Published online Dec 27, 2022. doi: 10.4240/wjgs.v14.i12.1375
Peer-review started: July 16, 2022
First decision: October 30, 2022
Revised: November 13, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: December 27, 2022
Processing time: 164 Days and 1.4 Hours
Transanal total mesorectal excision (TaTME) allows patients with ultralow rectal cancer to be treated with sphincter-saving surgery. However, accurate delineation of the distal resection margin (DRM), which is essential to achieve R0 resection for low rectal cancer in TaTME, is technically demanding.
To assess the feasibility of optical biopsy using probe-based confocal laser end
A total of 43 consecutive patients who were diagnosed with low rectal cancer and scheduled for TaTME were prospectively enrolled from January 2019 to January 2021. pCLE was used to determine the distal edge of the tumor as well as the DRM during surgery. The final pathological report was used as the gold standard. The diagnostic accuracy of pCLE examination was calculated.
A total of 86 pCLE videos of 43 patients were included in the analyses. The sensitivity, specificity and accuracy of real-time pCLE examination were 90.00% [95% confidence interval (CI): 76.34%-97.21%], 86.96% (95%CI: 73.74%-95.06%) and 88.37% (95%CI: 79.65%-94.28%), respectively. The accuracy of blinded pCLE reinterpretation was 86.05% (95%CI: 76.89%-92.58%). Furthermore, our results show satisfactory interobserver agreement (κ = 0.767, standard error = 0.069) for the detection of cancer tissue by pCLE. There were no positive DRMs (≤ 1 mm) in this study. The median DRM was 7 mm [interquartile range (IQR) = 5-10 mm]. The median Wexner score was 5 (IQR = 3-6) at 6 mo after stoma closure.
Real-time in vivo pCLE examination is feasible and safe for selecting the DRM during TaTME for low rectal cancer (clinical trial registration number: NCT04016948).
Core Tip: Transanal total mesorectal excision (TaTME) allows patients even with ultra-low rectal cancer to be treated with sphincter-saving surgery. However, low rectal cancer resection with sphincter preservation may lead to a positive distal resection margin (DRM), with a high risk for local recurrence. Confocal laser endomicroscopy (CLE) enables the real-time, in vivo optical biopsy of living tissue. Real-time in vivo probe-based CLE examination can provide optical biopsy and is feasible and safe for selecting the DRM during TaTME for low rectal cancer.
- Citation: Tan J, Ji HL, Hu YW, Li ZM, Zhuang BX, Deng HJ, Wang YN, Zheng JX, Jiang W, Yan J. Real-time in vivo distal margin selection using confocal laser endomicroscopy in transanal total mesorectal excision for rectal cancer. World J Gastrointest Surg 2022; 14(12): 1375-1386
- URL: https://www.wjgnet.com/1948-9366/full/v14/i12/1375.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i12.1375
Colorectal cancer is the third most prevalent type of cancer and the second primary cause of cancer-related mortality worldwide[1]. Low rectal carcinoma often requires abdominoperineal resection and permanent abdominal colostomy, and it places major economic and psychological burdens on patients[2]. Transanal total mesorectal excision (TaTME) is increasingly adopted by colorectal surgeons in the treatment of patients with low rectal cancer[3,4]. This technique gives patients, even those with ultralow rectal cancer, the opportunity to undergo sphincter-saving surgery. However, low rectal cancer resection with sphincter preservation may lead to a positive distal resection margin (DRM), with a high risk for local recurrence[5-7]. To date, no devices have been used in the surgical field to guide resection margin selection. Frozen biopsy is recommended during surgery for low rectal cancer to confirm a negative DRM. However, it cannot be used to guide selection of the resection margin in real time, and it is a time-consuming, irreversible, and traumatic process. Accordingly, accurate delineation of the DRM is essential to achieve R0 resection for low rectal cancer.
Recently, several studies have reported that confocal laser endomicroscopy (CLE) enables the real-time, in vivo optical biopsy of living tissue[8-12]. It has the potential to fundamentally change the role of biopsy in the gastrointestinal field, and a state-of-the-art classification system (Miami classification) has been proposed for normal and pathological states in gastrointestinal diseases based on probe-based CLE (pCLE)[13]. However, no studies have investigated the feasibility of optical biopsy using pCLE in the real-time in vivo selection of the DRM during TaTME for low rectal cancer. We hypothesize that real-time in vivo pCLE examination can help surgeons select the DRM accurately and might contribute to improving the oncological and functional prognosis of low rectal cancer after treatment with TaTME. The aim of this study was to evaluate the feasibility of optical biopsy using pCLE for selecting the DRM during TaTME in the treatment of low rectal cancer.
This was a prospective, single-center study that was approved by the Institutional Review Board of Nanfang Hospital of Southern Medical University. The study protocol was registered at Cli
Patients who were diagnosed with low rectal cancer by preoperative endoscopic biopsy and scheduled for TaTME were prospectively enrolled in this study. Written informed consent was obtained from each patient prior to participation. Inclusion criteria were as follows: Diagnosis of low rectal cancer (tumor lying within 5 cm from the anal verge) and planned treatment with TaTME; age of at least 18 years; and American Society of Anesthesiologists class 1-3. The exclusion criteria were as follows: (1) T4b cancer as determined by computed tomography, magnetic resonance imaging or endoscopic examination; (2) Emergent case with obstruction or perforation; (3) Coagulopathy (international normalized ratio > 1.5 or prothrombin time < 50%); (4) Impaired renal function (creatinine level > 1.2 mg/dL); (5) Pregnancy; (6) Breastfeeding; and (7) Past history of allergies.
During surgery, pCLE was used to determine the distal edge of the tumor as well as to examine the preselected DRM. pCLE was performed using the Cellvizio Endomicroscopy System [Mauna Kea Technologies (MKT), Paris, France]. The ColoFlex UHD probe, a flexible mini-probe with a lateral resolution of 1 μm, was used in our study. The pCLE imaging data were collected at a scan rate of 12 frames/s. The probe has a field of view of 240 μm and can image at a depth of 60 μm below the mucosal surface, and it allows optical biopsies with 1000 times magnification.
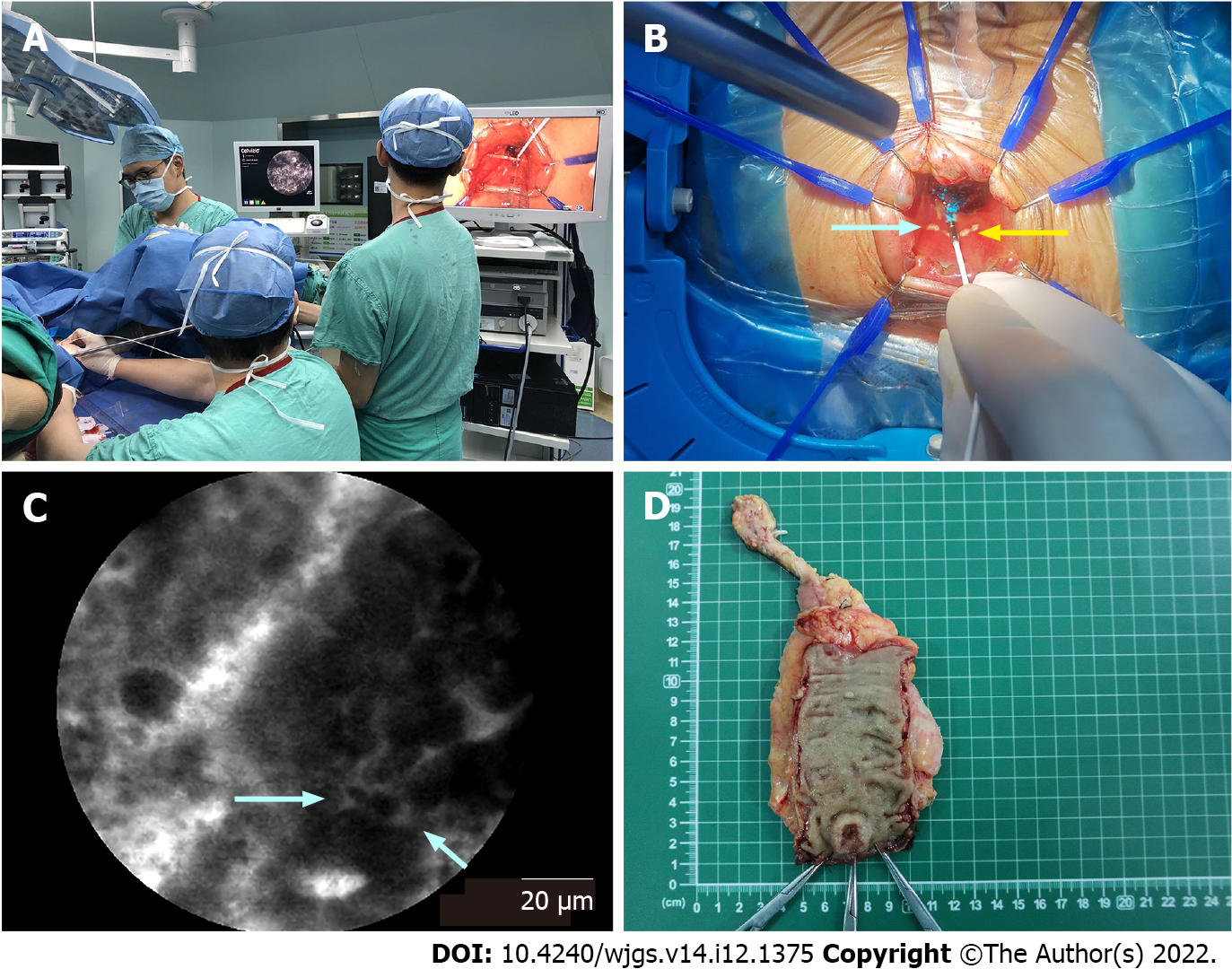
Before image acquisition, fluorescein sodium was injected intravenously. The fluorescent agent used was 10% fluorescein sodium (Baiyunshan Mingxing Pharmaceutical Company, Guangzhou, China). The fluorescein sodium (0.5 mL) hypersensitivity test was implemented 20 min before pCLE examination. Then, 2.5 mL of fluorescein sodium diluted with 2.5 mL of 0.9% sodium chloride was injected intravenously 5 min prior to pCLE imaging. After strict sterilization, one end of the probe was connected to the laser outlet of Cellvizio, and the other end was placed on the surgical table. Adequate exposure of the tumor lesion was achieved using a colorectal retractor (CooperSurgical Lone Star colorectal retractor, Beijing Xinya S&T Co., Ltd., Beijing, China), and pCLE imaging was performed by the surgeon under direct vision by using the probe in direct contact with the tissues (Figure 1). The pCLE video recording was initiated when the probe contacted the lesion, and it terminated when the probe moved away from the lesion. The pathologist analyzed the pCLE videos and evaluated the margin between the abnormal tissue of the lesion and the surrounding normal mucosa in real time. A dot was marked with an electric scalpel at the distal edge of the tumor as determined by pCLE (Figure 1B and 1C). If the pathologist could not determine the distal edge of the tumor, then the dot was marked at a nontumor site identified by pCLE imaging as the closest healthy tissue below the macroscopic lesion. Then, the DRM was located 5-10 mm below the marked dot (Figure 1B). Finally, pCLE examination was performed preceding the surgical resection to ensure a negative DRM. Conventional samples were collected for histology at the marked dot and the DRM (Figure 1D). Histopathological analysis of the samples and the final resection specimen was performed as the gold standard, and the diagnosis made by pCLE was compared with that of the final pathological reports. All pCLE videos were stored on a personal computer in the form of MKT files (proprietary format, MKT Software, Paris, France).
The pCLE optical biopsy diagnostic criteria were according to the “Miami criteria”[13] and Kuiper et al[14]’s diagnostic classification. Briefly, the diagnostic criteria include the crypt architecture and vessel architecture classification. The crypt architecture was divided into three types. Normal mucosa was scored as crypt type 1 and presented normal regular luminal openings, size, and distribution of crypts covered by a homogeneous layer of epithelial cells, including goblet cells. Hyperplastic polyps and inflammatory lesions were scored as crypt type 2 and presented regular-shaped or star-shaped luminal crypt openings with normal or reduced goblet cells. Neoplastic lesions were scored as crypt type 3, which included variable width of epithelial lining with tubular-shaped crypts and loss of goblet cells (striped dark epithelium) and irregular and decreased volume of lamina propria. For vessel architecture, normal mucosa was scored as vessel type 1 and presented a hexagonal, honeycomb appearance that presented a network of capillaries outlining the luminal openings of the crypts. Hyperplastic polyps and inflammatory lesions were scored as vessel type 2, presented hexagonal, honeycomb appearance with mild (or no) increase in the number of capillaries or increased amounts of normal vessels without leakage. Neoplasia was scored as vessel type 3, presenting dilated and distorted vessels with elevated leakage and irregular architecture with little or no orientation to adjunct tissue. We analyzed the pCLE imaging features and made relative diagnoses according to the above categories.
For the patients who received neoadjuvant chemoradiotherapy, we adopted a pCLE scoring classification system created by Safatle-Ribeiro et al[15], assigning one point to the presence of each feature as follows: Vascular features including fluorescein leakage and an increased vessel/crypt ratio; and epithelial features including dark irregular crypts, intratumoral budding, back-to-back glands, and a cribriform pattern. Hence, in our study, patients with 0-1 points were diagnosed with complete response (no residual neoplasia), and those with 2-6 points were diagnosed with partial response (residual neoplasia). Consequently, we analyzed the diagnostic accuracy of pCLE in patients with neoadjuvant chemoradiotherapy according to the above classification.
During the surgery, one pathologist (observer A) made a real-time interpretation of the findings of the pCLE examination. Then, reinterpretation of the same pCLE videos was performed by another pathologist (observer B) who was blinded to the real-time diagnosis and final histopathology report. Finally, the real-time and blinded interpretations of the pCLE videos were compared with the final pathological report. Both observers had been trained in the pCLE system and image interpretation and had read more than 100 pCLE images of the colorectum. The quality of all videos was evaluated, and the diagnosis was made according to the “Miami criteria”[13]. We also adopted the colonic crypt architecture and vessel architecture classification for pCLE established by Kuiper et al[14]. The sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV) and interobserver agreement of pCLE optical biopsy in distinguishing between normal and cancerous tissue were calculated.
The TME specimen quality should be assessed based on the following features. Grade 1 represents low quality: Incomplete mesorectum; mesorectum fascia defects deeper than 5 mm; conical gross specimen. Grade 2 represents moderate quality: Relatively intact mesorectum; mesorectum fascia defects deeper than 5 mm; no visible muscularis propria with adequate resection margin; approximately conical gross specimen. Grade 3 represents high quality: Intact mesorectum; no mesorectum fascia defects deeper than 5 mm; no visible muscularis propria; cylindrical specimen. A circumferential resection margin (CRM) was defined as positive when it was less than 1 mm, and a positive CRM or positive DRM was considered R1 resection. All TME specimens were evaluated by pathologists after surgery.
From January 2019 to June 2019, the average time for intraoperative diagnosis by frozen section was 25 ± 10 min in our hospital. We hypothesized that the average time of intraoperative diagnosis by pCLE would be 20 min, and 43 cases were determined. With this number of cases, the study would have 90% power to detect a difference between the two techniques to prove the superiority of pCLE (two-sided type I error = 0.05).
Patient demographic and clinical data and pCLE image characteristics were evaluated by descriptive statistics. The data of continuous variables are represented as the mean ± SD or median [interquartile range (IQR)], and the data of categorical variables are presented as numbers and frequencies. The intraobserver agreement was calculated by means of intraclass correlation coefficients (ICCs). Based on the 95% confidence interval (CI) of the ICC estimate, values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively. Cohen’s kappa (κ) was calculated to assess the interobserver agreement of the two observers. The κ value was graded as follows: Poor (0.01-0.20); fair (0.21-0.40); moderate (0.41-0.60); substantial (0.61-0.80); and excellent (0.81-1.00). Statistical Package for the Social Sciences software (Release 22.0, SPSS, Inc., 2012) was applied for statistical analyses.
From January 2019 to January 2021, a total of 43 consecutive patients were enrolled according to the predefined inclusion and exclusion criteria. Patient demographics and tumor characteristics are shown in Table 1. There were 29 males (67.4%) and 14 females (32.6%), with a median age of 57 (IQR = 47-65) years. The median tumor height (the height from the anal verge to the distal edge of the tumor) was 4 cm (IQR = 3.6-4.6 cm). Preoperative neoadjuvant chemoradiotherapy was administered to 21 patients (48.8%), three of whom showed a complete response (no viable cancer cells). Finally, 43 marked dots and 43 DRMs were analyzed by comparing the pCLE diagnosis with the pathological reports. All pCLE procedures were performed successfully and safely, and no adverse reactions were observed following fluorescein injection.
| Variable | n = 43 |
| Age: Median (IQR), yr | 57 (47-65) |
| Sex: Male/female, n | 29/14 |
| BMI: Median (IQR), kg/m2 | 22.40 (19.50-23.95) |
| ASA class, n (%) | |
| 1 | 8 (18.6) |
| 2 | 30 (69.8) |
| 3 | 5 (11.6) |
| 4 | 0 |
| Tumor size: Median (IQR), cm | 2.5 (2.0-3.8) |
| Tumor height1: Median (IQR), cm | 4.0 (3.6-4.6) |
| Histological subtype, n (%) | |
| Adenocarcinoma | 39 (90.7) |
| Mucinous adenocarcinoma/signet ring cell carcinoma | 4 (9.3) |
| Differentiation grade, n (%) | |
| Well | 7 (16.3) |
| Moderate | 32 (74.4) |
| Poor | 4 (9.3) |
| Neoadjuvant chemoradiotherapy, n (%) | 21 (48.8) |
| TRG2, n (%) | |
| Grade 0 | 3 (7.0) |
| Grade 1 | 10 (23.3) |
| Grade 2 | 7 (14.0) |
| Grade 3 | 1 (2.3) |
| T stage, n (%) | |
| T0 | 5 (11.6) |
| T1 | 4 (9.3) |
| T2 | 17 (39.5) |
| T3 | 13 (30.2) |
| T4 | 4 (9.3) |
| N stage, n (%) | |
| N0 | 30 (69.8) |
| N1 | 10 (23.3) |
| N2 | 3 (7.0) |
| M stage, n (%) | |
| M0 | 43 (100) |
| M1 | 0 |
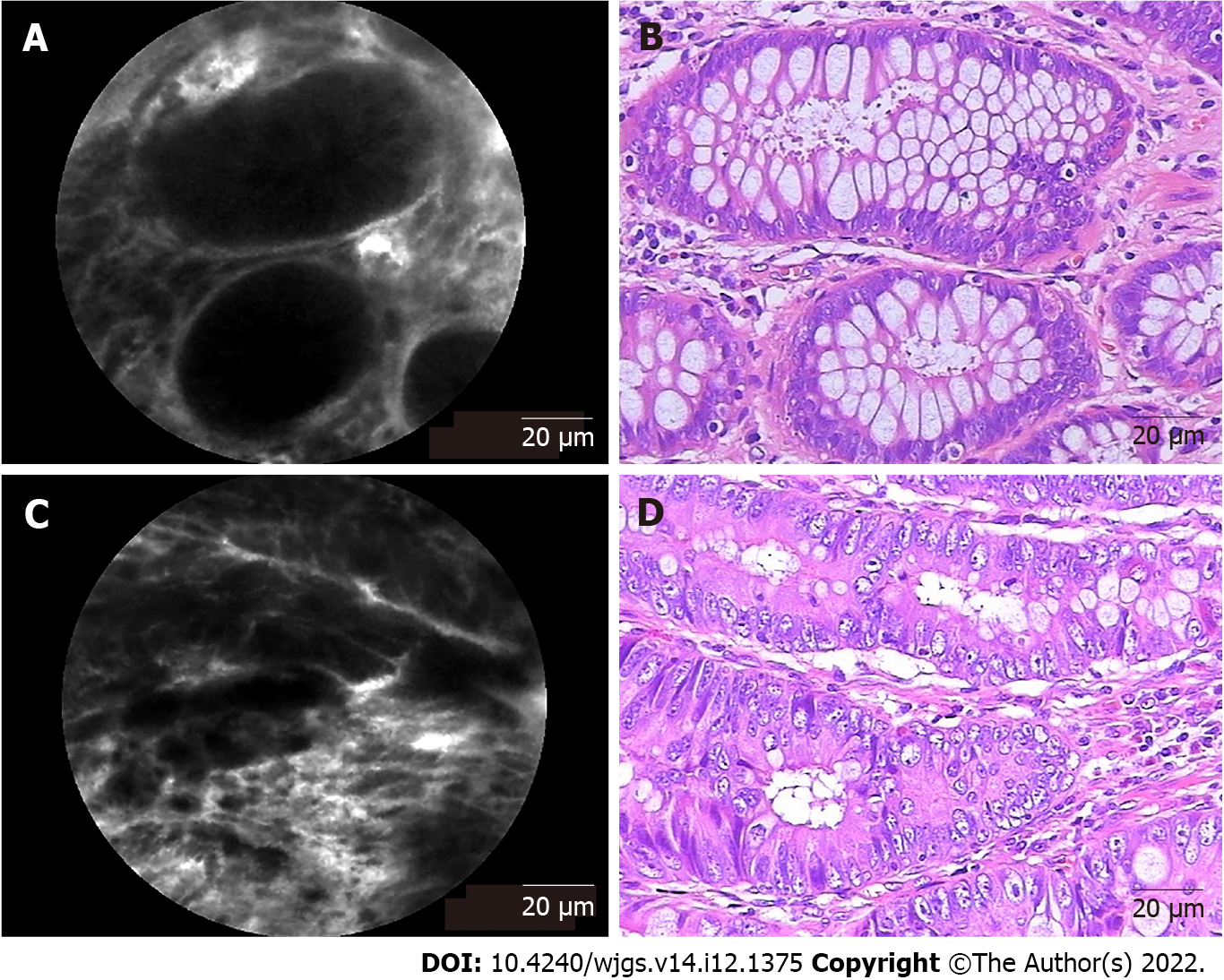
In total, 43 patients underwent pCLE examination, including 21 patients who underwent neoadjuvant chemoradiotherapy. Representative pCLE images and matched images of hematoxylin and eosin-stained rectal tissues are shown in Figure 2. In normal rectal tissues, pCLE images presented normal round crypt structures with regular luminal openings, covered by a homogeneous single-cell-layered epithelium with dark goblet cells, and regular narrow vessels with hexagonal, honeycomb appearance surrounding crypts (Figure 2A). In rectal neoplastic tissues, pCLE images presented dark and ir
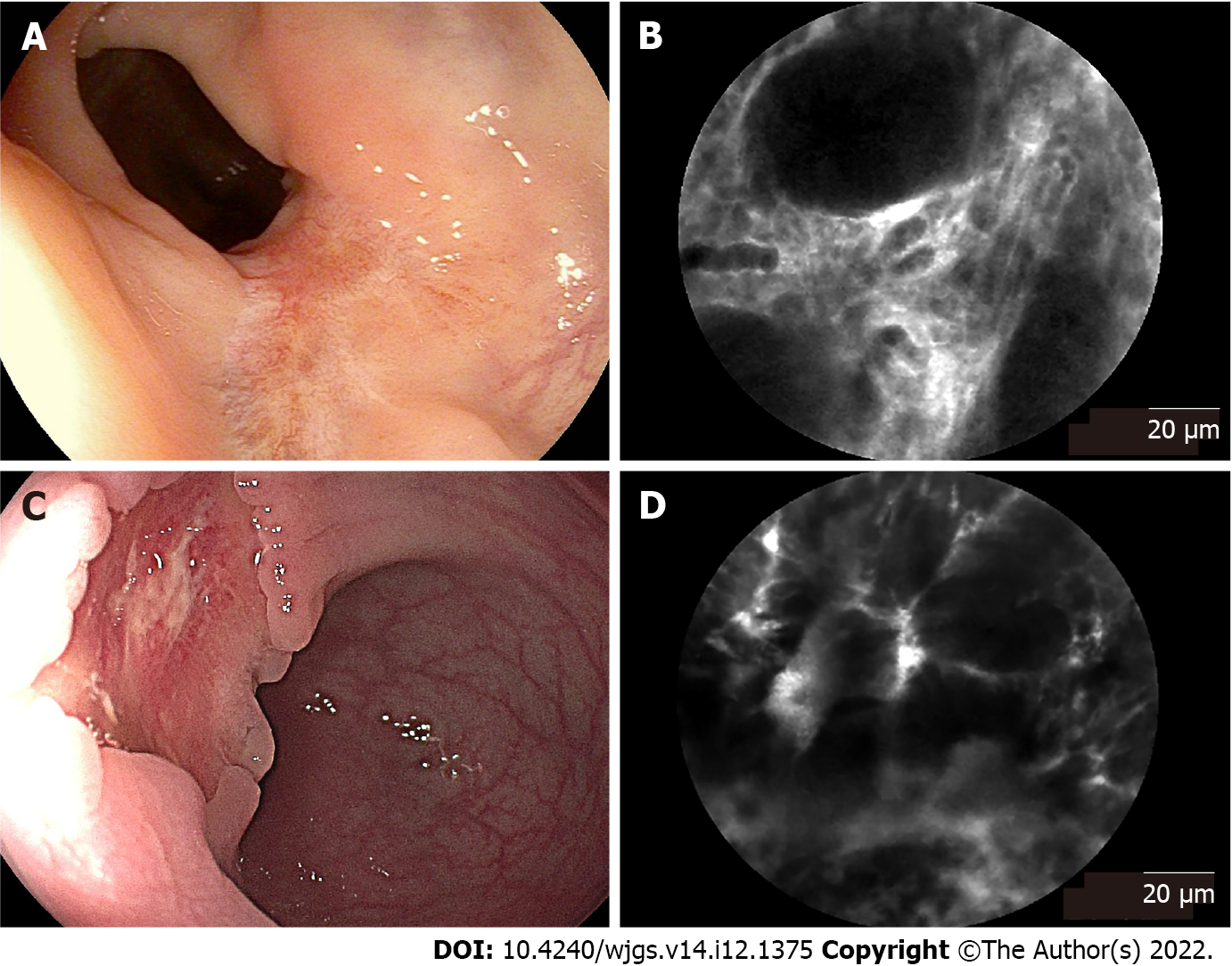
In 21 patients who underwent neoadjuvant chemoradiotherapy, 3 patients had a complete response, while 18 had a partial response after neoadjuvant chemoradiotherapy according to the pathological reports after surgery. Representative endoscopic images and corresponding pCLE images are shown Figure 3. All patients received endoscopic examination before surgery to evaluate the residual lesions. Seven patients’ endoscopic reports showed a complete response, presenting a residual scar (Figure 3A). Fourteen patients’ endoscopic reports showed a partial response, presenting a residual tumor lesion (Figure 3C). In complete response rectal tissues (no neoplastic features), the typical pCLE images showed regular crypts with thickening epithelium and enlarged vessels with fibrotic stroma (Figure 3B). The pCLE images of residual neoplasia showed atypical glands with dark and irregular crypts and enlarged twisty vessels (Figure 3D). The pCLE imaging correctly diagnosed 15 cases of residual neoplasia (scored in range 2-6 points) in 18 cases of pathological partial response.
A total of 86 pCLE videos from 43 patients were included in the analyses. The sensitivity, specificity, PPV, and NPV of real-time pCLE examination (observer A) in distinguishing between cancerous and normal tissue were 90.00% (95%CI: 76.34%-97.21%), 86.96% (95%CI: 73.74%-95.06%), 85.71% (95%CI: 71.46%-94.57%), and 90.91% (95%CI: 78.33%-97.47%), respectively (Table 2). The overall rate of accuracy was 88.37% (95%CI: 79.65%-94.28%). In the blinded pCLE reinterpretation after surgery (observer B), the sensitivity, specificity, PPV, NPV and accuracy of the pCLE examination were 87.50% (95%CI: 73.20%-95.81%), 84.78% (95%CI: 71.13%-93.66%), 83.33% (95%CI: 68.64%-93.03%), 88.64% (95%CI: 75.44%-96.21%), and 86.05% (95%CI: 76.89%-92.58%), respectively (Table 2).
| Observer A (real-time interpretation) | Observer B (blinded interpretation) | |||
| % | 95%CI | % | 95%CI | |
| Sensitivity | 90.00 | 76.34-97.21 | 87.50 | 73.20-95.81 |
| Specificity | 86.96 | 73.74-95.06 | 84.78 | 71.13-93.66 |
| Accuracy | 88.37 | 79.65-94.28 | 86.05 | 76.89-92.58 |
| PPV | 85.71 | 71.46-94.57 | 83.33 | 68.64-93.03 |
| NPV | 90.91 | 78.33-97.47 | 88.64 | 75.44-96.21 |
| Interobserver agreement | κ = 0.767, standard error = 0.069 | |||
The neoadjuvant chemoradiotherapy group showed a lower sensitivity (83.33% vs 95.45%, P = 0.485), specificity (77.27% vs 95.83%, P = 0.153), accuracy (80.00% vs 95.65%, P = 0.055), and PPV (75.00% vs 95.45%, P = 0.147) than the nonneoadjuvant treatment group (Table 3). In our study, the mean ICC was 0.839 (95%CI: 0.763-0.892), which means that the intraobserver agreement was good. The interobserver agreement was substantial for the detection of rectal cancer, with a mean κ of 0.767 (standard error = 0.069).
| Neoadjuvant group (n = 42) | Nonneoadjuvant group (n = 44) | P value | |||
| % | 95%CI | % | 95%CI | ||
| Sensitivity | 83.33 | 58.58-96.42 | 95.45 | 77.16-99.88 | 0.458 |
| Specificity | 77.27 | 54.63-92.18 | 95.83 | 78.88-99.89 | 0.153 |
| Accuracy | 80.00 | 64.35-90.95 | 95.65 | 85.16-99.47 | 0.055 |
| PPV | 75.00 | 50.90-91.34 | 95.45 | 77.16-99.88 | 0.147 |
| NPV | 85.00 | 62.11-96.79 | 95.83 | 78.88-99.89 | 0.473 |
The surgical and functional outcomes are shown in Table 4. No positive DRMs were detected in our study. All TME specimens were evaluated by pathologists after surgery. There were 40 specimens defined as grade 3 and 3 specimens defined as grade 2. The median distance from the lowest edge of the tumor to the DRM was 7 mm (IQR = 5-10 mm). The median operative duration was 240 min (IQR = 202-265 min), while the median intraoperative pCLE examination duration was 17 min (IQR = 15-18 min). The time required to select the DRM decreased over time.
| Variable | |
| Operative duration: Median (IQR), min | 240 (202-265) |
| pCLE examination duration: Median (IQR), min | 17 (15-18) |
| Estimated blood loss: Median (IQR), mL | 27 (20-50) |
| DRM distance: Median (IQR), mm | 7.0 (5.0-10.0) |
| Anastomotic leakage, n (%) | 2 (4.7) |
| Positive DRM, n (%) | 0 (0) |
| Wexner score1, median (IQR) | 5 (3-6) |
| Anastomotic stenosis, n (%) | 1 (2.3) |
| Recurrence, n (%) | 1 (2.3) |
| Metastasis, n (%) | 2 (4.7) |
The median Wexner score was 5 (IQR = 3-6), as evaluated at six months after stoma closure. The median follow-up period was 24 (range, 22-46) mo. One patient had anastomotic stenosis. Two patients had liver metastasis at 6 mo and 13 mo after surgery. One patient died one year after metastasis, and another died 18 mo after metastasis. Notably, one patient had cancer recurrence 18 mo after surgery.
Sphincter-saving low rectal cancer resection is technically challenging, especially in obese patients with large tumors. The narrow pelvis and the forward angle of the distal rectum restrict the laparoscopic view, making it difficult to perform laparoscopic procedures. TaTME provides an open approach from the anus to cancerous lesions and provides an excellent view of the surgical field, allowing the tumor to be seen directly from the bottom to the top. In our study, the transanal approach allowed the pCLE probe to directly contact the tissues without endoscopy. Therefore, pCLE can provide continuous and stable imaging of the tissue architecture and cellular morphology in the mucosal layer during TaTME. The pCLE analysis evaluated both epithelial and vascular patterns of malignancy, including the Cannizzaro-Spessotto scale, vessel/crypt ratio, stroma, dark crypts, budding, back-to-back glands and cribriform pattern[15]. To our knowledge, this is the first study of optical biopsy using pCLE to select the DRM in TaTME for low rectal cancer.
To date, surgeons only “experientially” determine the DRM using surgical instruments or macroscopic examination of the tumor margin, which may lead to an insufficient or excessive DRM. de Lacy et al[6] reported that patients with low rectal cancer treated with TaTME had a positive DRM rate of 7.8%. pCLE can provide in vivo microscopic imaging of the colorectal mucosa and submucosa, enabling the real-time histological diagnosis of superficial and submucosal cancer infiltration[8,10]. In some studies, pCLE showed high agreement with true histopathology, reaching an accuracy of 88%-94.44%[8,10,16]. In our study, the accuracy of real-time pCLE examination was 88.37% (95%CI: 79.65%-94.28%). A direct and stable plane can be provided in TaTME for pCLE examination without the use of endoscopy.
Our study demonstrates that pCLE examination can be useful for detecting cancer infiltration and selecting the DRM. In our study, the diagnosis made by pCLE showed a good correlation with that made by histopathology as the gold standard. Real-time pCLE examination could differentiate between cancerous and normal tissue with a favorable accuracy of 88.37%. In particular, the sensitivity (90.00%) and NPV (90.91%) were high, resulting in high accuracy in not selecting a positive DRM. Wijsmuller et al[17] reported that neoadjuvant chemoradiotherapy could significantly alter pCLE rendering due to subsequent inflammation, edema, fibrosis and crypt distortion. Our results show a lower sensitivity (83.33% vs 95.45%, P = 0.458), specificity (77.27% vs 95.83%, P = 0.153), accuracy (80.00% vs 95.65%, P = 0.055), and PPV (75.00% vs 95.45%, P = 0.147) in the neoadjuvant chemoradiotherapy group than in the nonneoadjuvant treatment group. However, these differences between the two groups were not statistically significant. Therefore, pCLE examination is suitable for patients with or without neoadjuvant chemoradiotherapy. It is undeniable that the response to neoadjuvant chemoradiotherapy may lead to crypt distortion with epithelial irregularities due to inflammation, edema and fibrosis (Figure 2C), and these may increase the incidence of diagnostic errors. Therefore, awareness of neoadjuvant chemoradiotherapy before pCLE examination may be helpful to improve the diagnostic accuracy.
Several studies have reported that a DRM of 1 cm or less did not compromise oncological safety[18,19]. Therefore, we were relatively liberal with the selection of the DRM as long as it was confirmed to be negative intraoperatively by pCLE. There were no positive DRMs in our study, confirming the feasibility of optical biopsy using pCLE as an accurate method to select a tumor-free DRM in TaTME. As reported in a recent study[20], the mean DRM distance of patients who underwent TaTME for the treatment of low rectal cancer was 17.7 mm, which was much longer than our result of 7 mm. Previous studies investigating anorectal function after anterior resection for rectal cancer have suggested that a shorter remaining rectum might contribute to more disordered postoperative anorectal function because the rectal anal inhibitory reflex is generally preserved with higher levels of anastomosis and a longer residual rectum[21,22]. In this study, the median Wexner score was 5 (range, 3-6) at 6 mo after stoma closure, which means that patients in our study had satisfactory anorectal function after surgery. In summary, real-time pCLE examination may help reduce the tumor-free DRM and potentially contribute to the postoperative restoration of anorectal function in patients with low rectal cancer.
In this study, we first used pCLE to evaluate the tumor margin and select the DRM with satisfactory accuracy. We recommend pCLE examination as a routine test to help surgeons select the DRM in TaTME and perform “tailored surgery” for low rectal cancer patients. The limitation of this study was based on a single center, and the sample size was relatively small, which might limit the power of the study. Therefore, a large-scale multicenter, prospective, randomized controlled trial needs to be performed. The cancer cells sometimes crawl mainly submucosa rather than the mucosal layer, such as poorly differentiated adenocarcinomas. Due to the limitation of current technology, the pCLE imaging depth is restricted to 60 μm. Therefore, in our experience, patients who have been diagnosed with poorly differentiated adenocarcinoma preoperatively should receive submucosal intraoperative frozen biopsy to ensure distal margin safety.
In conclusion, real-time in vivo pCLE examination is feasible and safe for selecting the DRM during TaTME for low rectal cancer, with high accuracy and a particularly high NPV. The pCLE examination is convenient, timesaving and easy for surgeons to perform and could thus be promoted as a regular examination for selecting the DRM during TaTME for low rectal cancer.
Transanal total mesorectal excision (TaTME) allows patients even with ultra-low rectal cancer to be treated with sphincter-saving surgery. However, accurate delineation of the distal resection margin (DRM), which is essential to achieve R0 resection for low rectal cancer in TaTME, is technically demanding. Probe-based confocal laser endomicroscopy (pCLE) enables the real-time, in vivo optical biopsy of living tissue, which means it might help making intraoperative real-time diagnosis for suspicious tumor lesions. Therefore, we investigated whether pCLE can provide optical biopsy for DRM selection and help tailored surgery in low rectal cancer.
No studies have investigated the feasibility of optical biopsy using pCLE in the real-time in vivo selection of the DRM during TaTME for low rectal cancer. This study aimed to explore whether real-time in vivo pCLE examination can help surgeons select the DRM accurately and contribute to improving the surgical outcome and oncological and functional prognosis of low rectal cancer after treatment with TaTME. To our knowledge, this is the first study of optical biopsy using pCLE to select the DRM in TaTME for low rectal cancer.
This study investigated whether real-time in vivo pCLE examination is feasible and safe for selecting the DRM during TaTME for low rectal cancer.
The pCLE exaination was used to determine the distal magin during surgery. The final pathological report was used as the gold standard. The diagnostic accuracy of pCLE examination was calculated.
Real-time in vivo pCLE examination is feasible and safe for selecting the DRM during TaTME for low rectal cancer, with high accuracy and a particularly high negative predictive value. The pCLE examination is convenient, timesaving and easy for surgeons to perform and could thus be promoted as a regular examination for selecting the DRM during TaTME for low rectal cancer.
Real-time in vivo pCLE examination can provide optical biopsy for distal margin selecting in TaTME for low rectal cancer.
Real-time in vivo pCLE can be used to determine the distal margin in TaTME surgical procedure for low rectal cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Enomoto H, Japan; Krishnan A, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15476] [Article Influence: 2579.3] [Reference Citation Analysis (2)] |
| 2. | Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, Rooney PS, Susnerwala S, Blower A, Saunders MP, Wilson MS, Scott N, O'Dwyer ST. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 562] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 3. | Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; TaTME Registry Collaborative. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg. 2017;266:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 4. | Francis N, Penna M, Mackenzie H, Carter F, Hompes R; International TaTME Educational Collaborative Group. Consensus on structured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc. 2017;31:2711-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ES, Sietses C, Tuynman JB, Lacy AM, Hanna GB, Bonjer HJ. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30:3210-3215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 6. | de Lacy FB, van Laarhoven JJEM, Pena R, Arroyave MC, Bravo R, Cuatrecasas M, Lacy AM. Transanal total mesorectal excision: pathological results of 186 patients with mid and low rectal cancer. Surg Endosc. 2018;32:2442-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Zeng WG, Liu MJ, Zhou ZX, Wang ZJ. A Distal Resection Margin of ≤1 mm and Rectal Cancer Recurrence After Sphincter-Preserving Surgery: The Role of a Positive Distal Margin in Rectal Cancer Surgery. Dis Colon Rectum. 2017;60:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Kim B, Kim YH, Park SJ, Cheon JH, Kim TI, Kim WH, Kim H, Hong SP. Probe-based confocal laser endomicroscopy for evaluating the submucosal invasion of colorectal neoplasms. Surg Endosc. 2017;31:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Buchner AM, Gomez V, Heckman MG, Shahid MW, Achem S, Gill KR, Jamil LH, Kahaleh M, Lo SK, Picco M, Riegert-Johnson D, Raimondo M, Sciemeca D, Wolfsen H, Woodward T, Wallace MB. The learning curve of in vivo probe-based confocal laser endomicroscopy for prediction of colorectal neoplasia. Gastrointest Endosc. 2011;73:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | De Palma GD, Staibano S, Siciliano S, Persico M, Masone S, Maione F, Siano M, Mascolo M, Esposito D, Salvatori F, Persico G. In vivo characterisation of superficial colorectal neoplastic lesions with high-resolution probe-based confocal laser endomicroscopy in combination with video-mosaicing: a feasibility study to enhance routine endoscopy. Dig Liver Dis. 2010;42:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Shahid MW, Buchner AM, Raimondo M, Woodward TA, Krishna M, Wallace MB. Accuracy of real-time vs. blinded offline diagnosis of neoplastic colorectal polyps using probe-based confocal laser endomicroscopy: a pilot study. Endoscopy. 2012;44:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Gómez V, Buchner AM, Dekker E, van den Broek FJ, Meining A, Shahid MW, Ghabril MS, Fockens P, Heckman MG, Wallace MB. Interobserver agreement and accuracy among international experts with probe-based confocal laser endomicroscopy in predicting colorectal neoplasia. Endoscopy. 2010;42:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Wallace M, Lauwers GY, Chen Y, Dekker E, Fockens P, Sharma P, Meining A. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy. 2011;43:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Kuiper T, van den Broek FJ, van Eeden S, Wallace MB, Buchner AM, Meining A, van Hee K, Fockens P, Dekker E. New classification for probe-based confocal laser endomicroscopy in the colon. Endoscopy. 2011;43:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Safatle-Ribeiro AV, Marques CFS, Pires C, Arraes L, Baba ER, Meirelles L, Kawaguti FS, da Costa Martins B, Lenz LT, de Lima MS, Gusmon-Oliveira CC, Ribeiro U Jr, Maluf-Filho F, Nahas SC. Diagnosis of Clinical Complete Response by Probe-Based Confocal Laser Endomicroscopy (pCLE) After Chemoradiation for Advanced Rectal Cancer. J Gastrointest Surg. 2021;25:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Liu Z, Luo X, Jiang W, Chen D, Chen W, Li K, Liu X, Cui Z, Li Z, Han Z, Liu S, Li G, Xu C, Yan J. Real-time in vivo optical biopsy using confocal laser endomicroscopy to evaluate distal margin in situ and determine surgical procedure in low rectal cancer. Surg Endosc. 2019;33:2332-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Wijsmuller AR, Ghnassia JP, Varatharajah S, Schaeffer M, Leroy J, Marescaux J, Ignat M, Mutter D. Prospective Trial on Probe-Based Confocal Laser Endomicroscopy for the Identification of the Distal Limit in Rectal Adenocarcinoma. Surg Innov. 2018;25:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Kiran RP, Lian L, Lavery IC. Does a subcentimeter distal resection margin adversely influence oncologic outcomes in patients with rectal cancer undergoing restorative proctectomy? Dis Colon Rectum. 2011;54:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Bujko K, Rutkowski A, Chang GJ, Michalski W, Chmielik E, Kusnierz J. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? Ann Surg Oncol. 2012;19:801-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Ren J, Liu S, Luo H, Wang B, Wu F. Comparison of short-term efficacy of transanal total mesorectal excision and laparoscopic total mesorectal excision in low rectal cancer. Asian J Surg. 2021;44:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Rasmussen OO, Petersen IK, Christiansen J. Anorectal function following low anterior resection. Colorectal Dis. 2003;5:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Kakodkar R, Gupta S, Nundy S. Low anterior resection with total mesorectal excision for rectal cancer: functional assessment and factors affecting outcome. Colorectal Dis. 2006;8:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |