Published online Dec 27, 2022. doi: 10.4240/wjgs.v14.i12.1329
Peer-review started: August 25, 2022
First decision: September 2, 2022
Revised: September 11, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: December 27, 2022
Processing time: 124 Days and 2 Hours
Patients with mesenteric ischemia frequently suffer from bowel necrosis even after revascularization. Hydrogen gas has showed promising effects for ischemia-reperfusion injury by reducing reactive oxygen species in various animal and clinical studies. We examined intestinal tissue injury by ischemia and reperfusion under continuous initiation of 3% hydrogen gas.
To clarify the treatment effects and target cells of hydrogen gas for mesenteric ischemia.
Three rat groups underwent 60-min mesenteric artery occlusion (ischemia), 60-min reperfusion following 60-min occlusion (reperfusion), or ischemia-reperfusion with the same duration under continuous 3% hydrogen gas inhalation (hydrogen). The distal ileum was harvested. Immunofluorescence staining with caspase-3 and leucine-rich repeat-containing G-protein-coupled 5 (LGR5), a specific marker of intestinal stem cell, was conducted to evaluate the injury location and cell types protected by hydrogen. mRNA expressions of LGR5, olfactomedin 4 (OLFM4), hairy and enhancer of split 1, Jagged 2, and Neurogenic locus notch homolog protein 1 were measured by quantitative polymerase chain reaction. Tissue oxidative stress was analyzed with immunostaining for 8-hydroxy-2'-deoxyguanosine (8-OHdG). Systemic oxidative stress was evaluated by plasma 8-OHdG.
Ischemia damaged the epithelial layer at the tip of the villi, whereas reperfusion induced extensive apoptosis of the cells at the crypt base, which were identified as intestinal stem cells with double immunofluorescence stain. Hydrogen mitigated such apoptosis at the crypt base, and the LGR5 expression of the tissues was higher in the hydrogen group than in the reperfusion group. OLFM4 was also relatively higher in the hydrogen group, whereas other measured RNAs were comparable between the groups. 8-OHdG concentration was high in the reperfusion group, which was reduced by hydrogen, particularly at the crypt base. Serum 8-OHdG concentrations were relatively higher in both reperfusion and hydrogen groups without significance.
This study demonstrated that hydrogen gas inhalation preserves intestinal stem cells and mitigates oxidative stress caused by mesenteric ischemia and reperfusion.
Core Tip: Distal ileum of rats was observed after 60-min mesenteric artery occlusion (ischemia), 60-min reperfusion following 60-min occlusion (reperfusion), or ischemia-reperfusion with the same duration under continuous 3% hydrogen gas inhalation (hydrogen). Immunofluorescence staining with caspase-3 and leucine-rich repeat-containing G-protein-coupled 5 (LGR5) (a specific marker of intestinal stem cell) identified ischemia damaged the epithelial layer at the tip of the villi, whereas reperfusion induced extensive apoptosis of intestinal stem cells that was mitigated by hydrogen. In addition, quantitative polymerase chain reaction revealed the LGR5 expression of the tissues was higher in rats with hydrogen inhalation than in those with reperfusion injury.
- Citation: Yamamoto R, Suzuki S, Homma K, Yamaguchi S, Sujino T, Sasaki J. Hydrogen gas and preservation of intestinal stem cells in mesenteric ischemia and reperfusion. World J Gastrointest Surg 2022; 14(12): 1329-1339
- URL: https://www.wjgnet.com/1948-9366/full/v14/i12/1329.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i12.1329
Mesenteric ischemia is caused by insufficient blood flow in the mesenteric artery to meet the metabolic demand of the visceral organs[1]. Despite the relatively low incidence of mesenteric ischemia, delayed diagnosis caused by vague symptoms sometimes results in devastating complications, such as intestinal necrosis, abdominal sepsis, and mortality[1-3]. While rapid recirculation of mesenteric vessels by endovascular or surgical intervention has been critical, increasing the arterial flow would be the only method of preserving the intestines when hemodynamic instability causes mesenteric ischemia[1,4,5]. Notably, some patients develop bowel necrosis even after revascularization of the mesenteric artery[6].
In the last decades, hydrogen gas (molecular hydrogen) has emerged as an attractive medicinal agent for various diseases, specifically for ischemic diseases that require revascularization[7-9]. Some animal studies have shown that hydrogen had anti-inflammatory effects and reduced the reactive oxygen species (ROS) that are produced during ischemia-reperfusion injury[7,8,10,11]. Hydrogen inhalation was associated with the reduction of necrotic tissues in the animal model of cerebral and myocardial ischemia[7,8]. Moreover, several clinical investigations have reported that hydrogen gas inhalation was beneficial on acute myocardial infarction and out-of-hospital cardiac arrest, which are typical ischemia-reperfusion injuries[12,13].
Furthermore, studies on the safety of hydrogen gas have identified that hydrogen can be supplied to patients using a simple device without any adverse events, suggesting its high feasibility for clinical use[12-14]. Although the physiological mechanisms of the possible therapeutic benefits remain unclear, recent studies have suggested that the antioxidant effects of hydrogen would be introduced by cell signal transduction, preventing cellular apoptosis[15,16]. Another study reported that oxidation-reduction reactions that involve molecular hydrogen occur only with strong fatal ROS rather than with weak or beneficial ROS[14].
Accordingly, as mesenteric ischemia with revascularization is an ischemia-reperfusion injury, we aimed to clarify the favorable effects of hydrogen gas inhalation for mesenteric ischemia as a novel noninvasive treatment. In this study, we examined the degree of tissue damage in the intestines following ischemia and reperfusion and the tissue-protective effects of continuous initiation of 3% hydrogen gas. Moreover, several cell markers were substantially measured for the differentiation of hydrogen-target cells to elucidate the pathophysiological mechanisms of hydrogen.
The protocol used in this study was approved by the Research Council and Animal Care and Use Committee of the Research Institute of Keio University in Tokyo, Japan (approval number 21013-0) and was performed in accordance with the guidelines for the care and use of laboratory animals established by the Japanese Pharmacological Society and the National Institutes of Health.
Eight-week-old male Sprague-Dawley rats (250-270 g) were purchased from Sankyo Labo Service Corporation, Inc. (Tokyo, Japan) and kept in a temperature- and light-controlled room (20 °C, 12-h light/dark cycle). The rats had free access to food and water. Before the procedure, they were intraperitoneally anesthetized with a combination of 0.3 mg/kg medetomidine, 2.0 mg/kg midazolam, and 2.5 mg/kg butorphanol. They were appropriately anesthetized throughout the procedure.
Hydrogen gas (3%) was prepared using a hydrogen gas supply device (Nihon Kohden Co., Tokyo, Japan)[14] and administered to rats at a rate of 0.2 L/min. The hydrogen gas stored in the device was mixed with air, and the targeted concentration was measured inside the supply device. The gas flow rate was adjusted at the output port of the device and validated with a flow meter attached to the respiratory circuit.
The rats were allocated to three groups: ischemia (control 1), reperfusion (control 2), and hydrogen groups. The ischemia group (n = 10) underwent a median laparotomy and dissection of the superior mesenteric artery. The artery was then occluded at the root by a double microclamp for 60 min. The marginal arteries of the intestines were also ligated at the ileocolic junction and at 15 cm proximal from the junction to achieve complete ischemia of the terminal ileum. The reperfusion group (n = 11) similarly underwent 60-min occlusion of the superior mesenteric artery and ligation of the marginal arteries. The occlusion was then released by declamping of the mesenteric artery, and reperfusion of the intestine was observed for 60 min without closing the abdomen[17,18]. The hydrogen group (n = 9) was connected to the respiratory circuit using a gas supply hood that covered the face and head of the rats, in which spontaneous respiration was maintained without using mechanical ventilation[14]. After hydrogen gas inhalation, the rats underwent the same surgical procedures as those in the reperfusion group.
In each rat, intestinal ischemia was confirmed by the paleness of the distal ileum and pulselessness of the mesenteric artery. The procedures of the tree groups were conducted simultaneously to reduce potential confounders by procedures. All animals were sacrificed immediately after the aforementioned procedures. A 2-cm-long ileum was then excised at 6 cm proximal from the ileocolic junction, which was processed for histological evaluation and ribonucleic acid (RNA) extraction. Blood samples were also obtained directly from the left ventricle[19]. Details of study protocol were not pre-registered nor published.
Tissues were fixed in 4% neutral-buffered paraformaldehyde, and 4 μm paraffin-embedded sections were prepared. Hematoxylin and eosin (H&E) staining was performed to evaluate histopathological changes that were visually compared between the three groups (ischemia, ischemia-reperfusion, and ischemia-reperfusion under hydrogen gas inhalation).
For immunostaining, samples were deparaffinized and rehydrated, and endogenous peroxidase activity was then suppressed using 0.3% hydrogen peroxide (H2O2) in methanol. Nonspecific binding was blocked with bovine serum albumin (BSA) for 30 min. Primary rabbit antirat antibody against active caspase-3 (CST 9661S; Cell Signaling Technology, Beverly, MA, United States) or against 8-hydroxy-2'-deoxyguanosine (8-OHdG) that is an oxidized nucleoside of DNA induced by ROS (N45.1; Japan Institute for the Control of Aging, Fukuroi, Shizuoka, Japan) was applied with 1:200 dilution and then incubated overnight at 4 °C. After washing, sections were incubated with biotinylated antirabbit antibodies (Vector Laboratories, Burlingame, CA, United States) diluted to 1:200 in a blocking serum for 30 min. Sections were subjected to peroxidase along with 3,3’-diaminobenzidine-tetrahydrochloride and H2O2 (Elite ABC Kit and DAB Substrate Kit; Vector Laboratories). Slides were washed and counterstained with Gill’s hematoxylin (Accustain; Sigma-Aldrich, St. Louis, MO, United States), and samples were microphotographed at Zeiss Axioscope2 (Carl Zeiss, Oberkochen, Germany).
Frozen sections of the harvested tissues were incubated with one of the following primary antibodies diluted in 0.1% BSA/phosphate-buffered saline (PBS): LGR5 as an intestinal stem cell marker[20] (1:200, bs-1117R Bioss Antibodies Inc., MA, United States) and active caspase-3 (1:200). After washing with PBS/0.1% Tween 20 (Wako Pure Chemical Industries, Japan), sections were incubated with secondary antibodies for 1 h at room temperature using Alexa Fluor 488-conjugated (1:500, Invitrogen, CA, United States) or TAS fluorescence systems (NEL 702, PerkinElmer Life Sciences Inc., MA, United States). After counterstaining with Hoechst 33258 (94403, Sigma-Aldrich, MO, United States) to visualize nuclei, images were obtained with a BZ9000 (Keyence, Osaka, Japan).
Total RNA was isolated using a miRNeasy Mini Kit (Qiagen, Valencia, CA, United States) and converted to cDNA using the High-Capacity Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, United States), according to the manufacturer’s instructions. Real-time polymerase chain reaction (PCR) was performed using a QuantiFast SYBR Green PCR Kit (Qiagen), according to the manufacturer’s instructions. The PCR primers used in this study were as follows: LGR5 forward, 5'-TGTCATGTGAGCTGGATGG-3' and reverse, 5'-ATGCAGGAGACTGGCAGGTA-3'; OLFM4 forward, 5'-GTGGACAGAAGGTGGTACTCTG-3' and reverse, 5'- GCTGGACATACTCCTTCACCTTA-3'; Hes1 forward, 5'-ATAAACCCTCAACTGCTCCGT-3' and reverse, 5'-CCATGATAGGCTTTGATGACTTTCT-3'; Jag2 forward, 5'-CCACACCAGATGAGGAGCTG-3' and reverse, 5'-CAGAACTTGTTGCAGGTGGC-3'; Notch1 forward, 5'-TGGTCTCAACTGCCAGAACC-3' and reverse, 5'-CACTCGCAGTGGTACTGTGT-3'; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5'-TTGTGCAGTGCCAGCCTC-3' and reverse, 5'-GGTAACCAGGCGTCCGATAC-3'. Expression levels were calculated using the 2−ΔΔCt method and normalized to levels of the internal control GAPDH[19,21,22]. Real-time PCR was performed by a researcher who was blinded to the group allocation.
Serum samples were prepared from blood using Nanosep® Centrifugal Devices with Omega™ Membrane 10K (Pall Corporation, NY, United States), according to the manufacturer’s instructions. The supernatant was used to determine 8-OHdG by a competitive enzyme-linked immunosorbent assay (Highly Sensitive 8-OHdG Check; Japan Institute for the Control of Aging, Fukuroi, Shizuoka, Japan)[20].
Descriptive statistics are presented as median (interquartile range) or number (percentage). Intergroup comparisons of mRNA expressions and 8-OHdG concentrations were performed using analysis of variance with Tukey-Kramer as post-hoc test and/or Kruskal-Wallis tests, as appropriate. All statistical tests used a α error rate of 0.05 and were two-sided. All statistical analyses were conducted using IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, NY, United States), and Microsoft Excel (Microsoft, Redmond, WA, United States).
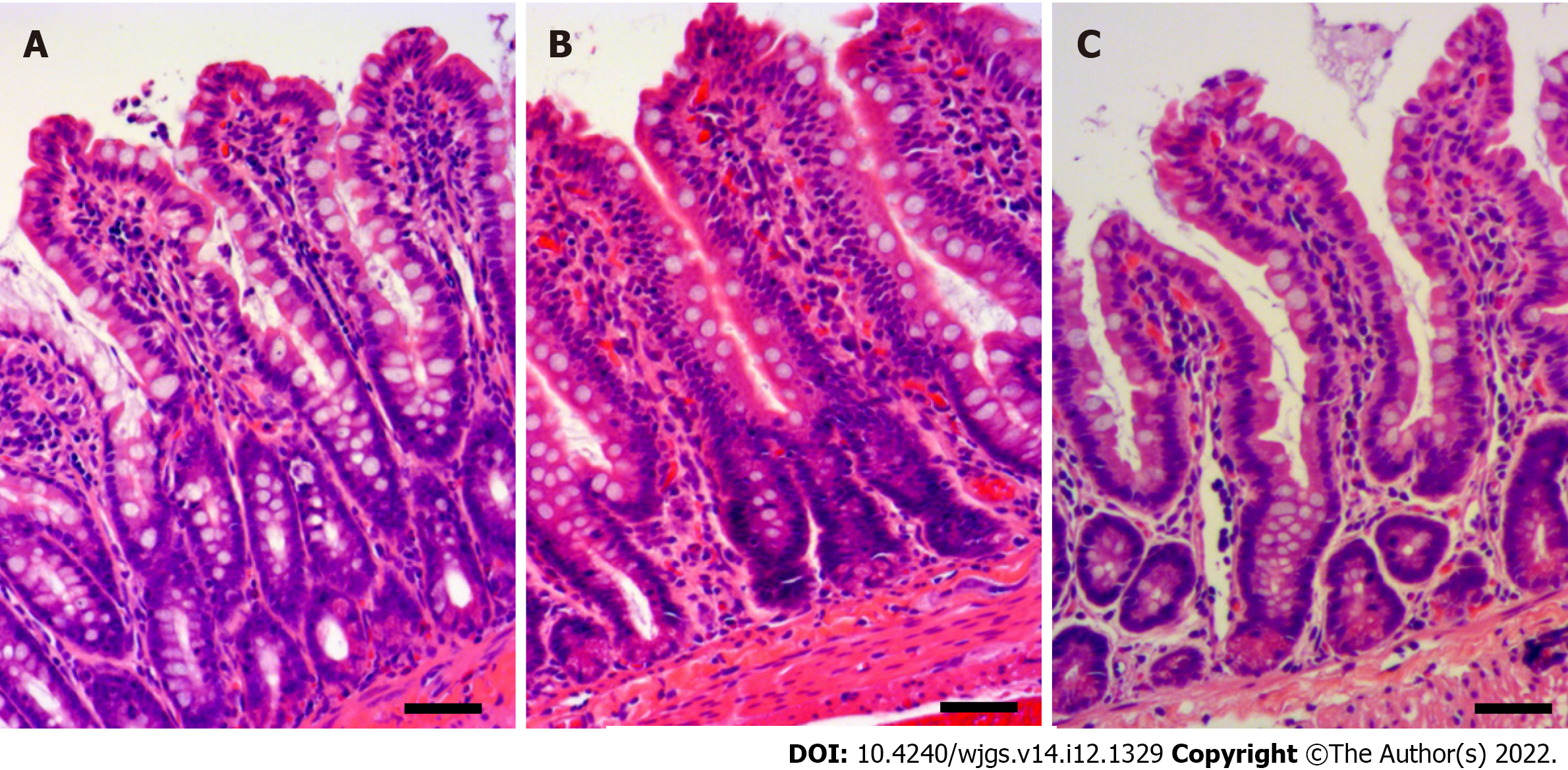
Histological sections with H&E staining showed morphologic changes in the intestinal mucosa of the ischemia group (Figure 1A), in which pyknosis was observed in the epithelial layer. Mucosa was more severely injured in the reperfusion group (Figure 1B), and extensive pyknosis in the epithelial layer, denudation of the tip of the villi, and capillary congestion were observed. Conversely, the hydrogen groups showed similar degree of injury to the ischemia group (Figure 1C), with mild epithelial pyknosis and denudation of the villi.
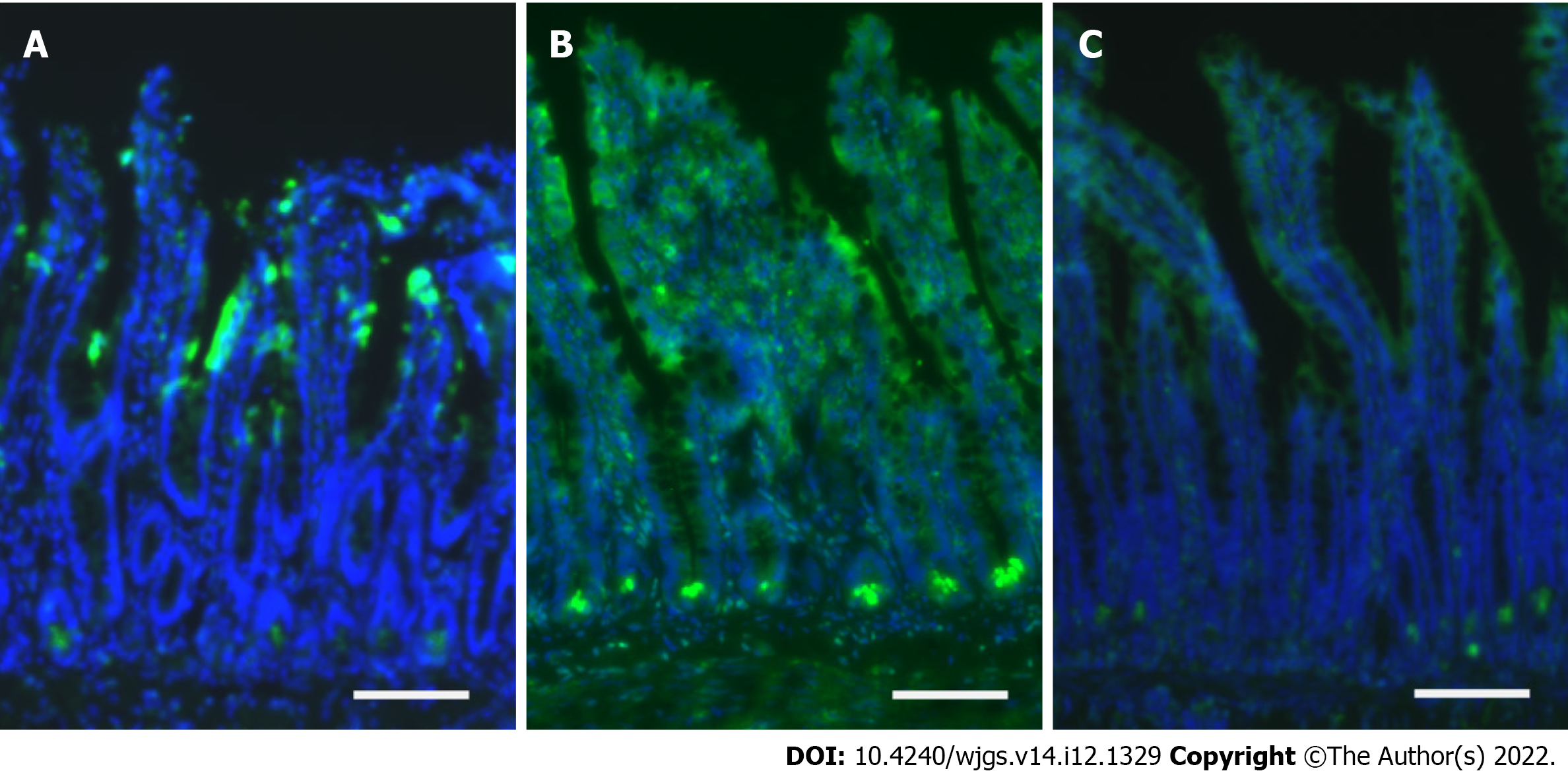
Immunofluorescence analyses with caspase-3 antibodies revealed that the intestinal mucosa of the ischemia group was mainly injured at the epithelial layer closed to the tip of the villi (Figure 2A). In the reperfusion group, apoptosis was extensively identified at the crypt base, where intestinal stem cells exist[23,24], in addition to injuries at the whole epithelial layer (Figure 2B). Apoptosis at the crypt base was limited in the hydrogen group (Figure 2C), whereas epithelial injury was observed at the villi with a half side of the digestive tract.
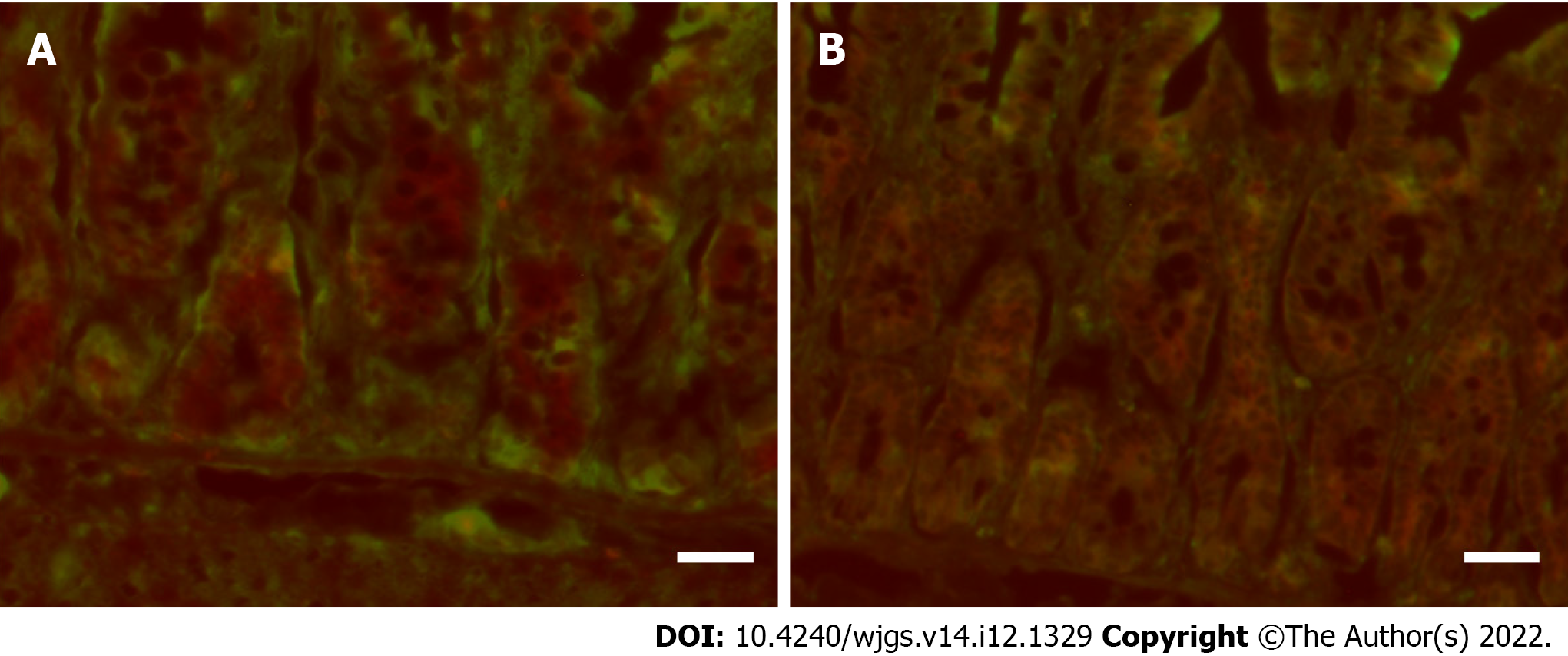
In the ischemia group, immunofluorescence analyses using simultaneous staining of caspase-3 and LGR5, a specific protein for intestinal stem cell, revealed that LGR5-positive cells at the crypt base did not undergo apoptosis [caspase-3 (red) and LGR5 (green) were separately stained; Figure 3A]. Conversely, multiple LGR5-positive cells underwent apoptosis after reperfusion (there were double-stained cells with caspase-3 and LGR5; Figure 3B).
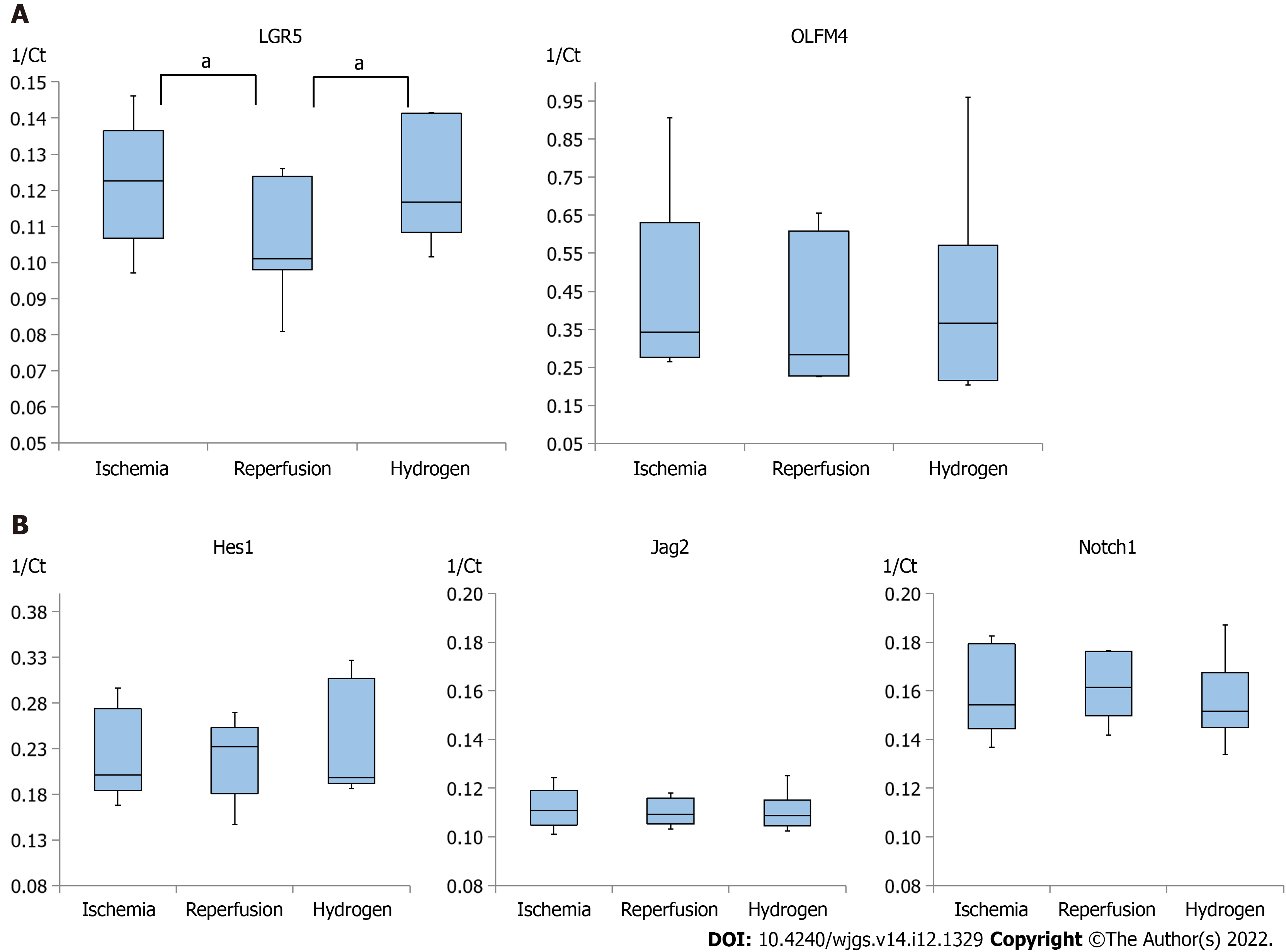
Quantitative analyses of RNA in the homogenized intestinal tissues were conducted on LGR5, OLFM4, Hes1, Jag2, and Notch1, and Figure 4 summarizes these RNA expressions in the ischemia, reperfusion, and hydrogen groups. The expression of LGR5 was significantly lower in the reperfusion group than in the ischemia group, whereas the expression of LGR5 was higher in the hydrogen group than in the reperfusion group (Figure 4A). In addition, the expression of OLFM4 was relatively higher in the hydrogen group than in the reperfusion group, although the differences were not significant.
Conversely, the expression of Hes1, a transcriptional repressor of genes, was relatively high in the reperfusion group than in the ischemia and hydrogen groups (Figure 4B). Expressions of Jag2 (a ligand in Notch signaling for cell fate decision) and Notch1 (a transmembrane receptor in Notch signaling) were comparable among the ischemia, reperfusion, and hydrogen groups (Figure 4B).
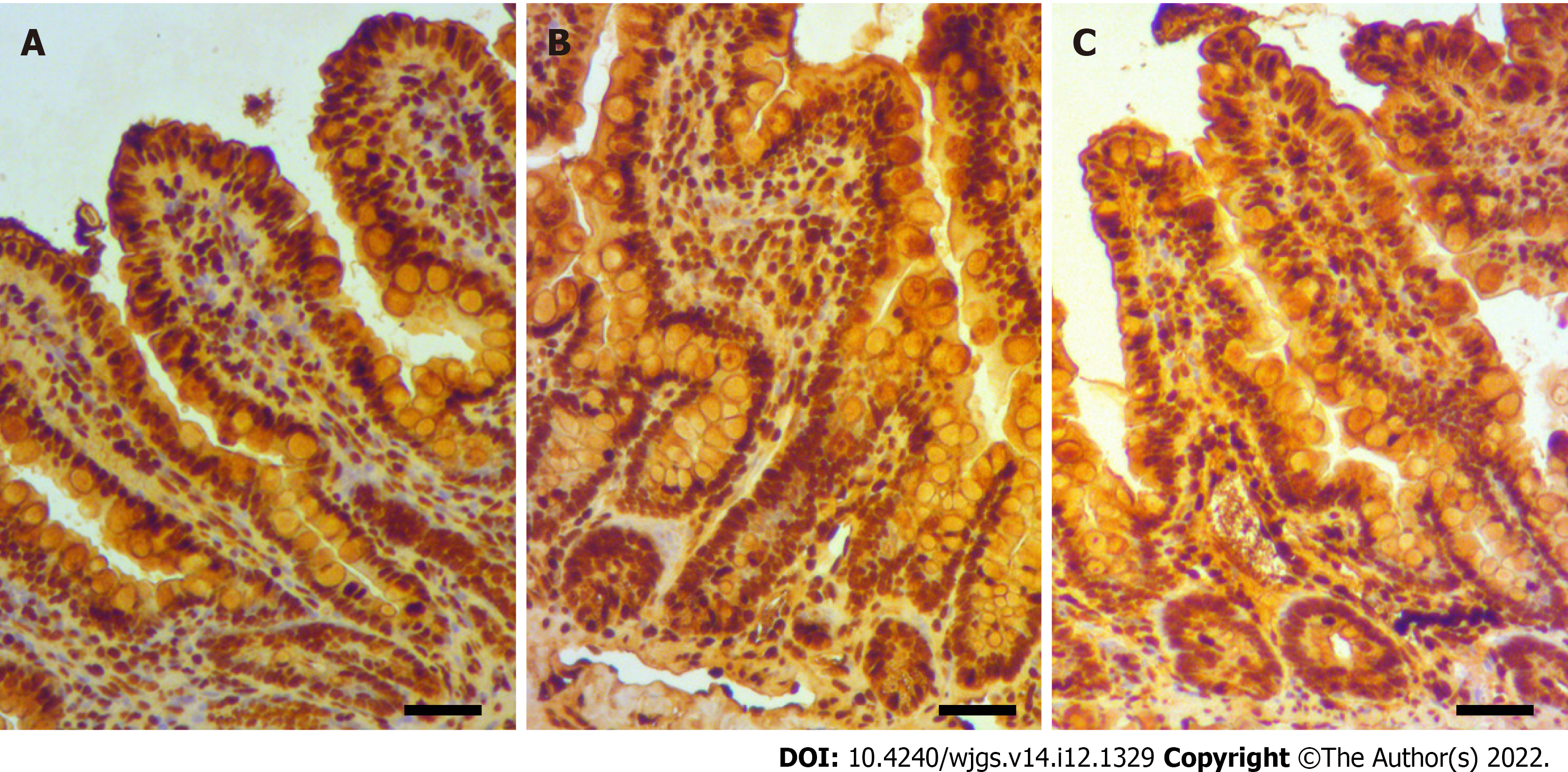
Immunostaining for 8-OHdG of intestinal tissue showed that the ischemia group had limited oxidative stress at the intestinal mucosa, although considerable pyknosis occurred in the epithelial layer, particularly at the tip of the villi (Figure 5A). Conversely, the reperfusion group had extensive oxidative injury throughout the epithelium, including at the crypt base (Figure 5B). The hydrogen group had mild-to-moderate oxidative injury at the mucosa of the crypt base, along with pyknosis with the denudation at the tip of the villi (Figure 5C).
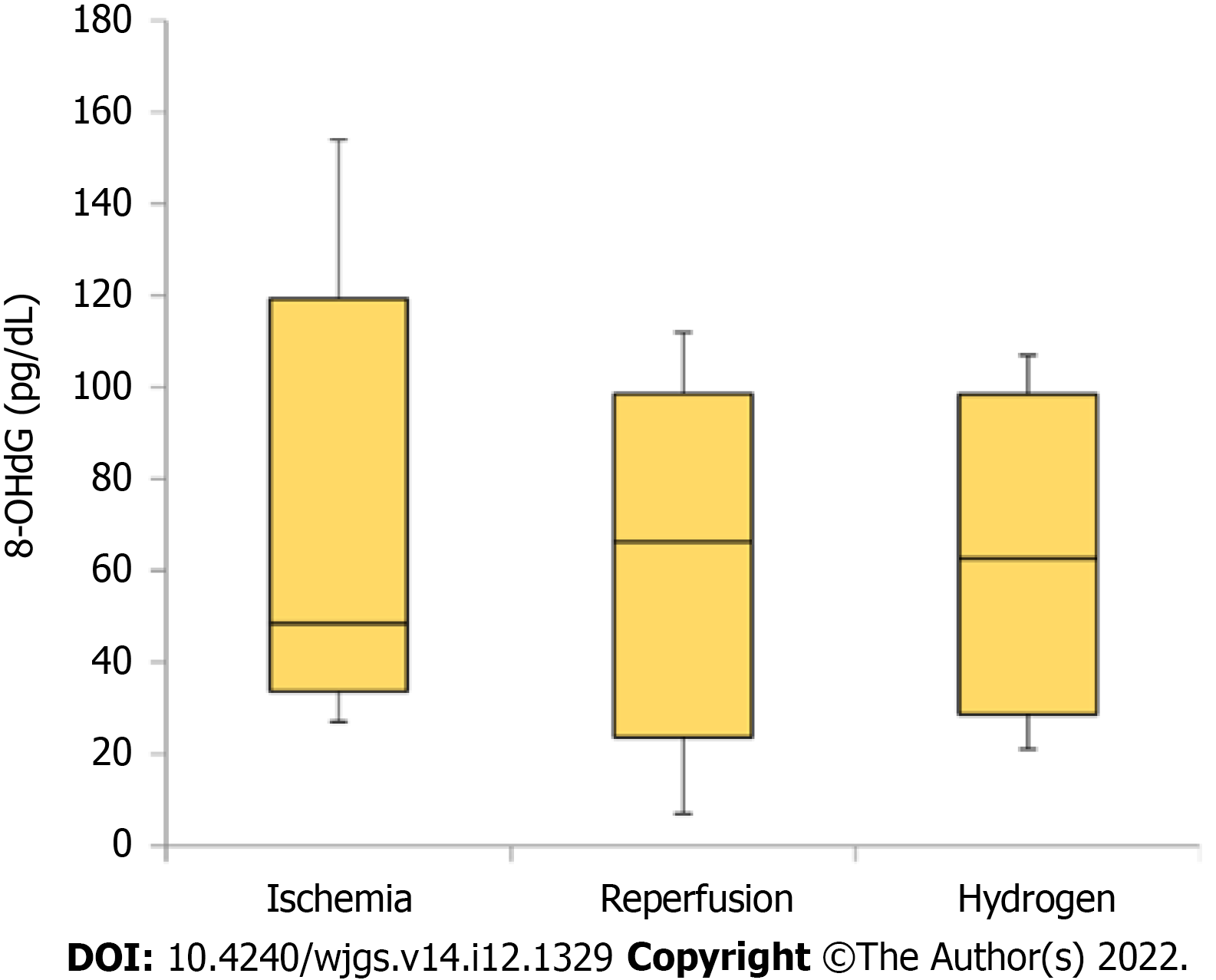
Regarding systematic oxidative injury, the serum 8-OHdG concentrations immediately after the intervention in each group (ischemia, ischemia and reperfusion, and ischemia and reperfusion under hydrogen gas inhalation) are summarized in Figure 6. Despite the lack of significance, 8-OHdG concentration was higher in the reperfusion and hydrogen groups than in the ischemia group.
In this study, the tissue-protective effects of continuous hydrogen gas inhalation were histologically identified in the model of ischemic-reperfusion injury at mesentery. In addition, hydrogen protected intestinal stem cells from oxidative stress following ischemia-reperfusion injury, which has not been reported as therapeutic effect of hydrogen in previous studies. Notably, the intestinal stem cells were not injured by ischemia alone (ischemia without reperfusion), and therefore, hydrogen would provide tissue-protective effect only when reperfusion happens, rather than only ischemic injury exists.
Previous clinical and animal studies have suggested that hydrogen gas has anti-oxidative and anti-inflammatory effects on several critical diseases, such as cerebral infarction, myocardial infarction, and post-cardiac arrest syndrome[7,10,12]. Although pathophysiological mechanisms underlying these therapeutic effects remain unclear, hydrogen would attenuate excessive neutrophil activation and reduce hydroxyl radicals produced following an ischemia-reperfusion injury[25,26]. In this study, fewer oxidized nucleosides of DNA (8-OHdG) were observed at the crypt base of the intestines during continuous inhalation of hydrogen gas, which suggests that hydrogen mitigated ROS toxicity.
Immunofluorescence assay using LGR5 suggested that the intestinal stem cells would be a target of the therapeutic effects of hydrogen, at least under mesenteric ischemia and reperfusion. Stem cells have unique features, one of which is self-proliferation under adequate ischemic stimuli, whereas differentiated enterocytes undergo apoptosis because of ischemia-induced energy depletion[23,27]. In a study on the association between ROS and intestinal stem cells, modest ROS following ischemia would signal proliferation and differentiation of stem cells[27]. However, the same study suggested that high levels of ROS can induce intestinal stem cell apoptosis, which is similar to the observations in this study. We showed relative preservation of intestinal stem cells with ischemic stress alone and extensive apoptosis of stem cells with reperfusion that would have introduced massive ROS. Therefore, our results might indicate that hydrogen reduces excessive ROS caused by ischemia-reperfusion stimuli and prevents apoptosis of intestinal stem cells.
The protective effects of hydrogen on intestinal stem cells are also indicated by the higher LGR5 expression with hydrogen gas inhalation in the quantitative measurement of RNA. OLFM4 is a robust marker of LGR5-positive stem cells[28], and in this study, its expression was relatively higher in the hydrogen group than in the reperfusion group. Jag2/Notch1/Hes1 expressions have been reported to increase with epithelial cell proliferation following ischemia-reperfusion injury in the intestines[19]. Expressions of Jag2 and Notch1 were comparable between the three groups, and the expression of Hes1 was slightly high in the reperfusion group, suggesting that proliferation signals at the epithelium under ischemia would be similar regardless of reperfusion or hydrogen inhalation.
Systemic oxidative stress was not different between the reperfusion and hydrogen groups. Therefore, hydrogen would not systematically affect the total amount of ROS in the body. Although hydrogen may reduce ROS in other tissues or organs in addition to the intestinal mucosa, such possible effects were not examined in this study. Moreover, the mechanisms of the reduction of ROS toxicity by hydrogen were not assessed. Future studies should focus on these topics to develop a noninvasive novel therapy using hydrogen gas.
This study reported on the tissue-protective effects of continuous hydrogen gas inhalation in ischemia-reperfusion injury in the intestines. The target cells of hydrogen might be intestinal stem cells, which are injured by excessive ROS caused by reperfusion following ischemia rather than by ischemic stress alone. The pathophysiological mechanisms for ROS reduction by hydrogen in stem cells should be further clarified in future studies.
Mesenteric ischemia introduces unfavorable clinical outcomes particularly when bowel necrosis is diagnosed, and it can happen even after revascularization. However, promising treatment has not been developed to prevent bowel necrosis after revascularization.
Hydrogen gas inhalation has showed tissue preserving effects for several ischemia-reperfusion injuries by reducing reactive oxygen species (ROS) in various animal and clinical studies. In addition, the safety of hydrogen gas was shown by clinical studies that examined the efficacy of hydrogen on myocardial infarction and post-cardiac arrest syndrome. Therefore, hydrogen gas for mesenteric ischemia can be a novel noninvasive treatment.
This study aimed to clarify the favorable effects of hydrogen gas inhalation for mesenteric ischemia and reperfusion. We hypothesized that the degree of tissue damage in the intestines following ischemia and reperfusion would be mitigated by continuous initiation of 3% hydrogen gas.
Rats were allocated to three groups: ischemia (control 1) that underwent 60-min occlusion of mesenteric artery by clamping under laparotomy, reperfusion (control 2) that underwent the ischemia procedure and 60-min release of occlusion, and hydrogen that underwent the ischemia and reperfusion under 0.3% hydrogen gas inhalation at a rate of 0.2 L/min. Then, the tissue damages at the ileum were histologically evaluated, using immunostaining against caspase-3, 8-hydroxy-2'-deoxyguanosine, and leucine-rich repeat-containing G-protein-coupled 5 (LGR5). Several mRNA, including LGR5, were quantitatively measured with RT-PCR.
The reperfusion procedure introduced intestinal tissue destruction, which was mitigated by hydrogen gas inhalation. In addition, the intestinal tissue injury by the reperfusion involved intestinal stem cell that was marked by LGR5, whereas the ischemia without reperfusion did not affect the stem cell. The expression of LGR5 was significantly lower in the reperfusion group than in the ischemia group, whereas the expression of LGR5 was higher in the hydrogen group than in the reperfusion group.
This study reported on the tissue-protective effects of continuous hydrogen gas inhalation in the ischemia and reperfusion injury at the intestine. The target cells of hydrogen might be intestinal stem cells that are injured by excessive ROS caused by reperfusion following ischemia.
Hydrogen may reduce ROS in other tissues in addition to the intestinal mucosa, which should be examined in the future study. Moreover, the mechanisms of the reduction of ROS toxicity by hydrogen should be revealed to validate the hydrogen as a noninvasive novel treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Turkey; de Oliveira I, Brazil S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med. 2016;374:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 329] [Article Influence: 36.6] [Reference Citation Analysis (1)] |
| 2. | Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Grootjans J, Lenaerts K, Buurman WA, Dejong CH, Derikx JP. Life and death at the mucosal-luminal interface: New perspectives on human intestinal ischemia-reperfusion. World J Gastroenterol. 2016;22:2760-2770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 4. | Park WM, Gloviczki P, Cherry KJ Jr, Hallett JW Jr, Bower TC, Panneton JM, Schleck C, Ilstrup D, Harmsen WS, Noel AA. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002;35:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Ryer EJ, Kalra M, Oderich GS, Duncan AA, Gloviczki P, Cha S, Bower TC. Revascularization for acute mesenteric ischemia. J Vasc Surg. 2012;55:1682-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Plumereau F, Mucci S, Le Naoures P, Finel JB, Hamy A. Acute mesenteric ischemia of arterial origin: importance of early revascularization. J Visc Surg. 2015;152:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, Makino S, Ohta S, Ogawa S, Fukuda K. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 8. | Ohta S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta. 2012;1820:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L, Wang G. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010;34:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Hayashida K, Sano M, Kamimura N, Yokota T, Suzuki M, Ohta S, Fukuda K, Hori S. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation. 2014;130:2173-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Homma K, Yoshida T, Yamashita M, Hayashida K, Hayashi M, Hori S. Inhalation of Hydrogen Gas Is Beneficial for Preventing Contrast-Induced Acute Kidney Injury in Rats. Nephron Exp Nephrol. 2015;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Katsumata Y, Sano F, Abe T, Tamura T, Fujisawa T, Shiraishi Y, Kohsaka S, Ueda I, Homma K, Suzuki M, Okuda S, Maekawa Y, Kobayashi E, Hori S, Sasaki J, Fukuda K, Sano M. The Effects of Hydrogen Gas Inhalation on Adverse Left Ventricular Remodeling After Percutaneous Coronary Intervention for ST-Elevated Myocardial Infarction - First Pilot Study in Humans. Circ J. 2017;81:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Tamura T, Hayashida K, Sano M, Suzuki M, Shibusawa T, Yoshizawa J, Kobayashi Y, Suzuki T, Ohta S, Morisaki H, Fukuda K, Hori S. Feasibility and Safety of Hydrogen Gas Inhalation for Post-Cardiac Arrest Syndrome - First-in-Human Pilot Study. Circ J. 2016;80:1870-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Yamamoto R, Homma K, Suzuki S, Sano M, Sasaki J. Hydrogen gas distribution in organs after inhalation: Real-time monitoring of tissue hydrogen concentration in rat. Sci Rep. 2019;9:1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Cai J, Kang Z, Liu WW, Luo X, Qiang S, Zhang JH, Ohta S, Sun X, Xu W, Tao H, Li R. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Itoh T, Fujita Y, Ito M, Masuda A, Ohno K, Ichihara M, Kojima T, Nozawa Y. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem Biophys Res Commun. 2009;389:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Mine Y, Fujita F, Murase T, Ito S, Takatsuki M, Ikematsu K, Eguchi S. Heat Shock Protein 70 Messenger RNA in Rat Leukocytes Elevates After Severe Intestinal Ischemia-Reperfusion. J Surg Res. 2019;242:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Strumwasser A, Bhargava A, Victorino GP. Attenuation of endothelial phosphatidylserine exposure decreases ischemia-reperfusion induced changes in microvascular permeability. J Trauma Acute Care Surg. 2018;84:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Chen G, Qiu Y, Sun L, Yu M, Wang W, Xiao W, Yang Y, Liu Y, Yang S, Teitelbaum DH, Ma Y, Lu D, Yang H. The jagged-2/notch-1/hes-1 pathway is involved in intestinal epithelium regeneration after intestinal ischemia-reperfusion injury. PLoS One. 2013;8:e76274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kim CK, Yang VW, Bialkowska AB. The Role of Intestinal Stem Cells in Epithelial Regeneration Following Radiation-Induced Gut Injury. Curr Stem Cell Rep. 2017;3:320-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Cai Y, Wang W, Liang H, Sun L, Teitelbaum DH, Yang H. Keratinocyte growth factor improves epithelial structure and function in a mouse model of intestinal ischemia/reperfusion. PLoS One. 2012;7:e44772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Chen G, Sun L, Yu M, Meng D, Wang W, Yang Y, Yang H. The Jagged-1/Notch-1/Hes-1 pathway is involved in intestinal adaptation in a massive small bowel resection rat model. Dig Dis Sci. 2013;58:2478-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Nalapareddy K, Nattamai KJ, Kumar RS, Karns R, Wikenheiser-Brokamp KA, Sampson LL, Mahe MM, Sundaram N, Yacyshyn MB, Yacyshyn B, Helmrath MA, Zheng Y, Geiger H. Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell Rep. 2017;18:2608-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4394] [Cited by in RCA: 5119] [Article Influence: 319.9] [Reference Citation Analysis (0)] |
| 25. | Sano M, Suzuki M, Homma K, Hayashida K, Tamura T, Matsuoka T, Katsumata Y, Onuki S, Sasaki J. Promising novel therapy with hydrogen gas for emergency and critical care medicine. Acute Med Surg. 2018;5:113-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Shirakawa K, Kobayashi E, Ichihara G, Kitakata H, Katsumata Y, Sugai K, Hakamata Y, Sano M. H2 Inhibits the Formation of Neutrophil Extracellular Traps. JACC Basic Transl Sci. 2022;7:146-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Morris O, Jasper H. Reactive Oxygen Species in intestinal stem cell metabolism, fate and function. Free Radic Biol Med. 2021;166:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Grover PK, Hardingham JE, Cummins AG. Stem cell marker olfactomedin 4: critical appraisal of its characteristics and role in tumorigenesis. Cancer Metastasis Rev. 2010;29:761-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |