Published online Dec 27, 2022. doi: 10.4240/wjgs.v14.i12.1418
Peer-review started: July 29, 2022
First decision: October 5, 2022
Revised: October 18, 2022
Accepted: November 20, 2022
Article in press: November 20, 2022
Published online: December 27, 2022
Processing time: 151 Days and 4.5 Hours
Differential diagnosis of colorectal intramucosal tumors from invasive adenocarcinoma is important in clinical practice due to the different risks of lymph node metastasis and different treatment options. The phenomenon of a colorectal adenoma with part of the gland entering the submucosa is known as pseudoinvasion of the adenoma, which is a major challenge for pathological diagnosis. It is essential to raise awareness of colorectal adenoma with submucosal pseudoinvasion clinically to avoid overtreatment.
We describe a case of rectal adenoma with submucosal pseudoinvasion in a 48-year-old man. The patient was admitted to Jinhua People's Hospital due to a change in stool habit for 5 d. We performed colonoscopy, and the results suggested a submucosal bulge approximately 1.0 cm × 1.0 cm in size in the rectum 8 cm from the anal verge, with red surface erosion. Ultrasound colonoscopy was also performed and a homogeneous hypoechoic mass about 0.52 cm × 0.72 cm in size was seen at the lesion, protruding into the lumen with clear borders and invading the submucosa. Endoscopic surgery was then performed and the pathological specimen showed a tubular adenoma with high-grade intraepithelial neoplasia (intramucosal carcinoma) involving the adenolymphatic complex. In addition, we performed a literature review of rectal tubular adenoma with submucosal pseudoinvasion to obtain a deeper understanding of this disease.
The aim of this study was to improve awareness of this lesion for clinicians and pathologists to reduce misdiagnosis.
Core Tip: Colorectal adenoma with submucosal pseudoinvasion has only been studied in a small number of small cases in the current national and international literature. At present, endoscopists diagnose our patient's lesion by electronic staining endoscopy (NBI), magnification endoscopy and ultrasound en
- Citation: Chen D, Zhong DF, Zhang HY, Nie Y, Liu D. Rectal tubular adenoma with submucosal pseudoinvasion misdiagnosed as adenocarcinoma: A case report. World J Gastrointest Surg 2022; 14(12): 1418-1424
- URL: https://www.wjgnet.com/1948-9366/full/v14/i12/1418.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i12.1418
Both neoplastic and non-neoplastic epithelium of the mucosa may enter the submucosa for some reason, a phenomenon known as pseudoinvasion or misplaced epithelium of the submucosa. When part of the gland of a colorectal adenoma mistakenly enters the submucosa, it is called pseudoinvasion of the adenoma[1]. The incidence of colorectal adenoma with submucosal pseudoinvasion is low, and those occurring in the rectal region are extremely rare, with few reports in the national and international literature. The definitive diagnosis relies mainly on pathological support[2]. If the pathologist misjudges, this will lead to overtreatment in clinical practice. Here we present a case of rectal adenoma with submucosa pseudoinvasionin order to benefit both patients andpractitioners.
A 48-year-old man was admitted to Jinhua People's Hospital on June 29, 2021 due to change in stool habit for 5 d.
His symptoms started 5 d previously and were accompanied by a change in stool habit (yellow, thin, pasty stools 3-5 times a day, without mucus and blood). No significant change in body weight was noted.
The patient had a history of surgery for hypofractionated adenocarcinoma of the stomach 6 mo ago. According to the World Health Organization (WHO) Classification of Digestive System Tumor, hypofractionated adenocarcinoma is defined as the cancer cells are short columnar or indefinite, arranged in small nests or strands, and basically without glandular tube structure.
The patient had no relevant personal and family history.
On examination, his abdomen was soft, old surgical scars were visible in the upper abdomen, and no pressure pain, rebound pain, or masses were found.
Laboratory examinationsshowed that routine blood, urine, stool, liver and kidney function, carcinoembryonic antigen, alpha-fetoprotein and carbohydrate antigen 199 were all within the normal range.
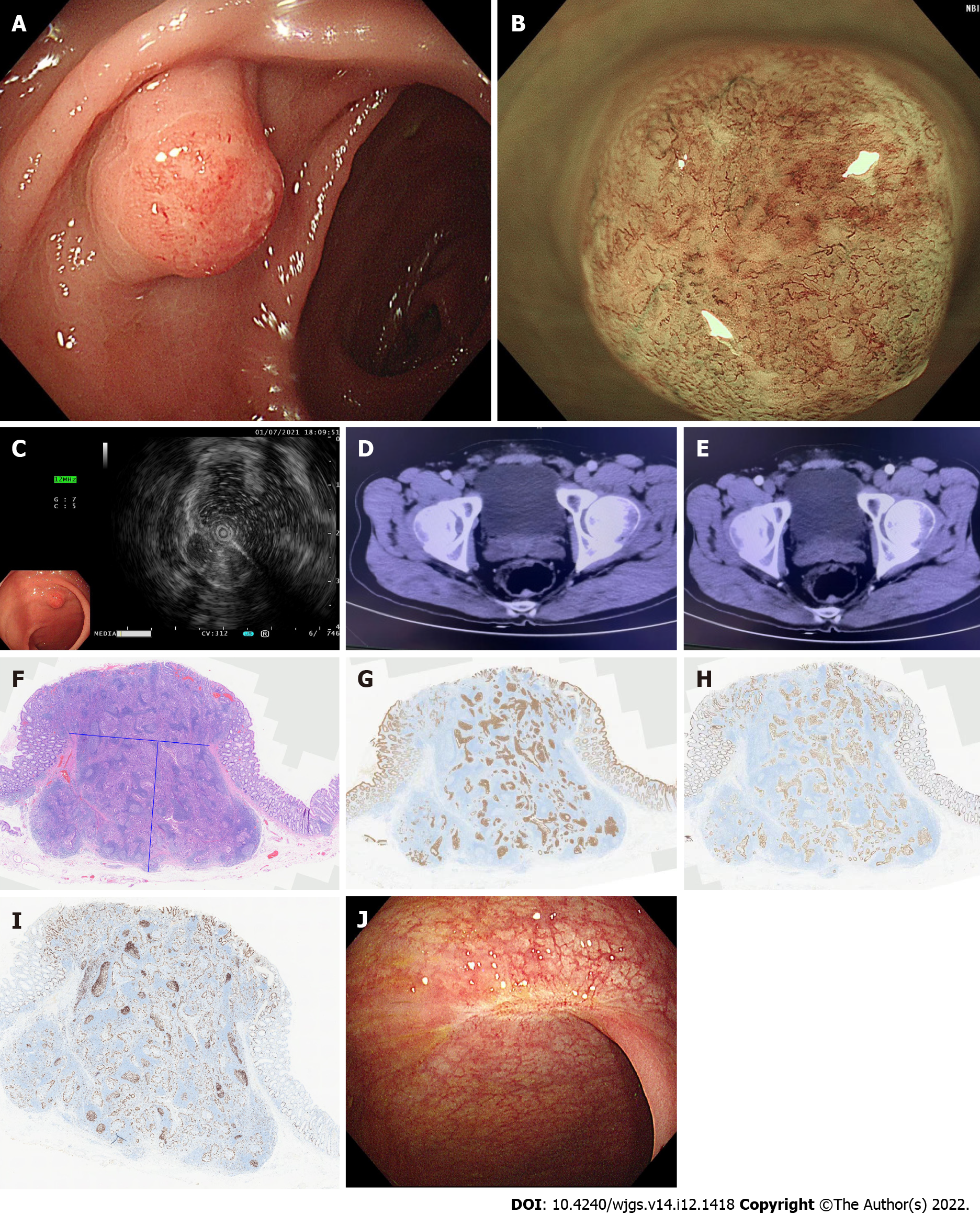
The patient was advised to undergo abdominal enhanced computed tomography (CT) and colonoscopy, and the results of colonoscopy on July 1, 2021 suggested a submucosal bulge approximately 1.0 cm × 1.0 cm in the rectum 8 cm from the anal verge, with red surface erosion (Figure 1A). Narrow band imaging (NBI) and magnification colonoscopy showed uneven caliber and distribution of blood vessels. Type2B was considered according to JNET staging (Figure 1B).
Ultrasound colonoscopy was performed on July 1, 2021 and a homogeneous hypoechoic mass about 0.52 cm × 0.72 cm in size was seen at the lesion, protruding into the lumen with clear borders and invading the submucosa (Figure 1C). CT on June 30, 2021 (enhancement of two sites) showed a nodule in the posterior rectal wall (Figure 1D and E).
Postoperative pathological results in our hospital [rectal endoscopic submucosal dissection (ESD) specimen] showed moderately differentiated adenocarcinoma with significant hyperplasia of lymphoid tissue, about 1.0 cm × 0.8 cm in size, infiltrated to the submucosa. No cancer thrombus was seen in the vasculature, and the surrounding cut edge was not involved approximately 200 µm from the basal cut edge. Immunohistochemical results were as follows: CD10 (-), CD56 (-), CDX2 (+), CgA (-), CK20 (+), CK7 (-), EGFR (1+), Ki67 (30%+), P53 (missense expression), and Syn (-).
In order to quickly improve the pathological understanding of early GI tumors in our hospital and better carry out our ESD surgery, the pathological specimen was sent to the Department of Pathology of the Second Affiliated Hospital of Zhejiang University School of Medicine and the results suggested (rectal ESD specimen) tubular adenoma with high-grade intraepithelial neoplasia (intramucosal carcinoma) involving the adenolymphatic complex. No clear vascular invasion was seen. The horizontal and vertical margins were negative (Figure 1F-I). Based on the above pathological findings, the patient was advised to undergo repeat colonoscopy. A follow-up colonoscopy on January 12, 2022 showed post-rectal scar formation and no local recurrence. A repeat pelvic MRI on April 12, 2022 suggested post-rectal changes and no lymphatic metastases were found.
After full communication with the patient and obtaining his consent, ESD was performed on July 5, 2021.
One year after ESD, colonoscopy showed a postoperative scar in the rectum without local recurrence (Figure 1J).
Pseudoinvasion or misplaced epithelium of the submucosa was first described by Muto et al[3] in 1972 and its characteristic histological manifestation was defined as the mislocation of non-neoplastic or adenomatous epithelium into the submucosa for some reasons. Its predilection is in the sigmoid colon, accounting for about 85% of cases, followed by the descending colon, accounting for about 10%, and the rectum is relatively uncommon[2]. The average age of patients is 60 years for men and 56 years for women, similar to the average age of patients with common adenomatous polyps, with a male to female ratio of 3:1. Single lesion resection or local excision was effective, with no recurrence or metastasis at follow-up[3]. In 2006, a national colorectal cancer screening program was launched in the United Kingdom, and as the program progressed, an increasing number of difficult cases emerged, among which the differential diagnosis of epithelial malposition of colonic adenoma and adenocarcinoma was the most difficult. Therefore, an Expert Board was established to analyze and discuss the pathologies with diagnostic doubts. The percentage of misplaced epithelium diagnosed by the original pathologists increased from 30.6% to 80.3%, indicating that pathologists lack sufficient knowledge of this pathology and a number of misdiagnoses occurred[4]. This is undoubtedly a major challenge in clinical and pathological diagnosis.
Colorectal adenoma with submucosal pseudoinvasion has only been studied in a small number of cases in the current national and international literature. The morphological patterns were sorted and categorized in a limited number of cases, and two patterns of pseudoinvasion were summarized[5,6]. One type is lobulated, defined as a submucosal neoplastic gland forming a lobulated or nested mass. This type is more prevalent in the sigmoid colon with leptomeningeal lesions. The mechanism of onset may be increased luminal pressure due to intestinal contractility and repeated physical injury due to traction, or tip torsion due to intestinal peristalsis, impaired blood flow, and subsequent entry of the gland into the submucosa through a relaxed mucosal muscle gap or weak area. Therefore, this type of pseudoinvasion may occur with ruptured glandular necrosis and hemorrhage, causing an inflammatory reaction and fibrosis[1,2,5,7]. The other type is lymphoglandular complex-like, in which the adenoma participates in the formation of a lymphoglandular complex into the submucosa, which can mimic invasive adenocarcinoma with lymphatic metastasis, with broad-based elevated lesions being the most common[5]. The available case studies show that almost all adenomas with pseudoinvasion have a maximum diameter greater than 10 mm, which means that there is a correlation between the size of the adenoma and the presence of submucosal pseudoinvasion. It can be inferred that even in the absence of excessive pressure or mechanical force, adenomas can easily enter the submucosa through the mucosal weak zone when they exceed a certain size[1,4,5,8]. When diagnosing colorectal adenoma with submucosal pseudoinvasion, it is important to be alert to the possibility that both patterns of pseudoinvasion components may have direct true infiltration in the submucosa, which should be treated as invasive adenocarcinoma, and therefore the pathology report needs to reflect the presence or absence of true infiltration[9]. In addition to the pathomorphological features of interest, in a small number of case studies of submucosal pseudoinvasion[6], immunohistochemical testing was performed and a Ki-67 positivity index of 25%-80% was found. P53 showed a wild-type pattern in positive cells within adenoma tissue and a missense mutation pattern in tumor tissue within a pooled lymph node that continued with a region of high-grade heterogeneous hyperplasia in one case.
The principles of the treatment options for colorectal adenoma with submucosal pseudoinvasion are consistent with those for colorectal intramucosal tumors, with endoscopic mucosal resection or ESD being the mainstay. Colorectal intramucosal tumors are defined as tumor infiltration confined to the mucosal layer (M-stage carcinoma). Those infiltrating into the submucosal layer without invading the intrinsic muscular layer are called submucosal carcinoma (SM-stage carcinoma)[10]. Those infiltrating into the upper 1/3, middle 1/3, and lower 1/3 of the submucosal layer are defined as SM1-stage carcinoma, SM2-stage carcinoma, and SM3-stage carcinoma, respectively[11]. The WHO classification of gastrointestinal tumors describes colorectal carcinoma as "epithelial malignant tumors originating from the colorectum which are diagnosed as cancer when they penetrate the mucosal muscle and infiltrate into the submucosa"[9]. Colorectal intramucosal tumors and invasive adenocarcinoma both have significantly different risks in terms of local recurrence and lymph node metastasis[12,13]. The absence of lymph node as well as vascular metastasis in intramucosal carcinoma is an absolute indication for endoscopic treatment. The percentage of lymph node metastasis from tumor infiltration to the superficial submucosa (SM1) is only 3.3%. Therefore, it can be a relative indication for endoscopic treatment. However, a rigorous pathological evaluation is required to determine whether lymphatic and vascular infiltration is present, and the need for additional surgical procedures will be determined on a situational basis. A previous report[14] showed no significant difference in the efficacy of endoscopic and surgical treatment for intramucosal and superficial submucosal carcinoma. For highly infiltrative submucosal lesions, additional surgery is required for submucosal infiltrations of 1000 µm or more[15].
Our patient's rectal lesion was diagnosed based on the morphology, vascular configuration, and surface structure in terms of pathological type by plain endoscopy, electronic staining endoscopy (NBI), and magnification endoscopy. A more direct diagnosis of the depth of infiltration was obtained vertically by ultrasound enteroscopy. Ultrasound can clearly display the structure of each layer of the colorectal wall and accurately determine the depth of lesion invasion and infiltration of surrounding organs[16-18]. Although crystal violet staining is suitable for the precise diagnosis of glandular duct openings in the pit pattern, it is currently not recommended in vivo due to its toxicity; therefore, it was not performed in this patient. After comprehensive evaluation, the preoperative lesion was considered to be superficial submucosal invasive carcinoma, and endoscopic ESD surgery is still indicated. Endoscopic surgery was performed after fully informing the patient of his condition and obtaining his consent. The postoperative pathological findings showed specific changes that led us to misdiagnose it as an invasive moderately differentiated adenocarcinoma. Fortunately, the pathological staging, basal cut margins, depth of submucosal invasion, and vascular invasion of this lesion did not suggest the need for additional surgery and did not result in overtreatment. The lesion was found to be a rare high-grade tubular adenoma of the rectum with pseudoinvasion of the submucosa only after late review of the pathology. To date, no effective clinical adjuvant examination has been availableto confirm whether the lesion is pseudoinvasion or not[2,4,14,19], and the definitive diagnosis still relies to a great extent on the pathologists' knowledge of its pathology.
The aim of this study was to improve the awareness of clinicians and pathologists regarding this type of lesion, in order to reduce the probability of misdiagnosis as invasive adenocarcinoma and avoid overtreatment.
The authors sincerely thank the participants for their help and willingness to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Azeem MAA, Egypt; Osera S, Japan; Yap RVC, Philippines S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Panarelli NC, Somarathna T, Samowitz WS, Kornacki S, Sanders SA, Novelli MR, Shepherd NA, Yantiss RK. Diagnostic Challenges Caused by Endoscopic Biopsy of Colonic Polyps: A Systematic Evaluation of Epithelial Misplacement With Review of Problematic Polyps From the Bowel Cancer Screening Program, United Kingdom. Am J Surg Pathol. 2016;40:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Shepherd NA, Griggs RK. Bowel cancer screening-generated diagnostic conundrum of the century: pseudoinvasion in sigmoid colonic polyps. Mod Pathol. 2015;28 Suppl 1:S88-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Muto T, Bussey HJ, Morson BC. Pseudo-carcinomatous invasion in adenomatous polyps of the colon and rectum. J Clin Pathol. 1973;26:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Griggs RK, Novelli MR, Sanders DS, Warren BF, Williams GT, Quirke P, Shepherd NA. Challenging diagnostic issues in adenomatous polyps with epithelial misplacement in bowel cancer screening: 5 years' experience of the Bowel Cancer Screening Programme Expert Board. Histopathology. 2017;70:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Lee HE, Wu TT, Chandan VS, Torbenson MS, Mounajjed T. Colonic Adenomatous Polyps Involving Submucosal Lymphoglandular Complexes: A Diagnostic Pitfall. Am J Surg Pathol. 2018;42:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Hou WH, Song SJ, Hou WD, Shen MQ, Ma LJ, Niu JW, Zhang XL, Wang GW, Jin ML. [Pathological characteristics of colorectal adenoma with submucosal pseudoinvasion]. Zhonghua Bing Li Xue Za Zhi. 2021;50:32-37. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Yantiss RK, Goldman H, Odze RD. Hyperplastic polyp with epithelial misplacement (inverted hyperplastic polyp): a clinicopathologic and immunohistochemical study of 19 cases. Mod Pathol. 2001;14:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Zhou S, Ma Y, Chandrasoma P. Inverted lymphoglandular polyp in descending colon. Case Rep Pathol. 2015;2015:646270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | WHO Classification of Tumours Editorial Board. WHO classification of tumours. Digestive system tumours. 5th ed. Lyon: IARC Press 2019: 60-95. |
| 10. | Intestinal Study Group; Chinese Society of Digestive Endoscopy; Jiang B, Liu SD. Chinese consensus: endoscopic diagnosis and treatment of early large bowel cancer. Zhonghua Xiaohua Neijing Zazhi. 2008;25:617-620. [DOI] [Full Text] |
| 11. | Endoscopic Submucosal Dissection Experts’ Cooperation Group. Consensus: Endoscopic submucosal dissection of Gastrointestinal mucous membrane. Zhonghua Weichang Waike Zazhi. 2012;15:1083-1086. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum. 2009;52:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Loughrey MB, Shepherd NA. Problematic Colorectal Polyps: Is It Cancer and What Do I Need to Do About It? Surg Pathol Clin. 2017;10:947-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Zhang YH, Sheng JQ, Chen ZM, Yan W, Yu DL, Li AQ, Wang HH, Han Y, Li SR. Clinical Evaluation of Traetment on Early-Stage Colorectal Cancer by Endoscopic Resection. Weichangbing Xue. 2007;12:5-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Shinei K, Meng N. Endoscopic treatment of neoplasms in colon and rectum. Liaoning Science and Technology Press 2007: 31-38. [DOI] [Full Text] |
| 16. | Zhou PH, Yao LQ, Xu MD, Liang Z, Qin XY. Endoscopic ultrasound diagnosis of colorectal cancer invasion depth and surrounding lymph nodes metastasis (with 60 cases report). Zhongguo Neijing Zazhi. 2001;7:12-14. [DOI] [Full Text] |
| 17. | Zhou PH, Yao LQ. The value of ultrasonic mini probe in the diagnosis of lesions of lower digestive tract. Zhonghua Xiaohua Neijing Zazhi. 2002;19:205-207. [DOI] [Full Text] |
| 18. | Xia L, Dai X, Liu HL, Lin MB, Yin L, Zhu Q. Endoscopic ultrasonography with combination of miniature probe and radial scanning in preoperative staging for rectal cancer. Zhonghua Xiaohua Neijing Zazhi. 2009;26:175-179. [DOI] [Full Text] |
| 19. | Kealy WF. Colonic lymphoid-glandular complex (microbursa): nature and morphology. J Clin Pathol. 1976;29:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |