Published online Mar 27, 2022. doi: 10.4240/wjgs.v14.i3.211
Peer-review started: April 19, 2021
First decision: June 13, 2021
Revised: July 25, 2021
Accepted: February 9, 2022
Article in press: February 9, 2022
Published online: March 27, 2022
Processing time: 340 Days and 6.4 Hours
Few series have reported the utility of fast-track protocols (FTP) in minimally invasive liver surgery.
To report the applicability of FTP in minimally invasive liver surgery and to correlate with difficulty scores.
The series of patients undergoing minimally invasive liver surgery from 2014 was analyzed. Iwate, Southampton and Gayet’s scores were compared as predictors of FTP adherence. Accomplishment of FTP was considered within 24-h, 48-h and 72-h. Multivariate models were performed to define discharge < 24 h, < 72 h, complications and readmissions.
From 160 cases, 78 were candidates for FTP, of which 22 (28.2%), 19 (24.4%) and 14 (17.9%) were discharged in < 24-h, 48-h and 72-h, respectively (total = 71.5%). Iwate, Southampton and Gayet’s scores achieved area under the receiver operating characteristic values for < 24-h stay of 0.780, 0.687 and 0.698, respectively. Sensitivity and specificity values for the best score (Iwate) were 87.7% and 66.7%, respectively (cutoff = 5.5). In multivariate models, < 72 h stay and complications revealed body mass index as a risk factor independent from difficulty scores.
The development of aggressive FTP is feasible and < 24-h stay can be achieved even in moderate and advanced complexity cases. Difficulty scores, including body mass index value, may be useful to predict which cases may adhere to these protocols.
Core Tip: The current manuscript shows how fast-track protocols on laparoscopic liver surgery can be accomplished according to difficulty scoring systems.
- Citation: Ciria R, Padial A, Ayllón MD, García-Gaitan C, Briceño J. Fast-track protocols in laparoscopic liver surgery: Applicability and correlation with difficulty scoring systems. World J Gastrointest Surg 2022; 14(3): 211-220
- URL: https://www.wjgnet.com/1948-9366/full/v14/i3/211.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i3.211
Minimally invasive liver surgery (MILS) has become wide spread in recent years. Nowadays, the feasibility and advantages of MILS have been widely demonstrated[1,2]. Enhanced recovery after surgery (ERAS) protocols are multimodal pathways developed to overcome the deleterious effect of perioperative stress after major surgery. The last guidelines published in 2016 from the ERAS Society performed a systematic review over more than 30 articles in which the classical 23 ERAS items validated for colorectal surgery were analyzed for liver surgery[3,4]. The conclusion was that the application of ERAS protocols in liver surgery could be beneficial although the available evidence was poor and prospective studies were encouraged.
Several difficulty scores have been reported to date in laparoscopic liver surgery (LLS). The primary endpoints of all of them are intraoperative complications, conversions and degree of difficulty. The most widely used is the Iwate score[5]. There are others like the Southampton score[6] and the Gayet’s score[7]. However, none of them have been tested to predict early recovery and/or completion of a fast-track protocol (FTP).
Our liver unit adopted MILS in 2014, and since the very beginning has implemented a very aggressive FTP leading to an innovative 1-d stay protocol. The main aim of our manuscript is to report a prospective validation of our FTP in MILS. As secondary aims, we analyzed our experience with 1-d stay surgery in LLS and the results of FTP grouped by the 3 scores of difficulty in LLS currently reported in literature in order to compare the capability of each score to predict FTP accomplishment.
All patients who underwent MILS since the adoption of the laparoscopic approach (October 2014) at the University Hospital Reina Sofía in Córdoba-Spain were included in the study. All patients signed informed consent for the approval of their personal data for research. All cases were included in a prospectively maintained database. Approval number of the institutional review board of the University Hospital Reina Sofia was 4380 (Code 0000-0002).
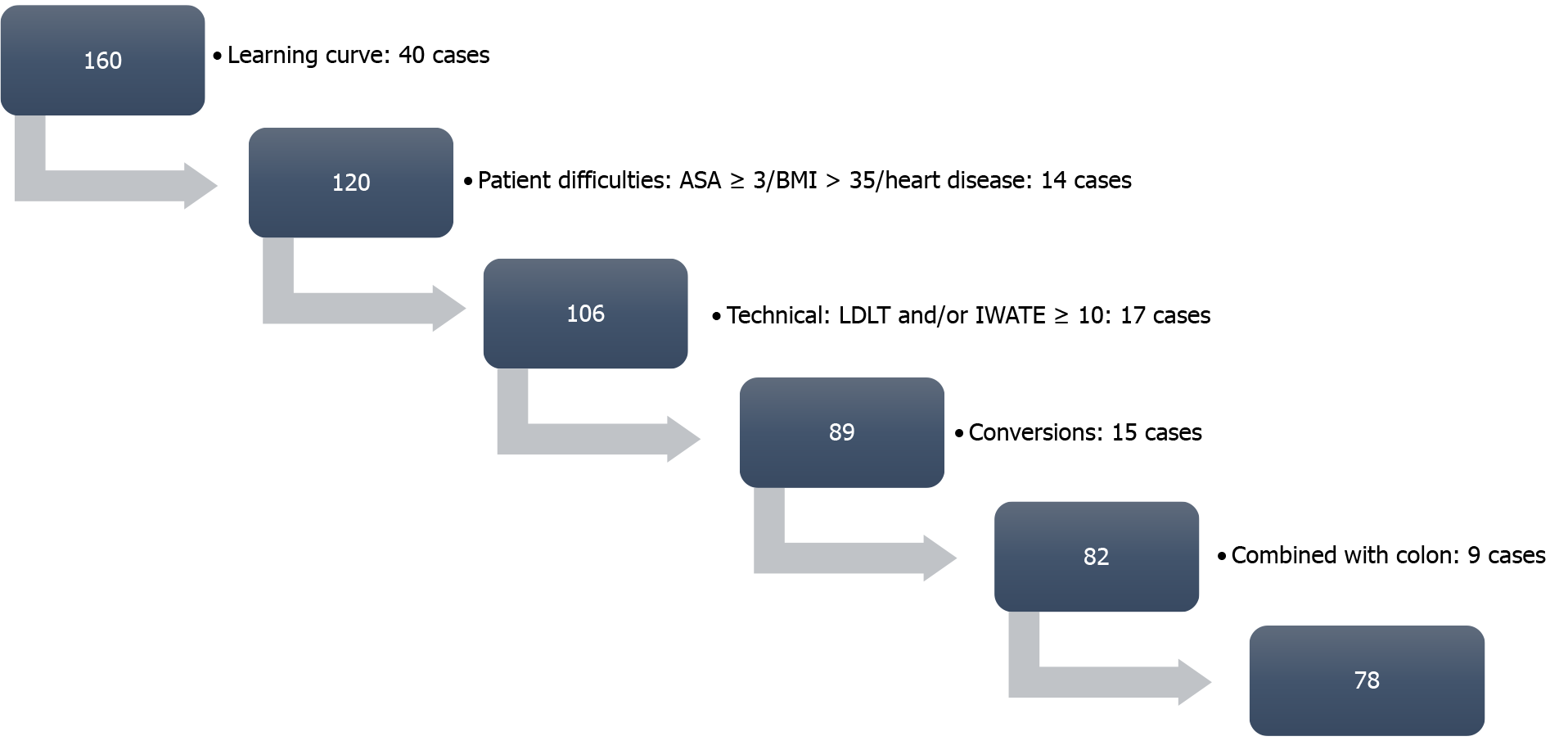
The inclusion criteria were unrestricted. We started our FTP at case 40 (to avoid learning curve). Regarding comorbidities, no significant heart disease, body mass index (BMI) < 35 and American Society of Anesthesiologists score < 4 were required. Regarding complexity, Iwate and Southampton scores ≥ 10, living donation and synchronic colorectal and liver resections were excluded. Conversions were also excluded from the analysis, being considered an “a posteriori” variable.
According to their original publications, we considered Iwate, Southampton and Gayet’s scores for the calculations and the testing. Iwate score was calculated according to its last update in which an “Expert” category was added and up to 12 points could be reached. Southampton and Gayet’s scores were calculated according to their original reports as no further modifications have been reported.
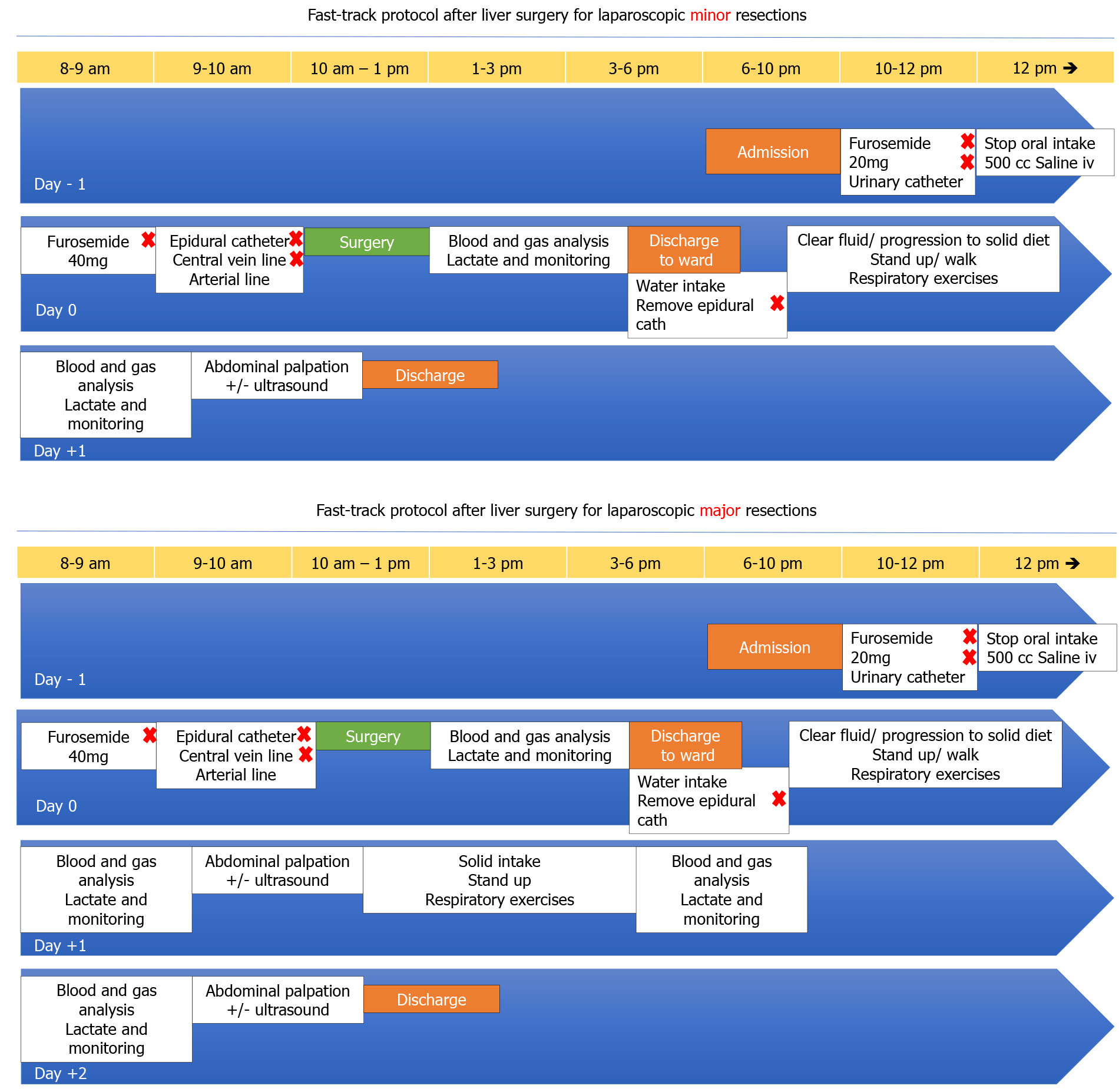
Since the decision of developing an FTP for LLS, two different protocols were considered for both minor and major resections, including a 24-h and a 48-h discharge protocol, respectively. The protocols are depicted in Figure 1A and B. Considering that the main aim is maintaining a low central venous pressure during the surgical procedure, most of the interventions in our protocol are focused on an aggressive preoperative emptying of the intravascular compartment, a fluid restriction during the operation and a rapid postoperative recovery with early intake.
Our laparoscopic liver resection is based on general principles of open and MILS. Our standard position of the patient is supine with tilt left 30º-45º in case of right posterior resections. Our main transection device is ultrasonic surgical aspiration irrigation device with bipolar sealing forceps for vascular structures. Main vessels are transected using endo-staplers. Following the surgery, the patients are admitted to a postoperative recovery area in which patients are monitored continuously by anesthesiologists. Immediately after arrival, blood tests are obtained, and unless abnormal the patient is discharged to our surgical ward in a 2-4 h period.
A prospectively maintained database was screened to obtain complexity scores and to identify potential variables not included in the previously reported scores that would increase their prediction capabilities. Comparisons were performed after normality tests (Shapiro-Wilk) using parametric or non-parametric tests, accordingly. Multivariate models were performed by logistic binary regression tests including variables within 0.1 significance in the major models. The final models included variables below 0.05 significance. Receiver operating curves were performed defining the best cutoff point of the complexity scores. The main endpoints of our study were: (1) Global results and discharge 24h/48h/72 h or FTP not accomplished; (2) Prediction capability of early discharge from difficulty scores; (3) Receiver operating curves for “early discharge” accomplishment of scores; and (4) Multivariate models: discharge-24 h/discharge-72 h/general complications/readmissions.
From a total of 160 LLS, the final dataset for the analysis was 78 cases. Exclusions were defined as depicted in Figure 2. Mean comprehensive complication index was 5.18 ± 11.52 for the group of patients within the FTP. A total of 23% had any kind of complication, from which only 5 cases (6.4%) were major complications (Dindo-Clavien III-IV). Comparisons with the group of patients that were not candidates to enter into a FTP showed that the selection procedure was adequate (Table 1). From the 78 cases of candidates for FTP, 22 (28.2%), 19 (24.4%) and 14 (17.9%) were discharged in less than 24-h, 48-h and 72-h, respectively (total = 71.5%). The rest (29.5%) did not accomplish any kind of FTP because of the following reasons: complication (26.1%), long distance from home > 200 km (17.3%), delay in the discharge from the recovery area > 12 h (34.8%) and weekender/no acceptance from the patients (21.7%). Readmission rate in the whole series was 7.5%. It was lower but did not reach statistical significance in the FTP group compared to the non-FTP group (7.7% vs 11.9%; P = not significant). In the FTP group, readmissions were related to the surgical procedure but could not be considered a direct consequence of the application of an FTP. One of the cases was a late evisceration that happened 8 d after the discharge.
| Whole series (160 cases) | Excluded learning curve (first 40 cases) | Non-candidate for FTP (42 cases) | Candidate for FTP (78 cases) | P (FTP vs no FTP) | |
| Baseline data | |||||
| Age | 59 ± 13 | 59 ± 14 | 58 ±15 | 59 ± 14 | NS |
| Sex (M/F ratio) | 83/77 | 66/54 | 29/13 | 37/41 | 0.023 |
| BMI | 27.56 ± 4.88 | 27.51 ± 5.03 | 27.69 ± 6.21 | 27.43 ± 4.44 | NS |
| Malignancy, n (%) | 122 (76.25) | 93 (77.50) | 32 (76.19) | 61 (78.20) | NS |
| Postoperative stay | 4.41 ± 4.68 | 4.50 ± 5.12 | 7.40 ± 7.17 | 2.94 ± 2.46 | 0.001 |
| Operative time | 253.81 ± 91.91 | 258.41 ± 89.81 | 294.19 ± 84.82 | 239.14 ± 86.95 | 0.001 |
| Tradit minor/major | 82/77 | 58/61 | 16/25 | 42/36 | NS |
| Iwate | 0.02 | ||||
| Low | 26 | 19 | 5 | 14 | |
| Intermediate | 60 | 43 | 9 | 34 | |
| Advanced | 54 | 43 | 13 | 30 | |
| Expert | 19 | 14 | 14 | 0 | |
| Iwate | 0.009 | ||||
| I | 68 | 48 | 13 | 35 | |
| II | 24 | 18 | 3 | 15 | |
| III | 67 | 53 | 25 | 28 | |
| Iwate | NS | ||||
| Low | 21 | 13 | 3 | 10 | |
| Moderate | 81 | 58 | 18 | 40 | |
| High | 52 | 43 | 15 | 28 | |
| Extremely high | 4 | 4 | 4 | 0 | |
| Complications | |||||
| CCI | 8.10 ± 17.53 | 4.91 ± 12.51 | 16.57 ± 26.34 | 5.18 ± 11.52 | 0.01 |
| No complications, n (%) | 117 (73.1) | 33 (82.5) | 24 (57.1) | 60 (76.9) | 0.024 |
| Redo surgery, n (%) | 8 (5.0) | 1 (2.5) | 6 (14.3) | 1 (1.3) | 0.04 |
| Readmission, n (%) | 12 (7.5) | 1 (2.5) | 5 (11.9) | 6 (7.7) | NS |
| Minor complications (I-II), n (%) | 30 (18.8) | 4 (10.0) | 13 (31.0) | 13 (16.7) | 0.024 |
| Major complications (IIIa, IIIb, IV) | 13 (8.1) | 3 (7.5) | 5 (11.9) | 5 (6.4) | NS |
| 6 IIIa | 1 IIIa | 4 IIIa | |||
| 2 IIIb | 1 IIIb | 1 IIIb | |||
| 2 Iva | 2 Iva | ||||
| 1 IVb | 1 IVb | ||||
| 2 V | 2 V |
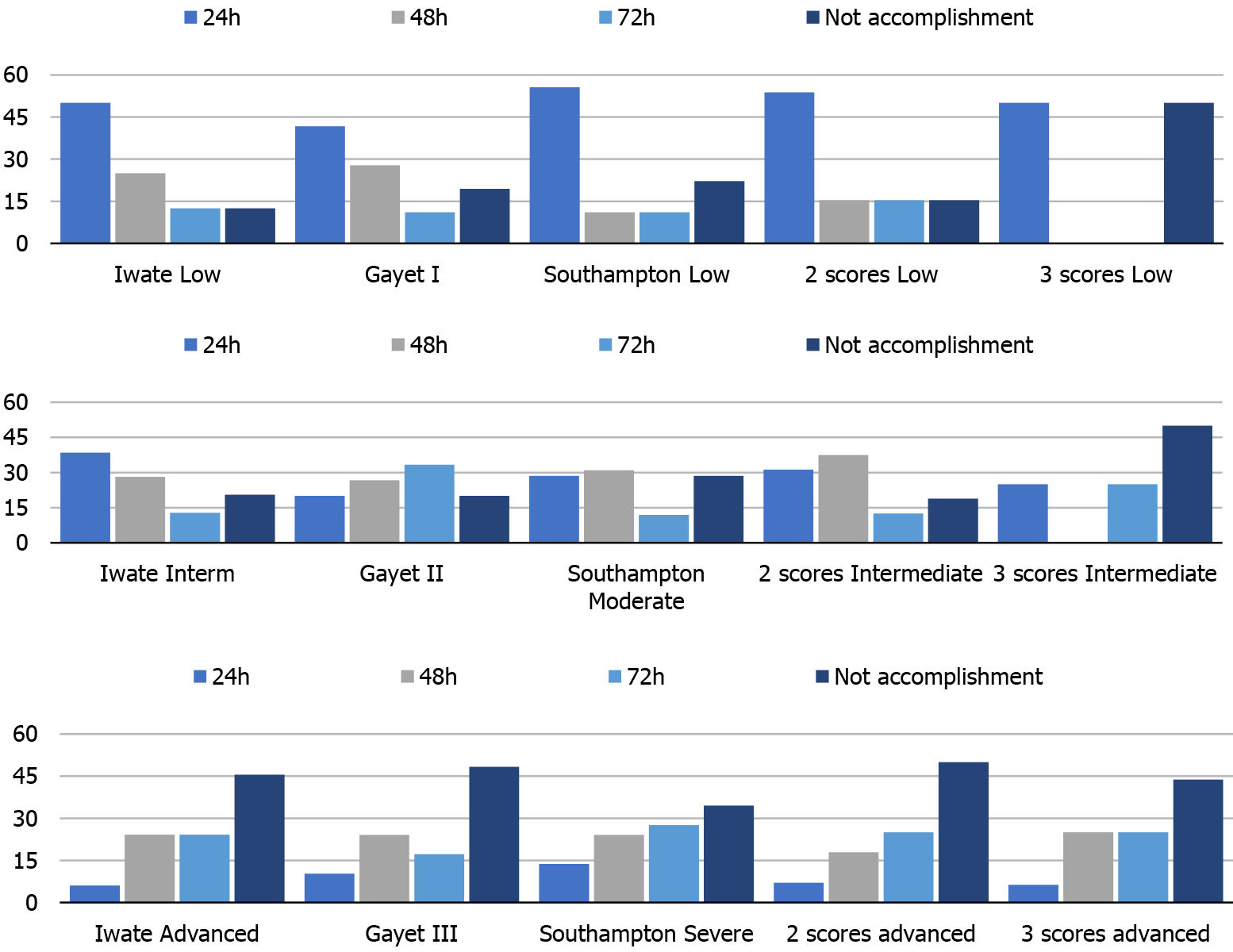
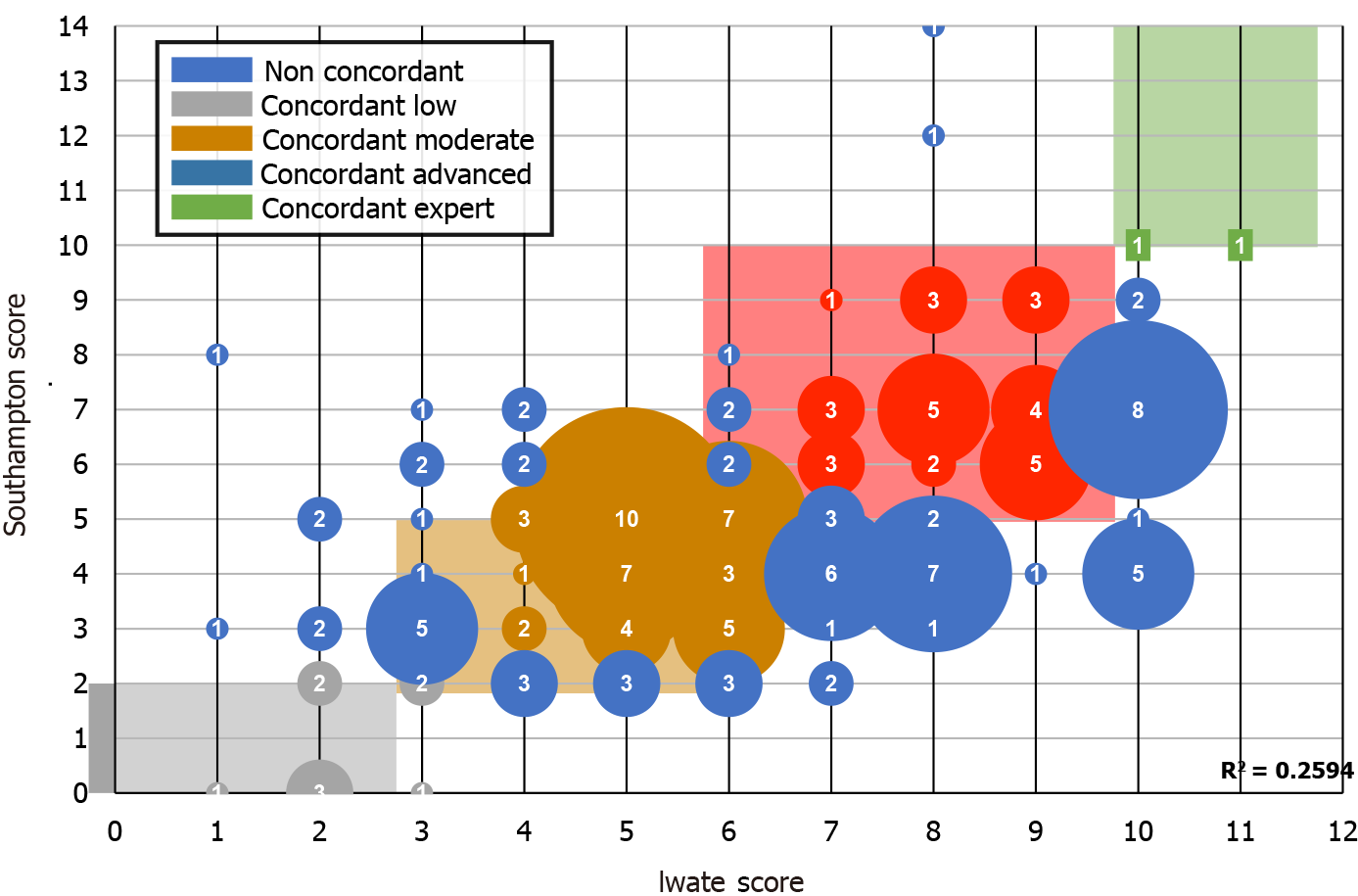
As observed in Figure 3, the accomplishment of an FTP is directly related to the difficulty of the LLS. It should be noted that a low punctuation in the scores predicted a low postoperative stay and that a high difficulty score predicted a non-accomplished FTP. We also analyzed the combination of 2 or 3 scores with equal punctuation in order to find out whether they would benefit and complement each other by adding homogeneity. However, the combination of the scores was lower in the prediction of accomplishment of an FTP. After these findings, a correlation test was performed in order to find out if Iwate and Southampton scores correlated linearly. An R2 = 0.2594 score was obtained. As observed in Figure 4, several cases were not concordant in their punctuation. Several high Iwate score cases were downgraded by the Southampton scoring system.
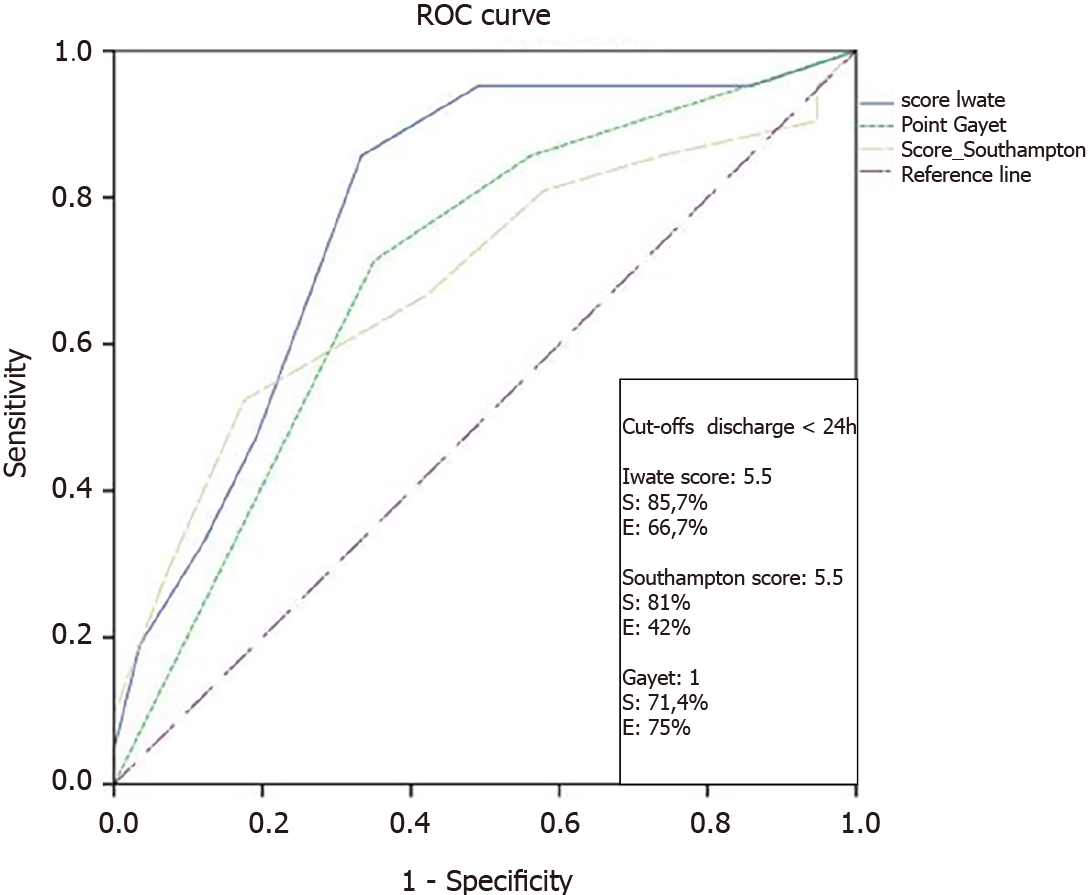
By performing receiver operating curves, it could be demonstrated that the difficulty scores could predict early discharge < 24 h. In this sense, it should be noted that the best cutoff points were equivalent for both Iwate and Southampton scores (score = 5.5). The best sensitivity was observed for the Iwate score (S = 85.7%), with a specificity of 66.7% (Figure 5).
Multivariate models were obtained to find out if complexity scores were independent predictors of early discharge, complications and/or readmissions. A model was performed for each of the complexity scores in order to avoid interactions. Age, BMI, sex, American Society of Anesthesiologists score, previous surgery, malignancy, bilobar spread and liver disease were added as variables. All patients in the series were included (160 cases). As observed in Table 2, in each model the complexity scores were independent risk scores. Interestingly, BMI was a persistent risk factor added to these scores in both the complications and discharge < 72 h models.
| Model | ||||||
| Iwate | ||||||
| Discharge 24 h | Sig | OR | Discharge 72 h | Sig | OR | |
| Iwate | 0.001 | 1.626 (1.2-2.18) | Iwate | 0.01 | 1.46 (1.09-1.95) | |
| BMI 25-30 | 0.033 | 6.39 (1.16-35.30) | ||||
| BMI 30-35 | 0.013 | 8.51 (1.57-46.14) | ||||
| Complications | Sig | OR | Readmissions | Sig | OR | |
| Iwate | 0.02 | 1.2 (1.03-1.41) | Iwate | 0.007 | 1.58 (1.13-2.22) | |
| BMI > 35 | 0.008 | 8.75 (1.76-43.44) | ||||
| Southampton | ||||||
| Southampton | 0.015 | 1.43 (1.07-1.92) | ||||
| BMI 25-30 | 0.032 | 6.08 (1.16-31.87) | ||||
| BMI 30-35 | 0.006 | 9.85 (1.91-50.70) | ||||
| Complications | Sig | OR | Readmissions | Sig | OR | |
| Southampton | 0.036 | 1.3 (1.01-1.68) | ||||
| BMI > 35 | 0.013 | 7.09 (1.52-33.04) | ||||
| Gayet | ||||||
| Discharge 24 h | Sig | OR | Discharge 72 h | Sig | OR | |
| Gayet | 0.004 | 1.85 (1.21-2.81) | Gayet | 0.043 | 1.53 (1.01-2.31) | |
| BMI 25-30 | 0.042 | 5.72 (1.06-30.86) | ||||
| BMI 30-35 | 0.014 | 8.08 (1.52-42.99) | ||||
| Complications | Sig | OR | Readmissions | Sig | OR | |
| Gayet | 0.008 | 1.48 (1.11-1.98) | Gayet | 0.027 | 2.08 (1.08-4.01) | |
| BMI > 35 | 0.009 | 8.56 (1.71-42.85) | ||||
Patients undergoing a standard laparoscopic liver resection may be considered as optimal candidates to be included into early recovery protocols, as the surgical procedure needs no anastomosis nor vascular reconstruction. The adoption of LLS by liver teams seems to be clearly exponential, and thus the recovery and postoperative comfort are improving. According to our results, adequate selection may lead to high rates of effectiveness in terms of early discharge, low readmission rates and reduced incidence of complications. Complexity scores may be helpful in the selection process.
Several complexity scores have been reported to date[5-14]. From a technical point of view, most of them have assessed the effect of variables such as tumor location or extent of liver resection. However, liver and patient status have not been considered as important in the scores, and only impaired liver function and previous liver surgery or preoperative chemotherapy have been marginally evaluated. Only Hasegawa et al[13] considered BMI score as valuable in a difficulty score. According to our results, BMI score may be considered as important because it may add relevant information about the prognosis of the patients and the potential adherence to an FTP. In our opinion, liver and patient status have not been adequately considered and should be re-evaluated into difficulty scores. Liver function parameters are only considered by traditional markers (such as Child, platelets or bilirubin). Western and eastern populations are different from a demographical and epidemiological point of view. The main disease, underlying liver impairment and a potential fatty liver or neoadjuvant chemotherapy may surely complement current scores.
A recent meta-analysis performed on 580 laparoscopic liver patients (292 early recovery vs 288 traditional) performed on 8 studies highlighted the potential benefit of these protocols in this type of surgery[15]. However, the risk of bias was too high as the authors did not report detailed randomization methods, allocation concealment or blind methods. Moreover, the included studies did not adopt a standard and unified clinical treatment of ERAS programs, and complexity of the resection was not included or controlled as a bias factor. A more recent meta-analysis on ERAS clinical pathways included 4 randomized trials showing several advantages like length of stay and lower complication rates[16]. However, according to the recent recommendations from the ERAS group, liver teams were encouraged to report other components or modifications that could improve results or help spread this clinical pathway.
Our protocol is probably the most aggressive perioperative protocol reported to date in LLS. Our main aim was to reach < 24 h stay in minor hepatectomies and < 48 h in major hepatectomies without any detrimental effect on postoperative outcome. As stated before, about 30% of the cases, if adequately selected, can be discharged in less than 24 h and up to 50% in less than 2 d. It should be noted that 74% of the cases that did not adhere to our FTP were due to non-medical issues or complications. The area to which our hospital gives assistance includes regions more than 200 km away from our city. We detected that people from there were reluctant to early discharge after a major abdominal operation as they felt “unsafe.”
The perioperative protocol has experienced some changes mainly due to increased experience. Our most recent cases have been performed without epidural catheter and some of them without central line. Similarly, we have stopped urinary catheterization the night before and discontinued furosemide 12 h before the surgery. These improvements are parallel to the better knowledge from our anesthesiologist, which have perfectly adapted the balance between central venous pressure, pneumoperitoneum pressure and airway pressure, making surgery a bloodless field[17]. It should be remarked that anesthesiologists are the cornerstone in our LLS. The intraoperative management based on boluses of inotropes rather than fluid administration is a difficult management that needs expertise and experience.
Some limitations of our research should be highlighted. First, the final population in the study was not extremely large; second, the results may have obviously changed according to our improved experience; and third, complexity has too changed, and thus applicability may be limited. However, we offer a homogeneous population in a brief period of time in a recently developed LLS team. This main advantage may be transferable to several liver teams worldwide and may help them face the same difficulties that we have had in a different way. Alternatively, our protocol is the first incorporating a full perioperative pathway within complexity scoring systems, making a 24 h early discharge possible in the setting of LLS.
In conclusion, it is feasible to develop aggressive FTP in LLS, even in high-complexity cases. In fact, our protocols are the first-reported to adequately predict and accomplish a postoperative hospital stay shorter than 24 h. Currently available difficulty scores are useful to define candidates for FTP and may predict a full completion even in aggressive postoperative stay formats. However, we consider that BMI has not been adequately considered and may be added to the scores in order to improve their prediction capabilities.
There is a lack of evidence regarding the correlation between laparoscopic liver surgery (LLS) difficulty scoring systems and accomplishment of fast-track protocols (FTP).
The main motivation is to identify if current difficulty scoring systems may be used to predict early discharging policies and development of complications after LLS within an FTP.
The main objectives are to define if difficulty scoring systems may predict accomplishment of FTPs in LLS and to determine variables that may complement these scoring systems to increase their prediction capabilities.
We analyzed out patients included in an FTP and compared Iwate, Southampton and Gayet’s scoring systems. Comparisons were also made in some sets of patients who were included in 24-h and 48-h early discharge protocols for both minor and major resections, respectively.
Our selection criteria was successful with more than 70% of our patients being discharged in less than 72 h. Iwate scoring system was the most accurate to predict 24-h discharge with an area under the receiver operating characteristic = 0.78 and 87.7% and 66.7% for sensitivity and specificity values, respectively, and a cutoff of 5.5 points.
Iwate difficulty score is the most accurate to predict adhesion to an FTP after LLS. Body mass index was considered as an independent risk factor that should be added to current scoring systems.
Incoming difficulty scoring systems may be further evaluated to include variables not considered to date.
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Surgery
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhou S S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Wu RR
| 1. | Ciria R, Gomez-Luque I, Ocaña S, Cipriani F, Halls M, Briceño J, Okuda Y, Troisi R, Rotellar F, Soubrane O, Abu Hilal M. A Systematic Review and Meta-Analysis Comparing the Short- and Long-Term Outcomes for Laparoscopic and Open Liver Resections for Hepatocellular Carcinoma: Updated Results from the European Guidelines Meeting on Laparoscopic Liver Surgery, Southampton, UK, 2017. Ann Surg Oncol. 2019;26:252-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Ciria R, Ocaña S, Gomez-Luque I, Cipriani F, Halls M, Fretland ÅA, Okuda Y, Aroori S, Briceño J, Aldrighetti L, Edwin B, Hilal MA. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc. 2020;34:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CH, Garden OJ, Farges O, Kokudo N, Vauthey JN, Clavien PA, Demartines N. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016;40:2425-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 407] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 4. | Dejong CH, van Dam RM. Enhanced recovery programs in liver surgery. World J Surg. 2014;38:2683-2684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. 2016;5:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 6. | Halls MC, Berardi G, Cipriani F, Barkhatov L, Lainas P, Harris S, D'Hondt M, Rotellar F, Dagher I, Aldrighetti L, Troisi RI, Edwin B, Abu Hilal M. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg. 2018;105:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 7. | Kawaguchi Y, Fuks D, Kokudo N, Gayet B. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg. 2018;267:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 8. | Ciria R, Ayllon MD, Briceño J. Difficulty scores in laparoscopic liver surgery-getting closer to a powerful and necessary tool. Hepatobiliary Surg Nutr. 2019;8:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Troisi RI, Montalti R, Van Limmen JG, Cavaniglia D, Reyntjens K, Rogiers X, De Hemptinne B. Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB (Oxford). 2014;16:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, Kaneko H, Wakabayashi G. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 11. | Cauchy F, Fuks D, Nomi T, Schwarz L, Barbier L, Dokmak S, Scatton O, Belghiti J, Soubrane O, Gayet B. Risk factors and consequences of conversion in laparoscopic major liver resection. Br J Surg. 2015;102:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Lee MK 4th, Gao F, Strasberg SM. Perceived complexity of various liver resections: results of a survey of experts with development of a complexity score and classification. J Am Coll Surg. 2015;220:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Hasegawa Y, Wakabayashi G, Nitta H, Takahara T, Katagiri H, Umemura A, Makabe K, Sasaki A. A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg Endosc. 2017;31:5356-5363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Tong Y, Li Z, Ji L, Wang Y, Wang W, Ying J, Cai X. A novel scoring system for conversion and complication in laparoscopic liver resection. Hepatobiliary Surg Nutr. 2018;7:454-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Yang R, Tao W, Chen YY, Zhang BH, Tang JM, Zhong S, Chen XX. Enhanced recovery after surgery programs versus traditional perioperative care in laparoscopic hepatectomy: A meta-analysis. Int J Surg. 2016;36:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Rouxel P, Beloeil H. Enhanced recovery after hepatectomy: A systematic review. Anaesth Crit Care Pain Med. 2019;38:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Kobayashi S, Honda G, Kurata M, Tadano S, Sakamoto K, Okuda Y, Abe K. An Experimental Study on the Relationship Among Airway Pressure, Pneumoperitoneum Pressure, and Central Venous Pressure in Pure Laparoscopic Hepatectomy. Ann Surg. 2016;263:1159-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |