Published online Apr 27, 2022. doi: 10.4240/wjgs.v14.i4.304
Peer-review started: December 7, 2021
First decision: January 12, 2022
Revised: January 15, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: April 27, 2022
Processing time: 138 Days and 3.8 Hours
The studies of laparoscopic-assisted transhiatal gastrectomy (LTG) in patients with Siewert type II adenocarcinoma of the esophagogastric junction (AEG) are scarce.
To compare the surgical efficiency of LTG with the open transhiatal gastrectomy (OTG) for patients with Siewert type II AEG.
We retrospectively evaluated a total of 578 patients with Siewert type II AEG who have undergone LTG or OTG at the First Medical Center of the Chinese People’s Liberation Army General Hospital from January 2014 to December 2019. The short-term and long-term outcomes were compared between the LTG (n = 382) and OTG (n = 196) groups.
Compared with the OTG group, the LTG group had a longer operative time but less blood loss, shorter length of abdominal incision and an increased number of harvested lymph nodes (P < 0.05). Patients in the LTG group were able to eat liquid food, ambulate, expel flatus and discharge sooner than the OTG group (P < 0.05). No significant differences were found in postoperative complications and R0 resection. The 3-year overall survival and disease-free survival performed better in the LTG group compared with that in the OTG group (88.2% vs 79.2%, P = 0.011; 79.7% vs 73.0%, P = 0.002, respectively). In the stratified analysis, both overall survival and disease-free survival were better in the LTG group than those in the OTG group for stage II/III patients (P < 0.05) but not for stage I patients.
For patients with Siewert type II AEG, LTG is associated with better short-term outcomes and similar oncology safety. In addition, patients with advanced stage AEG may benefit more from LTG in the long-term outcomes.
Core Tip: Our objective was to compare the surgical efficiency of laparoscopic-assisted transhiatal gastrectomy (LTG) with the open transhiatal gastrectomy in patients with Siewert type II adenocarcinoma of the esophagogastric junction. We found that LTG was associated with better short-term outcomes and similar oncology safety. In addition, patients with advanced stage adenocarcinoma of the esophagogastric junction may benefit more from LTG in 3-year overall survival and disease-free survival.
- Citation: Song QY, Li XG, Zhang LY, Wu D, Li S, Zhang BL, Xu ZY, Wu RLG, Guo X, Wang XX. Laparoscopic-assisted vs open transhiatal gastrectomy for Siewert type II adenocarcinoma of the esophagogastric junction: A retrospective cohort study. World J Gastrointest Surg 2022; 14(4): 304-314
- URL: https://www.wjgnet.com/1948-9366/full/v14/i4/304.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i4.304
In recent decades, the global incidence of gastric cancer has declined annually while the incidence of adenocarcinoma of the esophagogastric junction (AEG) has presented an upward trend, especially in Asian countries[1-5]. Although there are many controversies concerning the optimal treatment for AEG patients, surgery is still the cornerstone of therapeutic strategies[6]. According to the results of the nationwide clinical trial (JCOG 9502) in Japan, the transhiatal approach is recommended for Siewert type II/III AEG patients with esophageal invasion within 3 cm[7,8]. Since the first report of laparoscopic-assisted transhiatal gastrectomy (LTG) by Kitano et al[9] in 1994, LTG has developed rapidly worldwide. With the improvement of laparoscopic technology and the optimization of equipment, a large number of countries have successively carried out LTG for gastric cancer because it provides not only better short-term outcomes but also comparable oncologic safety and survival in comparison with open transhiatal gastrectomy (OTG), especially in early-stage and distal gastric cancer[10-13]. Conversely, due to the lack of scientific evidence, the feasibility of LTG in proximal gastric cancer is still controversial. Moreover, peripheral lymphatic drainage pathways of Siewert type II AEG are more complicated as the particularity of the anatomical location, and LTG surgery with D2 lymphadenectomy remains more challenging than other gastric cancer sites[14,15].
At present, the studies on the short-term and long-term clinical effects of Siewert type II AEG regarding LTG and OTG are limited[16-20]. Thus, this study retrospectively analyzed the clinical data of Siewert type II AEG patients in our hospital, compared the short-term and long-term outcomes of LTG and traditional OTG and aimed to explore the feasibility of LTG treatment of Siewert type II AEG.
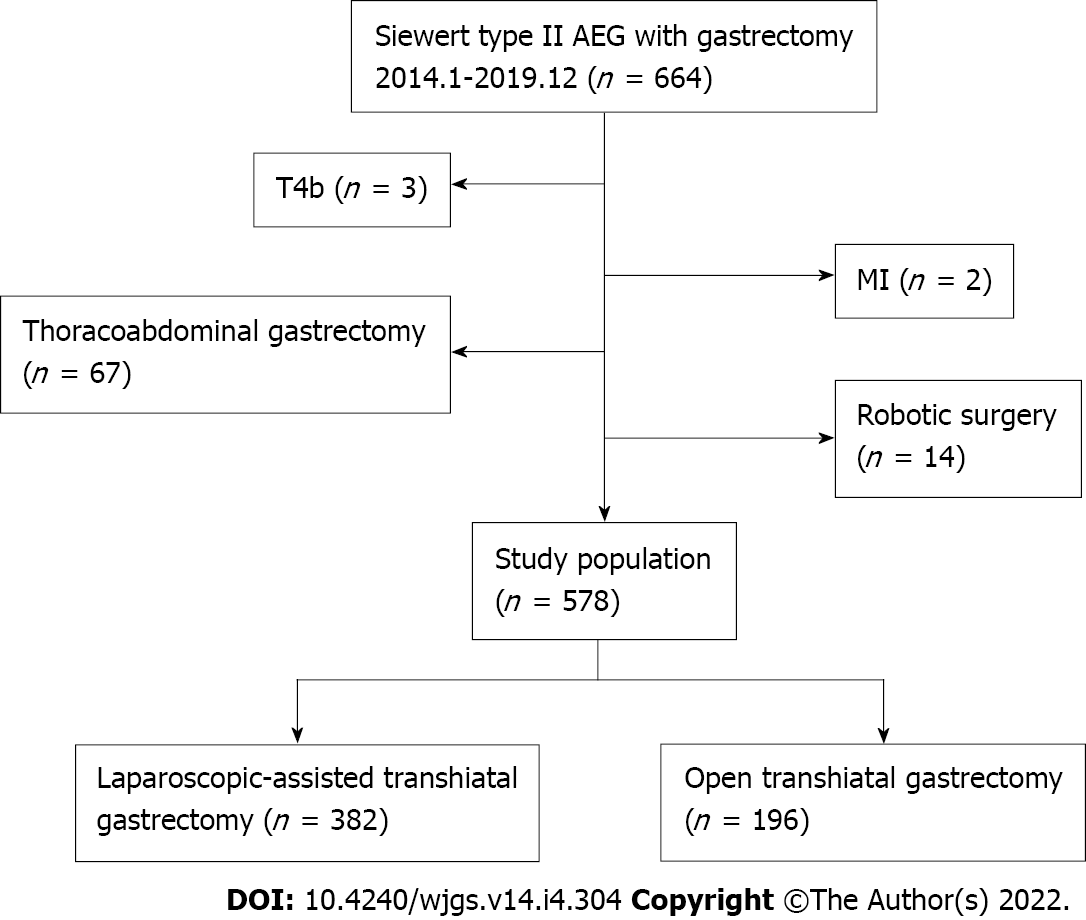
This work retrospectively reviewed patients with Siewert II AEG who have undergone gastrectomy at the First Medical Center of Chinese PLA General Hospital in China from January 2014 to December 2019. The inclusion criteria contained: (1) Histologically proven Siewert type II AEG; (2) Surgery via either OTG or LTG with total or proximal gastrectomy with D2 lymphadenectomy; (3) Staging T1-4a, N0-3, M0 (according to the 8th edition of the TNM staging system of the American Joint Committee on Cancer)[21]; and (4) Esophageal invasion < 3 cm. The exclusion criteria were presented as following: (1) Patients with a secondary malignancy within 5 years; (2) American Society of Anesthesiologists physical status score > 3; (3) Only underwent palliative resection or combined organ resection; and (4) Received preoperative chemotherapy of radiotherapy. Finally, a total of 578 patients were pooled into the study (LTG = 382, OTG = 196).
This study has been registered on Clinical-Trial.gov (ChiCTR2100053647) and approved by the Ethics Committee of Chinese PLA General Hospital.
LTG: The patient was placed in a supine position and given general anesthesia by employing a 5-hole method. After exploring the relevant positions of various tissues in the abdominal cavity and the location and size of the tumor, a radical total and proximal gastrectomy was performed in this study. Gastrectomy and D2-lymphadenectomy were completed. Then, a small incision was made in the middle of the abdomen to reconstruct the digestive tract. Gastric tube construction and esophagogastrostomy were often performed after proximal gastrectomy. After total gastrectomy, most patients underwent esophagojejunostomy and jejunojejunostomy (Roux-en-Y reconstruction).
OTG: The positioning and anesthesia of the patients remained the same as those of the LTG group. An incision was made in the middle of the abdomen to enter the abdominal cavity. Other operative details such as gastrectomy, lymphadenectomy and reconstruction were the same as those in the LTG group.
We retrospectively collected the following clinical and pathological factors available in our clinical database: Age, sex, body mass index, smoking/drinking history, American Society of Anesthesiologists score, tumor size, histopathological grade, TNM stage, operation time, intraoperative blood loss, length of abdominal incision, length of proximal margin, number of harvested lymph nodes (LNs), number of positive LNs, resection status (R-status) of margin, postoperative recovery (the time to liquid diet, ambulation, first flatus or defecation and discharge) and postoperative complications (anastomotic leakage, anastomotic stenosis, abdominal abscess, pneumonia, arrhythmia and wound infection). All postoperative complications were classified with the application of the Clavien-Dindo grading system[22].
In addition, postoperative patients were periodically followed up with blood tests, physical examinations and chest/abdominal computed tomography scans through outpatient visits. The follow-up interval was every 3–6 mo for the first 2 years and every 6–12 mo for the subsequent 3 years. All surviving patients were followed up annually thereafter until death. Overall survival (OS) was calculated from the time of surgery to death due to any cause or latest follow-up. Disease-free survival (DFS) was calculated as the time from surgery to first recurrence or death because of any reason.
Continuous data were presented as mean ± standard deviation with t test if normally distributed or as the median (interquartile range) with Mann-Whitney U test if not normally distributed. Dichotomous variables were compared with the χ2 test or Fisher test. Survival analysis was performed by the Kaplan-Meier curves based on the log-rank test. Statistical analysis was done by IBM SPSS (version 26.0.0.0). The figures were plotted with RStudio (version 1.4.1717). Bilateral P < 0.05 was considered to be statistically significant.
As shown in Figure 1, a total of 578 patients were eligible (512 male and 66 female) for our study, of which 382 (66.1%) patients underwent LTG and 196 (33.9%) patients underwent OTG. The demographic information of the participants was presented in Table 1. No significant difference could be observed in the distribution of baseline features between the two groups.
| Characteristics | LTG, n = 382 | OTG, n = 196 | P value |
| Age in yr | 64 (58, 69) | 63 (59, 69) | 0.816a |
| Sex, n (%) | |||
| Female | 44 (11.5) | 22 (11.2) | 1.000 |
| Male | 338 (88.5) | 174 (88.8) | |
| BMI (kg/m2) | 24.45 (22.10, 26.70) | 24.40 (22.50, 27.25) | 0.389a |
| Smoking history, n (%) | 0.635 | ||
| No | 280 (73.3) | 148 (75.5) | |
| Yes | 102 (26.7) | 48 (24.5) | |
| Drinking history, n (%) | 0.773 | ||
| No | 212 (55.5) | 112 (57.1) | |
| Yes | 170 (44.5) | 84 (42.9) | |
| ASA, n (%) | |||
| 1 | 201 (52.6) | 100 (51.0) | 0.396 |
| 2 | 164 (42.9) | 82 (41.8) | |
| 3 | 17 (4.5) | 14 (7.1) | |
| Tumor size (cm) | 3.49 ± 1.60 | 3.69 ± 1.62 | 0.161 |
| Grade, n (%) | 0.267 | ||
| 1-2 | 132 (34.6) | 58 (29.6) | |
| 3-4 | 250 (65.4) | 138 (70.4) | |
| T stage, n (%) | 0.860 | ||
| T1-T2 | 129 (33.8) | 64 (32.7) | |
| T3-4a | 253 (66.2) | 132 (67.3) | |
| N stage, n (%) | 0.602 | ||
| N0 | 168 (44.0) | 81 (41.3) | |
| N1-N3 | 214 (56.0) | 115 (58.7) | |
| TNM stage, n (%) | 0.544 | ||
| I | 107 (28.0) | 49 (25.0) | |
| II | 120 (31.4) | 70 (35.7) | |
| III | 155 (40.6) | 77 (39.3) |
Perioperative outcomes are shown in Table 2. The LTG group experienced a significantly longer operation time (230.14 ± 58.92 min vs 198.4 ± 56.76 min, P < 0.001) but significantly decreased blood loss (200.42 ± 304.34 mL vs 275.77 ± 384.72 mL, P = 0.010) and significantly shorter abdominal incision (9.66 ± 1.73 cm vs 18.12 ± 3.92 cm, P < 0.001) in comparison with the OTG group. Patients with LTG were sooner able to take a liquid diet (3.65 ± 2.56 d vs 4.62 ± 2.59 d, P < 0.001) and expel flatus or defecation (3.87 ± 2.17 d vs 5.62 ± 2.35 d, P < 0.001) after the operation, indicating the restoration of the intestinal function. Additionally, patients in the LTG group were able to ambulate after 2.93 ± 2.04 d, which is fewer days than the OTG group required (4.13 ± 2.55 d) (P < 0.001). In addition, the duration of postoperative hospitalization of the LTG group was significantly shorter than that in OTG groups [9 (8, 11) d vs 10 (9, 12) d, P < 0.001].
| LTG, n = 382 | OTG, n = 196 | P value | |
| Operation time in min | 230.14 ± 58.92 | 198.4 ± 56.76 | < 0.001 |
| Blood loss in m | 200.42 ± 304.34 | 275.77 ± 384.72 | 0.010 |
| Length of abdominal incision in cm | 9.66 ± 1.73 | 18.12 ± 3.92 | < 0.001 |
| Length of proximal margin in cm | 1.15 ± 0.72 | 1.16 ± 0.77 | 0.986 |
| R-status, n (%) | 0.879 | ||
| R0 | 380 (99.5) | 194 (99.0) | |
| R1/2 | 2 (0.5) | 2 (1.0) | |
| Number of harvested LNs | 28.81 ± 12.16 | 26.20 ± 12.23 | 0.015 |
| Number of positive LNs | 3.72 ± 6.33 | 3.61 ± 5.30 | 0.842 |
| Time to liquid diet in d | 3.65 ± 2.56 | 4.62 ± 2.49 | < 0.001 |
| Time to first flatus or defecation in d | 3.87 ± 2.17 | 5.62 ± 2.35 | < 0.001 |
| Time to ambulation in d | 2.93 ± 2.04 | 4.13 ± 2.55 | < 0.001 |
| Postoperative hospitalization in d | 9 (8, 11) | 10 (9, 12) | < 0.001a |
| Postoperative complication, n (%) | 19 (5.0) | 9 (4.6) | 0.840 |
| Clavien–Dindo ≥ IIIa | 18 (4.7) | 8 (4.1) | 0.729 |
| Anastomotic leakage | 13 (3.4) | 5 (2.6) | 0.577 |
| Abdominal abscess | 2 (0.5) | 1 (0.5) | 1.000 |
| Anastomotic stenosis | 2 (0.5) | 1 (0.5) | 1.000 |
| Pneumonia | 0 | 1 (0.5) | 0.339b |
| Arrhythmia | 1 (0.3) | 0 | 1.000b |
| Wound infection | 1 (0.3) | 1 (0.5) | 1.000b |
| Mortality | 0 | 0 |
Postoperative complications occurred in 5.0% of patients after LTG and in 4.6% of patients after OTG (P = 0.840). There existed no significant difference between the two groups in terms of anastomotic leakage, anastomotic stenosis, abdominal abscess, pneumonia, arrhythmia or wound infection (P > 0.05). Furthermore, the complications of Clavien-Dindo grade III or higher were comparable in both groups (P = 0.729). No mortality existed within 30 d postoperatively in either group. Further details are presented in Table 2.
According to the histopathological analysis, the rate of complete tumor resection (R0) could be achieved in 99.5% in the LTG group and 99.0% in the OTG group (P = 0.879). The number of the harvested LNs was significantly higher in the LTG groups (28.81 ± 12.16 vs 26.20 ± 12.23, P = 0.015). In addition, the number of positive LNs was similar in the two groups (P > 0.05). Apart from that, the length of the proximal margin was also comparable between the two groups (P = 0.597).
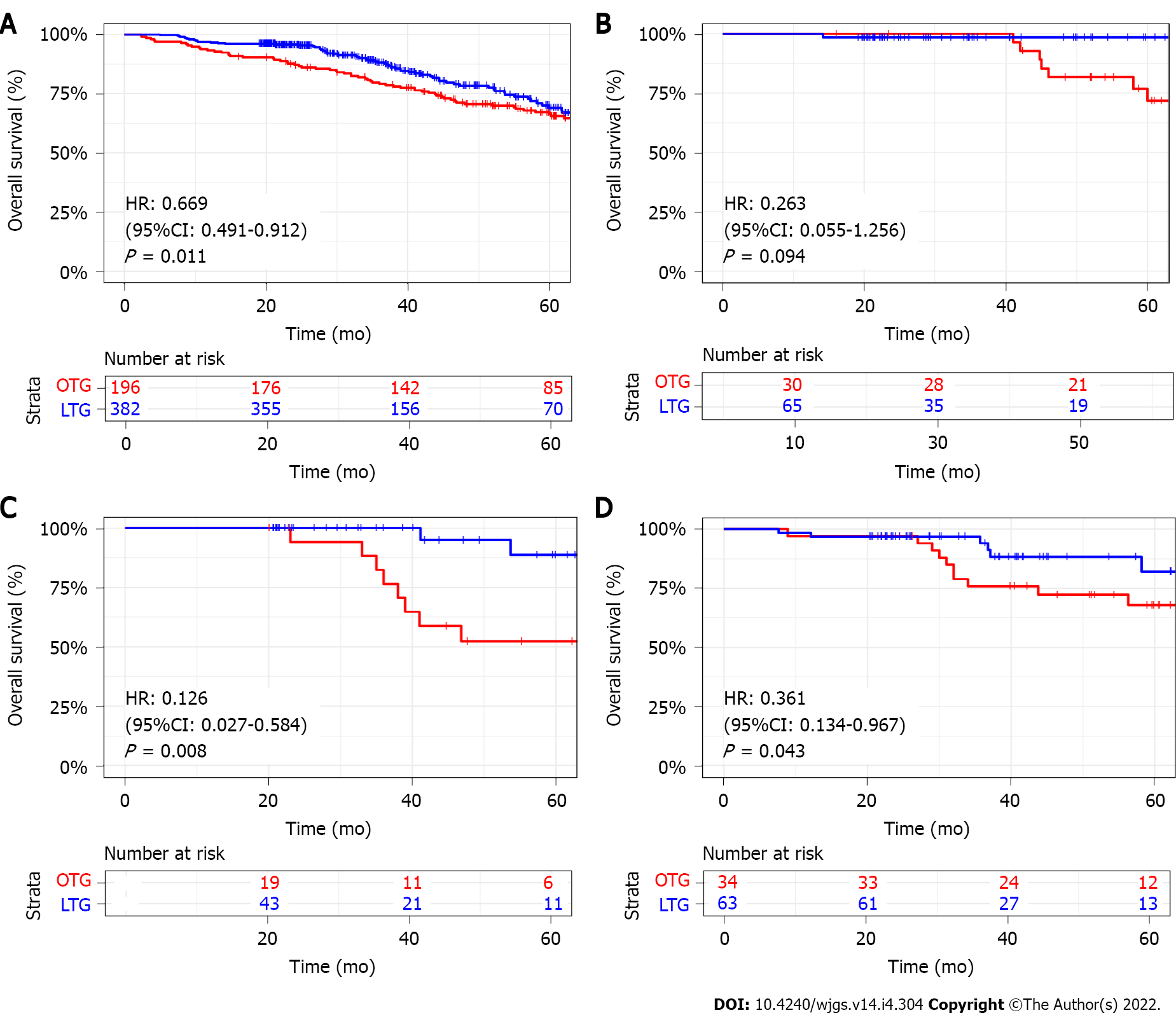
The median follow-up time was 38.94 mo (Interquartile range: 23.28-59.93) for all patients. In comparison with the OTG group, the LTG group showed a better 3-year OS (88.2% vs 79.2%, P = 0.011) (Figure 2A). Then, we performed a stratified analysis of survival according to the TNM stage. For patients with stage I, there existed no significant difference in 3-year OS between the two groups, but patients in the LTG group with stage II and stage III had a better 3-year OS compared with that of the OTG group [Stage II: hazard ratio (HR): 0.126, 95% confidence interval (CI): 0.027-0.584, P = 0.008; Stage III: HR: 0.361, 95%CI: 0.134-0.967, P = 0.043] (Figure 2B-D).
The rate of recurrence presented no significant difference in the LTG and OTG groups (12.8% vs 10.7%, P = 0.547). The patterns of recurrence were listed in Table 3. Distributions of recurrence for LTG were similar to that for OTG, and there existed no differences in organ metastasis (liver, lung, bone, brain, pancreas), anastomotic recurrence, peritoneal dissemination, lymph node metastasis or others (P > 0.05).
| LTG, n = 382 | OTG, n = 196 | P value | |
| Recurrence, n (%) | |||
| No | 333 (87.2) | 175 (89.3) | 0.547 |
| Yes | 49 (12.8) | 21 (10.7) | |
| Liver metastasis, n (%) | |||
| No | 372 (97.4) | 193 (98.5) | 0.590 |
| Yes | 10 (2.6) | 3 (1.5) | |
| Lung metastasis, n (%) | |||
| No | 376 (98.4) | 192 (98.0) | 0.941 |
| Yes | 6 (1.6) | 4 (2.0) | |
| Bone metastasis, n (%) | |||
| No | 377 (98.7) | 193 (98.5) | 1.000 |
| Yes | 5 (1.3) | 3 (1.5) | |
| Brain metastasis, n (%) | |||
| No | 380 (99.5) | 193 (98.5) | 0.445 |
| Yes | 2 (0.5) | 3 (1.5) | |
| Pancreas metastasis, n (%) | |||
| No | 381 (99.7) | 194 (99.0) | 0.555 |
| Yes | 1 (0.3) | 2 (1.0) | |
| Anastomotic recurrence, n (%) | |||
| No | 369 (96.6) | 189 (96.4) | 1.000 |
| Yes | 13 (3.4) | 7 (3.6) | |
| Peritoneal dissemination, n (%) | |||
| No | 377 (98.7) | 196 (100.0) | 0.257 |
| Yes | 5 (1.3) | 0 (0.0) | |
| Lymph node metastasis, n (%) | |||
| No | 377 (98.7) | 196 (100.0) | 0.257 |
| Yes | 5 (1.3) | 0 (0.0) | |
| Others, n (%) | |||
| No | 378 (99.0) | 196 (100.0) | 0.364 |
| Yes | 4 (1.0) | 0 (0.0) | |
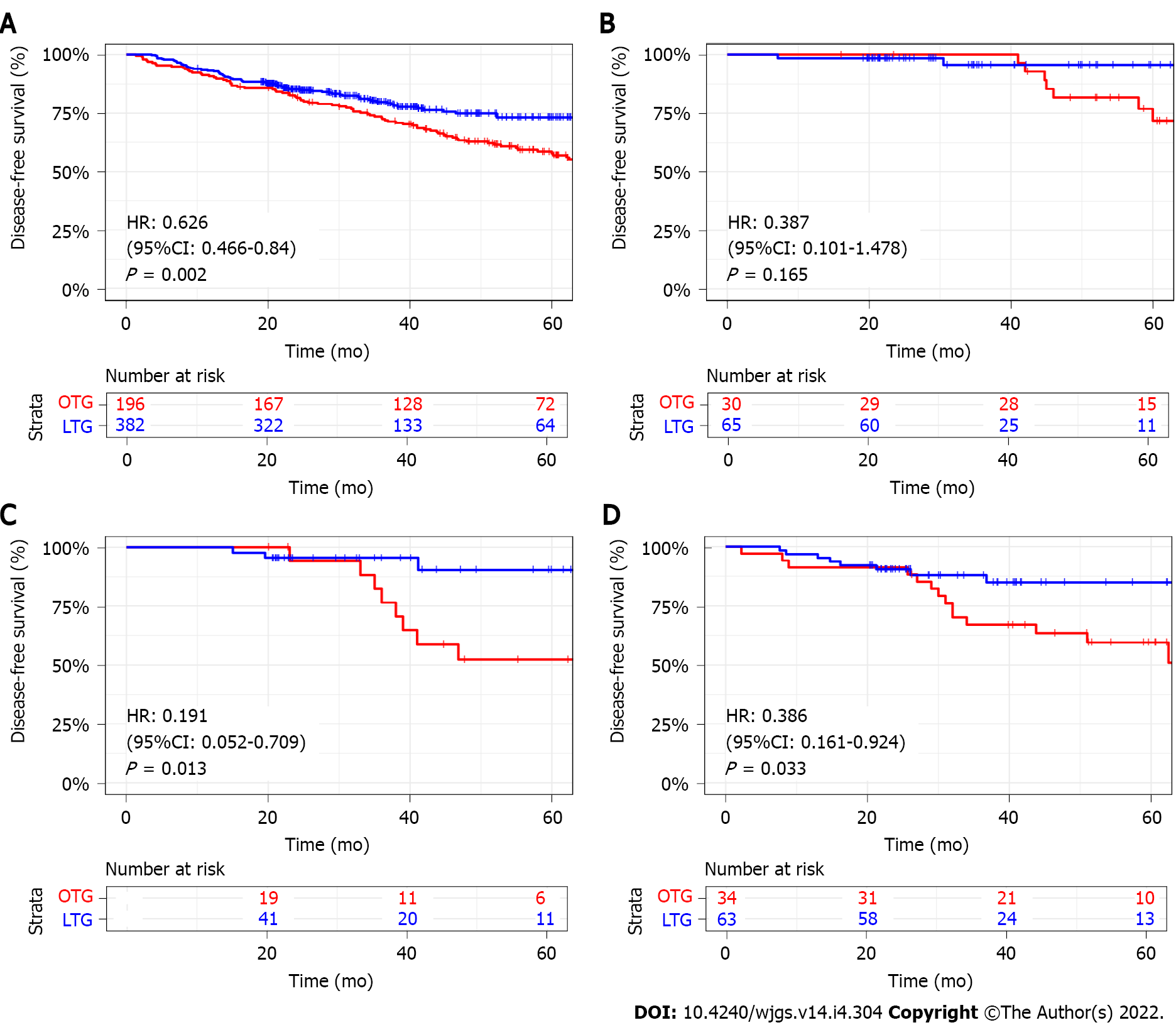
The 3-year DFS was significantly better in the LTG group than that in the OTG group (79.7% vs 73.0%, P = 0.002) (Figure 3A). After stratification by TNM stage, the 3-year DFS was similar between the two groups in stage I patients. However, for stage II and stage III patients, the 3-year DFS was better in the LTG group compared with that of OTG group with significant difference (Stage II: HR: 0.191, 95%CI: 0.052-0.709, P = 0.013; Stage III: HR: 0.386, 95%CI: 0.161-0.924, P = 0.033) (Figure 3B-D).
Recently, the prevalence of Siewert type II AEG has risen rapidly, and most patients are diagnosed as an advanced stage with a poor prognosis at the first visit[23]. Complete removal of the tumor and adequate regional LN resection remains the only curative treatment for AEG[6]. Since the first report of laparoscopic-assisted gastrectomy, laparoscopic techniques have developed quickly in gastrointestinal tumors[9,24]. However, due to the lack of scientific evidence, the safety and feasibility of LTG in the treatment of Siewert type II AEG still remain controversial[16,17]. In the present study, LTG for Siewert type II AEG showed longer operation times but less blood loss, shorter abdominal incision and faster recovery compared with OTG. The obtained results were similar to the previous studies[17,18,20]. A large number of studies have demonstrated that LTG was comparable for morbidity and mortality to OTG for gastric cancer while few of them were focused on AEG[25-28]. In this study, no significant difference was observed in postoperative complications between the LTG group and OTG group for Siewert type II AEG. Apart from that, the complications of Clavien-Dindo grade III or higher were comparable in both groups. These results suggested that LTG can be safely performed and provide better short-term outcomes for patients diagnosed with Siewert type II AEG.
Ensuring the safety of oncology is critical to the choice of surgical strategy. Shi et al[17] compared 132 patients with LTG and 264 patients with OTG. After propensity score matching, the number of harvested LNs showed no significant difference for AEG. By contrast, Sugita et al[18] suggested an increased number of dissected LNs in the LTG group compared with OTG for Siewert type II AEG[18]. In the current work, there existed a higher number of harvested LNs in the LTG group than that in the OTG group. The previous studies reported that the number of harvested LNs is an important prognostic factor for patients with AEG[29,30]. In addition, other oncological parameters in terms of length of proximal margin, R0 resection and the number of positive LNs were comparable between the two groups. As a result, the oncological safety of LTG is equivalent to OTG.
Regarding the long-term outcomes, we found that the distribution of recurrence patterns was similar in the two groups. Shi et al[17] reported that there existed no significant difference for OS between the LTG and OTG groups[17]. Nevertheless, their study population included not only Siewert type II but also type III AEG. In addition, Huang et al[19] and Sugita et al[16] suggested that Siewert type II patients in the LTG group had significantly better OS than that in the OTG group[16,19]. The existing limitations included short observation period and small population, respectively. We observed a better 3-year OS and DFS of LTG for Siewert type II AEG patients compared with those treated with OTG. Moreover, we conducted a stratified analysis based on the TNM stage. Patients with stage I exhibited no survival benefit from LTG, while patients with stage II and III also revealed better survival outcomes in the LTG group.
Undoubtedly, our study has some limitations. First, this study was a single-center, retrospective cohort study. In addition, the follow-up compliance of patients is limited, and the specific death and the patterns of recurrence of some patients remain unknown. Thus, prospective randomized controlled studies are still needed.
In conclusion, LTG is a safe and feasible treatment for Siewert type II AEG. Meanwhile, patients with advanced stage AEG may benefit more from LTG in the long-term outcomes.
Due to the lack of scientific evidence, the feasibility of laparoscopic-assist transhiatal gastrectomy (LTG) in patients with Siewert type II adenocarcinoma of the esophagogastric junction (AEG) is still controversial.
To compare the feasibility of LTG with the traditional open transhiatal gastrectomy (OTG) in patients with Siewert type II AEG.
We retrospectively evaluated and compared the short-term and long-term outcomes for patients with Siewert type II AEG treated with LTG and OTG and aimed to explore the feasibility of LTG treatment of Siewert type II AEG.
We retrospectively evaluated 578 patients with Siewert type II AEG who have undergone LTG or OTG at the First Medical Center of the Chinese People’s Liberation Army General Hospital from January 2014 to December 2019. The short-term and long-term outcomes were compared between the LTG (n = 382) and OTG (n = 196) groups.
Compared with the OTG group, the LTG group had less surgical trauma and a faster recovery after surgery. No significant difference was present between the two groups regarding oncological safety. The 3-year overall survival and disease-free survival were better in the LTG group than those in the OTG group (88.2% vs 79.2%, P = 0.011; 79.7% vs 73.0%, P = 0.002, respectively). In the stratified analysis, both overall survival and disease-free survival were better in the LTG group than those in the OTG group for stage II/III patients (P < 0.05) but not for stage I patients.
For patients with Siewert type II AEG, LTG is associated with better short-term outcomes and similar oncology safety. In addition, patients with advanced stage AEG may benefit more from LTG in the long-term outcomes.
Well-designed multicenter prospective randomized controlled studies are still needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Heath OM, United Kingdom; Levine Y, Israel; Ozawa S, Japan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 2. | Imamura Y, Watanabe M, Toihata T, Takamatsu M, Kawachi H, Haraguchi I, Ogata Y, Yoshida N, Saeki H, Oki E, Taguchi K, Yamamoto M, Morita M, Mine S, Hiki N, Baba H, Sano T. Recent Incidence Trend of Surgically Resected Esophagogastric Junction Adenocarcinoma and Microsatellite Instability Status in Japanese Patients. Digestion. 2019;99:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Dubecz A, Solymosi N, Stadlhuber RJ, Schweigert M, Stein HJ, Peters JH. Does the Incidence of Adenocarcinoma of the Esophagus and Gastric Cardia Continue to Rise in the Twenty-First Century? J Gastrointest Surg. 2013;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 531] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 5. | Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Hashimoto T, Kurokawa Y, Mori M, Doki Y. Surgical Treatment of Gastroesophageal Junction Cancer. J Gastric Cancer. 2018;18:209-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, Nashimoto A, Hiratsuka M; Japan Clinical Oncology Group (JCOG9502). Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Kurokawa Y, Sasako M, Sano T, Yoshikawa T, Iwasaki Y, Nashimoto A, Ito S, Kurita A, Mizusawa J, Nakamura K; Japan Clinical Oncology Group (JCOG9502). Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg. 2015;102:341-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 10. | Lee MS, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013;27:2598-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Lee JH, Lee CM, Son SY, Ahn SH, Park DJ, Kim HH. Laparoscopic versus open gastrectomy for gastric cancer: long-term oncologic results. Surgery. 2014;155:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 529] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 13. | Kim HI, Hur H, Kim YN, Lee HJ, Kim MC, Han SU, Hyung WJ. Standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC): a prospective, observational, multicenter study [NCT01283893]. BMC Cancer. 2014;14:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Kurokawa Y, Takeuchi H, Doki Y, Mine S, Terashima M, Yasuda T, Yoshida K, Daiko H, Sakuramoto S, Yoshikawa T, Kunisaki C, Seto Y, Tamura S, Shimokawa T, Sano T, Kitagawa Y. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors: A Prospective Nationwide Multicenter Study. Ann Surg. 2021;274:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 15. | Duan XF, Yue J, Tang P, Shang XB, Jiang HJ, Yu ZT. Lymph node dissection for Siewert II esophagogastric junction adenocarcinoma: A retrospective study of 3 surgical procedures. Medicine (Baltimore). 2017;96:e6120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Sugita S, Kinoshita T, Kuwata T, Tokunaga M, Kaito A, Watanabe M, Tonouchi A, Sato R, Nagino M. Long-term oncological outcomes of laparoscopic versus open transhiatal resection for patients with Siewert type II adenocarcinoma of the esophagogastric junction. Surg Endosc. 2021;35:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Shi Y, Li L, Xiao H, Guo S, Wang G, Tao K, Dong J, Zong L. Feasibility of laparoscopic gastrectomy for patients with Siewert-type II/III adenocarcinoma of the esophagogastric junction: A propensity score matching analysis. PloS One. 2018;13:e0203125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Sugita S, Kinoshita T, Kaito A, Watanabe M, Sunagawa H. Short-term outcomes after laparoscopic versus open transhiatal resection of Siewert type II adenocarcinoma of the esophagogastric junction. Surg Endosc. 2018;32:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Huang CM, Lv CB, Lin JX, Chen QY, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Cao LL, Lin M, Tu RH. Laparoscopic-assisted versus open total gastrectomy for Siewert type II and III esophagogastric junction carcinoma: a propensity score-matched case-control study. Surg Endosc. 2017;31:3495-3503. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Zhao Y, Zhang J, Yang D, Tang Z, Wang Q. Feasibility of laparoscopic total gastrectomy for advanced Siewert type Ⅱ and type Ⅲ esophagogastric junction carcinoma: A propensity score-matched case-control study. Asian J Surg. 2019;42:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4396] [Article Influence: 549.5] [Reference Citation Analysis (4)] |
| 22. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24776] [Article Influence: 1179.8] [Reference Citation Analysis (0)] |
| 23. | Huang Q, Sun Q, Fan XS, Zhou D, Zou XP. Recent advances in proximal gastric carcinoma. J Dig Dis. 2016;17:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Lee Y, Min SH, Park KB, Park YS, Ahn SH, Park DJ, Kim HH. Long-term Outcomes of Laparoscopic Versus Open Transhiatal Approach for the Treatment of Esophagogastric Junction Cancer. J Gastric Cancer. 2019;19:62-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Fan Y, Liu M, Li S, Yu J, Qi X, Tan F, Xu K, Zhang N, Yao Z, Yang H, Zhang C, Xing J, Wang Z, Cui M, Su X. Surgical and oncological efficacy of laparoscopic-assisted total gastrectomy versus open total gastrectomy for gastric cancer by propensity score matching: a retrospective comparative study. J Cancer Res Clin Oncol. 2021;147:2153-2165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Yoshikawa T, Cho H, Rino Y, Yamamoto Y, Kimura M, Fukunaga T, Hasegawa S, Yamada T, Aoyama T, Tsuburaya A. A prospective feasibility and safety study of laparoscopy-assisted distal gastrectomy for clinical stage I gastric cancer initiated by surgeons with much experience of open gastrectomy and laparoscopic surgery. Gastric Cancer. 2013;16:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Shehzad K, Mohiuddin K, Nizami S, Sharma H, Khan IM, Memon B, Memon MA. Current status of minimal access surgery for gastric cancer. Surg Oncol. 2007;16:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Choi YY, Bae JM, An JY, Hyung WJ, Noh SH. Laparoscopic gastrectomy for advanced gastric cancer: are the long-term results comparable with conventional open gastrectomy? J Surg Oncol. 2013;108:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Zhu M, Cao B, Li X, Li P, Wen Z, Ji J, Min L, Zhang S. Risk factors and a predictive nomogram for lymph node metastasis of superficial esophagogastric junction cancer. J Gastroenterol Hepatol. 2020;35:1524-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Xu J, Cao J, Wang L, Wang Z, Wang Y, Wu Y, Lv W, Hu J. Prognostic performance of three lymph node staging schemes for patients with Siewert type II adenocarcinoma of esophagogastric junction. Sci Rep. 2017;7:10123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |