Published online Dec 27, 2022. doi: 10.4240/wjgs.v14.i12.1387
Peer-review started: September 25, 2022
First decision: October 20, 2022
Revised: October 28, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: December 27, 2022
Processing time: 93 Days and 5.6 Hours
With the development of laparoscopic techniques, gallbladder cancer (GBC) is no longer a contraindication to laparoscopic surgery (LS). Although LS is recom
To evaluate the short- and long-term outcomes of LS in comparison to those of open surgery (OS) for stage T2 GBC.
We searched the PubMed, Embase, Cochrane Library, Ovid, Google Scholar, and Web of Science databases for published studies comparing the efficacy of LS and OS in the treatment of stage T2 GBC, with a cutoff date of September 2022. The Stata 15 statistical software was used for analysis. Relative risk (RR) and weighted mean difference (WMD) were calculated to assess binary and continuous outcome indicators, respectively. Begg’s test and Egger’s test were used for detecting publication bias.
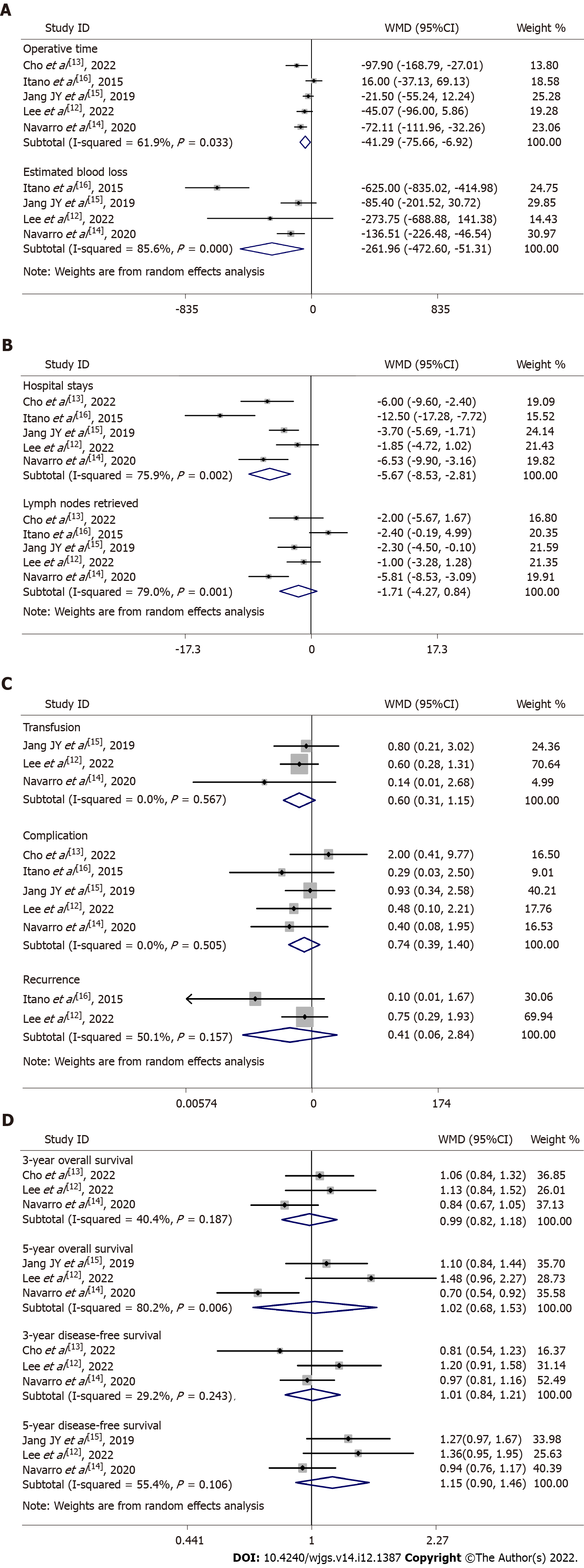
A total of five studies were included, with a total of 297 patients, 153 in the LS group and 144 in the OS group. Meta-analysis results showed that the LS group was better than the OS group in terms of operative time [WMD = -41.29, 95% confidence interval (CI): -75.66 to -6.92, P = 0.02], estimated blood loss (WMD = -261.96, 95%CI: -472.60 to -51.31, P = 0.01), and hospital stay (WMD = -5.67, 95%CI: -8.53 to -2.81, P = 0.0001), whereas there was no significant difference between the two groups in terms of blood transfusion (RR = 0.60, 95%CI: 0.31-1.15, P = 0.13), complications (RR = 0.72, 95%CI: 0.39-1.33, P = 0.29), number of lymph nodes retrieved (WMD = –1.71, 95%CI: -4.27 to -0.84, P = 0.19), recurrence (RR = 0.41, 95%CI: 0.06-2.84, P = 0.36), 3-year and 5-year overall survival (RR = 0.99, 95%CI: 0.82-1.18, P = 0.89 and RR = 1.02, 95%CI: 0.68-1.53, P = 0.92; respectively), and 3-year and 5-year disease-free survival (RR = 1.01, 95%CI: 0.84-1.21, P = 0.93 and RR = 1.15, 95%CI: 0.90-1.46, P = 0.26; respectively).
The long-term outcomes of LS for T2 GBC are similar to those of OS, but LS is superior to OS in terms of operative time, intraoperative bleeding, and postoperative hospital stay. Nevertheless, these findings should be validated via high-quality randomized controlled trials and longer follow-ups.
Core Tip: This study evaluated the safety and efficacy of laparoscopic surgery in comparison to those of open surgery for stage T2 gallbladder cancer. A total of five studies were included after retrieving various literature databases, with a cutoff date of September 2022. Meta-analysis results showed that the laparoscopic surgery group was better than the open surgery group in terms of operative time, estimated blood loss, and hospital stay, whereas there was no significant difference between the two groups in terms of blood transfusion, complications, number of lymph nodes retrieved, recurrence, and 3-year and 5-year overall and disease-free survival rates.
- Citation: Zhang W, Ouyang DL, Che X. Short- and long-term outcomes of laparoscopic vs open surgery for T2 gallbladder cancer: A systematic review and meta-analysis. World J Gastrointest Surg 2022; 14(12): 1387-1396
- URL: https://www.wjgnet.com/1948-9366/full/v14/i12/1387.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i12.1387
Gallbladder cancer (GBC) is one of the most common malignancies of the biliary system and has the sixth highest incidence among gastrointestinal tumors[1]. Radical resection is the only potentially curative treatment for GBC[2-4]. Traditional open extended cholecystectomy, including regional lymph node dissection and wedge resection of the gallbladder bed, is the standard radical surgery for stage T2 GBC[5,6]. Since the late 1980s, laparoscopic surgery (LS) has been widely used to treat benign gallbladder disease, and GBC has been considered a contraindication to LS[7,8]. With the continuous improvement of devices and techniques in recent years, curative resection of gastrocolic cancer and liver cancer in difficult sites and even pancreaticoduodenectomy can be conducted laparoscopically. Additionally, LS is increasingly employed in radical resection of stage T1a GBC, and thus GBC is no longer a contraindication to LS[9]. However, the short- and long-term outcomes of LS for stage T2 GBC are still controversial.
Although there are still concerns about the efficacy of laparoscopic radical surgery of stage T2 GBC, LS has already been exploratively applied to treat patients with T2 GBC, and even T3 GBC, at several large medical institutes. There has been a rapid increase in incidental GBC with the widespread use of laparoscopic techniques in benign gallbladder disease, especially in patients with T2 GBC[10,11]. It has become a point of debate whether LS is safe for the treatment of T2 GBC and whether open surgery (OS) is required.
Previous studies on T2 GBC have been limited to case reports or small sample retrospective single arm case series on the technical feasibility, safety, and oncological outcomes. Several recent studies have reported long-term outcomes of laparoscopic treatment of stage T2 GBC[12-16]. As there is still a lack of evidence from high-quality multicenter randomized controlled trials (RCTs), we believe that it is necessary to conduct a meta-analysis to provide an evidence-based reference for laparoscopic radical surgery of T2 GBC.
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[17]. The data used in this study were derived from published studies and are anonymous. This study did not need informed consent from patients or a review by an institutional ethics committee. This meta-analysis was registered under the registration number CRD42022367334 on the systematic review registration platform PROSPERO (https://www.crd.york.ac.uk/PROSPERO/). We also cited high-quality articles in Reference Citation Analysis (https://www.referencecitationanalysis.com).
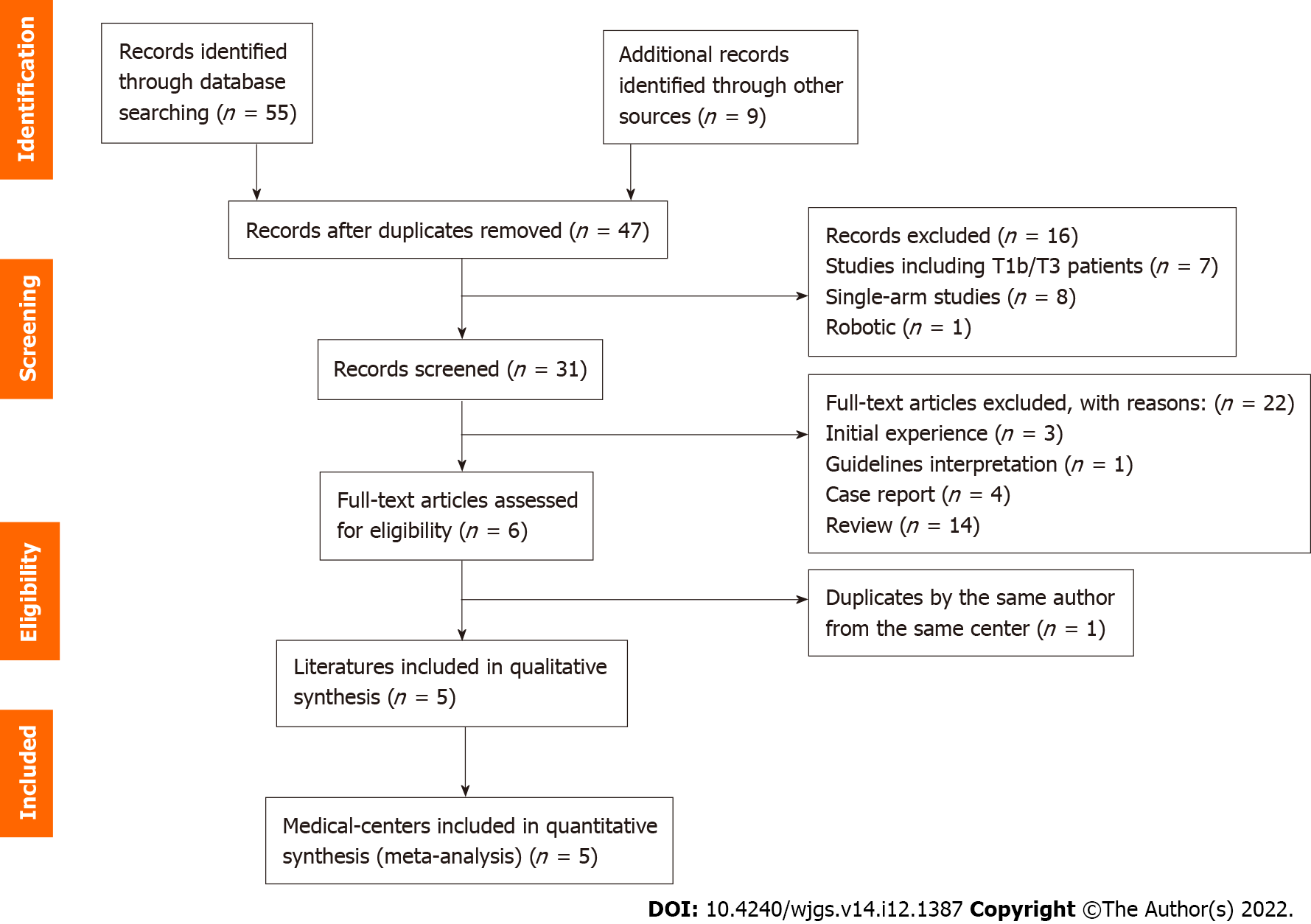
The PubMed, Medline, Cochrane Library, Ovid, Google Scholar, and Web of Science databases were searched with a cutoff date of September 2022. The search topics were “laparosco*”, “open”, “extended cholecystectomy”, “open surgery” and “T2 gallbladder cancer”. The search strategy for each database is described in the Supplementary material. We also conducted an expanded search based on the references of the retrieved publications. Table 1 lists the basic characteristics of the included studies.
| Ref. | Country | Type | Period | Case | Age | Sex (M/F) | Liver resection1 | Quality | |||
| LS vs OS | LS | OS | LS | OS | LS | OS | |||||
| Lee et al[12], 2022 | Korea | R | 2011-2018 | 20 vs 24 | 71.85 ± 9.11 | 68.08 ± 10.64 | 5/15 | 11/13 | 1/4/15 | 1/13/10 | 7 |
| Cho et al[13], 2022 | Korea | R (PSM) | 2010-2017 | 19 vs 19 | 69.9 ± 9.1 | 66.7 ± 7.8 | 8/11 | 12/7 | NA | NA | 6 |
| Navarro et al[14], 2020 | Korea | R (PSM) | 2005-2017 | 43 vs 43 | 66.7 ± 10.3 | 65.4 ± 7.6 | 25/18 | 28/15 | 38/5/0 | 23/12/8 | 6 |
| Jang et al[15], 2019 | Korea | R | 2004-2017 | 55 vs 44 | 70.1 ± 8.1 | 65.5 ± 10.5 | 19/36 | 23/21 | 38/16/1 | 9/32/3 | 8 |
| Itano et al[16], 2015 | Japan | R | 2003-2013 | 16 vs 14 | 68.1 ± 19.9 | 71.5 ± 13.2 | 9/7 | 5/9 | NA | NA | 7 |
(1) Population: Stage T2 GBC; (2) Intervention: LS; (3) Comparison: OS; (4) Study sample size: Unlimited; (5) Type of studies: RCTs and prospective or retrospective cohort studies; (6) Follow-up time: Unlimited; (7) Language type of the publications: Unlimited; (8) Study type: Human studies; and (9) Primary outcomes: Overall survival, disease-free survival, recurrence, and the number of lymph nodes removed. Secondary outcomes: Operative time, intraoperative blood loss, hospital stay, and postoperative complications.
(1) Studies with unknown follow-up times or incomplete data and no response from the contact author and those not peer-reviewed; (2) Single arm studies with LS or OS; and (3) Robots, reviews, case reports, and animal studies.
The quality of the cohort studies (retrospective or prospective) was assessed using the Newcastle-Ottawa Scale, which specifically included study population selection, comparability, and exposure evaluation or outcome evaluation. The RCTs were conducted for the risk assessment according to the “risk assessment tool” recommended by the Cochrane Collaboration Network[18-20].
The meta-analysis was performed using the STATA SE 13 software. Relative risk (RR) and weighted mean difference (WMD) were used to calculate the pooled statistics for binary and continuous data, respectively, and the 95% confidence interval (CI) was reported for each. Heterogeneity was assessed using the χ2 test, with the significance level set at P = 0.05. This meta-analysis was carried out using a random effects model. P < 0.05 was considered to indicate statistical significance[21]. Begg’s test and Egger’s test were performed using the Stata 15 software to quantitatively assess each outcome for publication bias. Funnel plots were drawn to qualitatively and visually assess the outcomes for publication bias.
After searching the publication databases and excluding duplications, 47 articles remained. We then excluded the reviews (including systematic reviews), case reports, and meta-analyses as well as the studies that were not relevant based on their titles or abstracts, finally leaving five publications to be employed in this meta-analysis. The detailed steps of the publication retrieval are shown in Figure 1. These five publications involved one study from Japan and four studies from South Korea. The basic characteristics of the included studies are shown in Table 1. The included studies were all cohort studies, and the quality was evaluated using the Newcastle-Ottawa Scale. The Newcastle-Ottawa Scale scores are attached to Supplementary Table 1.
We compared LS and OS for T2 GBC in 11 postoperative outcomes, each of which was analyzed for sensitivity. The results of the meta-analysis are summarized in Table 2. Random effects models were used to obtain the effect sizes.
| Measured outcomes | Studies, n | Heterogeneity test | Model | RR/WMD | 95%CI | P value | |
| I2 (%) | P value | ||||||
| Operative time | 5 | 62 | 0.03 | Random | -41.29 | -75.66, -6.92 | 0.02a |
| Intraoperative blood loss | 4 | 86 | 0.0001 | Random | -261.96 | -472.60, -51.31 | 0.01a |
| Hospital stays | 5 | 76 | 0.002 | Random | -5.67 | -8.53, -2.81 | 0.0001a |
| Lymph nodes retrieved | 5 | 79 | 0.0008 | Random | -1.71 | -4.27, 0.84 | 0.19 |
| Transfusion | 3 | 0 | 0.57 | Random | 0.60 | 0.31, 1.15 | 0.13 |
| Complication | 5 | 0 | 0.5 | Random | 0.72 | 0.39, 1.33 | 0.29 |
| Recurrence | 2 | 50 | 0.16 | Random | 0.41 | 0.06, 2.84 | 0.36 |
| 3-yr OS | 3 | 40 | 0.19 | Random | 0.99 | 0.82, 1.18 | 0.89 |
| 5-yr OS | 3 | 80 | 0.006 | Random | 1.02 | 0.68, 1.53 | 0.92 |
| 3-yr DFS | 3 | 29 | 0.24 | Random | 1.01 | 0.84, 1.21 | 0.93 |
| 5-yr DFS | 3 | 55 | 0.11 | Random | 1.15 | 0.90, 1.46 | 0.26 |
Operative time, intraoperative blood loss, and hospital stay: Five studies reported the operative time with moderate heterogeneity (WMD = -41.29, 95%CI: -75.66 to -6.92, P = 0.02)[12-16]. Four studies reported the intraoperative blood loss with moderate heterogeneity (WMD = -261.96, 95%CI: -472.60 to
Number of lymph nodes retrieved, recurrence, blood transfusion, and complications: Five studies reported the number of lymph nodes retrieved with high heterogeneity (WMD = -1.71, 95%CI: -4.27 to 0.84, P = 0.19). Three studies reported the intraoperative blood transfusion with low heterogeneity (RR = 0.56, 95%CI: 0.29-1.09, P = 0.09)[12,14,15]. Five studies reported the complication rate with low heterogeneity (RR = 0.72, 95%CI: 0.39-1.33, P = 0.29)[12-16]. Two studies reported the recurrence rate with moderate heterogeneity (RR = 0.41, 95%CI: 0.06-2.84, P = 0.36)[12,16]. There was no significant difference between the LS and OS groups in the number of lymph nodes retrieved, recurrence, blood transfusion, or complications (Figure 2B and 2C).
3-year and 5-year overall and disease-free survival rates: Three studies reported the 3-year overall survival rate with moderate heterogeneity (RR = 0.99, 95%CI: 0.82-1.18, P = 0.89)[12-14]. Three studies reported the 5-year overall survival rate with high heterogeneity (RR = 1.02, 95%CI: 0.68-1.53, P = 0.92)[12,14,15]. Three studies reported the 3-year disease-free survival rate with low heterogeneity (RR = 1.01, 95%CI: 0.84-1.21, P = 0.93)[12-14]. Three studies reported the 5-year disease-free survival rate with moderate heterogeneity (RR = 1.15, 95%CI: 0.90-1.46, P = 0.26)[12,14,15]. There was no statistical difference between the LS and OS groups in terms of 3-year and 5-year overall and disease-free survival rates (Figure 2D).
The sensitivity analysis showed that our meta-analysis was stable, and no reversal of the meta-analysis results was found. Publication bias was qualitatively assessed using funnel plots. The funnel plots were largely symmetrically distributed, with no significant extreme values (Supplementary Figure 1). Neither Begg’s test nor Egger’s test revealed any significant publication bias (Supplementary Table 2).
Recently, LS for patients with stage T2 GBC has become feasible in high-volume medical centers and has shown similar outcomes to those of OS[16,22-25]. However, the value of LS for T2 GBC remains controversial. The current guidelines, such as those of the National Comprehensive Cancer Network and the Japanese Society of Hepatobiliary and Pancreatic Surgery, do not recommend LS for T2 GBC[9]. Previous studies referenced by the guidelines have shown that LS is associated with a higher risk of tumor spread and incisional recurrence than OS[7,26,27]. However, tumor spread is not a complication specific to LS and can also occur in OS[28]. Currently, since specimens are often intraoperatively obtained using plastic internal bags, which can prevent tumor spread and incision site recurrence in GBC[29,30], there is no statistically significant difference in the incidence of incisional implants between LS and OS[31].
LS follows the principles of OS. Lymph node dissection and R0 rate are two important indicators to evaluate radical surgery for GBC. Studies found that the rate of lymph node metastasis in stage T2 GBC was 46%[32,33]. It has been suggested that LS is superior to OS for lymph node dissection because of the unique magnified surgical field of view[22]. However, the results of this meta-analysis showed no significant difference between the two procedures. R0 resection is also an important prognostic factor for postoperative patients. Among the analyzed studies, only the study by Lee et al[12] reported the R0 resection rate to be similar between the LS and OS groups, with no statistical difference.
Although oncological outcomes based on surgical procedures, such as R0 rates and number of lymph nodes removed, were not significantly different between the LS and OS groups, the therapeutic effect should be based on more direct clinical evidence, such as improved survival, improved quality of life, or reduced tumor-related symptoms. These clinical benefits sometimes cannot be assessed based on intraoperative or short-term outcomes. Therefore, we explored long-term survival and found that postoperative recurrence and 3-year and 5-year overall and disease-free survival rates are not significantly different between the LS and OS groups.
In addition, our findings suggest that LS is associated with lower operation time, intraoperative blood loss, and length of hospital stay than OS. Although a random effects model was used to combine the effect sizes, there was a high degree of heterogeneity in operative time, intraoperative bleeding, and length of hospital stay, which significantly weakens the explanatory effect of the results and may cause confounding bias. The high heterogeneity may be explained by the fact that surgeons are still at the learning curve stage. As these results are prone to bias, they need to be validated via high-quality RCTs.
LS for T2 GBC has similar long-term survival outcomes to those of OS but is superior to OS in terms of operative time, intraoperative bleeding, and length of hospital stay. Additional high-quality RCTs and long follow-ups are needed to further evaluate the effectiveness of LS for stage T2 GBC.
Although laparoscopic surgery (LS) is recommended for stage T1 gallbladder cancer (GBC), the value of LS for stage T2 GBC is still controversial.
This study evaluated the short- and long-term outcomes of LS in comparison to those of open surgery (OS) for stage T2 GBC.
As there is still a lack of evidence from high-quality multicenter randomized controlled trials, we believe that it is necessary to conduct a meta-analysis to provide an evidence-based reference for laparoscopic radical surgery of T2 GBC.
We searched the PubMed, Embase, Cochrane Library, Ovid, Google Scholar, and Web of Science databases for published studies, with a cutoff date of September 2022.
A total of 5 studies were included with a total of 297 patients, 153 in the LS group and 144 in the OS group. Meta-analysis results showed that the LS group was better than the OS group in terms of operative time, estimated blood loss, and hospital stay, whereas there was no significant difference between the two groups in terms of blood transfusion, complications, number of lymph nodes retrieved, recurrence, and 3-year and 5-year overall and disease-free survival.
The long-term outcomes of LS for T2 GBC are similar to those of OS, but LS is superior to OS in terms of operative time, intraoperative bleeding, and postoperative hospital stay.
Our meta-analysis is the first to assess the efficacy of the laparoscopic approach in the treatment of stage T2 GBC and to provide a reference for clinical management.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rahmati M, Iran; AF Liu, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 487] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 2. | Sternby Eilard M, Lundgren L, Cahlin C, Strandell A, Svanberg T, Sandström P. Surgical treatment for gallbladder cancer - a systematic literature review. Scand J Gastroenterol. 2017;52:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Wang JK, Ma WJ, Wu ZR, Yang Q, Hu HJ, Liu F, Li FY. Is combined extra-hepatic bile-duct resection justified for advanced gallbladder carcinoma? Gastroenterol Rep (Oxf). 2019;7:426-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Weiland ST, Mahvi DM, Niederhuber JE, Heisey DM, Chicks DS, Rikkers LF. Should suspected early gallbladder cancer be treated laparoscopically? J Gastrointest Surg. 2002;6:50-6; discussion 56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Yokomizo H, Yamane T, Hirata T, Hifumi M, Kawaguchi T, Fukuda S. Surgical treatment of pT2 gallbladder carcinoma: a reevaluation of the therapeutic effect of hepatectomy and extrahepatic bile duct resection based on the long-term outcome. Ann Surg Oncol. 2007;14:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Reddy YP, Sheridan WG. Port-site metastasis following laparoscopic cholecystectomy: a review of the literature and a case report. Eur J Surg Oncol. 2000;26:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Wullstein C, Woeste G, Barkhausen S, Gross E, Hopt UT. Do complications related to laparoscopic cholecystectomy influence the prognosis of gallbladder cancer? Surg Endosc. 2002;16:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Miyazaki M, Yoshitomi H, Miyakawa S, Uesaka K, Unno M, Endo I, Ota T, Ohtsuka M, Kinoshita H, Shimada K, Shimizu H, Tabata M, Chijiiwa K, Nagino M, Hirano S, Wakai T, Wada K, Isayama H, Okusaka T, Tsuyuguchi T, Fujita N, Furuse J, Yamao K, Murakami K, Yamazaki H, Kijima H, Nakanuma Y, Yoshida M, Takayashiki T, Takada T. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22:249-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Butte JM, Waugh E, Meneses M, Parada H, De La Fuente HA. Incidental gallbladder cancer: analysis of surgical findings and survival. J Surg Oncol. 2010;102:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, Adams RB, Staley CA, Trindade EN, Schulick RD, Choti MA, Capussotti L. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478-86; discussion 1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Lee JW, Kwon JH, Lee JW. Oncologic and Long-Term Outcomes of Laparoscopic and Open Extended Cholecystectomy for Gallbladder Cancer. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Cho JK, Kim JR, Jang JY, Kim HG, Kim JM, Kwag SJ, Park JH, Kim JY, Ju YT, Jeong CY. Comparison of the Oncological Outcomes of Open versus Laparoscopic Surgery for T2 Gallbladder Cancer: A Propensity-Score-Matched Analysis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Navarro JG, Kang I, Hwang HK, Yoon DS, Lee WJ, Kang CM. Oncologic safety of laparoscopic radical cholecystectomy in pT2 gallbladder cancer: A propensity score matching analysis compared to open approach. Medicine (Baltimore). 2020;99:e20039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Jang JY, Han HS, Yoon YS, Cho JY, Choi Y. Retrospective comparison of outcomes of laparoscopic and open surgery for T2 gallbladder cancer - Thirteen-year experience. Surg Oncol. 2019;29:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Itano O, Oshima G, Minagawa T, Shinoda M, Kitago M, Abe Y, Hibi T, Yagi H, Ikoma N, Aiko S, Kawaida M, Masugi Y, Kameyama K, Sakamoto M, Kitagawa Y. Novel strategy for laparoscopic treatment of pT2 gallbladder carcinoma. Surg Endosc. 2015;29:3600-3607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13317] [Article Influence: 832.3] [Reference Citation Analysis (0)] |
| 18. | Rahmati M, McCarthy JJ, Malakoutinia F. Myonuclear permanence in skeletal muscle memory: a systematic review and meta-analysis of human and animal studies. J Cachexia Sarcopenia Muscle. 2022;13:2276-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Rahmati M, Gondin J, Malakoutinia F. Effects of Neuromuscular Electrical Stimulation on Quadriceps Muscle Strength and Mass in Healthy Young and Older Adults: A Scoping Review. Phys Ther. 2021;101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Rahmati M, Malakoutinia F. Aerobic, resistance and combined exercise training for patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis. Physiotherapy. 2021;113:12-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Rahmati M, Keshvari M, Mirnasuri S, Yon DK, Lee SW, Il Shin J, Smith L. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: A systematic review and meta-analysis. J Med Virol. 2022;94:5112-5127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (2)] |
| 22. | Shirobe T, Maruyama S. Laparoscopic radical cholecystectomy with lymph node dissection for gallbladder carcinoma. Surg Endosc. 2015;29:2244-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Machado MA, Makdissi FF, Surjan RC. Totally Laparoscopic Hepatic Bisegmentectomy (s4b+s5) and Hilar Lymphadenectomy for Incidental Gallbladder Cancer. Ann Surg Oncol. 2015;22 Suppl 3:S336-S339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Gumbs AA, Hoffman JP. Laparoscopic completion radical cholecystectomy for T2 gallbladder cancer. Surg Endosc. 2010;24:3221-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Agarwal AK, Javed A, Kalayarasan R, Sakhuja P. Minimally invasive versus the conventional open surgical approach of a radical cholecystectomy for gallbladder cancer: a retrospective comparative study. HPB (Oxford). 2015;17:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Steinert R, Nestler G, Sagynaliev E, Müller J, Lippert H, Reymond MA. Laparoscopic cholecystectomy and gallbladder cancer. J Surg Oncol. 2006;93:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, Furuse J, Saito H, Tsuyuguchi T, Yamamoto M, Kayahara M, Kimura F, Yoshitomi H, Nozawa S, Yoshida M, Wada K, Hirano S, Amano H, Miura F; Japanese Association of Biliary Surgery; Japanese Society of Hepato-Biliary-Pancreatic Surgery; Japan Society of Clinical Oncology. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008;15:41-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Maker AV, Butte JM, Oxenberg J, Kuk D, Gonen M, Fong Y, Dematteo RP, D'Angelica MI, Allen PJ, Jarnagin WR. Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol. 2012;19:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Yoon YS, Han HS, Cho JY, Choi Y, Lee W, Jang JY, Choi H. Is Laparoscopy Contraindicated for Gallbladder Cancer? J Am Coll Surg. 2015;221:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Han HS, Yoon YS, Agarwal AK, Belli G, Itano O, Gumbs AA, Yoon DS, Kang CM, Lee SE, Wakai T, Troisi RI. Laparoscopic Surgery for Gallbladder Cancer: An Expert Consensus Statement. Dig Surg. 2019;36:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Fornara P, Zacharias M, Wagner S. Port-site metastases: fact or fiction? Urol Int. 2003;71:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Goetze TO. Gallbladder carcinoma: Prognostic factors and therapeutic options. World J Gastroenterol. 2015;21:12211-12217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 173] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 33. | Lee SE, Kim KS, Kim WB, Kim IG, Nah YW, Ryu DH, Park JS, Yoon MH, Cho JY, Hong TH, Hwang DW, Choi DW; Korean Association of Hepato-Biliary and Pancreas Surgery. Practical guidelines for the surgical treatment of gallbladder cancer. J Korean Med Sci. 2014;29:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |