Published online Apr 27, 2022. doi: 10.4240/wjgs.v14.i4.276
Peer-review started: October 16, 2021
First decision: December 3, 2021
Revised: December 18, 2021
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: April 27, 2022
Processing time: 189 Days and 15.6 Hours
Neuroendocrine neoplasms (NENs) of the gastroenteropancreatic system are rare and heterogeneous tumours, yet with increasing prevalence. The most frequent primary sites are the small intestine, rectum, pancreas, and stomach. For a localized disease, surgical resection with local lymph nodes is usually curative with good overall and disease free survival. More complex situation is the treatment of locally advanced lesions, liver metastases, and, surprisingly, small asymptomatic tumours of the rectum and pancreas. In this review, we focus on the current role of surgical management of gastroenteropancreatic NENs. We present surgical approach for the most frequent primary sites. We highlight the role of endoscopic surgery and the watch-and-wait strategy for selected cases. As liver metastases pose an important clinical challenge, we present current indications and contraindications for liver resection and a role of liver transplantation for metastatic NENs.
Core Tip: Neuroendocrine neoplasms of the gastroenteropancreatic system are a rare and heterogeneous group of tumours. Due to the advancement of the diagnostic methods like new serum biomarkers and more accurate imaging modalities (including positron emission tomography), its incidence is rising. We present a review focused on up-to-date recommended surgical management of these tumours. We discuss key points of treatment for the most frequent primary sites and liver metastases. Finally, we point areas where univocal consensus is still being achieved by presenting recommendations of various Oncological and Surgical Societies.
- Citation: Stankiewicz R, Grąt M. Current status of surgical management of patients with gastroenteropancreatic neuroendocrine neoplasms. World J Gastrointest Surg 2022; 14(4): 276-285
- URL: https://www.wjgnet.com/1948-9366/full/v14/i4/276.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i4.276
Neuroendocrine neoplasms (NENs) arise from the diffuse neuroendocrine cell system and may occur at many different sites. NENs constitute a heterogeneous group of malignancies with neural phenotype and capacity to secrete amines and hormones. The gastroenteropancreatic (GEP) system and lungs are the most common primary tumour sites[1]. In this review, we focus on GEP-NENs.
Histological diagnosis is mandatory in all patients and can be carried out on resection specimen or core biopsies in an advanced disease. GEP-NENs should be classified based on morphology and proliferation into well-differentiated neuroendocrine tumours (NETs) (G1 to G3) and poorly-differentiated neuroendocrine cancers (NECs) (always G3) as shown in Table 1[2].
GEP-NENs are rare tumours with an annual incidence of 6.98 per 100000 persons in 2012 in the United States. According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program, the rise in the annual number of cases can be observed in the last few decades with the most dramatic rise in patients older than 65 years (25.3 per 100000 persons). The order of frequency in NENs is the small intestine (1.05 per 100000), rectum (1.04 per 100000), and pancreas (0.48 per 100000)[3]. Hepatic metastases occur in 50%-75% of patients with NENs[4]. The most common primary sites in patients with NEN liver metastasis are the small intestine (56%), pancreas (10%), and colon (10%)[5]. The overall survival (OS) varies depending on primary site and grade. According to the SEER, the median OS for all patients is 9.3 years. NENs in the rectum and appendix had the best median OS, while NENs in the pancreas had the worst median OS. Localized, low grade (G1/G2) NETs have the best prognosis of long OS[3].
In this review, we focus on NENs of the GEP system and their step-by-step surgical management. We discuss tumours of the stomach, small intestine, rectum, and pancreas. Special emphasis is put on the treatment of hepatic metastases with the role of liver transplantation (LT).
Gastric NENs are slow growing, indolent tumours but with potential for aggressiveness and metastases. They are very often incidental findings with tendency to being multi-focal. Registries show a rising frequency in diagnosis of gastric NEN[6]. The SEER estimates an incidence of gastric NENs at 0.5 per 100000 persons[3].
There are three types of gastric NETs. Type I (70%-80%) is characterized by rare metastases and excellent prognosis and evolves on the background of chronic atrophic gastritis. Type II (5%-10%) is a result of Zollinger-Ellison syndrome and metastases to lymph nodes and the liver can be expected. The prognosis in patients with type II is very good. Type III (15%-20%) is a sporadic tumour with a very high prevalence of metastases either to lymph nodes (50%-100%) or the liver (22%-75%), and the prognosis is similar to that of gastric adenocarcinoma[7].
Endoscopic assessment of the lesions is crucial for further treatment. In addition to taking biopsies, the number and size of tumours should also be noted. Large lesions (> 1-2 cm) should also be assessed by endoscopic ultrasound (EUS) in terms of invasion depth and positive lymph nodes[8].
Surgical management of gastric NETs depends on several factors, such as tumour subtype, degree of differentiation, and presence or absence of invasion.
Treatments for type I and II gastric NETs are: (1) < 1 cm–endoscopic removal or monitoring by close endoscopic surveillance; (2) 1-2 cm and lesions with submucosal invasion (EUS)–snare polypectomy or endoscopic mucosal resection (EMR); and (3) > 2 cm–individualized strategy, either endoscopic resection (if possible) or surgical resection.
Treatments for type III gastric NETs are: Partial gastrectomy and lymph node dissection; in selected cases with lesions < 1-2 cm in size, EMR or endoscopic submucosal dissection (ESD) should be considered[9].
A potential alternative for patients with small type I lesions who cannot be managed endoscopically is treatment with somatostatin analogues (SSA)[10,11]. Also, netazepide (gastrin/cholecystokinin receptor antagonist) seems a promising option for patients with type I gastric NETs[12]. The downside of this agent though, is that if this treatment is stopped, tumours will regrow.
The small intestine is the most common primary site of NENs. The presence of carcinoid heart disease, mesenteric lymph node metastases, distal abdominal lymph node metastases, liver metastatic burden, extra-abdominal metastases, skeletal involvement, and peritoneal carcinomatosis are independent prognostic factors for OS[13].
Surgical strategy for any locoregional small intestine NENs (SI-NENs) should be en bloc resection with its lymphatic drainage field, including the mesentery[14]. The entire small and large intestines should be evaluated (pre- and intra-operatively), as up to 40% of SI-NENs may have more than one site of primary gastrointestinal tract malignancy. Therefore, open resection seems preferred over laparoscopic, unless the latter enables a thorough examination by palpation, i.e., by small incision[15].
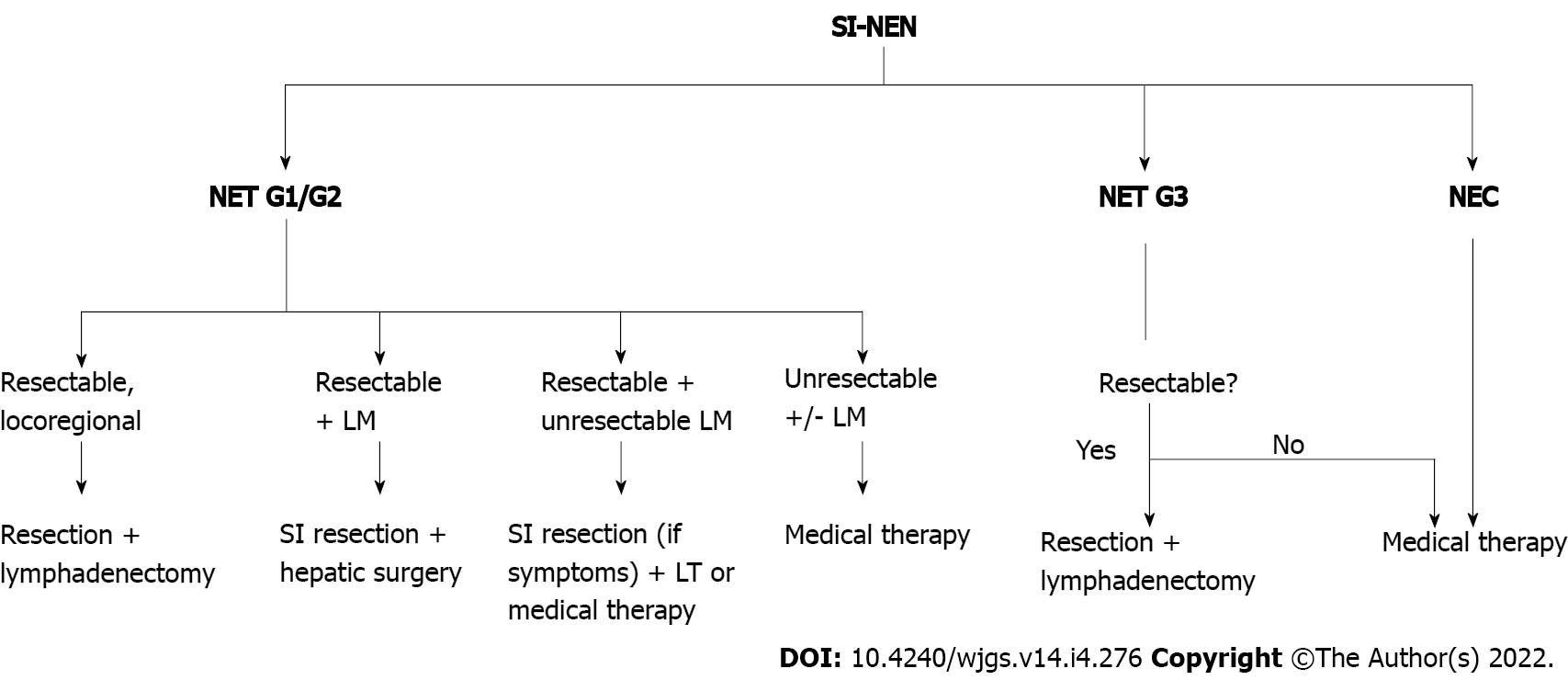
SI-NENs have a significant metastatic potential, and even for lesions < 1 cm, nodal and distant metastases can be found in 12% and 5% of cases, respectively[16]. The liver is the most common site of metastases. In the setting of resectable synchronous primary tumour and hepatic metastases, resection of the primary tumour and lymph nodes, with combination with liver metastases is warranted[14]. According to ESMO guidelines, patients qualified for synchronous resection must have a tumour with a Ki-67 index < 10% (or slow growing tumour) and metastases limited to the liver[17]. Those exceeding the above mentioned criteria should be qualified for medical therapy (Figure 1).
There are controversies over whether to resect or not the primary SI-NEN in the case of unresectable liver metastases. For symptomatic SI-NENs, resection with lymphadenectomy is advised[17]. ENETS guidelines acknowledge that the lack of prospective evidence does not permit a definite conclusion on any potential survival benefit in case of an asymptomatic disease–risk and benefit of the surgical intervention need to be considered individually[18]. In a systematic review, Capurso et al[19] presented benefit in survival (75-139 mo vs 50-88 mo) for patients who underwent primary site resection. This was based on six retrospective cohort studies which included a total number of 971 patients[19]. These findings were supported by the meta-analysis conducted by Almond et al[20]. They found an increase in median survival from 22 to 112 mo across six studies for patients who underwent primary site resection[20]. Conversely, a study by Daskalakis et al[21] based on the Swedish prospective database found no difference in terms of OS, morbidity, and 30-d mortality. Both groups of patients (161 underwent up-front locoregional surgery and 202 underwent delayed surgery) received systematic oncologic therapy for NENs (SSAs, interferon-alfa, liver-directed treatment, and peptide receptor radionuclide therapy)[21].
There is also some experience with intestinal transplantation for advanced local SI-NENs with unresectable mesenteric lymph node metastases[22]. This kind of therapy is still anecdotal and not accessible for all patients amenable for this treatment.
Rectal NENs are subepithelial lesions that are diagnosed with an increasing frequency. They constitute about 1% of rectal lesions, and are often accidental findings in colonoscopy[23]. Rectal NENs are usually small (< 1 cm in diameter) single lesions located 5-10 cm from the dental line[24]. Due to its typical macroscopic appearance, 95.9% of cases can be diagnosed on endoscopy alone[25]. Therefore, biopsy should only be considered in doubtful cases (atypical features) and in tumours that are more than 2 cm in size. Methods of treatment are either EMR, ESD, transanal endoscopic microsurgery, or surgery, depending on tumour size, grade, and lymph node and distant metastases. EUS is indicated for lesions more than 5 mm in size, to identify muscular layer invasion[23].
There is an accordance across the guidelines that all tumours larger than 2 cm should be removed surgically, either by low anterior resection or abdominoperineal resection. Tumours < 1 cm (G1, G2, and G3) should be removed by TEM or endoscopy. There are differences in the treatment strategy concerning lesions 1-2 cm in diameter. In general, those lesions with muscularis propria invasion should be resected surgically. Other lesions should be considered individually with tendency to TEM or endoscopy[14,23,26].
Pancreatic neuroendocrine neoplasms (PNENs) are a subgroup of NENs that have relatively distinct biological behavior and clinical management compared with pancreatic adenocarcinoma. Like other NENs, they have a capacity to produce amines and hormones. PNENs are believed to arise from islet cells precursors[27]. Tumours that overproduce hormones may be associated with various clinical syndromes and are referred to as functional. In contrast, those that do not secrete hormones or secrete peptides which do not result in an obvious syndrome are termed non-functional (70% of PNENs). The most common hormones produced by PNENs are: Insulin (insulinoma; 1-32 million per year), gastrin (gastrinoma; 0.5-21.5 million per year), vasoactive intestinal peptide (VIPoma; 0.05-.02 million per year), and glucagon (glucagonoma; 0.01-0.1 million per year)[28]. Most PNENs are malignant, and upwards of 60% of patients will have metastatic disease at the time of diagnosis[27]. Ten to twenty percent are associated with inherited cancer syndromes, such as multiple endocrine neoplasia type 1 (MEN-1), von Hippel-Lindau syndrome, and neurofibromatosis type 1 (NF-1)[29]. Detailed management of these syndromes is beyond the scope of this review.
Disease stage and tumour grade (Table 1) must be assessed along with hormonal activity (if symptoms occur). Computed tomography is the most commonly used modality for staging. It is quick and widely available, and provides excellent anatomic definition of the pancreas, and lymph node or liver metastases. Histological diagnosis is usually based on samples taken by fine-needle aspiration or biopsy under EUS guidance.
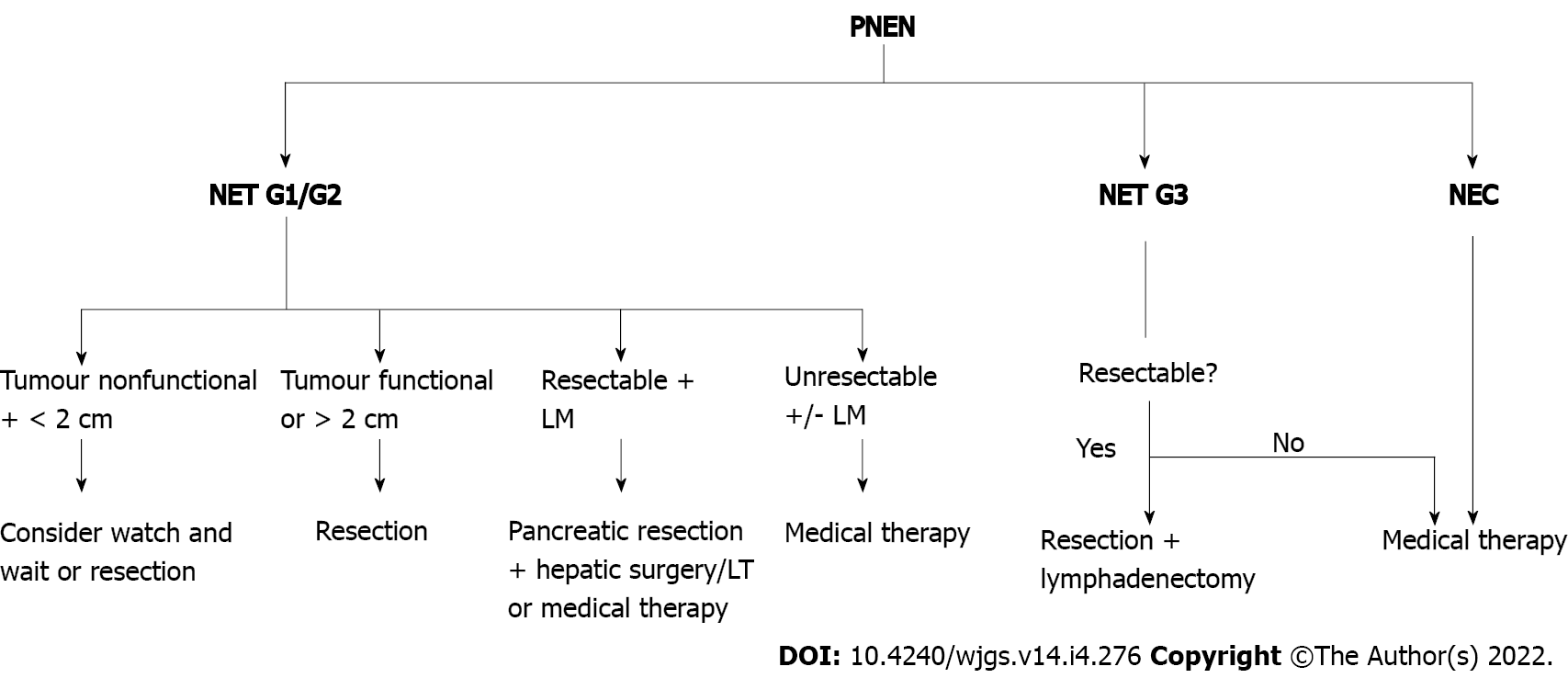
Patients with functional PNENs irrespective of their size, should be evaluated for surgery[30]. Typical resections (pancreaticoduodenectomy, distal pancreatectomy, or total pancreatectomy) or tumour enucleation may be used. The latter should be considered primarily for small (< 2 cm), peripheral insulinomas[14]. The advantage of enucleation over standard resection is that the former is associated with a lower rate of postoperative pancreatic insufficiency, shorter operative time, and less operative blood loss[31]. In high-grade PNETs or PNECs, only oncologic resection (pancreaticoduodenectomy or distal pancreatectomy with lymphadenectomy) should be considered[9] (Figure 2).
Non-functional PNETs < 2 cm can be managed either surgically or by the wait-and-watch approach. In the meta-analysis conducted by Sallinen et al[32], small, sporadic PNETs in 344 patients were observed with satisfactory results[32]. In only 22% of cases, tumour growth was observed and no metastases were reported. Twelve percent of patients had surgery, and the most common indications were tumour growth (47%) and patients’ preferences (31%). The same study showed more aggressive character of the small MEN-1 related PNETs. Over half of these patients had tumour growth during observation and in 9% of cases metastases were reported (distant and nodal). Opposite results come from the meta-analysis by Finkelstein et al[31]. Seven hundred and fourteen patients had tumours ≤ 2 cm, of which 587 underwent surgical resection and 127 were managed nonsurgically. Analysis showed an improved OS in the resection group at 1 year (P = 0.745), 3 years (P < 0.001), and 5 years (P < 0.001).
In the management of small (< 2 cm in size) PNENs, the malignant potential of the tumour (rather small in most of the cases) and consequences of the aggressive pancreatic surgery (about a 30% complication rate and 1.7% mortality) must be taken under consideration[33]. Each patient should be individually assessed and when conservative approach is decided, close follow-up is recommended[14,17].
GEP-NENs at diagnosis are metastatic in 40%-95% of cases[4]. The most common metastatic sites are the liver, other intraperitoneal sites, bone, and the lung. Of all liver metastases, over half are from the small intestine. In about 10% of patients with liver NEN metastases, the primary site remains unknown[5]. Liver metastases represent the most crucial prognostic factor, irrespective of the primary NEN site. As G3 NETs and NECs present with aggressive behaviour (multifocal or bilobar growth, and anticipated high recurrence rate), systemic therapy is more commonly used than resection of the metastases.
Despite a high recurrence rate after resection (80%-95% within 5 years[34]), surgery remains the most favorable approach for selected (G1 and G2 NET) patients with liver metastases. Surgical treatment comprises resection and cytoreductive surgery for symptom management and improvement of survival. For a few decades, debulking threshold of resection was debated. In the first series presented in 1977, the authors achieved good symptom control with a threshold of 95% for debulking[35]. After being confirmed by other authors, such a threshold of approximately 90% for debulking was a goal to achieve[34,36]. Graff-Baker et al[37] found no difference in progression free survival between groups with > 70% debulking and > 90% debulking, and the OS for the study population was 88%[37]. Adoption of this lower debulking threshold of > 70%, along with the use of parenchymal-sparing techniques, allowed for more than 75% of patients to undergo hepatic cytoreduction. Also, when > 70% debulking is achieved, despite less than complete resection (R1/R0), comparable survival outcomes are observed as for R0 resection with > 70% cytoreduction[37]. In patients with carcinoid syndrome, it is important to control the hypersecretion of serotonin with SSA prior to surgery, in order to prevent carcinoid crisis[18].
When evaluating patients with NET liver metastases for surgical treatment, one must remember that current imaging modalities are limited in detecting small lesions. Accuracy of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging is calculated to be only 24%, 38%, and 49%, respectively. Lesions smaller than 2 mm are not visible in the preoperative assessment[38].
In patients who cannot be qualified for partial liver resection, LT is an option for a improved survival for selected patients[39]. LT for metastatic NETs provides a 5-year OS rate between 47% and 71%[40]. Each patient should be considered individually for prognostic factors that would impact post-LT outcomes. These prognostic factors are: (1) Histologic grade. LT is reserved for G1 and G2 NETs[39,41]. Le Treut et al[42] found a difference in survival between well and poorly differentiated NENs in the European Liver Transplant Registry (ELTR), reaching almost 30% in 5-year OS[42]. The histologic grade can be different between primary and metastatic tumours in the liver, and treatment is guided by the worst grade in the available specimen; (2) Tumour burden. The cut-off < 50% for this factor was arbitrarily set by Mazzaferro et al[39]. Data from the ELTR found that the 5-year OS rate after LT was 42% when the estimated tumoral invasion was over 50%, while it was 61% for tumours under 50%[42]. Some data challenge this threshold of 50% tumour burden, stating underestimation of tumour burden in the pre-LT workup in the early, ELTR-based studies[43]; (3) Primary tumour site. While Mazzaferro et al[39] allowed only NET liver metastases originating from portal venous drainage to be suitable for LT, further analysis of ELTR data did not support this idea[39,42]. Among the patients in the ELTR study, the 5-year survival rate of patients with bronchial tree origin NETs was comparable to that of patients with GEP NETs (53% and 40%-62%, respectively); and (4) Surgical control of the primary tumour. It is recommended to resect primary tumour before LT. This is to monitor biologic response of the liver metastases and to avoid surgical complications from simultaneous surgeries. Data from the ELTR showed an inferior 5-year OS rate in cases where primary tumour was resected during LT compared to those cases where tumour was resected before LT (22% and 56%, respectively). The same study found that in 13% to 14% of cases of NETs with liver metastases, the primary tumour is unknown. The 5-year survival of this cohort was 54%[42]. As such, patients without identifiable primary tumour are still good candidates for LT.
There are two major, widely accepted patients selection criteria for LT in NET metastases. The group from Milan proposed their criteria in 2007 and revised them in 2016[39,44]. The Milan-NET selection criteria are: (1) Histologic grade G1 or G2; (2) Portal drainage of the primary tumour; (3) Pre-transplant curative resection of all extrahepatic lesions; (4) Hepatic tumour invasion under 50%; (5) Duration of stable disease over 6 mo; and (6) Age under 60 year (relative).
The Milan group reported 5-year OS and disease-free survival rates of 97% and 89%, respectively. However, only 15% of patients referred to LT underwent LT[44].
In the United States, the current guidelines regarding LT for NET liver metastases are based on the Milan-NET criteria[45] with the following additional criteria: (1) Unresectable liver metastases; (2) Radiographic characteristics of NET of the liver lesions; (3) Negative metastatic workup by positron emission tomography (PET) scan; (4) Lack of extrahepatic tumour recurrence during the past 3 mo; (5) In the presence of positive findings for lymph node metastases by PET scan, the finding should become negative for 6 mo before re-listing; and (6) In the presence of extrahepatic solid organ metastases, the case will be permanently delisted.
There is no uniformly accepted selection criteria for NET-LT. Some of the above mentioned factors are still debated and waiting for validation, i.e., patients age, primary resection before LT, hepatic tumour burden, and wait time for disease stabilization[45].
The high recurrence rate after NET-LT (31%-57%) remains an important clinical problem[40]. Available data on neoadjuvant or adjuvant therapy in NET-LT are scarce. Most of clinical experience comes from the series of patients who underwent liver resection[46-48].
For patients with unresectable primary GEP-NET and liver metastases, multivisceral transplantation (MVT) is also an option. Data on this treatment are limited by small case series and quality of the reported outcome. In the systematic review by Moris et al[40], the authors found that only 5.7% of patients from single center studies had MVT with various outcomes.
For patients with NET liver metastases beyond resection or LT, there is a number of liver-directed therapies. Ablative methods include microwave ablation, radiofrequency ablation, cryotherapy, and irreversible electroporation. Ischemia and necrosis of NET liver metastases can be achieved by occlusion of the arterial blood supply. Various methods are being used: Bland embolization, chemoembolization, drug eluting beads, and transarterial radioembolization (90Ytrium). Detailed application of these methods is beyond the scope of this review.
The most common metastatic NEN sites are the liver, other intraperitoneal sites, bone, and the lung. Liver metastases occur in 40%-95% of cases[4], but peritoneal metastases can be a part of the metastatic tumour load in approximately 20% of cases[13]. The most common primary site for peritoneal metastases is the small bowel. Presence of peritoneal metastases has an adverse impact on patient survival, irrespective of the hepatic metastases[49,50]. For patients with well-differentiated G1/G2 NETs, complete cytoreductive surgery can prolong overall and disease free survival. In a study from France, patients with peritoneal metastasis were treated by peritonectomy with or without partial hepatectomy[48]. The 5-year and 10-year OS rates were 69% and 52%, respectively, and the 5-year and 10-year disease free survival rates were 17% and 6%, respectively. The benefit from addition of hyperthermic intraperitoneal chemotherapy to complete cytoreductive surgery is questionable, according to the authors of that study. For high-grade NEN peritoneal metastases, only medical treatment is advised[17].
Recent WHO classification of the NEN (Table 1) distinguished two groups of high-grade NENs[2]. Those are well-differentiated NETs G3 with a Ki-67 index > 20% and poorly-differentiated NECs. The term NENs G3 covers both types of those malignancies. The NEN G3 patients are a heterogeneous group concerning prognosis and treatment benefit. GEP NECs are usually highly aggressive, with a propensity for early metastases and dismal prognosis[51]. In the SEER database, the median survival is 34 mo with localized disease, 14-16 mo with regional disease, and 5 mo with distant disease[52]. Data on the NET G3 subgroup are extremely scarce, and they are mainly located in the pancreas and have a better prognosis than NEC[51].
The treatment recommendations for NEN G3 patients are mostly expert consensus supported by heterogeneous retrospective studies. The opinion is that surgery alone is rarely curative and that patients with limited disease should receive multimodality based treatment. The 5-year survival for localized disease depends on the primary site; the best is for colorectal, stomach, and pancreas primaries (40%-50%)[52]. Metastatic surgery for GEP NEC is not recommended and the treatment is with systemic chemotherapy (etoposide and a platinum agent)[53].
A National Cancer Database Study summarized the treatment and outcome of 1861 patients with high-grade NENs[54]. Over 64% of patients was in stage IV of the disease at the moment of diagnosis. The most common primary site was the large bowel (26.6%). Only about 28% of the study population were amenable for surgery. The median survival was 9.3 mo. That study did not distinguish NETs G3 and NECs due to disparity of study period and the novel WHO classification.
Most of the ongoing or recently finished clinical trials examined medical therapies in advanced NENs, demonstrating prolongation of the progression free survival[55]. NEN clinical trials pose logistical challenges due to the relative rarity of NENs and the necessity of multi-centric collaboration to ensure adequate recruitment. This is especially relevant to the concept of surgical trials in metastatic NENs, where only a quarter of patients may be amenable for surgery.
There are four ongoing, still recruiting, NEN clinical trials with surgical intervention (diagnostic or curative) (Table 2)[56]. Two are observational. One of those studies gives medical or surgical treatment dependent of patients’ decision. Two studies are interventional and multicentric. None of those trials opens new surgical fields. For that to happen, new diagnostic and predictive tools must be developed. Clift et al[55] proposed three key areas: (1) The development of increasingly informative functional imaging; (2) The integration with imaging of real-time multianalyte genomic analysis of individual tumour; and (3) The application of system biology strategies to a multidimensional assessment of the relationship of the metabolome, the microbiome, and the proliferome to neuroendocrine neoplasia and the delineation of disease progression[55].
| Study title | Resection of metastatic PNETs after induction system treatment | Single-cell sequencing and establishment of models in NEN | Endoscopic ultrasound-guided radiofrequency ablation for the treatment | Prophylactic cholecystectomy in midgut NET patients who require primary tumor surgery |
| Primary site | Pancreas | GEP NEN | Pancreas | Jejunum, ileum, proximal colon |
| Study type | Observational | Observational | Interventional | Interventional |
| Multicentric | No | No | Yes | Yes |
| Primary purpose | NA | NA | Treatment | Treatment |
| Allocation | NA | NA | NA | Randomized |
| Estimated enrollment | 180 participants | 200 participants | 70 participants | 100 participants |
| Estimated study completion date | July 25, 2025 | December 2022 | June 1, 2021 | April 2025 |
Treatment of solitary NEN is often limited to tumour and local lymph node resection. When metastases appear, a multidisciplinary approach is often mandatory. A great variety of treatment modalities combined with a low incidence rate of NENs and their heterogeneity makes this group of tumours a clinical challenge. Patients should be treated in experienced centers with access to the above mentioned modalities. Even in advanced metastatic NETs, selected groups of patients can reach a 5-year OS rate over 50%.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Polish Transplant Society; Polish Society of Surgical Oncology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Cigrovski Berkovic M, Croatia; Liu L, China; Wang WQ, China; Yang Z, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Anaizi A, Rizvi-Toner A, Valestin J, Schey R. Large cell neuroendocrine carcinoma of the lung presenting as pseudoachalasia: a case report. J Med Case Rep. 2015;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 3. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2491] [Article Influence: 311.4] [Reference Citation Analysis (4)] |
| 4. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1183] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 5. | Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016;139:2679-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Grozinsky-Glasberg S, Alexandraki KI, Angelousi A, Chatzellis E, Sougioultzis S, Kaltsas G. Gastric Carcinoids. Endocrinol Metab Clin North Am. 2018;47:645-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Metz DC. Diagnosis of the Zollinger–Ellison syndrome. Clin Gastroenterol Hepatol. 2012;10:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC; North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 444] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 10. | Lau SC, Abdel-Rahman O, Cheung WY. Improved survival with higher doses of octreotide long-acting release in gastroenteropancreatic neuroendocrine tumors. Med Oncol. 2018;35:123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Massironi S, Zilli A, Fanetti I, Ciafardini C, Conte D, Peracchi M. Intermittent treatment of recurrent type-1 gastric carcinoids with somatostatin analogues in patients with chronic autoimmune atrophic gastritis. Dig Liver Dis. 2015;47:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 12. | Boyce M, Moore AR, Sagatun L, Parsons BN, Varro A, Campbell F, Fossmark R, Waldum HL, Pritchard DM. Netazepide, a gastrin/cholecystokinin-2 receptor antagonist, can eradicate gastric neuroendocrine tumours in patients with autoimmune chronic atrophic gastritis. Br J Clin Pharmacol. 2017;83:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Norlén O, Stålberg P, Öberg K, Eriksson J, Hedberg J, Hessman O, Janson ET, Hellman P, Åkerström G. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg. 2012;36:1419-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, Giordano T, Halfdanarson TR, Halperin D, He J, Heaney A, Heslin MJ, Kandeel F, Kardan A, Khan SA, Kuvshinoff BW, Lieu C, Miller K, Pillarisetty VG, Reidy D, Salgado SA, Shaheen S, Soares HP, Soulen MC, Strosberg JR, Sussman CR, Trikalinos NA, Uboha NA, Vijayvergia N, Wong T, Lynn B, Hochstetler C. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:839-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 330] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 15. | Pasquer A, Walter T, Hervieu V, Forestier J, Scoazec JY, Lombard-Bohas C, Poncet G. Surgical Management of Small Bowel Neuroendocrine Tumors: Specific Requirements and Their Impact on Staging and Prognosis. Ann Surg Oncol. 2015;22 Suppl 3:S742-S749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol. 2005;89:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 698] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 18. | Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, Öberg K; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 743] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 19. | Capurso G, Rinzivillo M, Bettini R, Boninsegna L, Delle Fave G, Falconi M. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg. 2012;99:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Almond LM, Hodson J, Ford SJ, Gourevitch D, Roberts KJ, Shah T, Isaac J, Desai A. Role of palliative resection of the primary tumour in advanced pancreatic and small intestinal neuroendocrine tumours: A systematic review and meta-analysis. Eur J Surg Oncol. 2017;43:1808-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Daskalakis K, Karakatsanis A, Hessman O, Stuart HC, Welin S, Tiensuu Janson E, Öberg K, Hellman P, Norlén O, Stålberg P. Association of a Prophylactic Surgical Approach to Stage IV Small Intestinal Neuroendocrine Tumors With Survival. JAMA Oncol. 2018;4:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Frilling A, Giele H, Vrakas G, Reddy S, Macedo R, Al-Nahhas A, Wasan H, Clift AK, Gondolesi GE, Vianna RM, Friend P, Vaidya A. Modified liver-free multivisceral transplantation for a metastatic small bowel neuroendocrine tumor: a case report. Transplant Proc. 2015;47:858-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 24. | Chablaney S, Zator ZA, Kumta NA. Diagnosis and Management of Rectal Neuroendocrine Tumors. Clin Endosc. 2017;50:530-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. The effect of preceding biopsy on complete endoscopic resection in rectal carcinoid tumor. J Korean Med Sci. 2014;29:512-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O'Dorisio TM, Warner RR, Wiseman GA, Benson AB 3rd, Pommier RF; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | Schimmack S, Svejda B, Lawrence B, Kidd M, Modlin IM. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch Surg. 2011;396:273-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ, Hou BH. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020;26:2305-2322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (5)] |
| 29. | Scott AT, Howe JR. Evaluation and Management of Neuroendocrine Tumors of the Pancreas. Surg Clin North Am. 2019;99:793-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Maurizi A, Partelli S, Falconi M. Pancreatic Surgery. Front Horm Res. 2015;44:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Finkelstein P, Sharma R, Picado O, Gadde R, Stuart H, Ripat C, Livingstone AS, Sleeman D, Merchant N, Yakoub D. Pancreatic Neuroendocrine Tumors (panNETs): Analysis of Overall Survival of Nonsurgical Management Versus Surgical Resection. J Gastrointest Surg. 2017;21:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Sallinen V, Le Large TY, Galeev S, Kovalenko Z, Tieftrunk E, Araujo R, Ceyhan GO, Gaujoux S. Surveillance strategy for small asymptomatic non-functional pancreatic neuroendocrine tumors - a systematic review and meta-analysis. HPB (Oxford). 2017;19:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Jung JG, Lee KT, Woo YS, Lee JK, Lee KH, Jang KT, Rhee JC. Behavior of Small, Asymptomatic, Nonfunctioning Pancreatic Neuroendocrine Tumors (NF-PNETs). Medicine (Baltimore). 2015;94:e983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 534] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 35. | Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg. 1977;22:1-342. [PubMed] |
| 36. | Que FG, Nagorney DM, Batts KP, Linz LJ, Kvols LK. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169:36-42; discussion 42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 278] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Graff-Baker AN, Sauer DA, Pommier SJ, Pommier RF. Expanded criteria for carcinoid liver debulking: Maintaining survival and increasing the number of eligible patients. Surgery. 2014;156:1369-76; discussion 1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Elias D, Lefevre JH, Duvillard P, Goéré D, Dromain C, Dumont F, Baudin E. Hepatic metastases from neuroendocrine tumors with a "thin slice" pathological examination: they are many more than you think. Ann Surg. 2010;251:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 40. | Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, Beal EW, Felekouras E, Vernadakis S, Fung JJ, Pawlik TM. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery. 2017;162:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 41. | Shimata K, Sugawara Y, Hibi T. Liver transplantation for unresectable pancreatic neuroendocrine tumors with liver metastases in an era of transplant oncology. Gland Surg. 2018;7:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Le Treut YP, Grégoire E, Klempnauer J, Belghiti J, Jouve E, Lerut J, Castaing D, Soubrane O, Boillot O, Mantion G, Homayounfar K, Bustamante M, Azoulay D, Wolf P, Krawczyk M, Pascher A, Suc B, Chiche L, de Urbina JO, Mejzlik V, Pascual M, Lodge JP, Gruttadauria S, Paye F, Pruvot FR, Thorban S, Foss A, Adam R; For ELITA. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: a 213-case European liver transplant registry study. Ann Surg. 2013;257:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 43. | Olausson M, Friman S, Herlenius G, Cahlin C, Nilsson O, Jansson S, Wängberg B, Ahlman H. Orthotopic liver or multivisceral transplantation as treatment of metastatic neuroendocrine tumors. Liver Transpl. 2007;13:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Mazzaferro V, Sposito C, Coppa J, Miceli R, Bhoori S, Bongini M, Camerini T, Milione M, Regalia E, Spreafico C, Gangeri L, Buzzoni R, de Braud FG, De Feo T, Mariani L. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases From Neuroendocrine Tumors. Am J Transplant. 2016;16:2892-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 45. | OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. Briefing Paper: Liver Review Board Guidance Documents. 2017. [cited 14 December 2021]. Available from: https://optn.transplant.hrsa.gov/media/2175/Liver_boardreport_guidance_201706.pdf. |
| 46. | Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain V, Davidson B, Siegler M, Caplin M, Solcia E, Schilsky R; Working Group on Neuroendocrine Liver Metastases. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 368] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 47. | Sowa-Staszczak A, Pach D, Chrzan R, Trofimiuk M, Stefańska A, Tomaszuk M, Kołodziej M, Mikołajczak R, Pawlak D, Hubalewska-Dydejczyk A. Peptide receptor radionuclide therapy as a potential tool for neoadjuvant therapy in patients with inoperable neuroendocrine tumours (NETs). Eur J Nucl Med Mol Imaging. 2011;38:1669-1674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Maire F, Hammel P, Kianmanesh R, Hentic O, Couvelard A, Rebours V, Zappa M, Raymond E, Sauvanet A, Louvet C, Lévy P, Belghiti J, Ruszniewski P. Is adjuvant therapy with streptozotocin and 5-fluorouracil useful after resection of liver metastases from digestive endocrine tumors? Surgery. 2009;145:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Wright MF, Cates J, Gonzalez RS, Das S, Berlin JD, Shi C. Impact of Peritoneal Metastasis on Survival of Patients With Small Intestinal Neuroendocrine Tumor. Am J Surg Pathol. 2019;43:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Das S, Shi C, Koyama T, Huang Y, Gonzalez R, Idrees K, Bailey CE, Berlin J. Peritoneal Carcinomatosis in Well-Differentiated Small-Intestinal Neuroendocrine Tumors with Mesenteric Tumor Deposits. J Med Surg Pathol. 2019;4:1-10. [PubMed] |
| 51. | Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, Barriuso J, Pavel M, O'Toole D, Walter T; other Knowledge Network members. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 52. | Dasari A, Mehta K, Byers LA, Sorbye H, Yao JC. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer. 2018;124:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 53. | Patta A, Fakih M. First-line cisplatin plus etoposide in high-grade metastatic neuroendocrine tumors of colon and rectum (MCRC NET): review of 8 cases. Anticancer Res. 2011;31:975-978. [PubMed] |
| 54. | Alese OB, Jiang R, Shaib W, Wu C, Akce M, Behera M, El-Rayes BF. High-Grade Gastrointestinal Neuroendocrine Carcinoma Management and Outcomes: A National Cancer Database Study. Oncologist. 2019;24:911-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Clift AK, Kidd M, Bodei L, Toumpanakis C, Baum RP, Oberg K, Modlin IM, Frilling A. Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology. 2020;110:444-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 56. | NIH. ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. [cited 14 December 2021]. Available from: https://clinicaltrials.gov/. |