Published online Mar 27, 2022. doi: 10.4240/wjgs.v14.i3.260
Peer-review started: November 30, 2021
First decision: December 26, 2021
Revised: January 8, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: March 27, 2022
Processing time: 115 Days and 13.8 Hours
Hepatocellular carcinoma (HCC) with massive portal vein tumor thrombosis (PVTT) and distant metastasis is considered unresectable. However, due to recent developments in systemic chemotherapy, successful cases of conversion therapy for unresectable diseases have been reported. Herein, we report a successful multidisciplinary approach for treatment of multi-visceral recurrence with sequential multikinase inhibitor and laparoscopic surgery.
A 63-year-old woman with chronic hepatitis B virus infection was diagnosed with HCC. Subsequently, she underwent two rounds of laparoscopic partial hepatectomy, laparoscopic left adrenalectomy, and transcatheter arterial chemoembolization plus sorafenib for recurrence. Four years after initial hepatectomy, she presented with a 43-mm mass in the spleen and tumor thrombus involving the main portal vein trunk with ascites. Her liver function was Child-Pugh B (8), and protein induced by vitamin K absence or antagonist II (PIVKA II) levels were elevated up to 46.291 mAU/mL. Since initial treatment with regorafenib for three months was unsuccessful, the patient was administered lenvatinib. Ten months post-treatment, there was no contrast enhancement of PVTT or splenic metastasis. Chemotherapy was discontinued due to severe diarrhea. Afterward, splenic metastasis became viable, and PIVKA II increased. Therefore, hand-assisted laparoscopic splenectomy was performed. She experienced no clinical recurrence 14 mo after resection.
Conversion surgery after successful multikinase inhibitor treatment might be considered an effective treatment option for advanced HCC.
Core Tip: A 63-year-old woman had chronic hepatitis B virus infection and previous treatment history of hepatocellular carcinoma. She developed a 43-mm splenic mass and tumor thrombus involving the right portal branch and an umbilical portion extending down to the main trunk with severe ascites. She was initially treated with regorafenib and then lenvatinib. Ten months post-treatment, there was no contrast enhancement of portal vein tumor thrombosis or splenic metastases. However, after lenvatinib discontinuation due to severe diarrhea, splenic metastases showed partial contrast enhancement. Subsequently, hand-assisted laparoscopic splenectomy was performed with no remarkable postoperative complications. She experienced no recurrence for 14 mo.
- Citation: Endo Y, Shimazu M, Sakuragawa T, Uchi Y, Edanami M, Sunamura K, Ozawa S, Chiba N, Kawachi S. Successful treatment with laparoscopic surgery and sequential multikinase inhibitor therapy for hepatocellular carcinoma: A case report. World J Gastrointest Surg 2022; 14(3): 260-267
- URL: https://www.wjgnet.com/1948-9366/full/v14/i3/260.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i3.260
Treatment strategy recommendations for hepatocellular carcinoma (HCC) have been introduced in various guidelines. These guidelines include the Clinical Practice Guidelines for Hepatocellular Carcinoma in Japan[1], Barcelona Clinic Liver Cancer (BCLC) Guidelines[2], and American Association for the Study of the Liver Diseases Guidelines[3]. According to these guidelines, indications for liver resection are limited by tumor progression. Moreover, many cases with distant metastasis or local major vessel invasion are not eligible for resection. Recently, development of effective molecular-targeted agents, including sorafenib[4], regorafenib[5], ramucirumab[6], and lenvatinib (LEN)[7] has prolonged patient survival and occasionally enabled multidisciplinary treatments combined with chemotherapy and liver resection for HCC. Among these agents, LEN, which is an oral multikinase inhibitor targeting kinases, is known to achieve a higher rate of objective response rate (ORR)[7]. These kinases include vascular endothelial growth factor receptor 1-3, fibroblast growth factor receptor (FGFR) 1-4, platelet-derived growth factor receptor-α (PDGFR), RET, and KIT. Therefore, there have been a limited number of reports on conversion surgery after LEN treatment[8-17]. However, to the best of our knowledge, there are only a few reports on long-term remission with portal vein tumor thrombus[8,16].
Herein, we report a successful multidisciplinary approach for treatment of unresectable HCC recurrence with sequential multikinase inhibitor therapy and laparoscopic surgery.
A 63-year-old woman with chronic hepatitis B virus infection was referred to our clinic due to incidental detection of a hepatic mass. Alpha-fetoprotein and protein induced by vitamin K absence or antagonist II (PIVKA II) levels were 25.24 ng/mL and 3021 mAU/mL, respectively. The patient was diagnosed with HCC in December 2014. Thereafter, she underwent hand-assisted laparoscopic partial hepatectomy for a solitary tumor with 5 cm in diameter in the right posterior sector. Pathological findings showed that the lesion was 40 mm in size, moderately differentiated, solitary HCC without any macroscopic vascular invasion (T1bN0M0 and stage IB, based on the 8th Union for International Cancer Control staging of HCC). Liver fibrosis was evident during initial surgery (METVIR F2-3).
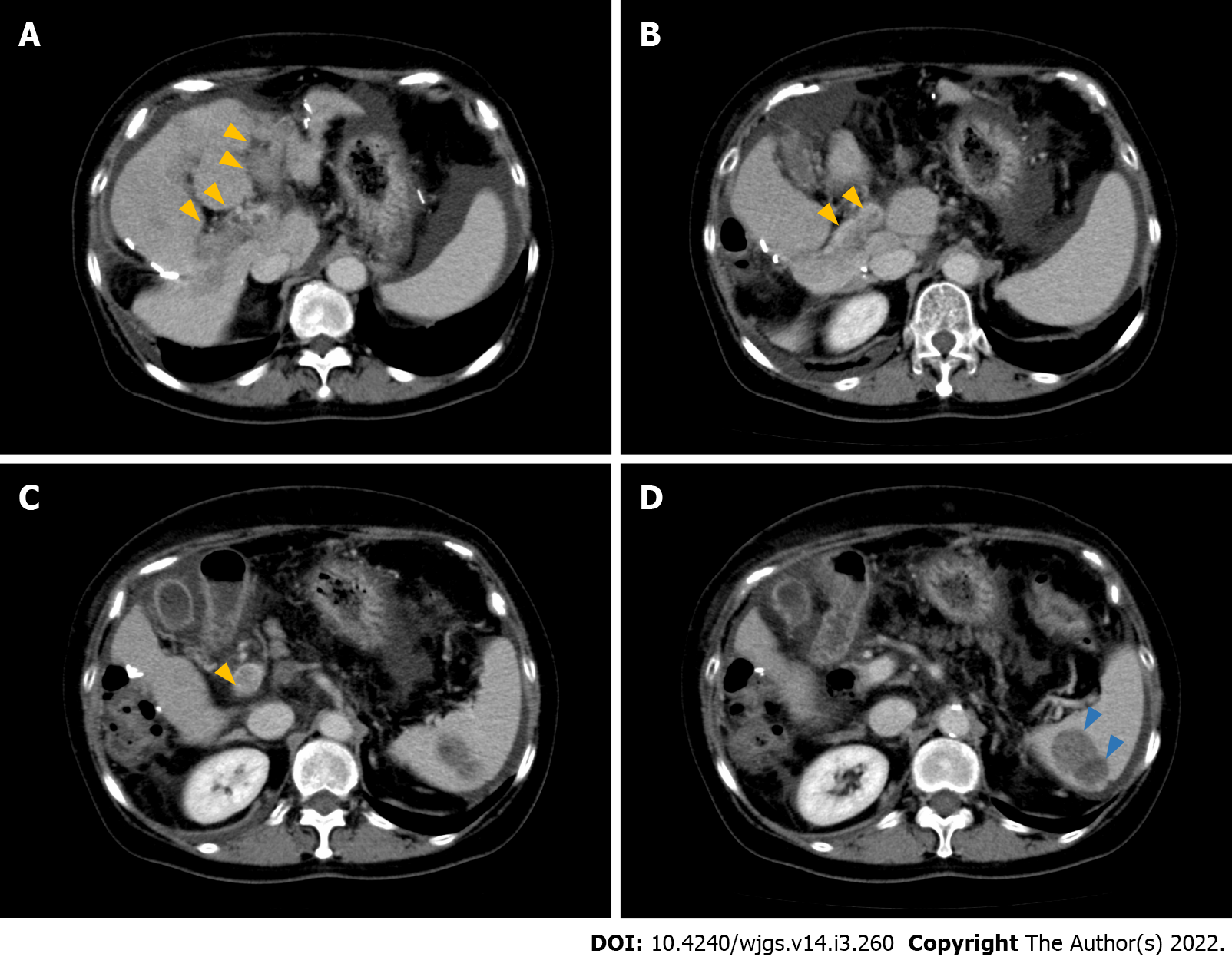
Six months after initial surgery, multiple recurrent lesions in the liver were observed. Consequently, the patient was treated with lipiodol-transcatheter arterial chemoembolization (TACE). After this successful TACE, sorafenib (400 mg per day) was administered. Six months later, she underwent laparoscopic left adrenalectomy for adrenal metastasis (pathology revealed metastatic, moderately differentiated HCC). Eight months after the adrenalectomy, the patient underwent laparoscopic partial hepatectomy for a solitary recurrence in the lateral sector (pathology revealed moderately differentiated HCC, background liver condition; METAVIR F3). Eight months after the second hepatectomy, the patient was treated with sorafenib (400 mg per day, followed by 600 mg per day) for increased PIVKA II levels. Despite 9-mo treatment with sorafenib, she was found to have a 43-mm mass in the spleen and portal vein tumor thrombosis (PVTT) that involved both the right and left portal branches down to the main trunk (Vp4) on computed tomography (CT) (Figure 1).
Hepatitis B infection.
Her personal and family history was unremarkable.
Her vital signs were normal. There were no remarkable findings other than abdominal distention.
PIVKA II levels increased tremendously up to 46.291 mAU/mL. The BCLC staging system classified the patient into stage C. Aspartate aminotransferase, alanine aminotransferase, and platelet count were 49 IU/L, 40 IU/L, and 14.7 × 104/μL, respectively. The FIB-4 index was calculated as 3.71, suggesting that she was likely to be cirrhotic. Her cirrhosis was classified into Child-Pugh B (8) and modified albumin-bilirubin grade 1.
CT findings revealed moderate ascites, which indicated portal hypertension due to tumor thrombosis. This also demonstrated irregularity of the external contour of the left lobe of the liver, suggesting cirrhosis.
On contrast enhanced CT scan, a hypodense mass with a size of 43 mm in the spleen and PVTT that involved the right anterior, posterior, and left portal branches down to main trunk (Vp4) were seen. Moreover, moderate ascites was observed. No obvious liver masses were recognized.
HCC with PVTT and splenic metastases, which led to massive ascites, possibly due to portal hypertension, was observed.
Initially, she was treated with regorafenib (400 mg/d) and tolvaptan for ascites.
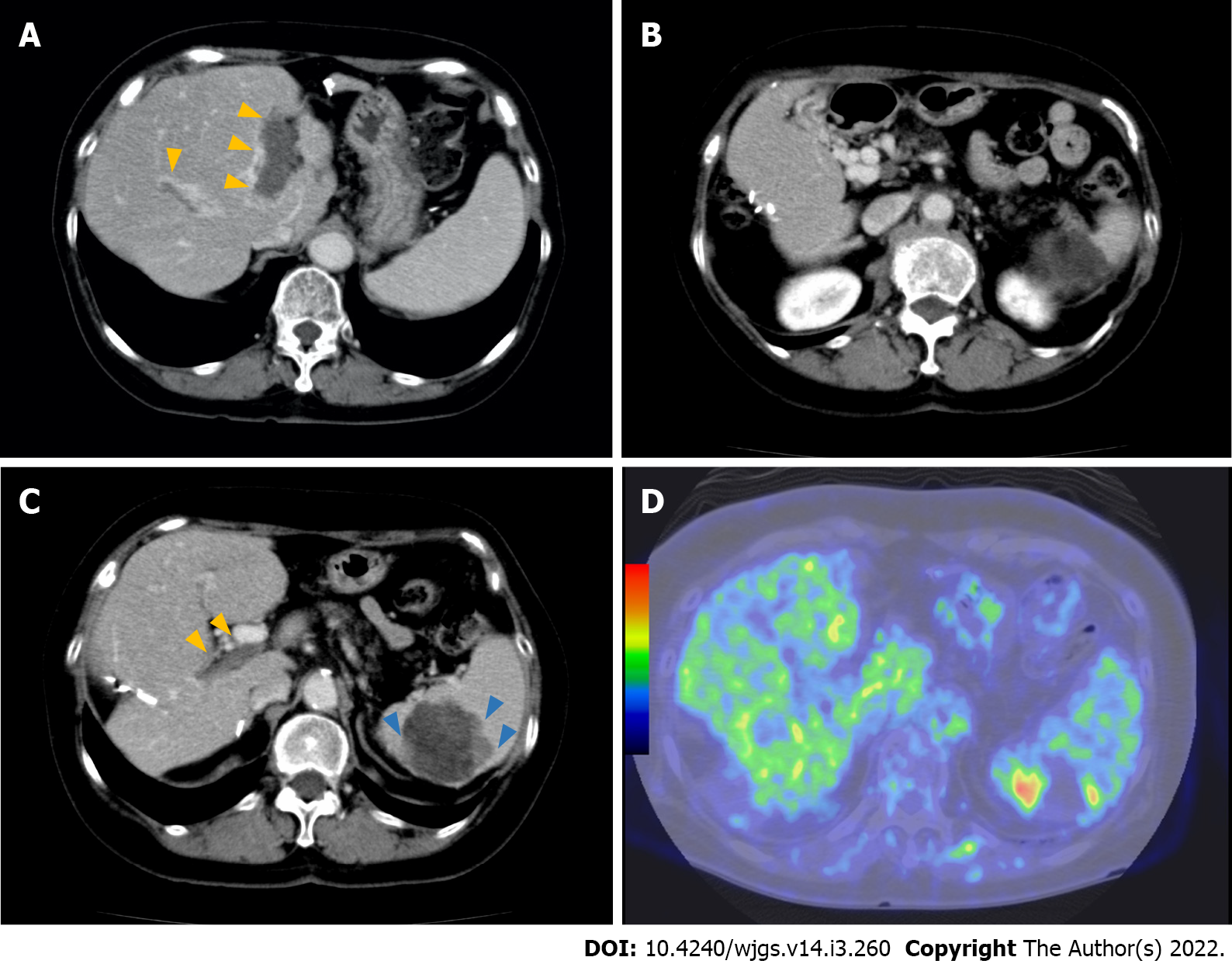
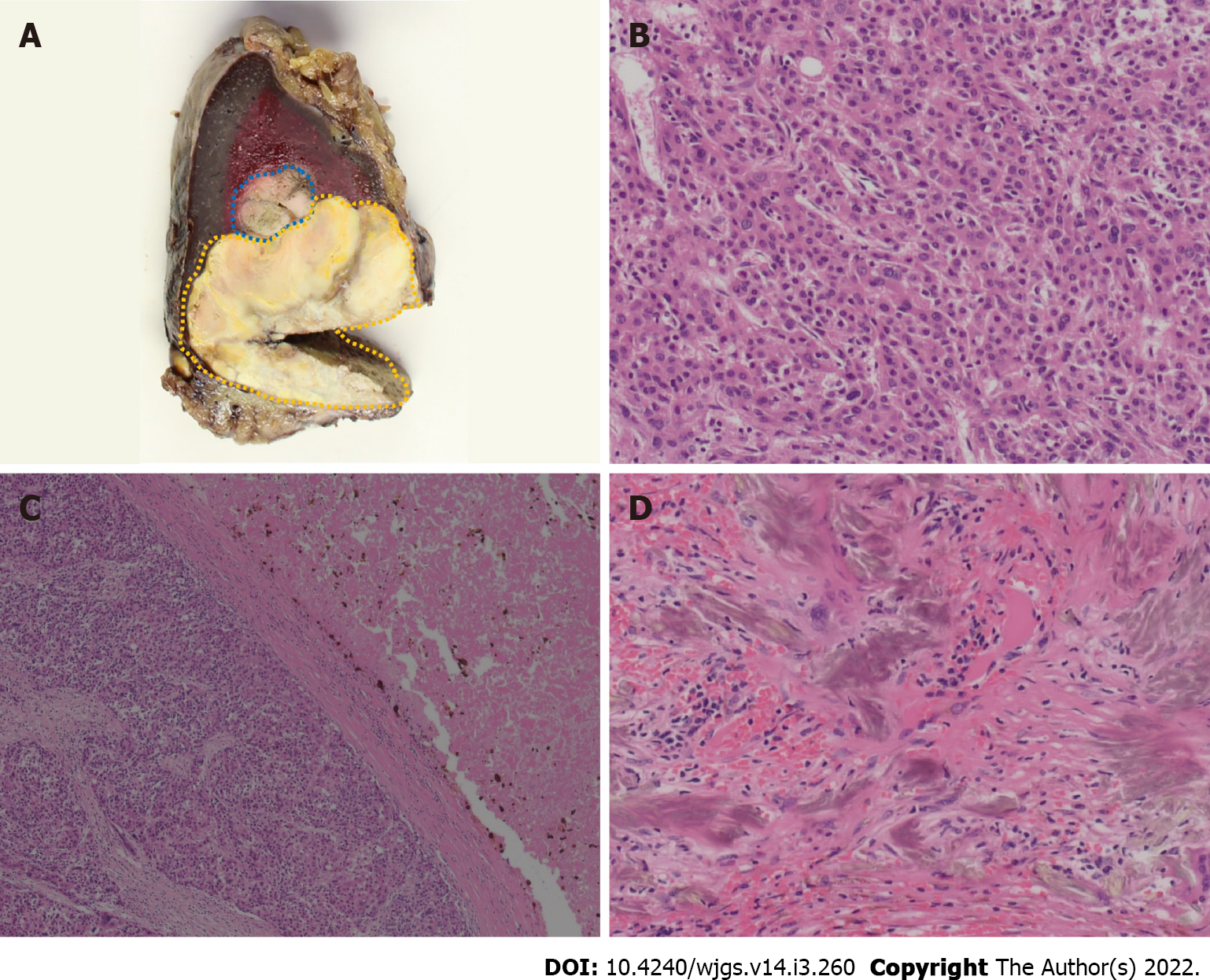
Three months after initiation of regorafenib treatment, the tumor thrombus and spleen metastasis continued to increase in size with elevated PIVKA II levels (129.815 mAU/mL). However, improvement of liver function by resolution of portal hypertension due to cavernous transformation occurred. Her ascites and liver function improved [Child-Pugh A (5)]. Therefore, LEN was orally administered at a dose of 8 mg/d. No severe side effects were observed, except for grade 2 hypertension and anorexia. Ten months after initiation of LEN therapy, the patient had a clinically complete response, according to radiological findings (Figure 2A and B). Additionally, PIVKA II level markedly decreased from 1.637 to 4 mAU/mL and was sustained within the normal range with continued therapy. After 18 mo, LEN treatment was ceased because the patient developed severe diarrhea. At that time, a follow-up CT examination revealed that the tumor burden had significantly decreased. However, after 7 mo, PIVKA II levels increased again, with contrast-enhancement of the splenic lesion on CT and positron emission tomography (PET) findings (Figure 2C and D). Splenectomy was required to control the disease. Therefore, a hand-assisted laparoscopic splenectomy was performed for solitary spleen metastasis. The patient’s postoperative course was uneventful. Macroscopic and microscopic histopathological examinations showed necrosis of HCC with slightly viable tumor cells. Surgical margins were negative (Figure 3). There was no clinical evidence of recurrence 14 mo after splenectomy and 81 mo after initial hepatectomy. Levels of PIVKA II remained within the normal range.
Based on our experience, LEN therapy could successfully lead to a hypovascular status of PVTT 10 mo after its initiation. In addition, conversion surgery was performed effectively for progression of solitary splenic metastasis after LEN discontinuation. To the best of our knowledge, there have been few reports regarding successful conversion surgery after multikinase inhibitor treatment for HCC with massive tumor thrombus[8,16].
We experienced good control of PVTT with LEN administration. In our case, PVTT became hypovascular 10 mo after LEN administration, along with a necrosis of the splenic lesion. After LEN discontinuation, PVTT continued to be hypovascular, whereas the splenic lesion progressed. There have been two case reports showing disappearance of PVTT[8,16]. Takeda et al[8] reported a female patient with advanced HCC and PVTT who was treated with LEN monotherapy and experienced a long-term antitumor effect. Rapidly, LEN caused hypovascularity in the main hypervascular target lesion, and PVTT became undetectable 11 mo after LEN initiation. Takahashi et al[16] also reported a 59-year-old male patient with a recurrent liver mass diffusely located at the lateral segment with a massive Vp4 PVTT extending from the umbilical portion to the main and contralateral third-order portal branches. Three months after starting LEN, PVTT critically regressed and retreated to the contralateral first-order portal branch. After LEN cessation for 7 d, radical left lobectomy and PVTT thrombectomy were performed. The majority of PVTT cases showed necrosis. They argued that LEN may have a relatively strong antitumor effect not only on main tumor, but also on PVTT, which is attributed to an antiangiogenic effect. According to two previous reports, LEN exerts both immediate antiangiogenic and long-term antitumor effects on PVTT. According to previous basic studies[18-20], FGFR plays an important role in this antitumor effect via inhibition of FGF19-FGFR autocrine loop and antiangiogenic effects through inhibition of FGFR/PDGFR. This explains why PVTT and hepatic lesions became hypovascular in 10 mo and continued to be in a hypovascular status approximately 2 years after LEN cessation in our patient.
Importantly, the safety of LEN administration for main PVTT (Vp4) has not been established. Kuzuya et al[21] compared the outcomes of advanced HCC with Vp3/4 between sorafenib and LEN as the first-line systemic therapy. The ORR was significantly higher in the LEN group than in the sorafenib group (53.8% vs 14.3%, P = 0.0193), and the median overall survival (OS) and time to progression were significantly longer in the LEN group than in the sorafenib group. None of these patients discontinued LEN treatment due to treatment-related adverse events in their series. Chuma et al[22] recently have reported the safety and efficacy of LEN treatment in highly advanced HCC. In this report, 20 patients with Vp4 HCC were included, and 12 patients (60%) experienced grade ≥ 3 adverse effects. The ORRs were 26.7% in patients with Child-Pugh A and 0% in those with Child-Pugh B. These findings suggest that LEN administration with close monitoring of patients’ live conditions would be acceptable.
It was notable that regorafenib, which has also anti-angiogenic properties did not have any impact on cavernous transformation of the portal vein and portal vein thrombosis. Although they have not been fully elucidated, the various reactions of regorafenib and LEN may originate from the different mechanisms of action between the two agents. The genes downregulated by regorafenib might be different from those manipulated by LEN. That would lead to their different effects. There have been few cases regarding regorafenib and conversion therapy for HCC with PVTT, despite REFLECT trial included patients with macrovascular invasion[5].
Since metastatic splenic lesions became viable after LEN cessation, splenectomy was necessary to control the disease. There have been a few cases of spleen metastases resection[23-26]. The spleen is an important organ in the immune system, and metastases to this organ usually involve multiple lesions, and solitary splenic metastasis seems rare. According to previous reports[23-25], splenectomy for spleen metastases led to favorable outcomes, despite some patients having dismal outcomes (OS, 2-84 mo). Kim et al[26] have reported lesions detected by fluorodeoxyglucose-PET, which was similar to those in our patient. It has been assumed that splenic metastasis could be transformed into poor differentiation through multiple treatments.
We report the rare case of a patient with advanced HCC in whom LEN monotherapy showed long-term antitumor activity. Clinicians should be aware of radiological changes suggestive of intratumoral vascularity during treatment with the novel antiangiogenic agent LEN in patients with advanced HCC. Further studies are needed to elucidate the background of patients’ favorable outcomes.
We would like to thank Dr. Hiromi Serizawa from the Department of Pathology, Hachioji Medical Center Tokyo Medical University for their insightful comment on pathological findings; Dr. Seishi Nakatsuka from the Department of Radiology, Keio University for his precise comment on radiological findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo XZ, China; Mukthinuthalapati VVPK, United States; Nenu I, Romania S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2874] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 3. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3023] [Article Influence: 431.9] [Reference Citation Analysis (3)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10264] [Article Influence: 603.8] [Reference Citation Analysis (2)] |
| 5. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2710] [Article Influence: 338.8] [Reference Citation Analysis (0)] |
| 6. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1246] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 7. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3819] [Article Influence: 545.6] [Reference Citation Analysis (1)] |
| 8. | Takeda H, Nishijima N, Nasu A, Komekado H, Kita R, Kimura T, Kudo M, Osaki Y. Long-term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol Res. 2019;49:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Sato N, Beppu T, Kinoshita K, Yuki H, Suyama K, Chiyonaga S, Motohara T, Komohara Y, Hara A, Akahoshi S. Conversion Hepatectomy for Huge Hepatocellular Carcinoma With Arterioportal Shunt After Chemoembolization and Lenvatinib Therapy. Anticancer Res. 2019;39:5695-5701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Liu Z, Fu Z, Li G, Lin D. Downstaging of Recurrent Advanced Hepatocellular Carcinoma After Lenvatinib Treatment: Opportunities or Pitfalls? Onco Targets Ther. 2020;13:10267-10273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Matsuki R, Kawai K, Suzuki Y, Kogure M, Nakazato T, Naruge D, Okano N, Seki S, Ohmori Y, Kawamura N, Kamma H, Hisamatsu T, Shibahara J, Mori T, Furuse J, Sakamoto Y. Pathological Complete Response in Conversion Hepatectomy Induced by Lenvatinib for Advanced Hepatocellular Carcinoma. Liver Cancer. 2020;9:358-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Ohya Y, Hayashida S, Tsuji A, Kuramoto K, Shibata H, Setoyama H, Hayashi H, Kuriwaki K, Sasaki M, Iizaka M, Nakahara O, Inomata Y. Conversion hepatectomy for advanced hepatocellular carcinoma after right portal vein transection and lenvatinib therapy. Surg Case Rep. 2020;6:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Tomonari T, Sato Y, Tanaka H, Tanaka T, Taniguchi T, Sogabe M, Okamoto K, Miyamoto H, Muguruma N, Saito Y, Imura S, Bando Y, Shimada M, Takayama T. Conversion therapy for unresectable hepatocellular carcinoma after lenvatinib: Three case reports. Medicine (Baltimore). 2020;99:e22782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Yokoo H, Takahashi H, Hagiwara M, Iwata H, Imai K, Saito Y, Matsuno N, Furukawa H. Successful hepatic resection for recurrent hepatocellular carcinoma after lenvatinib treatment: A case report. World J Hepatol. 2020;12:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, Zhuang W, Chen X, Chen H, Xu B, Lai J, Guo W. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 16. | Takahashi K, Kim J, Takahashi A, Hashimoto S, Doi M, Furuya K, Hashimoto R, Owada Y, Ogawa K, Ohara Y, Akashi Y, Hisakura K, Enomoto T, Shimomura O, Noguchi M, Oda T. Conversion hepatectomy for hepatocellular carcinoma with main portal vein tumour thrombus after lenvatinib treatment: A case report. World J Hepatol. 2021;13:384-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 17. | Yang X, Xu H, Zuo B, Yang X, Bian J, Long J, Wang D, Zhang J, Ning C, Wang Y, Xun Z, Lu X, Mao Y, Sang X, Zhao H. Downstaging and resection of hepatocellular carcinoma in patients with extrahepatic metastases after stereotactic therapy. Hepatobiliary Surg Nutr. 2021;10:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Okamoto K, Ikemori-Kawada M, Jestel A, von König K, Funahashi Y, Matsushima T, Tsuruoka A, Inoue A, Matsui J. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett. 2015;6:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 19. | Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 20. | Kanzaki H, Chiba T, Ao J, Koroki K, Kanayama K, Maruta S, Maeda T, Kusakabe Y, Kobayashi K, Kanogawa N, Kiyono S, Nakamura M, Kondo T, Saito T, Nakagawa R, Ogasawara S, Suzuki E, Ooka Y, Muroyama R, Nakamoto S, Yasui S, Tawada A, Arai M, Kanda T, Maruyama H, Mimura N, Kato J, Zen Y, Ohtsuka M, Iwama A, Kato N. The impact of FGF19/FGFR4 signaling inhibition in antitumor activity of multi-kinase inhibitors in hepatocellular carcinoma. Sci Rep. 2021;11:5303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Sorafenib vs. Lenvatinib as First-line Therapy for Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis. Anticancer Res. 2020;40:2283-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Chuma M, Uojima H, Hiraoka A, Kobayashi S, Toyoda H, Tada T, Hidaka H, Iwabuchi S, Numata K, Itobayashi E, Itokawa N, Kariyama K, Ohama H, Hattori N, Hirose S, Shibata H, Tani J, Imai M, Tajiri K, Moriya S, Wada N, Iwasaki S, Fukushima T, Ueno M, Yasuda S, Atsukawa M, Nouso K, Fukunishi S, Watanabe T, Ishikawa T, Nakamura S, Morimoto M, Kagawa T, Sakamoto M, Kumada T, Maeda S. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: A multicenter analysis. Hepatol Res. 2021;51:201-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Hanada K, Saito A, Nozawa H, Haruyama K, Hayashi N, Yamada M, Katagiri S, Katsuragawa H, Otsubo T, Takasaki K. Histopathologically-diagnosed splenic metastasis in a hepatocellular carcinoma case with adrenal metastasis. Intern Med. 2004;43:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Iwaki K, Ohta M, Ishio T, Kai S, Iwashita Y, Shibata K, Himeno K, Seike M, Fujioka T, Kitano S. Metastasis of hepatocellular carcinoma to spleen and small intestine. J Hepatobiliary Pancreat Surg. 2008;15:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Sanefuji K, Fukuzawa K, Okamoto M, Kai S, Takaki H, Hirotoshi Y, Motohiro A, Wakasugi K. Long-term surviving patient with inferior vena cava tumor thrombus and extrahepatic metastasis after spontaneous ruptured hepatocellular carcinoma. Clin J Gastroenterol. 2011;4:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Kim DH, Jeong SY, Lee SW, Lee J, Ahn BC. Solitary splenic metastasis from hepatocellular carcinoma detected on (18)F-FDG PET/CT. Clin Nucl Med. 2015;40:e325-e327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |