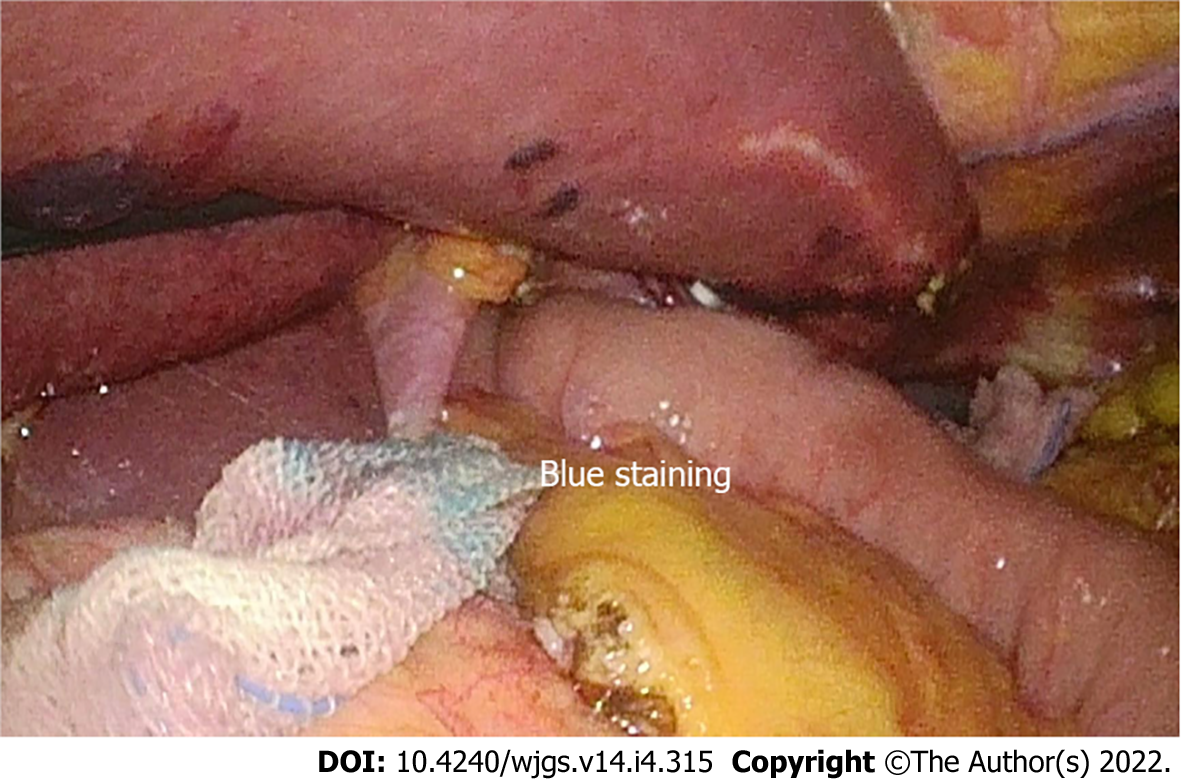
For the patients that underwent totally laparoscopic total gastrectomy, we performed IMBT as follows (Figure 1A): After the digestive tract reconstruction (Figure 2A and A’), the nasogastric tube (18F) was delivered 5 cm from the distal end of the anastomotic stoma, gauze was wrapped around the anastomosis, and then the jejunum was clamped using an intestinal clamp 5 cm distal to the anastomosis. Next, normal saline was injected through the nasogastric tube to rinse and observe whether continuous bright red liquid flowed out of the nasogastric tube when pumping back. If the liquid was detected, we looked for and stopped the bleeding and then flushed repeatedly until the clear liquid was pumped back out. Next, we dissolved 2 mL (20 mg) of methylene blue into 50 mL of normal saline and injected it through the nasogastric tube in order to make the methylene blue liquid disperse evenly around the anastomosis (Figure 2B and B’). Finally, we observed whether the gauze around the anastomosis was stained blue; if there was blue staining (Figure 3), we identified the leak according to the blue-stained site, sutured it, and then changed the gauze and repeated the process.
For the patients who underwent totally laparoscopic distal gastrectomy, IMBT was performed as follows (Figure 1B): The nasogastric tube (18F) was indwelled 5 cm from the distal end of the anastomotic stoma after the digestive tract reconstruction (Figure 2C and C’). Next, we wrapped the anastomosis with gauze, and closed it with clamps 5 cm distal to the anastomosis. Then, the anastomosis was flushed with normal saline through the nasogastric tube; the needle was pumped back to observe whether there was bright red liquid flowing out of the nasogastric tube. If red liquid was present, we looked for and stopped the bleeding. The flushing was repeated until the clear liquid was extracted from the nasogastric tube. Next, 5 mL (50 mg) of methylene blue was dissolved into 500 mL of normal saline and injected through the nasogastric tube in order to evenly distribute the methylene blue liquid around the anastomosis (Figure 2D and D’). Finally, if blue liquid was present, we repeated the above procedures.
We defined preoperative complications as one or more of the following: Anemia, malnutrition, diabetes, or pulmonary dysfunction. The World Health Organization's definition of anemia was used to define anemia: Hb concentration of < 12 g/dL in women and < 13 g/dL in men[29]. Malnutrition was defined by the European Society of Clinical Nutrition and Metabolism (ESPEN) criteria[30], which suggested two methods used to diagnose malnutrition: Method one: Body mass index (BMI) < 18.5 kg/m2; method two: Unintentional weight loss combined with a low age-related BMI (< 20 kg/m2 in subjects < 70 years or < 22 kg/m2 in those ≥ 70 years) or low fat-free mass index (FFMI) (< 17 kg/m2 in men and < 15 kg/m2 in women). Positive IMBT was defined as the visualization of methylene blue on the gauze surrounding the anastomosis. PAL was defined as meeting one of the following criteria: (1) Gastrointestinal contents or bile-like fluid drained from the abdominal drainage tube; (2) Gastrointestinal radiography showed leakage of the contrast medium from the drainage tube; (3) Methylene blue was extracted from the abdominal drainage tube after the oral administration of methylene blue; (4) Abdominal CT examination showed that the gastrointestinal wall was incomplete, revealing gas and fluid leaks around the anastomosis; and (5) Anastomotic leaks were found under endoscopy after surgery.
Analyses were performed with statistic software SPSS for Windows Version 25.0 (SPSS Inc., Chicago, Illinois, United States). Measurement data are expressed as the mean ± SD (normal distribution) or median (non-normal distribution). Count data are expressed as cases (rate). Univariate analysis was performed by the Chi-square test or a Fisher’s exact test when appropriate. Variables with P < 0.05 in the univariate analysis were included in multivariate analysis, which was conducted using the logistic regression model. P < 0.05 was considered statistically significant.
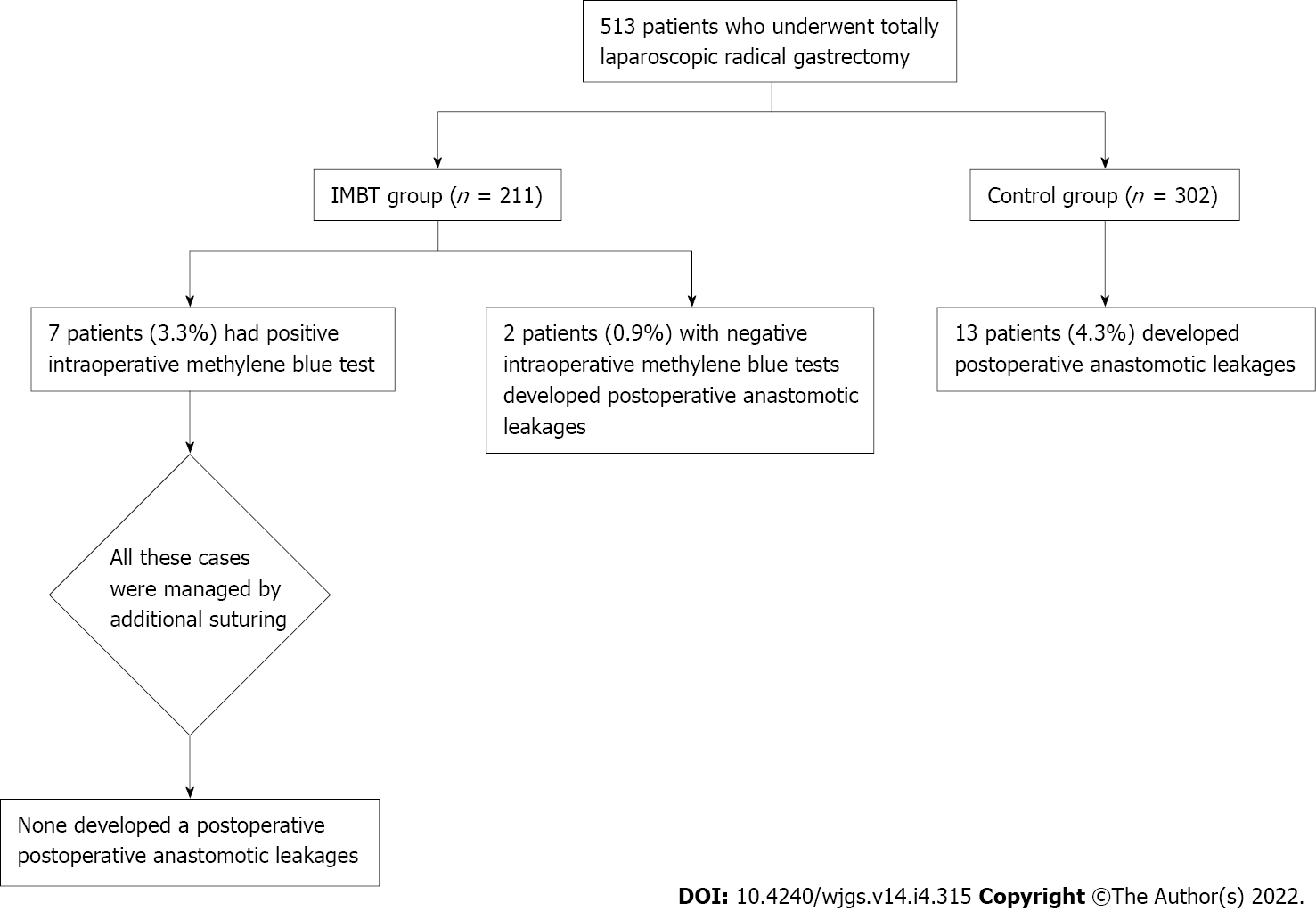
From January 2017 to December 2019, a total of 513 patients that underwent totally laparoscopic radical gastrectomy were analyzed retrospectively (211 patients in the IMBT group and 302 patients in the control group). Complete data of the intraoperative and postoperative findings are shown in Figure 4. The baseline data of the patients in the two groups are consistent, as shown in Table 1.
| Variable | IMBT group (211 cases) | Control group (302 cases) | χ2 | P value | |
| Gender | Male | 130 | 182 | 0.095 | 0.759 |
| Female | 81 | 120 | |||
| Age (yr) | < 75 | 143 | 196 | 0.457 | 0.499 |
| ≥ 75 | 68 | 106 | |||
| BMI (kg/m2) | < 25 | 155 | 211 | 0.784 | 0.376 |
| ≥ 25 | 56 | 91 | |||
| Preoperative complications | Present absent | 88123 | 111191 | 1.282 | 0.257 |
| Neoadjuvant chemotherapy | Present absent | 43168 | 61241 | 0.003 | 0.960 |
| Degree of tumor differentiation | High | 72 | 92 | 0.785 | 0.672 |
| Medium | 95 | 142 | |||
| Low | 44 | 68 | |||
| Postoperative tumor pathological stage1 | I | 32 | 62 | 3.298 | 0.192 |
| II | 62 | 94 | |||
| III | 117 | 146 | |||
| Surgeon’s experience | < 50 cases | 21 | 24 | 0.624 | 0.429 |
| ≥ 50 cases | 190 | 278 | |||
| Mode of surgery | TLTG | 101 | 146 | 0.025 | 0.875 |
| TLDG | 111 | 156 | |||
| Operation time | < 4h | 143 | 189 | 1.465 | 0.226 |
| ≥ 4h | 68 | 113 | |||
| Amount of bleeding ≥ 400 mL | Present | 79 | 100 | 1.024 | 0.312 |
| Absent | 132 | 202 |
Seven patients (3.3%) had positive IMBT in the IMBT group, as detailed in Table 2. These cases were managed by additional suturing, none had a PAL, and the mean postoperative hospital stay was 10.3 ± 1.1 d. Univariate analysis showed that surgeons with insufficient surgical experience (< 50 cases of totally laparoscopic radical gastrectomy) were associated with a higher rate of positive IMBT (14.3% vs 2.1%, P = 0.021). Other risk factors included operation time > 4 h, neoadjuvant chemotherapy, and a body mass index (BMI) > 25 kg/m2 (P = 0.008, 0.033, and 0.021, respectively), as shown in Tables 3 and 4. Multivariate analysis identified BMI > 25 kg/m2, operation time > 4 h, and insufficient surgical experience as independent risk factors for positive IMBT (P = 0.009, 0.002, and 0.010, respectively), as detailed in Table 5.
| Patient No. | Location of leak on anastomotic wall | Operation model | Dehiscence | Management | PAL | Postoperative hospital stays (d) |
| 1 | Posterior wall | TLTG | Present | Suturing | No | 10 |
| 2 | Posterior wall | TLTG | Absent | Suturing | No | 9 |
| 3 | Posterior wall | TLTG | Absent | Suturing | No | 11 |
| 4 | Joint opening | TLTG | Absent | Suturing | No | 10 |
| 5 | Joint opening | TLTG | Absent | Suturing | No | 11 |
| 6 | Left wall | TLDG | Absent | Suturing | No | 12 |
| 7 | Left wall | TLDG | Present | Suturing | No | 9 |
| Variable | IMBT group | IMBT | P value | Control group | PAL | P value | ||
| Negative | Positive (%) | Negative | Positive (%) | |||||
| Cases | 211 | 204 | 7 (3.3) | - | 302 | 289 | 13(4.3) | - |
| Gender | ||||||||
| Male | 130 | 126 | 4 (3.1) | 1.0 | 182 | 173 | 9 (4.9) | 0.575 |
| Female | 81 | 78 | 3 (3.7) | 120 | 116 | 4 (3.3) | ||
| Age (yr) | ||||||||
| < 75 | 143 | 139 | 4 (2.8) | 0.541 | 196 | 191 | 5 (2.6) | 0.70 |
| ≥ 75 | 68 | 65 | 3 (4.4) | 106 | 98 | 8 (7.5) | ||
| BMI (kg/m2) | ||||||||
| < 25 | 155 | 153 | 2 (1.3) | 0.021 | 211 | 206 | 5 (2.4) | 0.025 |
| ≥ 25 | 56 | 51 | 5 (8.9) | 91 | 83 | 8 (8.8) | ||
| Preoperative complications | ||||||||
| Absent | 123 | 120 | 3 (2.4) | 0.454 | 191 | 187 | 4 (2.0) | 0.018 |
| Present | 88 | 84 | 4 (4.5) | 111 | 102 | 9 (8.1) | ||
| Neoadjuvant chemotherapy | ||||||||
| Absent | 168 | 165 | 3 (1.8) | 0.033 | 241 | 234 | 7 (2.9) | 0.028 |
| Present | 43 | 39 | 4 (9.3) | 61 | 55 | 6 (9.8) | ||
| Degree of tumor differentiation | ||||||||
| High | 72 | 70 | 2 (2.8) | 0.784 | 92 | 88 | 4 (4.3) | 1.000 |
| Medium | 95 | 92 | 3 (3.2) | 142 | 136 | 6 (4.2) | ||
| Low | 44 | 42 | 2 (4.5) | 68 | 65 | 3 (4.6) | ||
| Postoperative tumor pathological stage1 | ||||||||
| I | 32 | 30 | 2 (6.3) | 0.493 | 62 | 59 | 3 (4.8) | 0.754 |
| II | 62 | 60 | 2 (3.2) | 94 | 89 | 5 (5.3) | ||
| III | 117 | 114 | 3 (2.6) | 146 | 141 | 5 (3.4) | ||
| Variable | IMBT group | IMBT | P value | Control group | PAL | P value | ||
| Negative | Positive (%) | Negative | Positive (%) | |||||
| Cases | 211 | 204 | 7 (3.3) | - | 302 | 289 | 13 (4.3) | - |
| Operation time (h) | ||||||||
| < 4 | 143 | 142 | 1 (0.7) | 0.008 | 184 | 177 | 7 (4.0) | 0.577 |
| ≥ 4 | 68 | 62 | 6 (8.8) | 118 | 112 | 6 (5.1) | ||
| Amount of bleeding (mL) | ||||||||
| < 400 | 132 | 130 | 2 (1.5) | 0.136 | 202 | 194 | 8 (4.0) | 0.765 |
| ≥ 400 | 79 | 74 | 5 (6.3) | 100 | 95 | 5 (5.0) | ||
| Mode of operation | ||||||||
| TLTG | 100 | 95 | 5 (5.0) | 0.200 | 146 | 136 | 10 (6.8) | 0.046 |
| TLDG | 111 | 109 | 2 (1.8) | 156 | 153 | 3 (1.9) | ||
| Surgeon’s experience (cases) | ||||||||
| < 50 | 21 | 18 | 3(14.3) | 0.021 | 24 | 21 | 3 (12.5) | 0.074 |
| ≥ 50 | 190 | 186 | 4 (2.1) | 278 | 268 | 10 (3.6) | ||
| Variable | B | Standard deviation | Wald | Exp(B) | Odds ratio (95%CI) | P value | |
| Lower limit | Upper limit | ||||||
| IMBT | |||||||
| BMI ≥ 25 kg/m2 | 2.123 | 0.810 | 6.862 | 8.357 | 1.707 | 40.922 | 0.009 |
| Neoadjuvant chemotherapy | 1.326 | 0.805 | 2.715 | 3.767 | 0.778 | 18.245 | 0.099 |
| Operation time ≥ 4 h | 4.023 | 1.319 | 9.303 | 55.881 | 4.212 | 741.381 | 0.002 |
| Inexperienced surgeons | 2.727 | 1.052 | 6.719 | 15.286 | 1.944 | 120.167 | 0.010 |
| PAL | |||||||
| BMI > 25 kg/m2 | 1.289 | 0.858 | 2.259 | 3.630 | 0.676 | 19.498 | 0.133 |
| Preoperative complications | 2.575 | 1.081 | 5.671 | 13.128 | 1.577 | 109.268 | 0.017 |
| Neoadjuvant chemotherapy | 1.967 | 0.740 | 7.063 | 7.150 | 1.676 | 30.506 | 0.008 |
| TLTG | 2.206 | 1.091 | 4.083 | 9.075 | 1.069 | 77.070 | 0.043 |
PAL occurred in 15 (2.9%) patients, including 2 in the IMBT group and 13 in the control group. The rate of PALs was significantly lower in the IMBT group than in the control group [2 of 211 patients (0.9%) vs 13 of 302 patients (4.3%), P = 0.0026].
The clinical characteristics of the patients with anastomotic leaks are shown in Table 6. The diagnosis time of PALs was 5.8 ± 2.0 d after surgery, postoperative hospital stay was 19.3 ± 3.5 d, and the abdominal drainage tube placement time was 17.3 ± 3.2 d. All 15 patients improved and were discharged from the hospital, and no one died. In the univariate analysis, patients with BMI > 25 kg/m2 (8.8% vs 2.4%, P = 0.025), preoperative complications (8.1% vs 2.0%, P = 0.018), totally laparoscopic total gastrectomy (6.8% vs 1.9%, P = 0.046), and neoadjuvant chemotherapy (9.8% vs 2.9%, P = 0.028) were associated with PALs, as shown in Tables 3 and 4. Multivariate analysis showed that preoperative complications (hazard ratio [HR] = 13.128, P = 0.017), totally laparoscopic total gastrectomy (HR = 9.075, P = 0.043), and neoadjuvant chemotherapy (HR = 7.150, P = 0.008) were independent risk factors for PALs (Table 5).
| Patient No. | Group | Day of diagnosis after surgery (d) | TLTG or TLDG | Tumor staging1 | Procedure used for patients | Time of placement of abdominal drainage tube (d) | Postoperative Hospital stays (d) |
| 1 | IMBT group | 6 | TLTG | IIB | Drainage | 15 | 16 |
| 2 | IMBT group | 8 | TLTG | IIIA | Second surgery + Drainage | 20 | 21 |
| 3 | Control group | 4 | TLTG | IA | Drainage | 18 | 19 |
| 4 | Control group | 5 | TLTG | IIA | Drainage | 13 | 15 |
| 5 | Control group | 9 | TLTG | IIB | Drainage | 19 | 21 |
| 6 | Control group | 8 | TLTG | IIB | Drainage | 12 | 14 |
| 7 | Control group | 5 | TLTG | IIIC | Drainage | 18 | 20 |
| 8 | Control group | 3 | TLTG | IIIC | Drainage | 16 | 18 |
| 9 | Control group | 8 | TLTG | IIB | Second surgery + Drainage | 21 | 24 |
| 10 | Control group | 7 | TLTG | IIIB | Second surgery + Drainage | 22 | 25 |
| 11 | Control group | 7 | TLTG | IIIC | Second surgery + Drainage | 17 | 21 |
| 12 | Control group | 5 | TLDG | IIIA | Drainage | 12 | 14 |
| 13 | Control group | 3 | TLDG | IIA | Second surgery + Drainage | 17 | 18 |
| 14 | Control group | 3 | TLDG | IIIA | Second surgery + Drainage | 19 | 20 |
| 15 | Control group | 6 | TLDG | IIIC | Second surgery + Drainage | 20 | 23 |
Anastomotic leaks are among the most common and severe complications after totally laparoscopic radical gastrectomy and are the main risk factor for patients' postoperative death[8-10]. The integrity of the anastomosis, which is closely related to the anastomotic technique, is a prerequisite for tissue healing and is essential for preventing anastomotic leaks[6,12]. In totally laparoscopic radical gastrectomy, we used IMBT to check the integrity of the anastomosis. The results showed that IMBT reduces the incidence of PALs, which is consistent with the IMBT results in open total gastrectomy[14].
Several methods are available to assess the integrity of the anastomosis. An intraoperative air leak test was proposed by Kanaji to check anastomotic integrity during open radical gastrectomy[6] and showed that this test reduces the occurrence of postoperative anastomotic leaks; however, the intraoperative air leak test did not show the exact site of the leaks and only depicted the approximate area. Celik et al[14] showed a low incidence of anastomotic leaks in the methylene blue testing group (3.7% vs 14.4%, P = 0.007) in which methylene blue is injected via a nasogastric tube to check the integrity of the anastomosis during an open total gastrectomy. Some researchers[31] who performed an intraoperative endoscopic examination during laparoscopic gastric bypass surgery showed a low incidence of anastomotic leaks (0 vs 8%, P = 0.0412) and a low reoperation rate (0 vs 8%, P = 0.0412). However, it is a challenge to find gastroscopic instruments as well as an experienced endoscopist. Our study confirmed that IMBT is an important method for assessing anastomotic integrity in totally laparoscopic radical gastrectomy, which detects anastomoses and pinpoints the areas of the leaks. Furthermore, we examined the anastomosis during totally laparoscopic distal gastrectomy, whereas previous studies focused on esophagojejunal anastomotic leaks after total gastrectomy.
This study found seven IMBT-positive patients whose anastomosis was reinforced with sutures, and none of them developed PALs. Our study indicated that patients with an operative time > 4 h, those with a BMI > 25 kg/m2, and insufficient surgical experience were associated with a higher risk of positive IMBT. Previous studies have shown that technically relevant factors such as prolonged operative time, excessive BMI, and inexperience of the surgeon are strongly associated with the occurrence of PALs[6,32-33]. Therefore, we recommend performing IMBT in patients with these high-risk factors.
However, two patients (0.9%) with negative IMBT developed PALs in this study, meaning that the cause of the anastomotic leaks is complex. This study found that patients with preoperative complications, totally laparoscopic total gastrectomy, and neoadjuvant chemotherapy are at a higher risk for PALs. Previous studies have indicated that anemia, malnutrition, and pulmonary insufficiency are also strongly associated with the occurrence of PALs[13,32,34], and are consistent with the results of our study. Kawamura et al[35] showed that the rate of anastomotic leaks is significantly higher in the laparoscopic total gastrectomy group (5.0 %) than in the laparoscopic distal gastrectomy group (1.2%), which is consistent with our study. However, there is still controversy about whether neoadjuvant chemotherapy leads to PALs. Gorur et al[36] reported that chemotherapy affects cell proliferation and the formation of collagenous fiber, which is a key component of anastomotic healing. Some studies reported that neoadjuvant chemotherapy does not increase the risk of PALs[37,38]. Our study suggested that neoadjuvant chemotherapy is a risk factor for PALs. We hypothesized that patients undergoing neoadjuvant chemotherapy have increased tissue toughness and adhesion within the abdominal cavity, resulting in increased surgical damage, thus leading to PALs. Therefore, we should pay close attention to patients with the above-mentioned risk factors.
This study has its limitations. First, it is a single-center retrospective study, which needs to be further confirmed by a multicenter, randomized controlled study with a larger sample size. Second, our study did not compare the IMBT, intraoperative air leak test, and intraoperative endoscopy. Finally, the methylene blue testing could not prevent PALs caused by non-technical factors.
In summary, IMBT can find technical defects within an anastomosis, and suturing can reduce the incidence of anastomotic leaks after totally laparoscopic radical gastrectomy. Independent risk factors associated with PALs include preoperative complications, totally laparoscopic total gastrectomy, and neoadjuvant chemotherapy.
We hypothesized that intraoperative methylene blue testing (IMBT) could reduce the incidence of postoperative anastomotic leaks (PALs) in totally laparoscopic radical gastrectomy.
IMBT, air leak testing, or endoscopy is used to assess the anastomotic integrity of esophagojejunostomy during open total gastrectomy for gastric cancer. To the best of our konwledge, this is the first study to assess the anastomotic integrity during totally laparoscopic radical gastrectomy.
To explore the effects of IMBT on the incidence of PALs and identify the risk factors for PALs in totally laparoscopic radical gastrectomy.
The difference in the incidence of PALs was compared between the IMBT group and the control group. Logistic regression analysis was used to clarify the risk factor for positive IMBT and PALs.
Positive IMBT was shown in 7 patients (3.3%) in the IMBT group, and no PAL occurred in these patients after suture reinforcement. Moreover, 15 patients (2.9%) developed PALs, and the rate of PALs was significantly lower in the IMBT group than in the control group [2 of 211 patients (0.9%) vs 13 of 302 patients (4.3%), P = 0.0026]. Further analysis demonstrated that preoperative complications (hazard ratio [HR] = 13.128, P = 0.017), totally laparoscopic total gastrectomy (HR = 9.075, P = 0.043), and neoadjuvant chemotherapy (HR = 7.150, P = 0.008) were independent risk factors for PALs.
IMBT can find technical defects within an anastomosis, and suturing can reduce the incidence of PALs in totally laparoscopic radical gastrectomy. Independent risk factors associated with PAL include preoperative complications, totally laparoscopic total gastrectomy, and neoadjuvant chemotherapy.
Randomized controlled trials are expected to be conducted to measure the effects of IMBT.
We thank Zhu JF for his review of our statistics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Surlin VM, Romania; van Helsdingen CP, Netherlands S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Wu YXJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64104] [Article Influence: 16026.0] [Reference Citation Analysis (174)] |
| 2. | Lee JH, Nam BH, Ryu KW, Ryu SY, Park YK, Kim S, Kim YW. Comparison of outcomes after laparoscopy-assisted and open total gastrectomy for early gastric cancer. Br J Surg. 2015;102:1500-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg. 2016;263:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 491] [Article Influence: 54.6] [Reference Citation Analysis (1)] |
| 4. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 678] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 5. | Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, Kwon OK, Kong SH, Kim HI, Lee HJ, Kim W, Ryu SW, Jin SH, Oh SJ, Ryu KW, Kim MC, Ahn HS, Park YK, Kim YH, Hwang SH, Kim JW, Cho GS. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 6. | Kanaji S, Ohyama M, Yasuda T, Sendo H, Suzuki S, Kawasaki K, Tanaka K, Fujino Y, Tominaga M, Kakeji Y. Can the intraoperative leak test prevent postoperative leakage of esophagojejunal anastomosis after total gastrectomy? Surg Today. 2016;46:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Katai H, Mizusawa J, Katayama H, Kunisaki C, Sakuramoto S, Inaki N, Kinoshita T, Iwasaki Y, Misawa K, Takiguchi N, Kaji M, Okitsu H, Yoshikawa T, Terashima M; Stomach Cancer Study Group of Japan Clinical Oncology Group. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer. 2019;22:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 8. | Aurello P, Cinquepalmi M, Petrucciani N, Moschetta G, Antolino L, Felli F, Giulitti D, Nigri G, D'Angelo F, Valabrega S, Ramacciato G. Impact of Anastomotic Leakage on Overall and Disease-free Survival After Surgery for Gastric Carcinoma: A Systematic Review. Anticancer Res. 2020;40:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Sierzega M, Kolodziejczyk P, Kulig J; Polish Gastric Cancer Study Group. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br J Surg. 2010;97:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Song KY. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J Surg Oncol. 2011;104:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Kim SH, Son SY, Park YS, Ahn SH, Park DJ, Kim HH. Risk Factors for Anastomotic Leakage: A Retrospective Cohort Study in a Single Gastric Surgical Unit. J Gastric Cancer. 2015;15:167-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Trapani R, Rausei S, Reddavid R, Degiuli M; ITALIAN RESEARCH GROUP FOR GASTRIC CANCER (GIRCG) Clinical Investigators. Risk factors for esophago-jejunal anastomosis leakage after total gastrectomy for cancer. A multicenter retrospective study of the Italian research group for gastric cancer. Eur J Surg Oncol. 2020;46:2243-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Migita K, Takayama T, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Nakajima Y. Risk factors for esophagojejunal anastomotic leakage after elective gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Celik S, Almalı N, Aras A, Yılmaz Ö, Kızıltan R. Intraoperatively Testing the Anastomotic Integrity of Esophagojejunostomy Using Methylene Blue. Scand J Surg. 2017;106:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Nishikawa K, Yanaga K, Kashiwagi H, Hanyuu N, Iwabuchi S. Significance of intraoperative endoscopy in total gastrectomy for gastric cancer. Surg Endosc. 2010;24:2633-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Alkatout I, Dhanawat J, Ackermann J, Freytag D, Peters G, Maass N, Mettler L, Pape JM. Video Feedback and Video Modeling in Teaching Laparoscopic Surgery: A Visionary Concept from Kiel. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Pinzon D, Byrns S, Zheng B. Prevailing Trends in Haptic Feedback Simulation for Minimally Invasive Surgery. Surg Innov. 2016;23:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carli F, Demartines N, Griffin SM, Lassen K; Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101:1209-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 499] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 19. | Liu H, Jin P, Quan X, Xie YB, Ma FH, Ma S, Li Y, Kang WZ, Tian YT. Feasibility of totally laparoscopic gastrectomy without prophylactic drains in gastric cancer patients. World J Gastroenterol. 2021;27:4236-4245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Son T, Sun J, Choi S, Cho M, Kwon IG, Kim HI, Cheong JH, Choi SH, Noh SH, Woo Y, Fong Y, Park S, Hyung WJ. Multi-institutional validation of the 8th AJCC TNM staging system for gastric cancer: Analysis of survival data from high-volume Eastern centers and the SEER database. J Surg Oncol. 2019;120:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Ko CS, Choi NR, Kim BS, Yook JH, Kim MJ. Totally laparoscopic total gastrectomy using the modified overlap method and conventional open total gastrectomy: A comparative study. World J Gastroenterol. 2021;27:2193-2204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Yanagimoto Y, Omori T, Fujiwara Y, Demura K, Jeong-Ho M, Shinno N, Yamamoto K, Sugimura K, Miyata H, Ushigome H, Takahashi Y, Nishimura J, Yasui M, Asukai K, Yamada D, Wada H, Takahashi H, Ohue M, Yano M, Sakon M. Comparison of the intracorporeal triangular and delta-shaped anastomotic techniques in totally laparoscopic distal gastrectomy for gastric cancer: an analysis with propensity score matching. Surg Endosc. 2020;34:2445-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1910] [Article Influence: 238.8] [Reference Citation Analysis (1)] |
| 24. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakamura K, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakamura T, Shimosegawa T. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? J Gastroenterol. 2017;52:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Park JY, Kim YJ. Uncut Roux-en-Y Reconstruction after Laparoscopic Distal Gastrectomy Can Be a Favorable Method in Terms of Gastritis, Bile Reflux, and Gastric Residue. J Gastric Cancer. 2014;14:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 429] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 27. | Nakamura M, Nakamori M, Ojima T, Iwahashi M, Horiuchi T, Kobayashi Y, Yamade N, Shimada K, Oka M, Yamaue H. Randomized clinical trial comparing long-term quality of life for Billroth I vs Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Br J Surg. 2016;103:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Kim MS, Kwon Y, Park EP, An L, Park H, Park S. Revisiting Laparoscopic Reconstruction for Billroth 1 Versus Billroth 2 Versus Roux-en-Y After Distal Gastrectomy: A Systematic Review and Meta-Analysis in the Modern Era. World J Surg. 2019;43:1581-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | World Health Organization Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. [cited 21 June 2021]; Available from: https://www.who.int/vmnis/indicators/haemoglobin/en/. |
| 30. | Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, de van der Schueren MA, Singer P. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015;34:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1165] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 31. | Valenzuela-Salazar C, Rojano-Rodríguez ME, Romero-Loera S, Trejo-Ávila ME, Bañuelos-Mancilla J, Delano-Alonso R, Moreno-Portillo M. Intraoperative endoscopy prevents technical defect related leaks in laparoscopic Roux-en-Y gastric bypass: A randomized control trial. Int J Surg. 2018;50:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Deguchi Y, Fukagawa T, Morita S, Ohashi M, Saka M, Katai H. Identification of risk factors for esophagojejunal anastomotic leakage after gastric surgery. World J Surg. 2012;36:1617-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Chen HN, Chen XZ, Zhang WH, Yang K, Chen XL, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. The Impact of Body Mass Index on the Surgical Outcomes of Patients With Gastric Cancer: A 10-Year, Single-Institution Cohort Study. Medicine (Baltimore). 2015;94:e1769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Gong W, Li J. Combat with esophagojejunal anastomotic leakage after total gastrectomy for gastric cancer: A critical review of the literature. Int J Surg. 2017;47:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Kawamura Y, Satoh S, Suda K, Ishida Y, Kanaya S, Uyama I. Critical factors that influence the early outcome of laparoscopic total gastrectomy. Gastric Cancer. 2015;18:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Gorur M, Sozutek A, Irkorucu O, Karakaya B. The influence of platelet-rich plasma (PRP) on colonic anastomosis healing impaired by intraperitoneal 5-flourouracil application. An experimental study. Acta Cir Bras. 2020;35:e202000504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Haskins IN, Kroh MD, Amdur RL, Ponksy JL, Rodriguez JH, Vaziri K. The Effect of Neoadjuvant Chemoradiation on Anastomotic Leak and Additional 30-Day Morbidity and Mortality in Patients Undergoing Total Gastrectomy for Gastric Cancer. J Gastrointest Surg. 2017;21:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, Ott K, Hoelscher A, Schneider PM, Bechstein W, Wilke H, Lutz MP, Nordlinger B, Van Cutsem E, Siewert JR, Schlag PM. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |