Published online Feb 27, 2022. doi: 10.4240/wjgs.v14.i2.185
Peer-review started: July 29, 2021
First decision: October 3, 2021
Revised: October 14, 2021
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: February 27, 2022
Processing time: 208 Days and 12.7 Hours
Obesity is a chronic and multifactorial disease with a variety of potential treatment options available. Currently, there are several multidisciplinary therapeutic options for its management, including conservative, endoscopic, and surgical treatment.
To clarify indications, technical aspects, and outcomes of bariatric endoscopy.
Narrative review of current literature based on electronic databases including MEDLINE (PubMed), Cochrane Library, and SciELO.
Bariatric endoscopy is in constant development and comprises primary and revisional treatment options as well as management of surgical complications. Various devices act upon different mechanisms of action, which may be individualized to each patient. Despite favorable results for the endoscopic treatment of obesity, prospective randomized studies with long-term follow-up are required to fully validate primary and revisional endoscopic therapies. Regarding the management of bariatric surgery complications, endoscopic therapy may be considered the procedure of choice in a variety of situations. Still, as there is no standardized algorithm, local experience should be considered in decision-making.
The treatment of patients with obesity is complex, and a multidisciplinary approach is essential. Bariatric endoscopy has shown impressive results both in the treatment of obesity and its surgical complications, and therefore, must be part of the armamentarium in the fight against this disease.
Core Tip: Obesity is a chronic and recurrent disease with multiple treatment options available. Currently, there are several multidisciplinary therapeutic options for its management, including conservative, endoscopic, and surgical treatment. This study aims to clarify indications, technical aspects, and results of bariatric endoscopy based upon a detailed literature review and individual authors’ experience.
- Citation: de Moura DTH, Dantas ACB, Ribeiro IB, McCarty TR, Takeda FR, Santo MA, Nahas SC, de Moura EGH. Status of bariatric endoscopy–what does the surgeon need to know? A review. World J Gastrointest Surg 2022; 14(2): 185-199
- URL: https://www.wjgnet.com/1948-9366/full/v14/i2/185.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i2.185
Obesity is defined as a body weight disorder resulting from a long-term positive energy balance and is characterized by excess adiposity. This disorder significantly increases the risk for developing many obesity-associated co-morbidities. It is a chronic, multifactorial disease resulting in a global pandemic associated with several comorbidities–most notably type 2 diabetes and hypertension–and an increase in all-cause mortality. Data from the Centers for Disease Control and Prevention (CDC) illustrates that the prevalence of obesity in the United States is 42.4%[1]. In Latin America, more specifically in Brazil, recent data from the National Health Survey (PNS) released by the Brazilian Institute of Geography and Statistics (IBGE) demonstrates that six out of 10 Brazilian adults are overweight, representing approximately 96 million individuals. If we exclusively consider those with body mass index (BMI) greater than 30 kg/m2, one in every four Brazilians has obesity[2].
Treatment for obesity includes lifelong lifestyle modifications including behavioral, dietary, and exercise changes, pharmacotherapy, endoscopic therapies, and surgery. The treatment of obesity should be individualized and tailored to specific patients, taking into account several factors such as the degree of obesity (i.e., class of obesity), individual associated comorbid conditions (i.e., health risks), psychobehavioral and metabolic characteristics, as well as proper assessment of previous weight loss strategies. As obesity is a multifactorial disease, treatment must also be multidisciplinary[3].
Although diet, exercise, and pharmacotherapy are the least invasive and most widely utilized methods, it is clear that long-term results are unsatisfactory. Surgery, on the other hand, is proven to be the most effective and durable method for sustained weight loss and control of obesity-associated comorbidities[4]. However, while surgery is highly effective, this strategy is the most invasive option and may be associated with perioperative complications in about 0.5% to 9.6% of patients[5,6]. Additionally, approximately 50% of patients will develop some degree of long-term weight regain, requiring complex clinical management[7].
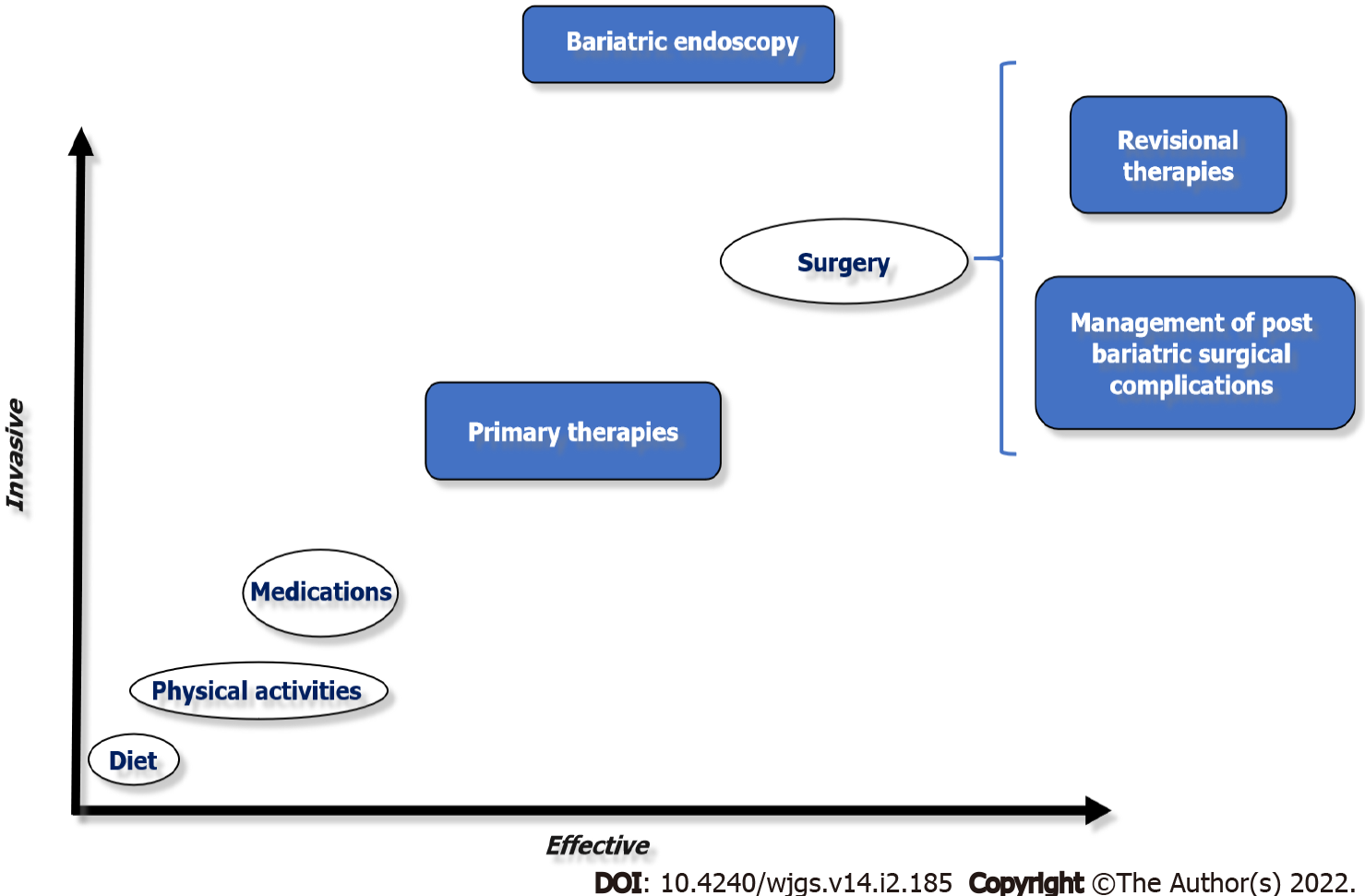
In this sense, the treatment for obesity and its associated comorbidities have recently expanded into the field of bariatric endoscopy: (1) via primary therapies, bridging a gap between less invasive therapies (lifestyle modification and/or pharmacological therapy) and bariatric surgery; (2) By optimizing the treatment of weight regain after bariatric surgery through revision therapies; and (3) Or in the management of postoperative bariatric surgery complications (Figure 1). In this review, we discuss the current state of bariatric endoscopy and highlight currently available treatments, including primary and revisional therapies
This is a narrative review including all available literature data obtained through electronic databases including MEDLINE (via PUBMED), Cochrane Library, and SciELO. This study was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The search study time period was from inception until January 15, 2022, using the search “bariatric endoscopy” AND “obesity”.
Primary therapies include intragastric balloons (IGBs), endoscopic suturing, and botulinum toxin injection.
IGBs: IGBs are indicated for patients with a BMI > 27 kg/m2 who have not achieved or maintained weight loss with conservative measures. Other qualifying patients include those with a BMI > 35 kg/m2 with comorbidities or > 40 kg/m2 in patients who have contraindications or do not wish to undergo bariatric surgery. Additional indications include patients with BMI > 50 kg/m2 as a bridge therapy for surgery. Absolute contraindications include active peptic ulcer disease, previous gastric surgery, large hiatal hernia, and patients with underlying eating disorders.
IGB models approved for use in most countries include “traditional” fluid filled (6 mo and one year), adjustable fluid filled (one year), and air filled (6 mo). The mechanism of action of IGBs is not yet fully established; however, it is believed that it is related to three factors: (1) Mechanical restriction, decreasing gastric capacity and leading to an increase in gastric emptying time, resulting in early satiety; (2) Hormonal changes due to direct contact with the gastric fundus, leading to a decrease in ghrelin and an increase in cholecystokinin, altering appetite and gastric emptying; and (3) Neurogenic, via central stimulation of the paraventricular nucleus of the solitary tract through vagal stimulation[8].
The IGB is the most widely adopted endoscopic method with proven efficacy and safety[9,10]. In a meta-analysis including only randomized studies evaluating fluid filled IGBs, the average difference in BMI loss was 1.41 kg/m2 with an absolute weight loss of 3.55 kg between the IGB group vs the control group[10]. However, most studies do not support the effectiveness of IGBs in long-term follow-up[11]. Despite being considered a safe method, close monitoring of the patient is essential to avoid serious adverse events (AEs), such as gastrointestinal obstruction, digestive hemorrhage, pancreatitis, gastric necrosis, and perforation[9-12].
Fluid filled IGB ("Traditional"): The "traditional" IGB should be filled with 400 to 750 mL of saline and methylene blue (to alert the patient when their urine appears greenish in case of leak or rupture of the IGB). In addition to the efficacy known in the short-term follow-up, this type of IGB has the advantage of a low rate of AEs. It should be noted; however, that fluid filled IGBs possess a higher rate of early withdrawal since the initial volume cannot be changed and patients may be unable to tolerate the balloon due to nausea and vomiting, especially within three days after placement[9-11].
Fluid filled adjustable balloon: The adjustable IGB (aIGB) may be filled up to 900 mL of fluid, having the advantage of adjusting the volume of liquid contained in the balloon. This may result in a lower rate of early withdrawal due to patient intolerance and supposedly greater weight loss after adjustment with increasing volume after initial implantation. However, due to the presence of the catheter used for the adjustment, this IGB is associated with a higher rate of ulcerations and abdominal discomfort. In a randomized study comparing the aIGB with lifestyle intervention vs lifestyle intervention alone, the aIGB group presented a mean total weight loss (TWL) at 32 wk of 15% compared to 3.3% of the control group. Adjustments to the aIGB occurred among 80% of patients for weight loss plateau or intolerance. Upward volume adjustment facilitated an additional mean of 5.2% TWL. Downward volume adjustment allowed 75% of patients in the aIGB group to complete the full duration of therapy. Intolerance caused early removal of the device in 17% of patients. Severe AEs were observed in 4% of patients[12].
Air balloon: The air IGB is traditionally known for being well tolerated and associated with fewer AEs including nausea and vomiting. However, air IGBs are also associated with less %TWL compared to the fluid filled IGBs, and air filled IGB removal is often challenging as these balloons may more rigid than the other types of IGBs[13,14].
Endoscopic suturing (endoscopic sleeve gastroplasty): Endoscopic sleeve gastroplasty (ESG) aims to restrict gastric volume by performing full-thickness sutures in the gastric body. At this time, there are several suture patterns which have been performed; however, the most commonly utilized pattern is the “U” stitch pattern. This pattern is characterized by suturing initially along the anterior wall towards the greater curvature and the posterior wall, with the turn through the posterior wall along the greater curvature and ending at the anterior wall. Often 6 to 8 sutures are performed in the “U” pattern. With this endoscopic technique, the gastric fundus is not sutured, maintaining a reservoir that contributes to the promotion of early satiety. Although the mechanism of action of ESG is not fully understood, circumferential and longitudinal reduction in the size of the stomach as well as delayed gastric emptying time are believed to promote early satiety[15-18].
ESG has been shown to be highly effective and safe in the management of patients with a BMI classified > 25 kg/m2 (overweight) and > 30 kg/m2 (obesity). A recent meta-analysis demonstrated a %TWL of 16.1% and 16.8% and an %EWL of 60% and 73% at 1-year and 18 mo follow-up, respectively[16]. Currently, there are still many unknowns regarding the long-term efficacy of primary endoscopic therapies, especially ESG[15-17]; however, a recent study showed satisfactory results during 5-year follow-up after ESG[18]. ESG has also been shown to be superior to IGB in terms of weight loss and side effect profile, solidifying this treatment strategy as an effective and safe option for individuals who do not quality for surgery or among patients who wish to avoid a traditional surgical approach. In a recent systematic review comparing ESG and IGB strategies, %TWL was superior in the ESG group (%TWL: 15.34% at 6 mo; 17.51% at 12 mo, and 17.85% at 24 mo) compared to the IGB group (%TWL: 12.16% at 6 mo; 10.35% at 12 mo; and 6.89% at 24 mo)[19]. This suggests improved initial weight loss as well as an improved ability to maintain that weight loss. While additional long-term data is needed, these results suggest a promising role for ESG in the management of obesity.
Unlike IGB, which is associated with nausea and vomiting in immediate post-procedure setting, post-ESG patients may experience abdominal pain as a primary symptom. However, like the initial symptoms of IGB, symptoms associated with ESG rapidly improve within three to five days post-procedure[19]. The safety of ESG has also been confirmed in a meta-analysis, demonstrating the rate of severe AEs to be 0.8%, and the rate of total AEs to be 2.3%[16]. Although safe, care during the procedure is essential to minimize complication. Therefore, we recommend use of CO2 for insufflation, general anesthesia with endotracheal intubation, proper patient positioning in the left lateral position, and specialized training to ensure adequate provider knowledge of the device, technique, and understanding of anatomy[15].
Botulinum toxin injection: Botulinum toxin injection of the gastric wall works via the inhibition of acetylcholine in the cholinergic neuromuscular endings, promoting delay in gastric emptying, thereby leading to early satiety. However, most randomized studies and meta-analyses have not demonstrated the effectiveness of the method in the treatment of obesity[20].
Weight recidivism (more commonly described as weight regain) does not have a standardized definition. The most widely accepted definition is considered regain of 50% of the weight loss with initial bariatric surgery (i.e., increase from the nadir weight) or regain of 20% of the nadir weight associated with the recurrence or development of an obesity-associated comorbidity. Weight regain is a multifactorial condition, including hormonal factors, the balance between expenditure and caloric intake, as well as behavioral, genetic, and anatomical factors[21,22]. While all of these factors are essential to providing complete care and ensuring success after bariatric surgery, bariatric endoscopy seeks to primarily alleviate or treat factors related to anatomical changes after bariatric surgery[21-23].
Among the currently available revisional therapies, endoscopic bariatric treatments include use of argon plasma coagulation (APC) and endoscopic suturing[24,25]. These two treatment modalities are typically used in the management of those who have undergone prior Roux-en-Y Gastric Bypass (RYGB) and aim to achieve reduction of the gastric pouch, gastrojejunal anastomosis (GJA), and in some cases, successful closure of gastrogastric fistulas (GGFs) when present. Furthermore, endoscopic suturing has recently been used to treat patients with weight regain after sleeve gastrectomy (sometimes referred to as a sleeve-in-sleeve procedure).
Reduction of the gastric pouch and gastrojejunal anastomosis after RYGB: APC: APC is performed circumferentially around the edge of the GJA (gastric face-about 1 to 1.5 cm). As a result, scarring and fibrosis of this area occurs, resulting in a reduction in the diameter of the GJA. In some cases, more than one session may be necessary to achieve the goal of reducing the diameter of the GJA to approximately 10 to 12 mm. A recent randomized study demonstrated no superiority of the group that underwent APC + suturing compared APC alone in terms of weight loss or complications between the techniques at one-year follow-up[25].
Endoscopic suturing (transoral outlet reduction): Endoscopic suturing for patients with a history of RYGB is typically undertaken using a transoral outlet reduction (TORe) technique. The reduction in the diameter of the GJA may also be performed using an endoscopic suturing technique–often performed after APC of the GJA since the combination of methods may result in better weight loss results compared to a suturing alone[24]. The APC technique alone is more widely used due to shorter procedure times, decreased need for deep sedation or endotracheal intubation, and cost-savings. However, endoscopic suturing also allows for the possibility of reducing the gastric pouch, which in selected cases may help to promote better weight loss results. Additionally, pursestring suturing of the GJA is likely a superior strategy to APC alone. Despite limited data at this time, TORe appears to be a highly effective and safe procedure[24].
Modified endoscopic submucosal dissection + APC + endoscopic suturing: Another strategy, based upon TORe as described above, is a modified endoscopic submucosal dissection (ESD)-TORe procedure. This treatment involves making a modified submucosal dissection around the circumference of the GJA, to expose the muscular propria. Once this accomplished, traditional APC is performed around the GJA and a pursestring, endoscopic suture pattern is made around the outlet with bites taken through the exposed muscle layer (minimizing the drawbacks of a non-full thickness or superficial bite that may occur with a traditional TORe technique). This technique was recently described and demonstrated encouraging results in a retrospective study, where the association of a modified ESD technique with APC and endoscopic suturing was superior to APC and suturing alone. In this study, both in the 6-mo follow-up (13.4% vs 8.5%; P = 0.045) and 1 year follow-up (12.1% vs 7.5%; P = 0.036) demonstrated greater %TWL in the modified ESD-TORe cohort[26]. However, the increase in costs and procedure time, as well as the need of previous experience in submucosal dissection may limit more widespread adoption of this technique. As such, this technique is likely to continue to only be available at highly specialized centers.
Treatment of gastrogastric fistula: Common endoscopic therapies such as APC, clips, and endoscopic suturing are associated with a low clinical success rate in the treatment of GGF. In a study including 29 patients with GGF, despite 100% technical success, clinical success after 1 year was only 17.1%[27]. The use of the cardiac septal defect occluder (CSDO) for the treatment of GGF has also been described. However, more studies are needed to prove the effectiveness and safety profile of this novel approach. In our experience, endoscopic management of GGF may be effective only in GGF smaller than 10 mm.
Reduction of gastric volume after sleeve gastrectomy: After sleeve gastrectomy, the ability to perform endoscopic suturing via a modified ESG technique is a promising method in the management of patients with postoperative weight regain. In a multicenter study, this technique demonstrated results similar to primary ESG, with %TWL in one year of 14.2%, 19.3%, 17.5%, and 20.4%, for overweight and patients with obesity class I, II, and III, respectively. Perhaps most importantly, in this study, no AEs were reported[28]. The use of a plication device via a USGI platform has also been described with promising results[29].
Complications of revisional therapies: Adverse effects after revisional endoscopic therapies are uncommon, occurring in approximately 3% to 7% of cases. The most common events reported include nausea and vomiting, abdominal discomfort, post-procedure bleeding, and the development of intraluminal strictures[22]. Additionally, a reverse Barrett´s esophagus, characterized as receding of the squamous columnar junction into the gastric pouch among patients with RYGB has also been described[30]. While the mechanism of action is not fully understood, management of these patients is typically conservative and a vast majority of other complications are managed endoscopically[22,24,31].
Endoscopic treatment of bariatric surgery complications is considered by many as the gold standard due to its high efficacy and minimally invasive nature with a low rate of AEs. Endoscopic treatment can be used for treating intraluminal bleeding, leaks, fistulas, and stenoses. Despite the fear of anastomotic and staple line dehiscence during an endoscopic exam in the very early post-operative period, endoscopic techniques are safe and have been well-documented to be effective in the literature[32-35].
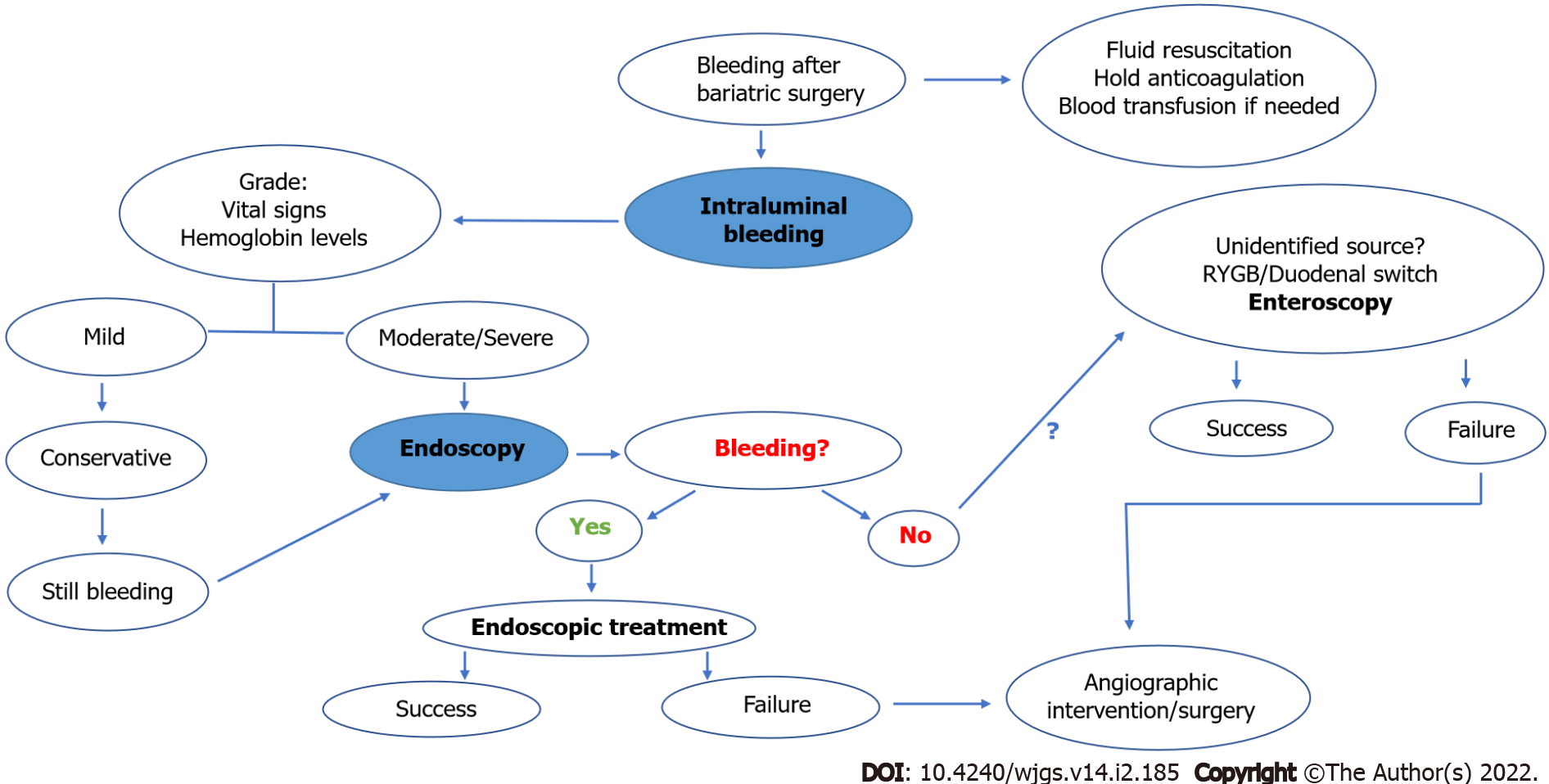
Hemorrhage: In patients with early bleeding after bariatric surgery, initial supportive measures such as volume resuscitation, temporary cessation of anticoagulation, and blood transfusion when necessary, should be performed. While highly effective, it should be noted that endoscopic management is only plausible in cases of intraluminal bleeding, especially along gastric suture lines. The main signs of intraluminal hemorrhage include hematemesis, enterorrhagia, or melena. In addition to assistance with diagnosis, endoscopy may provide therapy through a variety of mechanisms–via injection, mechanical, thermal, and topical therapies. If endoscopic treatment fails, angiography therapy or emergency surgery may be indicated[34,35]. The proposed algorithm for the management of early bleeding after bariatric surgery is described in Figure 2.
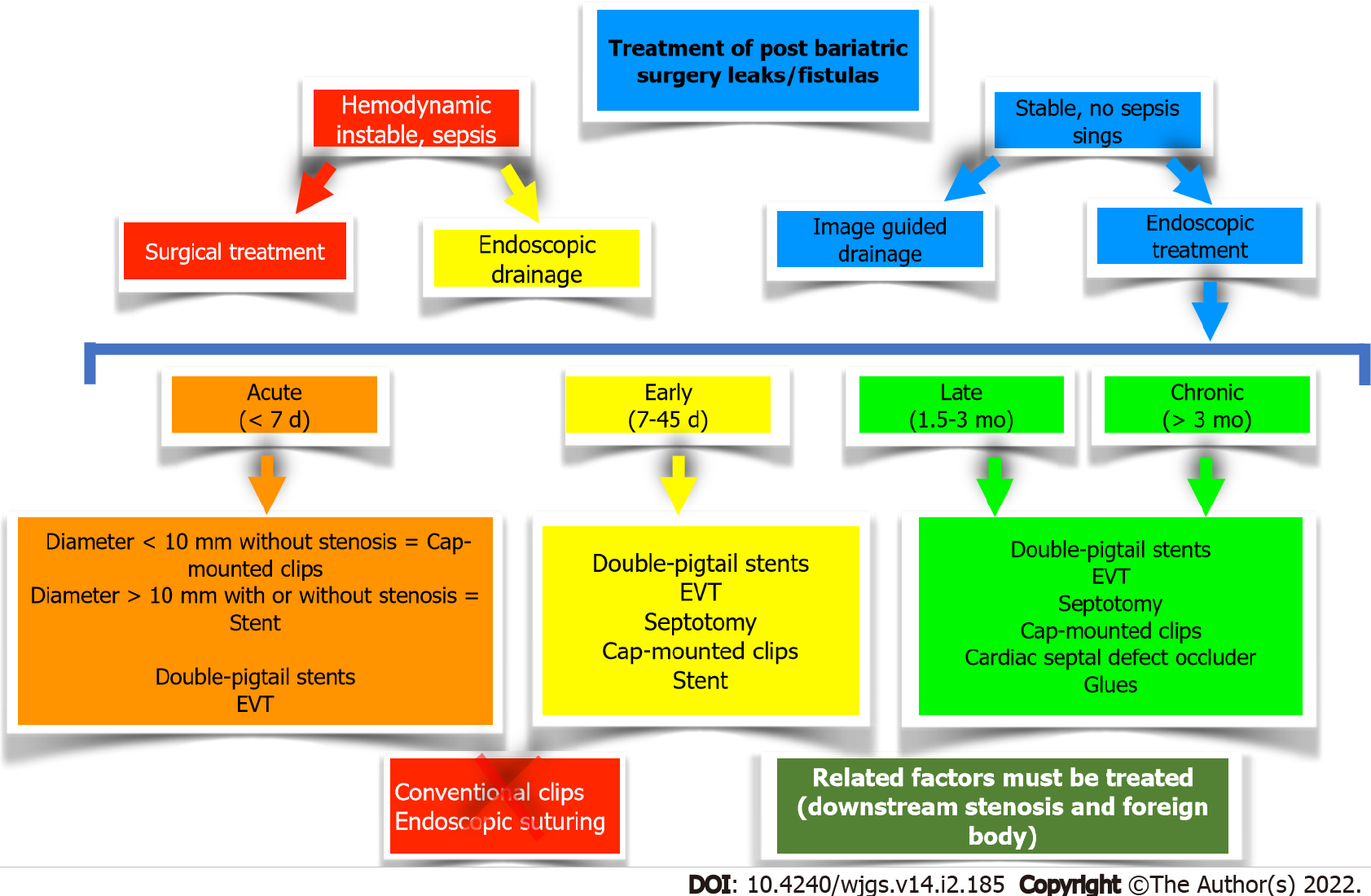
Treatment of postoperative leaks and fistulas: The endoscopic treatment of postoperative leaks and fistulas includes a wide variety of techniques and devices, with therapeutic options that aim to close (glues, clips, and sutures), cover (stents and CSDO), and drain [double-pigtails stents (DPS)], septotomy, and endoscopic vacuum therapy (EVT)[32,35] (Tables 1 and 2). It is imperative to understand that early treatment is the key to success. Additionally, basic surgical principles such as drainage of associated collections (by endoscopic, radiologic, or surgical approaches) and treatment of related factors such as distal strictures as well as removal of foreign bodies (preferably by endoscopic techniques), are essential for the successful treatment of postoperative leaks and fistulas[32,35]. These therapies are described individually below, and a proposed algorithm for the management of leaks and fistulas after bariatric surgery is highlighted in Figure 3.
| Endoscopic technique | Indications/advantages | Not indicated/disadvantages | Our experience |
| Glues (Closure) | (1) Acute/early/late/chronic; (2) Low-debit (< 200 mL/24 h); (3) Diameter < 10 mm; and (4) Safe | (1) Multiple sessions are usually required; (2) High costs; (3) Need for external drainage; and (4) Variable efficacy | (1) Low efficacy; (2) Multiple sessions; (3) High costs; (4) Late/chronic; (5) Combined approach; and (6) Can be used in select cases |
| Cap-mounted clips (Closure) | (1) Acute/early/late/chronic; (2) Small orifices (< 2 cm); and (3) Safe | (1) > 2 cm orifice; (2) Need for external drainage; and (3) Variable efficacy | (1) Low efficacy; (2) Late/chronic; and (3) Can be used in select cases |
| Suturing (Closure) | (1) Acute/early/late/chronic; and (2) Safe | (1) Need for external drainage; (2) Challenging-need previous experience with the device; (3) Low efficacy; and (4) High cost | (1) High cost; (2) Very poor long-term clinical success; and (3) We do not recommend it! |
| Stents (conventional esophageal or specific design for LSG) (over) | (1) Acute and early; (2) Very popular; (3) Efficacy > 70%; (4) Conventional = bariatric stent; (5) Early oral intake; and (6) Lower number of repeat procedures | (1) High rates of migration (up to 30%); (2) Need for external drainage; (3) Symptoms related to the stent; (4) Late and Chronic; and (5) You may have a “surprise” when remove it | (1) High rates of migration; (2) Partially covered > fully-covered (challenging to remove-do not keep it for more than 3 wk!); and (3) Bariatric stents: Similar efficacy, more SAE; Symptoms related to the stent (pre pyloric); More migration (post pyloric); We´re avoiding stents, specially Bariatric Stents |
| Cardiac septal defect occlude (Cover) | (1) Late and chronic; (2) Efficacy > 95%; and (3) Safe | (1) Off-label use; (2) Acute and Early; (3) High cost; and (4) Need for external drainage | (1) High efficacy in late/chronic fistulas with epithelialized tract without associated collection; (2) Safe; and (3) Good option after failure of conventional techniques |
| Endoscopic technique | Indications/advantages | Not indicated/disadvantages | Our experience |
| Septotomy | (1) You must do it when a septum is identified; (2) Early, late and chronic; (3) High efficacy: 80%-100%; and (4) Safe | It is just performed when a septum is identified! | (1) Very high clinical success rates; and (2) Septum is the cause of most late and chronic leaks/fistulas treated in a center without experience |
| EVT | (1) Acute, early, late and chronic; (2) High efficacy (> 90%) in leaks with or without associated collection; (3) No need of external drainage; and (4) Superior to stent in upper GI tract | (1) Patient discomfort related to NGT; (2) Usually repeat procedures are needed (sponge); (3) Respiratory/Cutaneous fistula; (4) Longer hospital stay (?); and (5) High costs (?) | (1) Very high clinical success rates; (2) Modified EVT: Easy placement, reduction in procedure time and need for repeat procedures, lower costs and Aes; and (3) Modified trelumina EVT: Drainage and nutrition with one tube through the nares |
| DPS | (1) Acute, early, late and chronic; (2) High efficacy (> 85%) in leaks/fistulas with associated collection; (3) Easy placement (7fr-gastroscope); (4) No need of external drainage; and (5) Short hospital stay | (1) Longer period for complete healing; (2) Risk of migration and bleeding; (3) No place to accommodate the stent in small collections; and (4) Usually fluoroscopy is needed | (1) Very high clinical success rates; (2) Shorter hospital stay; (3) Faster oral intake (clear liquids); and (4) Better patient acceptance–no symptoms |
Endoscopic glue: The use of “glue” such as cyanoacrylate, tissue adhesive, or sealants and the acellular fibrogenic matrix is unpopular in Brazil due to its high associated cost and heterogeneous results in the literature. Additionally, multiple sessions are typically required, further making this a less than ideal strategy for many patients. The best results for endoscopic glue use are described in chronic fistulas, typically smaller than 10 mm, with low drain output (traditionally < 200 mL in 24 h), and when used in conjunction with other therapeutic options (i.e., use with endoscopic suturing for oversewing marginal ulcerations). In a national study, the clinical success of the acellular fibrogenic matrix was 80%; however, this was a combined percentage accounting for a 30% success with the initial session, 55% success with the second treatment, and 15% success for patients requiring a third session[36].
Clips: The use of conventional clips are not indicated for the treatment of surgical leaks or fistulas, as this material does not have adequate tension to approximate tissue in these conditions[32]. However, cap-mounted clips can be effective as these devices approximate transmural defects with more tension than the conventional clips and have been proven to adequate close mucosal defects in longer term studies. In a recent systematic review, the effectiveness of the cap-mounted clip was 72.6% for anastomotic leaks and 55.8% for fistulas. It is important to understand that this device can only be used in fistulous orifices up to 20 mm due to its size, being best situated and utilized for small transmural defects not associated with intracavitary collections[37].
Endoscopic suturing: Endoscopic suturing is another strategy that allows for closure of leaks or fistulas via the use of transmural sutures, typically using a running mattress pattern to provide successful closure and reduce the risk or recurrent leak. However, despite the high technical success and safety, the clinical success is less than ideal, especially when adapted to fistula close. In a study including 56 patients with fistula, clinical success after one year was 17.1% for GGFs and 31.4% for other types of fistulas. Thus, due to the high cost of the device and unsatisfactory results, its use has been limited for this indication at this time in most countries[27,32,38].
Stents: Esophageal stents are traditionally used with satisfactory closure rates. However, these conventional (esophageal) stents have been associated with some AEs, ranging from symptoms such as pain, reflux, and nausea, to more severe complications such as perforation and migration[32,39]. In a meta-analysis including only post-bariatric surgery leaks and fistulas, clinical success was 72.8% and migration rate was 28.2%[40]. Due to the high rate of AEs, stents specifically designed for leaks and fistulas after bariatric surgery (i.e., sleeve gastrectomy) have been developed. In a multicenter study, including 37 patients, the clinical success rate with these novel stents was 78.27% (similar to the conventional stents), but the rate of the complications including migrations and perforations remained high. Therefore, based upon the literature and our own experience, these novel stents do not appear to be superior to conventional (esophageal) stents and should be utilized with caution–utilized mostly in centers with expertise in the management of this condition[41]. A recent meta-analysis also did not show any advantage of these customized stents when compared to conventional stents for the treatment of sleeve leaks and fistulas[42].
Cardiac septal defect occluder: The CSDO is a shape-memory, self-expanding double-disc closure device composed of nitinol and polyester, with impressive expansion force[43]. Traditionally used to provide closure for atrial or ventricular septal cardiac defects, off-label use has expanded to the realm of gastroenterology. In a multicenter study evaluating its off-label use in fistulas after bariatric surgery, the rate of clinical success in late and chronic fistulas was 97.1%[44]. At present, the use of CSDO devices is recommended for chronic fistulas due to the presence of an epithelialized tract; however, it is important to understand that CSDO device should not be used in acute and early leaks or fistulas as these can increase the size of the orifice due to the significant expansion force[43,44].
Endoscopic internal drainage with double pigtail plastic stents: Endoscopic internal drainage with DPS of perigastric collections after bariatric surgery has also been widely employed. This technique has demonstrated satisfactory results associated with less need for prolonged hospital stay and few AEs. The principles of the DPS method are similar to that of transgastric drainage of pancreatic pseudocysts, providing adequate internal drainage and closure of the tract around the pigtail catheter[45]. The vast majority of current studies have shown an efficacy greater than 85% and endoscopic drainage with DPSs have been associated with a low rate of AEs, including stent migration, bleeding, and perforation[46,47].
Septotomy: Endoscopic septotomy is another technique that is currently used worldwide and has a similar principle to the treatment of Zenker's diverticulum. When most helpful, the septotomy technique is beneficial where the septum must be sectioned to match the pressure of the leak or fistula site within the gastric chamber[48]. The septotomy must be performed up to the depth of the suture line, but not exceed this limit to avoid perforation. In a study involving 27 patients after bariatric surgery, including patients with RYGB, sleeve gastrectomy, and duodenal switch, the clinical success rate after septotomy was 100%, with an average treatment time of 18.11 days and the need for one to six procedures[48]. As such, this septotomy strategy can be highly effective when individual patient factors warrant this technique.
Endoscopic vacuum therapy: EVT is traditional strategy used in Europe, though its use and adoption has more recently spread across the world. The technique is performed by placing a sponge (or gauze covered by surgical adhesive when using a modified technique) on the distal end of a nasogastric tube, which is positioned in the perigastric collection (intracavitary) or in the lumen (intraluminal). Next, this nasogastric tube is connected to a vacuum machine or wall suction with continuous negative pressure (between-125 and -175 mmHg). The EVT system positioning is based on endoscopic findings, and the intracavitary position should be used whenever there is an associated collection. Mechanisms of action include microdeformation and macrodeformation, improvement of perfusion (angiogenesis), control of local edema, and bacterial clearance[49-51].
Several studies have reported high efficacy and low AE rates associated with EVT. Nonetheless, the need for repeated procedures, every seven days for traditional (polyurethane) EVT sponge, and patient discomfort due to continuous and prolonged use of a nasogastric tube are considered limiting factors by some centers. The advantages of the recently described low-cost modified EVT system includes easy placement (through nares), decreased procedure time, longer interval between EVT system exchanges, and decreased AEs[49,50]. In a meta-analysis comparing EVT vs stent placement for the treatment of upper gastrointestinal transmural defects, EVT was superior in closing transmural defects, associated with decreased treatment time, and found to have a lower associated mortality rate[52].
Treatment of stenoses: Stenosis after RYGB: Dilation with a hydrostatic balloon for stenoses after RYGB is a well-established method, with clinical success rates up to 100% after one to five treatment sessions. In addition to its effectiveness, balloon dilation is considered safe with low rates of AEs–perforation (4.9% of cases) is the most common[53]. It is important to acknowledge that the presence of an ischemic segment is associated with therapeutic failure and an increased risk of complications[53,54]. In recent years, metallic stents with lumen apposition (lumen apposing metal stents) have been used for cases refractory to dilation–with technical success rates of 100%, high clinical success rates in the short follow-up, and infrequent AEs compared to esophageal stent placement, including decreased migration, pain, recurrent stenosis, and bleeding. However, the need for re-intervention in long-term follow-up continues to be considered high[54]. In addition to the use of self-expandable metallic stents for refractory cases, incisional therapy and corticosteroid injection are less expensive options and may be performed in specialized centers.
Ring slippage after RYGB: Slipping of the ring may cause stenosis of the gastric pouch or even in the jejunal limb, leading to food intolerance. The endoscopic treatment of this condition can be carried out through pneumatic balloon dilation (using an achalasia balloon) or self-expandable stent (plastic or metal) placement. Patients who underwent pneumatic balloon dilation, aiming to stretch or rupture the ring, achieved high rates of clinical success after one to four sessions, usually with no recurrence of symptoms or perioperative complications[55]. Likewise, in a study evaluating the use of self-expandable stents in 41 patients, removal of the ring was possible in all cases. However, it should be noted that 22% of patients developed post-procedure stenosis due to local fibrosis, requiring endoscopic balloon dilation[56]. Despite these complications, reoperation or deaths are extremely rare after these approaches[55,56]. Due to the higher rate of stenosis after using self-expandable stents, we recommend treatment with pneumatic dilation as a first-line strategy whenever possible.
Erosion of the ring after RYGB: Ring erosion after RYGB is traditionally treated by endoscopy due to its ease and minimally invasive nature. Endoscopic removal of the ring is indicated with minimal intraluminal extrusion of 30%. This can be performed through the ring section, either with endoscopic scissors (silastic ring) or with APC [polypropylene (marlex) ring], followed by ring removal using a foreign body forceps or a polypectomy snare[56].
Erosion of the gastric band: Erosion of the gastric band has been noted to less frequently occur due to the more recent shift away from this surgical technique in clinical practice. Endoscopic band cutting is performed by passing a guidewire through the intragastric fragment of the band, followed by cutting using a lithotripter device. Then, with a polypectomy snare, the device is removed. The subcutaneous port must be removed before the endoscopic removal. Technical and clinical success rates are extremely high, with a low rate of AEs, mainly pneumoperitoneum. Most of these cases may be treated conservatively through decompression with an abdominal puncture[57,58]. While less individuals are undergoing the laparoscopic adjustable gastric band procedure, provider knowledge of potential complications and appropriate understanding of endoscopic treatment remain critically important.
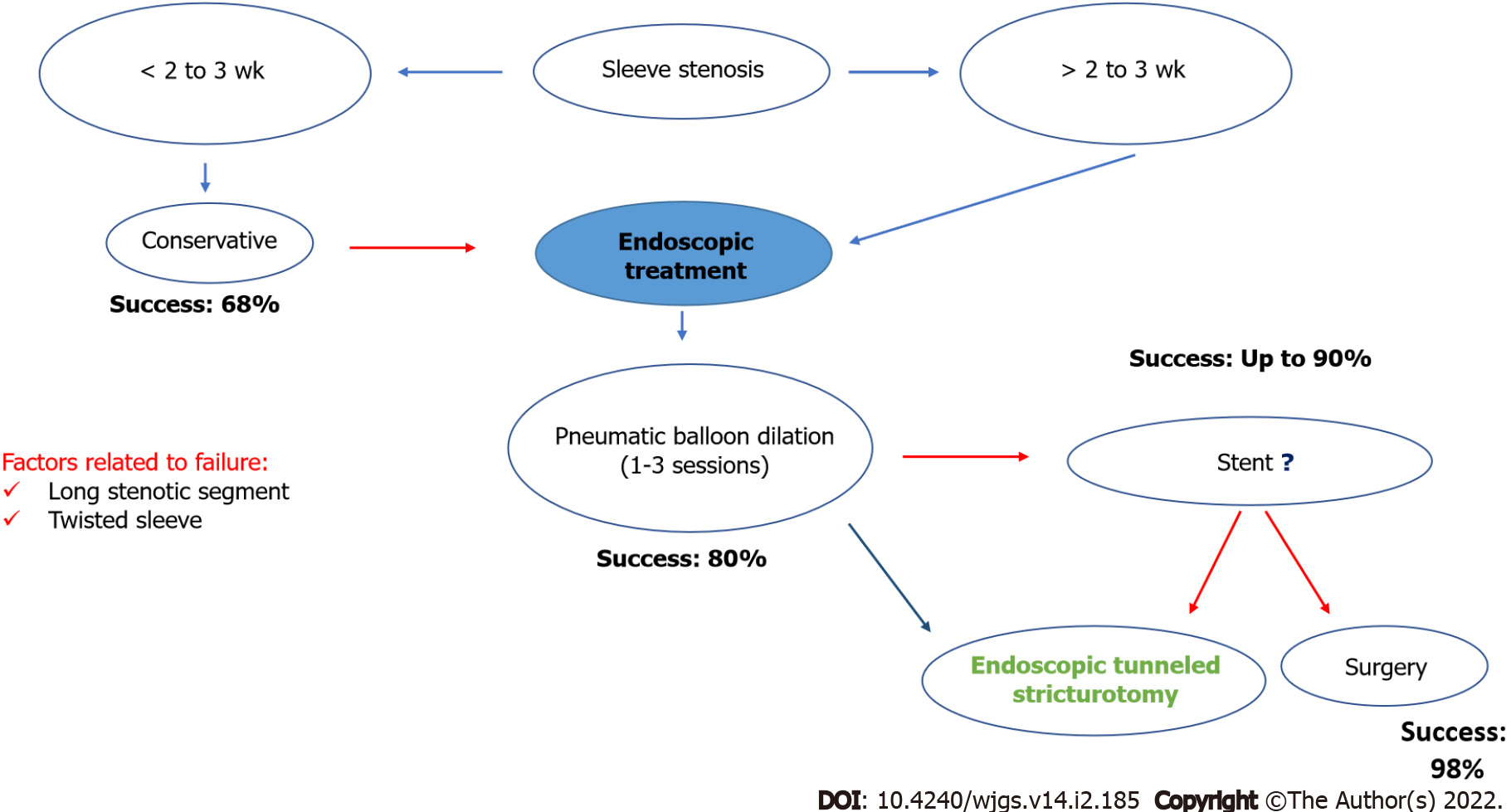
Treatment of stenosis after sleeve gastrectomy: Several algorithms for the management of stenosis after sleeve gastrectomy have been described. Our experience is similar to the results of a recent meta-analysis, where conservative management in the first two to three weeks is recommended, with improvement in obstructive symptoms in 68.8% of cases. If patients remain symptomatic and are refractory to conservative management, endoscopic treatment is therefore recommended, with success rates approaching 82%, via dilation with a pneumatic balloon (one to three sessions), starting with dilation up to 30 mm, followed by dilation up to 35 mm. Dilatation up to 40 mm can be performed; however, this is not usually recommended due to the high risk of complications. Some groups report success using hydrostatic balloons (up to 20 mm) in selected cases, but it is rarely used in our practice due to limited long-term relief and symptom and stenosis recurrence.
Another endoscopic option is the use of self-expanding metal stents; however, these are indicated mainly for patients that remain refractory to pneumatic dilation. Primary surgical treatment may also be performed, but it is more invasive and results are not superior to endoscopy. In cases refractory to endoscopic therapy, surgical treatment is traditionally indicated, with a 98% success rate[59]. Recently, the endoscopic tunneled stricturotomy technique has been described with promising results in refractory cases, becoming another minimally invasive alternative to surgery[60]. The algorithm proposed by our group is shown in Figure 4.
The exponential growth of bariatric surgery in the last decades has evolved inseparably from the advances in the field of endoscopy. In this article, we have reviewed the main therapeutic options of bariatric endoscopy that should be known by general, digestive, and bariatric surgeons. These have been summarized above and divided into three main areas: Primary treatments for obesity, revisional therapies, as well as the management of complications after bariatric surgery.
Among the primary treatments, the IGB is the most widely used, with satisfactory results in the short-term when appropriately indicated. Endoscopic suturing has been utilized with promising results and considerable weight loss; however, the evidence with long-term follow-up remains scarce. When a patient seeks a surgeon in demand for these techniques, we must emphasize that these procedures are not a substitute for bariatric surgery, and we should highlight three aspects: Adequate indication; expectation of realistic results within the BMI profile and associated comorbidities; and safety and quality of the procedure when performed by a specialized endoscopist in an appropriate medical facility. Furthermore, like other treatments for obesity, the support of a specialized multidisciplinary team and regular adherence to follow-up is necessary to ensure an optimal long-term result.
Weight regain has become a challenge due to the cumulative increase in the number of patients undergoing bariatric surgery. Mechanisms for weight regain are complex and again require a multidisciplinary approach–taking into account factors outside of just anatomic changes. In most cases mechanism of weight regain are multifactorial. Therefore, the initial step in treating these patients is a comprehensive assessment of the patient by a multidisciplinary team. For individuals with appropriate indications, endoluminal therapies are safe, reproducible and effective in treating patients with weight regain and as a less invasive therapy then revisional surgery. Therefore, endoscopic bariatric treatment should be utilized as a first line intervention to manage this condition.
When considering surgical complications, the management of postoperative bariatric surgery patients is challenging and, to achieve a positive outcome, again requires a multidisciplinary approach. Didactic knowledge, technical mastery, and good communication between the surgery, endoscopy, and interventional radiology teams remains essential. In this manner, it is also key to have a collaborative hospital structure and environment since minimally invasive treatment by endoscopic therapy may be used as first-line therapy to avoid more invasive procedures in the treatment of acute postoperative complications.
When diagnosing a leak or fistula, endoscopic treatment may be considered an early therapeutic option. As shown in Figure 3 and Table 2, the surgeon may rely upon endoscopic treatment even for severe cases. Endoscopic adjuncts to traditional surgical cases, such as peritonitis and sepsis, may include placement of an enteral feeding tube or, more recently, endoscopic internal drainage therapies such as EVT intraoperatively. Regarding late complications, bariatric endoscopy should be considered a first-line strategy for diagnosis and treatment along with an upper gastrointestinal series. This is essential for assessing patients with recurrent nausea, vomiting, reflux, or regurgitation. Additionally, endoscopy may often be used as an option for the treatment of stenosis and ring/band erosions, avoiding reoperations which include greater complexity and risk, since these are patients with long-standing surgeries, many by open access, and presenting with malnutrition due to recurrent vomiting.
As obesity treatment algorithms evolve, bariatric endoscopy procedures and their devices have been gradually adopted. However, it is important to note that there are still significant limitations due to its high associated costs and even restrictions for authorization and/or importation of these devices.
Despite being a comprehensive review of the literature, this article is not without limitations. As this is a recent topic, most studies are small or uncontrolled series, and more prospective and randomized studies are needed to establish the best therapeutic options for each situation. Also, many of these studies were carried out in large referral centers, with a team and structure dedicated to this patient profile. In this manner, not all the therapeutic options reviewed here can be applied to the reality of all services and hospitals.
Obesity and weight regain are multifactorial disorders, and, therefore, multidisciplinary treatment is essential. Bariatric and metabolic endoscopic therapies are in constant development, including devices with a wide variety of mechanisms of action. Available endoscopic approaches have been shown to be effective and safe in the management of obesity and in patients with weight regain. However, as there is no gold standard method for managing these patients, the assessment must be individualized. Despite the favorable results, randomized studies with long-term follow-up are still required for complete validation of primary and revisional endoscopic bariatric therapies.
Regarding the management of complications after bariatric surgery, it is essential to underscore the complexity of patient care, where follow-up with a multidisciplinary team is critical. Endoscopic therapies are associated with high rates of clinical success in the management of intraluminal bleeding conditions, stenoses, leaks and fistulas, especially when performed early in the post-operative period. To date, there is no precise algorithm for the management of these patients, and therefore, local experience and device availability should be considered when choosing a therapy. Institutions without specialized staff should consider referring these patients to a center of excellence.
Obesity is a chronic and recurrent disease resulting in a global pandemic associated with several associated comorbidities. Current treatments include lifestyle modifications including behavioral, dietary, exercise changes, and medications which are associated with less than ideal long-term outcomes. Bridging the gap between these therapies and traditional bariatric surgery is the field of bariatric endoscopy, which seeks to provide less invasive therapies to treat primary obesity, treat weight regain after bariatric surgery, and manage complications of bariatric surgery.
To review the current literature of bariatric endoscopy and highlight the field of to colleagues from other disciplines such as surgeons, endocrinologists, and primary care physicians.
Discuss the current state of bariatric endoscopy, including primary therapies, endoscopic management of weight regain, and the management of complications after bariatric surgery including hemorrhage, stenoses, and leaks and fistulas.
Narrative review including available literature data obtained through electronic databases and authors’ experience.
Bariatric endoscopy is in constantly evolving field which comprises primary and revisional treatment as well as the management of surgical complications. While longer-term, randomized studies are still warranted to fully validate primary and revisional endoscopic therapies, the field provides a high effective and safe means to treat patients with obesity and associated comorbid conditions. Regarding endoscopic treatment of post bariatric surgery complications, endoscopic management remains a first-line strategy to avoid the morbidity and mortality associated with repeat surgical operations.
Bariatric and metabolic endoscopic therapies are in constant development, including devices with a wide variety of mechanisms of action. Available endoscopic approaches have proved to be effective and safe for a variety of obesity associated treatments. In this manuscript, we have highlighted these indications, provided a detailed review of the literature, and summarized our own experience to improve the management and care of patients with obesity.
The advances in the bariatric endoscopy field have the unique opportunity to improve the quality of life and health outcomes for patients with obesity and associated comorbid conditions. The field as a whole as the ability to bridge the gap between lifestyle modifications and conventional surgery to provide treatment to a wide range of individuals, offering a minimally invasive approach for conditions and complications that previously required surgery.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Zhang H S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | (CDC) C for DC and P. Overweight & Obesity - Adult Obesity Facts [Internet]. 2021. [cited 4 January 2021]. Available from: https://www.cdc.gov/obesity/data/adult.html. |
| 2. | IBGE. Um em cada quatro adultos do país estava obeso em 2019; Atenção Primária foi bem avaliada [Internet]. [cited 4 January 2021]. Available from: https://agenciadenoticias.ibge.gov.br/agencia-noticias/2012-agencia-de-noticias/noticias/29204-um-em-cada-quatro-adultos-do-pais-estava-obeso-em-2019. |
| 3. | Santo MA, Pajecki D, Riccioppo D, Cleva R, Kawamoto F, Cecconello I. Early complications in bariatric surgery: incidence, diagnosis and treatment. Arq Gastroenterol. 2013;50:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Zilberstein B, Santo MA, Carvalho MH. Critical analysis of surgical treatment techniques of morbid obesity. Arq Bras Cir Dig. 2019;32:e1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Elias AA, Roque-de-Oliveira M, Campos JM, Sasake WT, Bandeira ÁA, Silva LB, Ferreira B, Ito RM, Shirozaki HY, Benetti FA, Paiva LDS, Garrido Júnior AB. Robotic-assisted bariatric surgery: case series analysis and comparison with the laparoscopic approach. Rev Col Bras Cir. 2018;45:e1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Ferraz ÁAB, Vasconcelos CFM, Santa-Cruz F, Aquino MAR, Buenos-Aires VG, Siqueira LT. Surgical site infection in bariatric surgery: results of a care bundle. Rev Col Bras Cir. 2019;46:e2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Pajecki D, Kawamoto F, Dantas ACB, Andrade PC, Brasil NC, Junqueira SM, Oliveira FMP, Ribeiro RA, Santo MA. Real-world evidence of health outcomes and medication use 24 mo after bariatric surgery in the public healthcare system in Brazil: a retrospective, single-center study. Clinics (Sao Paulo). 2020;75:e1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Kotzampassi K, Shrewsbury AD. Intragastric balloon: ethics, medical need and cosmetics. Dig Dis. 2008;26:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Cho JH, Bilal M, Kim MC, Cohen J; Study Group for Endoscopic Bariatric and Metabolic Therapies of the Korean Society of Gastrointestinal Endoscopy. The Clinical and Metabolic Effects of Intragastric Balloon on Morbid Obesity and Its Related Comorbidities. Clin Endosc. 2021;54:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Moura D, Oliveira J, De Moura EG, Bernardo W, Galvão Neto M, Campos J, Popov VB, Thompson C. Effectiveness of intragastric balloon for obesity: A systematic review and meta-analysis based on randomized control trials. Surg Obes Relat Dis. 2016;12:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Sander BQ, Alberti LR, Moura DTH, Scarparo JIB, Arantes VN. “Analysis of Long-Term Weight Regain in Obese Patients Treated with Intragastric Balloon”. Acta Sci Gastro Dis. 2019;08-10. [DOI] [Full Text] |
| 12. | Abu Dayyeh BK, Maselli DB, Rapaka B, Lavin T, Noar M, Hussan H, Chapman CG, Popov V, Jirapinyo P, Acosta A, Vargas EJ, Storm AC, Bazerbachi F, Ryou M, French M, Noria S, Molina D, Thompson CC. Adjustable intragastric balloon for treatment of obesity: a multicentre, open-label, randomised clinical trial. Lancet. 2021;398:1965-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 13. | Caglar E, Dobrucali A, Bal K. Gastric balloon to treat obesity: filled with air or fluid? Dig Endosc. 2013;25:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Borges AC, Almeida PC, Furlani SMT, Cury MS, Gaur S. Intragastric balloons in high-risk obese patients in a Brazilian center: initial experience. Rev Col Bras Cir. 2018;45:e1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | de Moura DTH, de Moura EGH, Thompson CC. Endoscopic sleeve gastroplasty: From whence we came and where we are going. World J Gastrointest Endosc. 2019;11:322-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | de Miranda Neto AA, de Moura DTH, Ribeiro IB, Khan A, Singh S, da Ponte Neto AM, Madruga Neto AC, do Monte Junior ES, Tustumi F, Bernardo WM, de Moura EGH. Efficacy and Safety of Endoscopic Sleeve Gastroplasty at Mid Term in the Management of Overweight and Obese Patients: a Systematic Review and Meta-Analysis. Obes Surg. 2020;30:1971-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Itani MI, Farha J, Sartoretto A, Abbarh S, Badurdeen D, de Moura DTH, Kumbhari V. Endoscopic sleeve gastroplasty with argon plasma coagulation: A novel technique. J Dig Dis. 2020;21:664-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Sharaiha RZ, Hajifathalian K, Kumar R, Saunders K, Mehta A, Ang B, Skaf D, Shah S, Herr A, Igel L, Dawod Q, Dawod E, Sampath K, Carr-Locke D, Brown R, Cohen D, Dannenberg AJ, Mahadev S, Shukla A, Aronne LJ. Five-Year Outcomes of Endoscopic Sleeve Gastroplasty for the Treatment of Obesity. Clin Gastroenterol Hepatol. 2021;19:1051-1057.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 19. | Singh S, de Moura DTH, Khan A, Bilal M, Chowdhry M, Ryan MB, Bazarbashi AN, Thompson CC. Intragastric Balloon Versus Endoscopic Sleeve Gastroplasty for the Treatment of Obesity: a Systematic Review and Meta-analysis. Obes Surg. 2020;30:3010-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Vargas EJ, Bazerbachi F, Calderon G, Prokop LJ, Gomez V, Murad MH, Acosta A, Camilleri M, Abu Dayyeh BK. Changes in Time of Gastric Emptying After Surgical and Endoscopic Bariatrics and Weight Loss: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:57-68.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Berti LV, Campos J, Ramos A, Rossi M, Szego T, Cohen R. POSITION OF THE SBCBM - NOMENCLATURE AND DEFINITION OF OUTCOMES OF BARIATRIC AND METABOLIC SURGERY. Arq Bras Cir Dig. 2015;28 Suppl 1:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Hourneaux De Moura DT, Thompson CC. Endoscopic management of weight regain following Roux-en-Y gastric bypass. Expert Rev Endocrinol Metab. 2019;14:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Staudenmann DA, Sui Z, Saxena P, Kaffes AJ, Marinos G, Kumbhari V, Aepli P, Sartoretto A. Endoscopic bariatric therapies for obesity: a review. Med J Aust. 2021;215:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Dhindsa BS, Saghir SM, Naga Y, Dhaliwal A, Ramai D, Cross C, Singh S, Bhat I, Adler DG. Efficacy of transoral outlet reduction in Roux-en-Y gastric bypass patients to promote weight loss: a systematic review and meta-analysis. Endosc Int Open. 2020;8:E1332-E1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Brunaldi VO, Farias GFA, de Rezende DT, Cairo-Nunes G, Riccioppo D, de Moura DTH, Santo MA, de Moura EGH. Argon plasma coagulation alone vs argon plasma coagulation plus full-thickness endoscopic suturing to treat weight regain after Roux-en-Y gastric bypass: a prospective randomized trial (with videos). Gastrointest Endosc. 2020;92:97-107.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Jirapinyo P, de Moura DTH, Thompson CC. Endoscopic submucosal dissection with suturing for the treatment of weight regain after gastric bypass: outcomes and comparison with traditional transoral outlet reduction (with video). Gastrointest Endosc. 2020;91:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Mukewar S, Kumar N, Catalano M, Thompson C, Abidi W, Harmsen W, Enders F, Gostout C. Safety and efficacy of fistula closure by endoscopic suturing: a multi-center study. Endoscopy. 2016;48:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | de Moura DTH, Barrichello S Jr, de Moura EGH, de Souza TF, Dos Passos Galvão Neto M, Grecco E, Sander B, Hoff AC, Matz F, Ramos F, de Lima JHF, Teixeira L, Dib V, Falcão M, Potti H, Baretta G, Jirapinyo P, Thompson CC. Endoscopic sleeve gastroplasty in the management of weight regain after sleeve gastrectomy. Endoscopy. 2020;52:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 29. | Jirapinyo P, de Moura DTH, Thompson CC. Sleeve in sleeve: endoscopic revision for weight regain after sleeve gastrectomy. VideoGIE. 2019;4:454-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Hourneaux de Moura DT, Hathorn KE, Thompson CC. You Just Got Burned! What Is Wrong With This Gastric Pouch? Gastroenterology. 2019;156:2139-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | de Moura DTH, Sachdev AH, Lu PW, Ribeiro IB, Thompson CC. Acute bleeding after argon plasma coagulation for weight regain after gastric bypass: A case report. World J Clin Cases. 2019;7:2038-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | de Moura DTH, Sachdev AH, Thompson CC. Endoscopic Full-Thickness Defects and Closure Techniques. Curr Treat Options Gastroenterol. 2018;16:386-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | García-García ML, Martín-Lorenzo JG, Torralba-Martínez JA, Lirón-Ruiz R, Miguel Perelló J, Flores Pastor B, Pérez Cuadrado E, Aguayo Albasini JL. Emergency endoscopy for gastrointestinal bleeding after bariatric surgery. Therapeutic algorithm. Cir Esp. 2015;93:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Susmallian S, Danoch R, Raskin B, Raziel A, Barnea R, Dvora P. Assessing Bleeding Risk in Bariatric Surgeries: A Retrospective Analysis Study. Dig Dis. 2020;38:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Kumbhari V, Cummings DE, Kalloo AN, Schauer PR. AGA Clinical Practice Update on Evaluation and Management of Early Complications After Bariatric/Metabolic Surgery: Expert Review. Clin Gastroenterol Hepatol. 2021;19:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Maluf-Filho F, Lima MS, Hondo F, Giordano-Nappi JH, Garrido T, Sakai P. [Endoscopic placement of a "plug" made of acellular biomaterial: a new technique for the repair of gastric leak after Roux-en-Y gastric bypass]. Arq Gastroenterol. 2008;45:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Bartell N, Bittner K, Kaul V, Kothari TH, Kothari S. Clinical efficacy of the over-the-scope clip device: A systematic review. World J Gastroenterol. 2020;26:3495-3516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (6)] |
| 38. | Chan SM, Auyeung KKY, Lam SF, Chiu PWY, Teoh AYB. Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas. Dig Endosc. 2022;34:43-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Nedelcu M, Manos T, Noel P, Gagner M, Palermo M, Danan M, Nedelcu A, Vilallonga R. Aortic Injuries Following Stent Deployments in Bariatric Surgery-Review of Literature. J Laparoendosc Adv Surg Tech A. 2021;31:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Okazaki O, Bernardo WM, Brunaldi VO, Junior CCC, Minata MK, de Moura DTH, de Souza TF, Campos JM, Santo MA, de Moura EGH. Efficacy and Safety of Stents in the Treatment of Fistula After Bariatric Surgery: a Systematic Review and Meta-analysis. Obes Surg. 2018;28:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | de Moura DTH, de Moura EGH, Neto MG, Jirapinyo P, Teixeira N, Orso I, Quadros LG, Amorim A, Medeiros F, Neto DR, de Siqueira Neto J, Albano A, de Sousa LH, Almeida D, Marchetti IA, Ivano F, de Lima JHF, Falcão M, Thompson CC. Outcomes of a novel bariatric stent in the management of sleeve gastrectomy leaks: a multicenter study. Surg Obes Relat Dis. 2019;15:1241-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Hamid HKS, Emile SH, Saber AA, Dincer M, de Moura DTH, Gilissen LPL, Almadi MA, Montuori M, Vix M, Perisse LGS, Quezada N, Garofalo F, Pescarus R. Customized bariatric stents for sleeve gastrectomy leak: are they superior to conventional esophageal stents? Surg Endosc. 2021;35:1025-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | De Moura DTH, Baptista A, Jirapinyo P, De Moura EGH, Thompson C. Role of Cardiac Septal Occluders in the Treatment of Gastrointestinal Fistulas: A Systematic Review. Clin Endosc. 2020;53:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Baptista A, Hourneaux De Moura DT, Jirapinyo P, Hourneaux De Moura EG, Gelrud A, Kahaleh M, Salinas A, Sabagh LC, Ospina A, Rincones VZ, Doval R, Bandel JW, Thompson CC. Efficacy of the cardiac septal occluder in the treatment of post-bariatric surgery leaks and fistulas. Gastrointest Endosc. 2019;89:671-679.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Farias GFA, Bernardo WM, De Moura DTH, Guedes HG, Brunaldi VO, Visconti TAC, Gonçalves CVT, Sakai CM, Matuguma SE, Santos MELD, Sakai P, De Moura EGH. Endoscopic vs surgical treatment for pancreatic pseudocysts: Systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e14255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 46. | Fuentes-Valenzuela E, García-Alonso FJ, Tejedor-Tejada J, Nájera-Muñoz R, de Benito Sanz M, Sánchez-Ocaña R, de la Serna Higuera C, Pérez-Miranda M. Endoscopic internal drainage using transmural double-pigtail stents in leaks following upper gastrointestinal tract surgery. Rev Esp Enferm Dig. 2021;113:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Sánchez-Luna SA, Guimãraes Hourneaux De Moura E, Sena de Medeiros F, Turiani Hourneaux De Moura D. Does it matter which plastic stents we use for the treatment of post-surgical leaks? Rev Esp Enferm Dig. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Baretta G, Campos J, Correia S, Alhinho H, Marchesini JB, Lima JH, Neto MG. Bariatric postoperative fistula: a life-saving endoscopic procedure. Surg Endosc. 2015;29:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | de Moura DTH, de Moura BFBH, Manfredi MA, Hathorn KE, Bazarbashi AN, Ribeiro IB, de Moura EGH, Thompson CC. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc. 2019;11:329-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (6)] |
| 50. | de Moura DTH, Hirsch BS, Do Monte Junior ES, McCarty TR, de Medeiros FS, Thompson CC, de Moura EGH. Cost-effective modified endoscopic vacuum therapy for the treatment of gastrointestinal transmural defects: step-by-step process of manufacturing and its advantages. VideoGIE. 2021;6:523-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Markus A, Henrik BJ, Benedikt R, Alexander H, Thomas B, Clemens S, Jan-Hendrik E. Endoscopic vacuum therapy in salvage and standalone treatment of gastric leaks after bariatric surgery. Langenbecks Arch Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | do Monte Junior ES, de Moura DTH, Ribeiro IB, Hathorn KE, Farias GFA, Turiani CV, Medeiros FS, Bernardo WM, de Moura EGH. Endoscopic vacuum therapy vs endoscopic stenting for upper gastrointestinal transmural defects: Systematic review and meta-analysis. Dig Endosc. 2021;33:892-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Ukleja A, Afonso BB, Pimentel R, Szomstein S, Rosenthal R. Outcome of endoscopic balloon dilation of strictures after laparoscopic gastric bypass. Surg Endosc. 2008;22:1746-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Bazerbachi F, Heffley JD, Abu Dayyeh BK, Nieto J, Vargas EJ, Sawas T, Zaghlol R, Buttar NS, Topazian MD, Wong Kee Song LM, Levy M, Keilin S, Cai Q, Willingham FF. Safety and efficacy of coaxial lumen-apposing metal stents in the management of refractory gastrointestinal luminal strictures: a multicenter study. Endosc Int Open. 2017;5:E861-E867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Joo MK. Endoscopic Approach for Major Complications of Bariatric Surgery. Clin Endosc. 2017;50:31-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Marins Campos J, Moon RC, Magalhães Neto GE, Teixeira AF, Jawad MA, Bezerra Silva L, Neto MG, Ferraz ÁA. Endoscopic treatment of food intolerance after a banded gastric bypass: inducing band erosion for removal using a plastic stent. Endoscopy. 2016;48:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Klimczak T, Szewczyk T, Janczak P, Jurałowicz P. Laparoscopic Adjustable Gastric Band (LAGB) Migration - Endoscopic Treatment Modalities. Pol Przegl Chir. 2016;88:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Dellaportas D, Nastos C, Theodosopoulos T, Fragulidis G, Polydorou A, Vezakis A. Novel Endoscopic Management of Eroding Laparoscopic Adjustable Gastric Band: A Case Series. Case Rep Gastrointest Med. 2018;2018:2747852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Brunaldi VO, Galvao Neto M, Zundel N, Abu Dayyeh BK. Isolated sleeve gastrectomy stricture: a systematic review on reporting, workup, and treatment. Surg Obes Relat Dis. 2020;16:955-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | de Moura DTH, Jirapinyo P, Aihara H, Thompson CC. Endoscopic tunneled stricturotomy in the treatment of stenosis after sleeve gastrectomy. VideoGIE. 2019;4:68-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |