Published online Oct 27, 2022. doi: 10.4240/wjgs.v14.i10.1169
Peer-review started: July 10, 2022
First decision: August 19, 2022
Revised: September 3, 2022
Accepted: October 18, 2022
Article in press: October 18, 2022
Published online: October 27, 2022
Processing time: 107 Days and 6.6 Hours
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated condition characterized by abundant IgG4 positive plasma cells and fibrosis in the affected tissues. It affects most parts of the body; however, there are not many reports on IgG4-RD involving the colon.
A 50-year-old man complaining of intermittent fever for more than two years was referred to our hospital. Based on various investigations before surgery, we diagnosed him with chronic perforation of the sigmoid colon caused by inflammatory change or tumor. IgG blood tests before the operation suggested IgG4-RD, and postoperative pathology confirmed this prediction.
We present a patient with IgG4-RD with colon involvement, which is an uncommon site. This report will expand the understanding of IgG4-RD in unknown tissues.
Core Tip: Immunoglobulin G4-related disease (IgG4-RD) is characterized by abundant IgG4 positive plasma cells and fibrosis in the affected tissues. It can affect most parts of the body, but there were not many reports of IgG4-RD in the intestines. This patient was an IgG4-RD case to be reported in the colon, which was identified by computed tomography, magnetic resonance imaging, pathology and blood tests of IgGs. This case report will help expand the understanding of IgG4-RD in some unknown tissues.
- Citation: Zhan WL, Liu L, Jiang W, He FX, Qu HT, Cao ZX, Xu XS. Immunoglobulin G4-related disease in the sigmoid colon in patient with severe colonic fibrosis and obstruction: A case report. World J Gastrointest Surg 2022; 14(10): 1169-1178
- URL: https://www.wjgnet.com/1948-9366/full/v14/i10/1169.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i10.1169
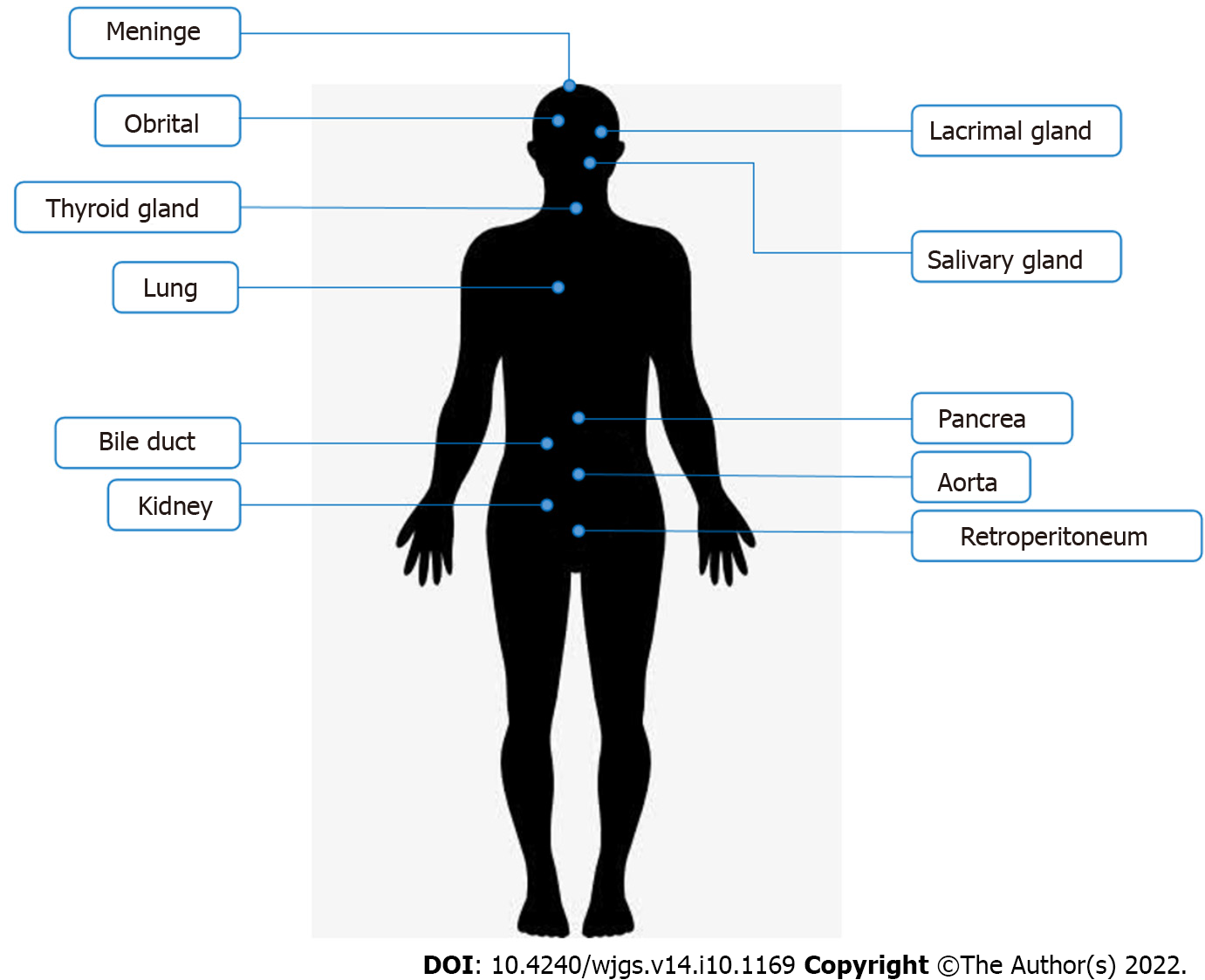
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated condition characterized by infiltration of IgG4 positive plasma cells and fibrosis in the affected tissues[1]. It was first identified as a distinct disease in 2003[2]. Due to lack of understanding in the past, this condition was misdiagnosed or could not be diagnosed. In recent years, awareness of the disease has increased over the past 20 years. The American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) IgG4-RD criteria were also formed and published[3]. Hence, many patients were diagnosed with IgG4-RD and received personalized treatment. Although it can affect almost any part of the body, it shows a strong preference for some organs (Figure 1), including salivary glands, lacrimal glands and orbitals, pancreas and biliary ducts, lungs, kidneys, aorta and retroperitoneum, meninges, and thyroid gland[4,5]. Typical manifestations of IgG4-RD are enlarged salivary and lacrimal glands, orbital pseudotumor, pancreatitis, sclerosing cholangitis, retroperitoneal fibrosis, tubular interstitial nephritis, and Liddell's thyroiditis[6].
Histopathology is still required to confirm the diagnosis, with the main histological features being IgG4-positive plasma cell infiltration, storiform fibrosis, and obliterative phlebitis[1]. Increased IgG4-positive plasma cells are seen in nearly all affected tissues; however, there is no specific clinical manifestation. Certain diseases such as lymphoma, vasculitis, and inflammatory bowel disease may also exhibit increased IgG4-positive plasma cells without storiform fibrosis and obliterative phlebitis[7,8]. In addition, the latter two features are not evident in the bone marrow and lymph nodes, making these two sites undesirable for histopathological investigations[9]. Additionally, the imaging features of IgG4-RD are as follows: A diffusely enlarged pancreas surrounded by capsule-like edema (“sausage-shaped” pancreas) and the anterolateral aorta wrapped by soft tissue in the case of retroperitoneal fibrosis[10-12]. However, isolated radiological findings are insufficient for diagnosis. 18Fluorodeoxyglucose-positron emission tomography (PET) can scan the entire body for staging and help find the sampling site[13].
IgG4-RD’s incidence is still underestimated because the disease is not clinically manifested and rarely leads to immediate organ failure[14]. Many patients are diagnosed accidentally or through histo
Intestinal involvement is not common in IgG4-RD, although sporadic reports have been published[16-19]. Therefore, more cases are needed to demonstrate that IgG4-RD can involve the gut. In this article, we will report the data of a patient with IgG4-RD involving the sigmoid colon, hoping to increase the understanding of IgG4-RD disease.
A 50-year-old man presented with intermittent fever for more than two years.
Intermittent fever occurred for more than two years. The body temperature remained at approximately 37.5 °C, and the fever usually occurred in the afternoon for 3–4 d a week. The fever was rarely high and often resolved without any treatment. The patient had been to the department of infectious diseases for treatment; however, no specific infection was found.
Six months ago, he had frequent and urgent micturition and increased nocturia. He went to the urology department, where prostatic hyperplasia was considered after ultrasound detection, but there was no cystoscopy or biopsy to be done. Then he was treated with tamsulosin hydrochloride and the symptoms, although partially alleviated, did not disappear fully.
Two months ago, he began to experience increased bowel movements and a feeling of incomplete bowel movements, accompanied by a bloated lower abdomen. So he came to the Gastrointestinal Surgery Clinic, and we ordered a colonoscopy and admitted him to the hospital.
Our patient had a 30-year-old history of hepatitis B without blatant virus replication. He also had allergic rhinitis for > 20 years, with the onset usually in August and September every year and relieved by itself after the season. He was allergic to ragweed pollen in 2017 and was cured by mometasone furoate nasal spray combined with cetirizine hydrochloride. He has never developed sinusitis.
The main drugs used in the patient’s medical records were interferon, cetirizine hydrochloride, mometasone furoate, and tamsulosin hydrochloride. He did not have a smoking and alcohol consumption history.
His family had no apparent history of colon tumors and autoimmune disease. Both his father and mother had died of cardiovascular diseases. Additionally, his mother had a history of hepatitis B.
Physical examination showed mild tenderness above the symphysis pubis in the lower abdomen without rebound pain. No other abnormality was seen.
The white blood cells count was normal as the baseline level, and hemoglobin was 92 g/L. The biochemical blood tests were within the normal range, including transaminases, bilirubin, amylase, and lipase. The tumor markers carcinoembryonic assay, cancer antigen 19-9, cancer antigen 72-4, and prostate-specific antigen were normal, while the urine culture and tuberculosis screening tests were negative. Nevertheless, this patient had a positive fecal occult blood test.
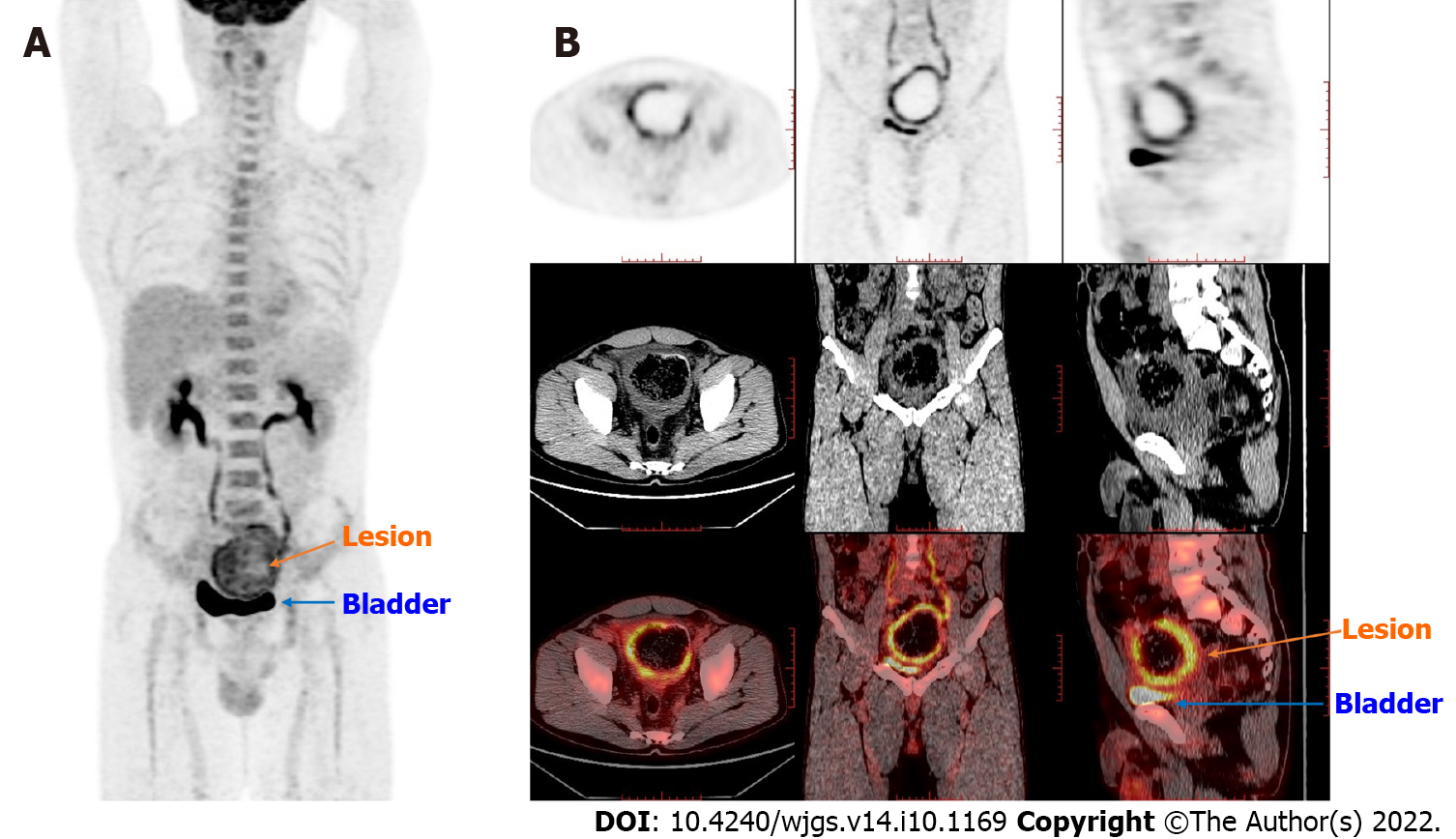
PET/computed tomography (CT) revealed a thick-walled cystic structure above the bladder, which was approximately 8.1 cm × 7.1 cm in size with increased radioactive uptake, and the maximum standard uptake value (SUVmax) was 6.0. Thus, a diagnosis of a tumor or chronic infection-related mass was made. PET-CT examination did not find high uptake in the parotid gland, pancreas, biliary tract, and prostate (Figure 2).
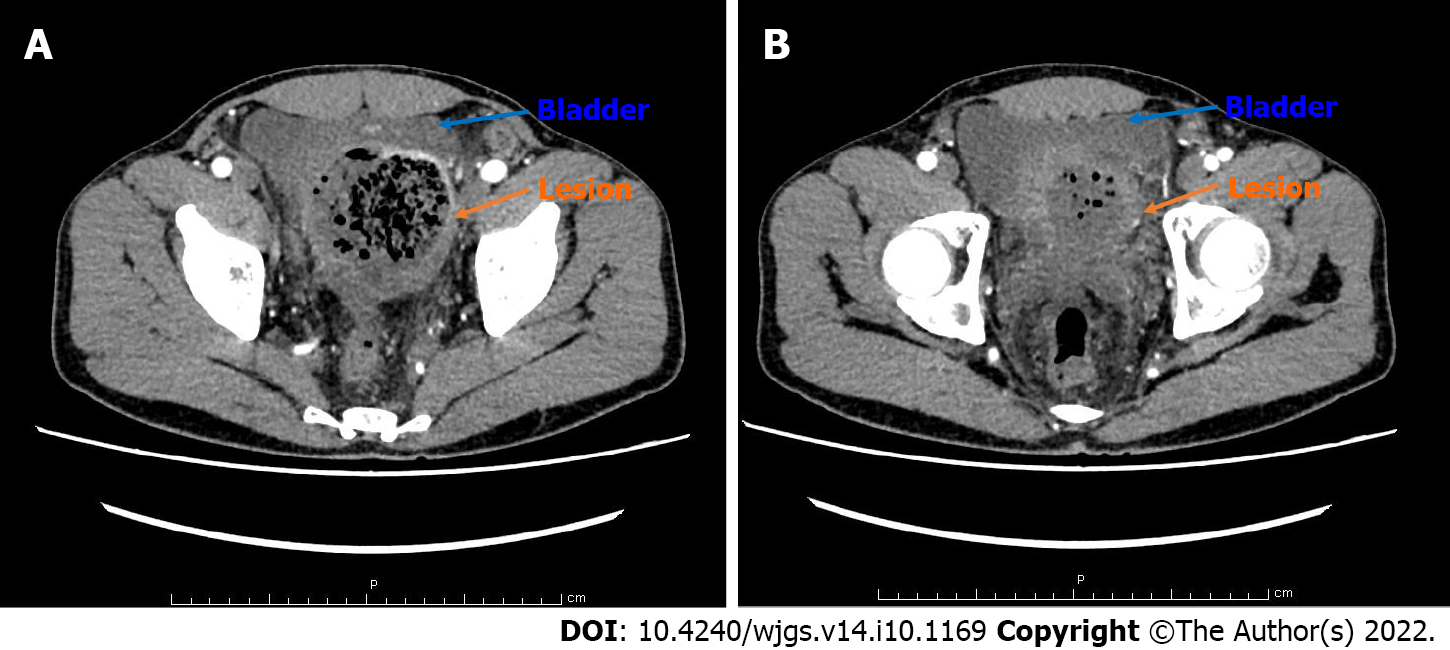
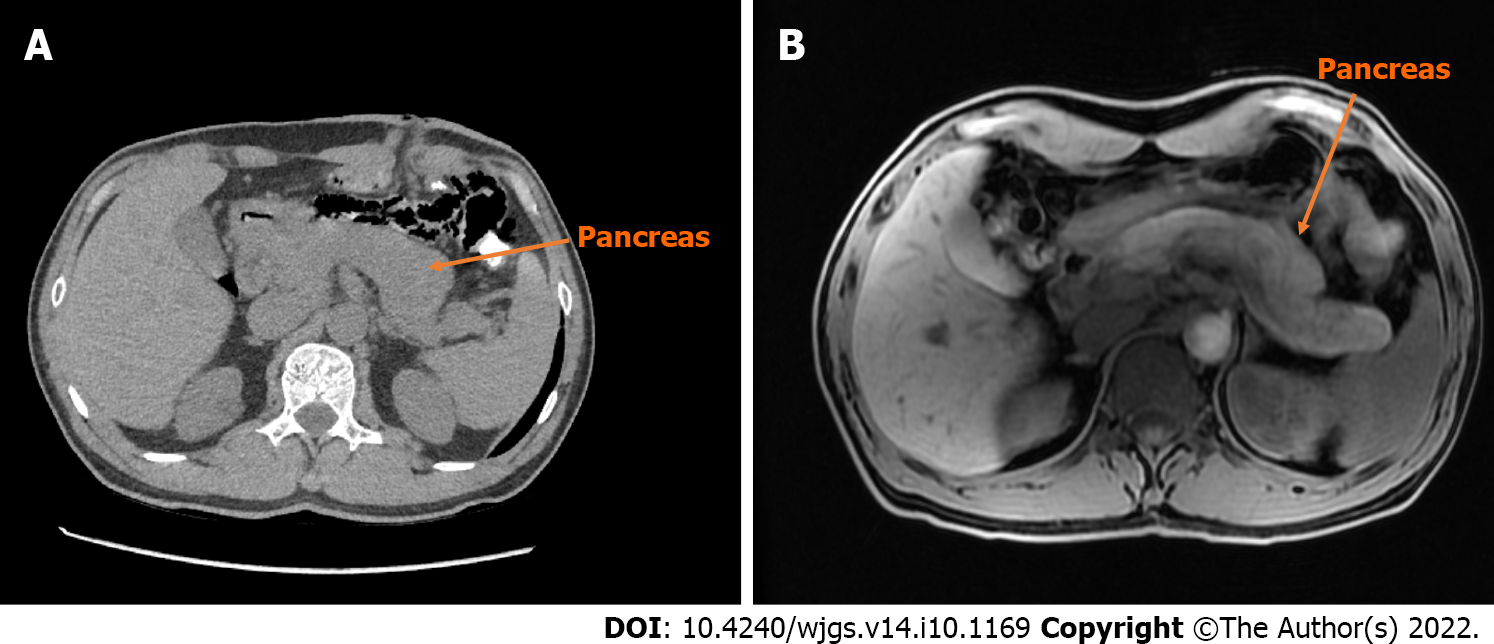
An enhancement CT scan of the entire abdomen showed a noticeable expansion of the proximal sigmoid colon with significant intestinal content. The intestinal wall was thickened with contrast enhancement, and the boundary between the sigmoid colon and bladder was not evident in the apparent bladder compression (Figure 3). Hence, the possibility of the colonic diverticulum with infection or tumor was considered. We scanned the pancreas by CT and magnetic resonance imaging (Figure 4) and found an enlarged pancreas similar to the “sausage-shaped” pancreas finding in IgG4-RD.
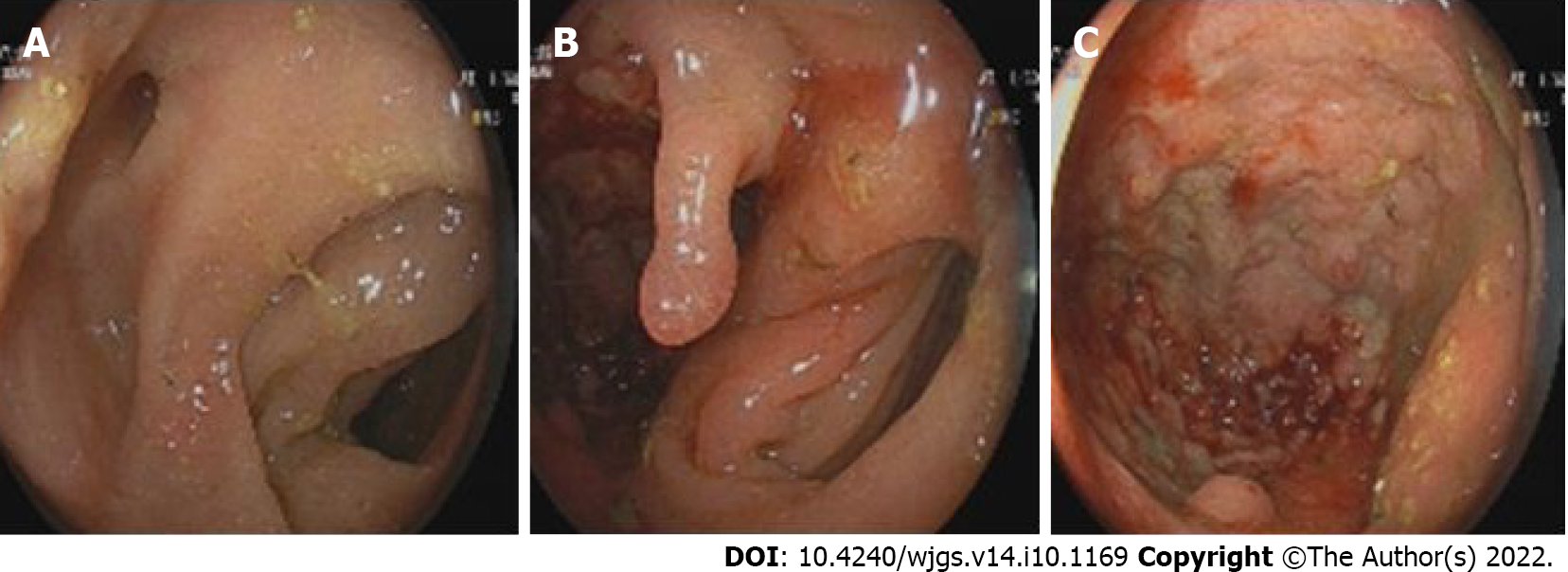
In the electronic colonoscopy, the lesion was located at the sigmoid colon. It was about 40 cm away from the anus with a blind cavity of 6 cm in diameter. The intestinal cavity is next to the blind hole with a large number of feces. Therefore, the colonoscope could no longer observe upwards (Figure 5).
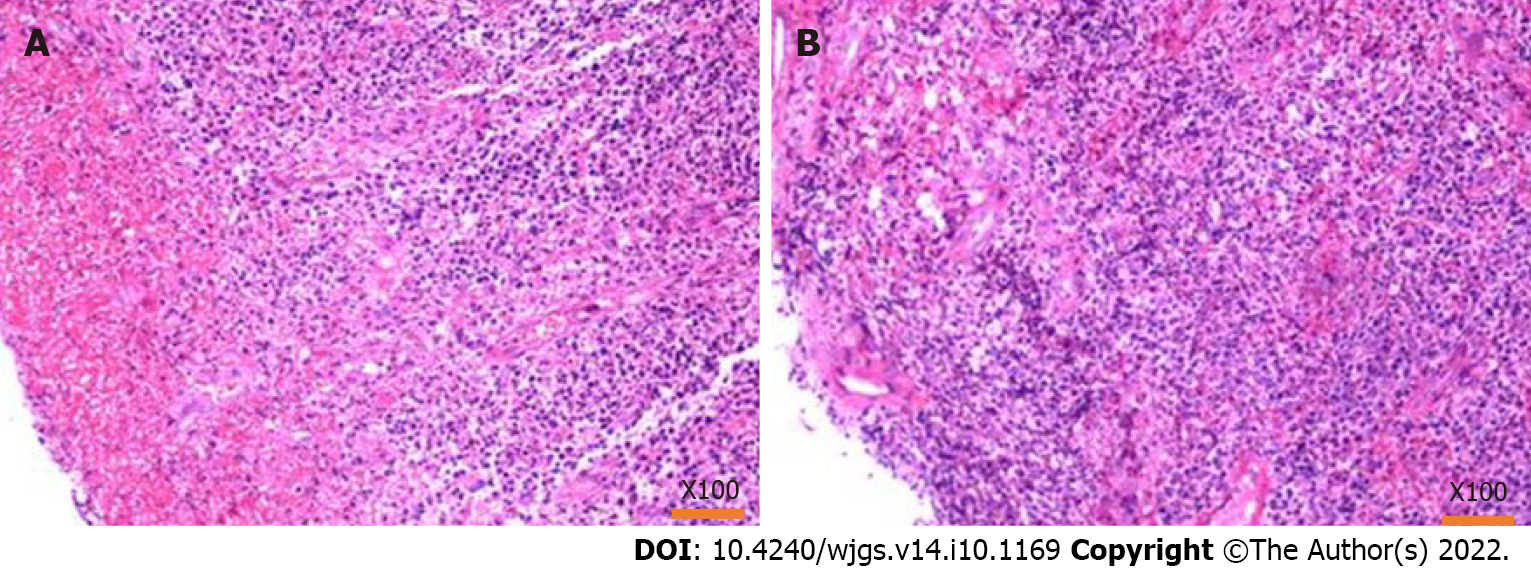
A tissue biopsy was performed on the thickened and enlarged part of the sigmoid colon. While the intestinal wall of the biopsy was hard, the bleeding was negligible. The biopsy pathology chiefly showed inflammatory necrotic and granulomatous tissue; no defined tumorous cells were seen (Figure 6).
Based on all the preoperational investigations, the initial diagnosis was an inflammatory disease of the sigmoid colon with chronic intestinal perforation, with a localized pelvic abscess formed between the sigmoid colon and bladder. However, the possibility of sigmoid colon tumors could not be ruled out.
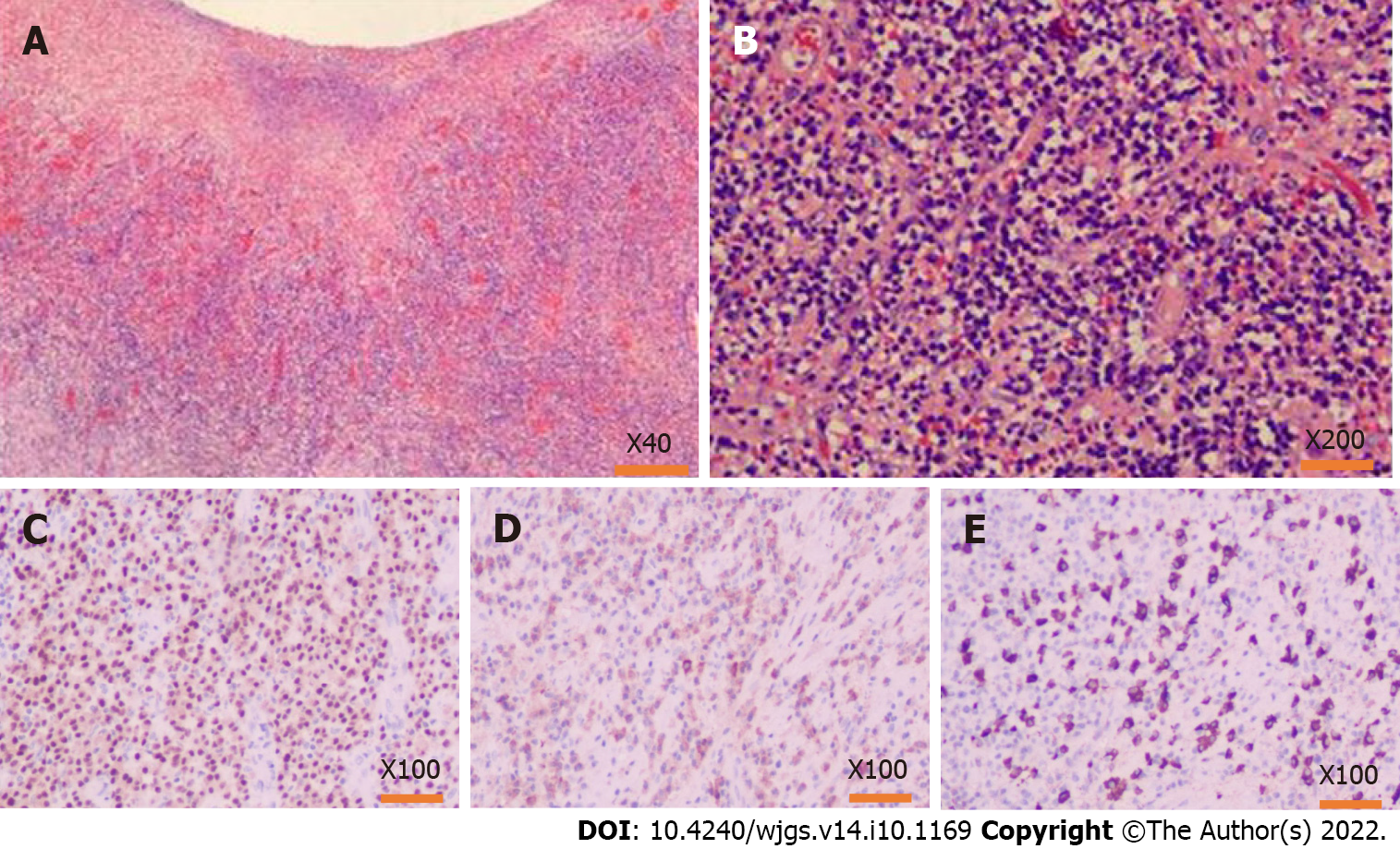
After the operation, pathological results (Figure 7) showed multiple ulcers distributed in the diseased intestinal wall. The ulcers were surrounded by obliterative phlebitis, widespread inflammatory granulation, and fibrous tissue. Many plasma cells and neutrophils infiltrated the lesion tissues. Furthermore, immunohistochemistry showed high IgG4-positive cells in the diseased tissues (IgG4+/IgG+ ratio was about 60%, IgG4+/HPF was about 110 cells). Therefore, the pathological diagnosis was IgG4-RD in the sigmoid colon.
Immunology-related blood indices (Tables 1 and 2) showed that the IgG4 level was 1. 830 g/L, which was higher than normal (for IgG4-RD, the cut-off value is > 1. 35 g/L).
| Test items | Results | Reference interval |
| Antinuclear antibody | Negative | Negative |
| Anti-nuclear chromatin antibody | 1.0 | ≤ 4.0 negative |
| Anti-RNP-A antibody | < 0.2 | < 1.0 negative |
| Anti-RNP-68 antibody | < 0.2 | < 1.0 negative |
| Anti-Sm/nRNP antibody | < 0.2 | < 1.0 negative |
| Anti-Sm antibody | < 0.2 | < 1.0 negative |
| Anti-SS-A antibody | < 0.2 | < 1.0 negative |
| Anti-Ro-52 antibody | < 0.2 | < 1.0 negative |
| Anti-SS-B antibody | < 0.2 | < 1.0 negative |
| Anti-Sci-70 antibody | < 0.2 | < 1.0 negative |
| Anti-Jo-1 antibody | < 0.2 | < 1.0 negative |
| Anti-ribosomal p protein antibody | < 0.2 | < 1.0 negative |
| Anti-centromere b protein antibody | < 0.2 | < 1.0 negative |
| Antineutrophil cytoplasmic antibody | Negative | Negative |
| Anti-protease 3 antibody | < 0.2 | < 1.0 negative |
| Anti-myeloperoxidase antibody | < 0.2 | < 1.0 negative |
| Anti-glomerular basement membrane antibody | < 0.2 | < 1.0 negative |
| Test items | Results | Reference interval |
| IgG | 19.2 | 7.51-15.6 |
| IgA | 3.23 | 0.82-4.53 |
| IgM | 0.64 | 0.46-3.04 |
| Complement 3 | 0.89 | 0.65-1.39 |
| Complement 4 | 0.24 | 0.16-0.38 |
| IgG1 | 10.20 | 4.05-10.11 |
| IgG2 | 8.26 | 1.69-7.86 |
| IgG3 | 0.368 | 0.11-0.85 |
| IgG4 | 1.890 | 0.03-2.01 for IgG4-RD, cutoff value > 1.35 |
Combined with pathology results, immunohistochemistry, and blood IgG indices, we diagnosed this patient with IgG4-RD involved sigmoid colon and pancreas.
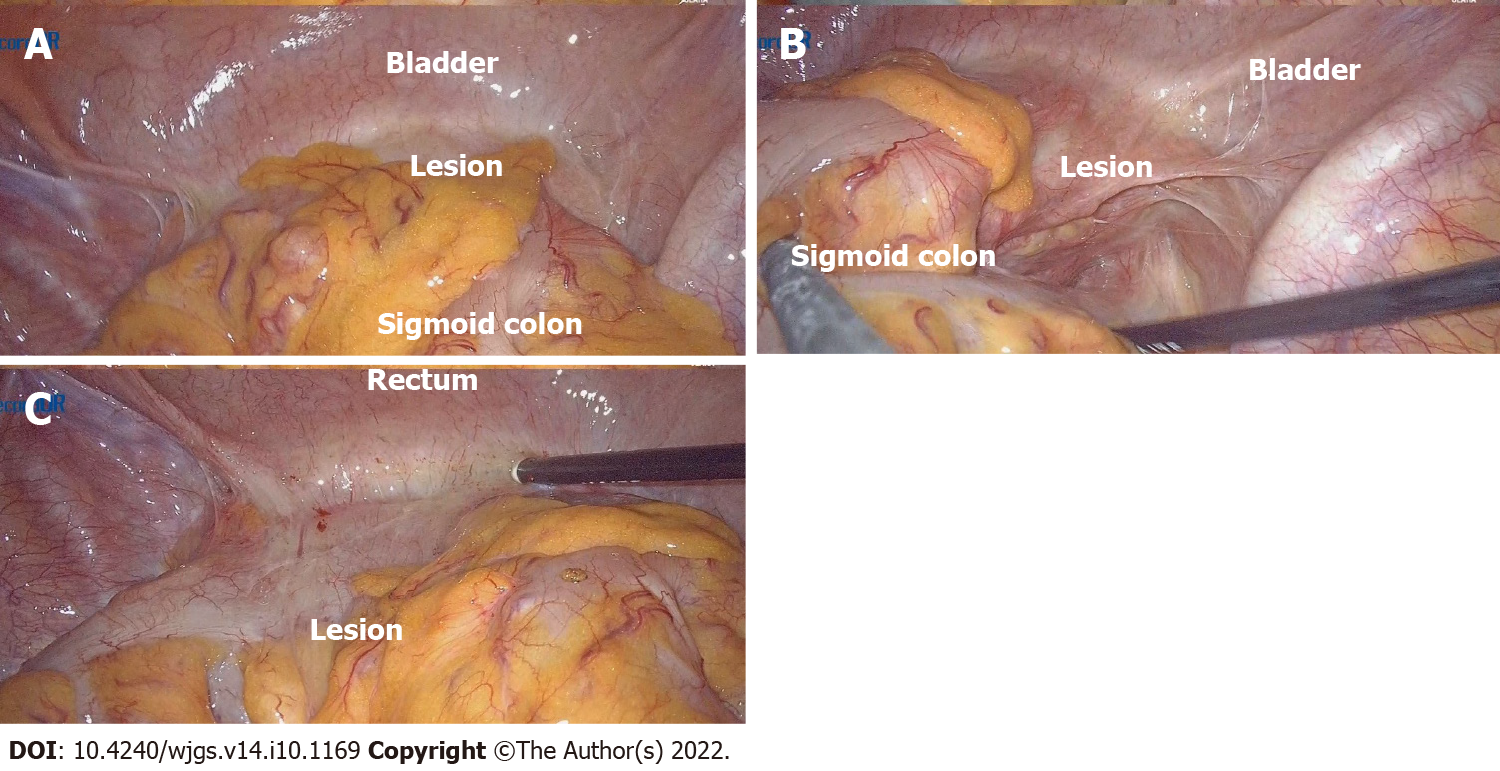
After signing the informed consent from the patient, we performed exploratory laparoscopic surgery, and during the surgery, we found that the patient’s sigmoid colon was too long, and the proximal sigmoid colon moved down and attached to the left side of the bladder, forming a 6 cm × 6 cm mass. There were no apparent abnormalities in the stomach, small intestine, and the rest of the colon and rectum. We tried to separate the periphery of the mass with a harmonic ultrasonic knife; however, the mass was very hard and closely adherent to the bladder. Therefore, the separation could not be completed under laparoscopy (Figure 8). We then switched to open surgery and used an electric knife to separate the sigmoid colon and mass from the bladder carefully. However, the electrosurgical resection was extremely difficult because the mass's boundary was unclear. Unlike the wall in common chronic abscesses, it was tough and similar to severe fibrotic tissue. After careful separation, we performed sigmoid resection plus descending colorectal anastomosis, and in order to prevent anastomotic leakage, we also performed transverse colostomy. The mass (mainly was the thicken colon wall, about 6 cm × 6 cm) and approximately 20 cm sigmoid colon were removed; however, many feces remained in the proximal colon, which might be caused by stenosis of the intestinal cavity and incomplete obstruction due to the lesion mass compression.
The patient recovered quickly after the operation and underwent a transverse colostomy three months later. We asked the rheumatology department and immunology doctors to provide a glucocorticoid treatment plan as follow-up treatment. After three months of treatment, the serum IgG4 level decreased to near the normal level. The patient did not have any abdominal discomfort and urination problems and also gained 5 kg in weight relative to before the operation.
The 2019 ACR and EULAR classification criteria for IgG4-RD (2019 ACR/EULAR IgG4-RD Criteria) are essential in understanding and treating IgG4-RD[3]. Although they list intestinal involvement as an exclusion criterion[3], we reported colonic involvement, which is a unique finding in our patient. Although the patient had a history of allergic rhinitis, the IgG4 level in the blood was increased, exceeding the critical value for IgG4-RD. In the histochemistry detection, we found the plasma cells and IgG4-positive cells infiltration, IgG4+/IgG+ ratio up to 60%, 110 IgG4+/HPF cells, obliterative phlebitis, and fibrous tissue in the lesion colon. According to the 2019 ACR/EULAR IgG4-RD Criteria[3], if case meets entry criteria and does not meet any exclusion criteria, proceed to step 3 evaluation, this patient could get a score of 42 (Dense lymphocytic infiltrate and storiform fibrosis with or without obliterative phlebitis, +13; Immunostaining, the IgG4+:IgG+ ratio is 41%–70% and IgG4+ cells/HPF is ≥ 10, +14; Serum IgG4 concentration, > Normal but < 2× upper limit of normal, +4; Diffuse pancreas enlargement and capsule-like rim with decreased enhancement, +11). Therefore, this patient could be diagnosed as IgG4-RD is involved in the colon and pancreas.
When considering IgG4-RD, serum protein electrophoresis and IgG subclass tests should be used as initial investigations because approximately 70% of patients showed elevated serum IgG4 levels[7]. However, since IgG4 levels are not necessary for diagnosing IgG4-RD[8], serological testing, a simple, non-invasive method, can provide essential clues.
Steroids are the first-line treatment for IgG4-RD, and most patients respond well to glucocorticoid therapy[20]. In the 2019 ACR/EULAR IgG4-RD Criteria, failure to respond to glucocorticoids is a vital exclusion criterion for IgG4-RD, which illustrates their significance[3]. However, patients with incomplete remission and recurrence do exist[21]. Additionally, some patients' glucocorticoid-induced hyperglycemia coupled with secondary pancreatic fibrosis complicates the treatment due to these toxic effects[22].
Significantly, due to hypergammaglobulinemia and plasma cell amplification in IgG4-RD, the clinical efficacy of rituximab (which targets CD20 and depletes B cells) was also remarkable[23]. The use of immunosuppressive drugs such as cyclophosphamide, mycophenolate, leflunomide, and tacrolimus and their combination with glucocorticoids in IgG4-RD remains to be further studied[24-27]. Early identification of IgG4-RD and treatment with glucocorticoids, rituximab, or other immunosuppressive therapies are critical because patients usually respond well to these treatments in the early stages of the disease. Importantly, when chronic pancreatitis and fibrotic disease occur, they are often irreversible[28].
In summary, this is a patient with colonic involvement with IgG4-RD. However, recognizing a disease is a gradual process, and the appearance of the disease in rare locations should not be ignored. Since the IgG4 levels in the resected tissue and serological tests were significantly increased, coupled with fibrosis and obliterative phlebitis in the resected colon, the diagnosis of IgG4-RD is reliable. Treatment of IgG4-RD with other immunosuppressive drugs should be further researched. This patient thus provided a rare aspect of IgG4-RD, which may help us further understand this disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ashihara N, Japan; Cai W, China; Vujasinovic M, Sweden; S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1773] [Article Influence: 136.4] [Reference Citation Analysis (0)] |
| 2. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 984] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 3. | Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, Hart PA, Inoue D, Kawano M, Khosroshahi A, Lanzillotta M, Okazaki K, Perugino CA, Sharma A, Saeki T, Schleinitz N, Takahashi N, Umehara H, Zen Y, Stone JH; Members of the ACR/EULAR IgG4-RD Classification Criteria Working Group. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 2020;79:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (1)] |
| 4. | Sekiguchi H, Horie R, Kanai M, Suzuki R, Yi ES, Ryu JH. IgG4-Related Disease: Retrospective Analysis of One Hundred Sixty-Six Patients. Arthritis Rheumatol. 2016;68:2290-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, Stone JH. IgG4-Related Disease: Clinical and Laboratory Features in One Hundred Twenty-Five Patients. Arthritis Rheumatol. 2015;67:2466-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 460] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 6. | Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol. 2014;9:315-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Cheuk W, Chan JK. Lymphadenopathy of IgG4-related disease: an underdiagnosed and overdiagnosed entity. Semin Diagn Pathol. 2012;29:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Chang SY, Keogh KA, Lewis JE, Ryu JH, Cornell LD, Garrity JA, Yi ES. IgG4-positive plasma cells in granulomatosis with polyangiitis (Wegener's): a clinicopathologic and immunohistochemical study on 43 granulomatosis with polyangiitis and 20 control cases. Hum Pathol. 2013;44:2432-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Chen LYC, Mattman A, Seidman MA, Carruthers MN. IgG4-related disease: what a hematologist needs to know. Haematologica. 2019;104:444-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 10. | Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J Gastroenterol. 2007;42 Suppl 18:39-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol. 2020;16:702-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 12. | Perugino CA, Wallace ZS, Meyersohn N, Oliveira G, Stone JR, Stone JH. Large vessel involvement by IgG4-related disease. Medicine (Baltimore). 2016;95:e3344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Ebbo M, Grados A, Guedj E, Gobert D, Colavolpe C, Zaidan M, Masseau A, Bernard F, Berthelot JM, Morel N, Lifermann F, Palat S, Haroche J, Mariette X, Godeau B, Bernit E, Costedoat-Chalumeau N, Papo T, Hamidou M, Harlé JR, Schleinitz N. Usefulness of 2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography for staging and evaluation of treatment response in IgG4-related disease: a retrospective multicenter study. Arthritis Care Res (Hoboken). 2014;66:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Masamune A, Kikuta K, Hamada S, Tsuji I, Takeyama Y, Shimosegawa T, Okazaki K; Collaborators. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J Gastroenterol. 2020;55:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 15. | Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 16. | Ciccone F, Ciccone A, Di Ruscio M, Vernia F, Cipolloni G, Coletti G, Calvisi G, Frieri G, Latella G. IgG4-Related Disease Mimicking Crohn's Disease: A Case Report and Review of Literature. Dig Dis Sci. 2018;63:1072-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 17. | Fujita K, Naganuma M, Saito E, Suzuki S, Araki A, Negi M, Kawachi H, Watanabe M. Histologically confirmed IgG4-related small intestinal lesions diagnosed via double balloon enteroscopy. Dig Dis Sci. 2012;57:3303-3306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Notohara K, Kamisawa T, Uchida K, Zen Y, Kawano M, Kasashima S, Sato Y, Shiokawa M, Uehara T, Yoshifuji H, Hayashi H, Inoue K, Iwasaki K, Kawano H, Matsubayashi H, Moritani Y, Murakawa K, Oka Y, Tateno M, Okazaki K, Chiba T. Gastrointestinal manifestation of immunoglobulin G4-related disease: clarification through a multicenter survey. J Gastroenterol. 2018;53:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Abe A, Manabe T, Takizawa N, Ueki T, Yamada D, Nagayoshi K, Sadakari Y, Fujita H, Nagai S, Yamamoto H, Oda Y, Nakamura M. IgG4-related sclerosing mesenteritis causing bowel obstruction: a case report. Surg Case Rep. 2016;2:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Masaki Y, Matsui S, Saeki T, Tsuboi H, Hirata S, Izumi Y, Miyashita T, Fujikawa K, Dobashi H, Susaki K, Morimoto H, Takagi K, Kawano M, Origuchi T, Wada Y, Takahashi N, Horikoshi M, Ogishima H, Suzuki Y, Kawanami T, Kawanami Iwao H, Sakai T, Fujita Y, Fukushima T, Saito M, Suzuki R, Morikawa Y, Yoshino T, Nakamura S, Kojima M, Kurose N, Sato Y, Tanaka Y, Sugai S, Sumida T. A multicenter phase II prospective clinical trial of glucocorticoid for patients with untreated IgG4-related disease. Mod Rheumatol. 2017;27:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Inoue D, Yoshida K, Yoneda N, Ozaki K, Matsubara T, Nagai K, Okumura K, Toshima F, Toyama J, Minami T, Matsui O, Gabata T, Zen Y. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore). 2015;94:e680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 22. | Omar D, Chen Y, Cong Y, Dong L. Glucocorticoids and steroid sparing medications monotherapies or in combination for IgG4-RD: a systematic review and network meta-analysis. Rheumatology (Oxford). 2020;59:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 24. | Buechter M, Klein CG, Kloeters C, Schlaak JF, Canbay A, Gerken G, Kahraman A. Tacrolimus as a reasonable alternative in a patient with steroid-dependent and thiopurine-refractory autoimmune pancreatitis with IgG4-associated cholangitis. Z Gastroenterol. 2014;52:564-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Li K, Gao D, Luo G, Zhao Y, Wang X, Zhang J, Jin J, Zhao Z, Yang C, Zhu J, Huang F. Combination therapy of leflunomide and glucocorticoids for the maintenance of remission in patients with IgG4-related disease: a retrospective study and literature review. Intern Med J. 2017;47:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Yunyun F, Yu C, Panpan Z, Hua C, Di W, Lidan Z, Linyi P, Li W, Qingjun W, Xuan Z, Yan Z, Xiaofeng Z, Fengchun Z, Wen Z. Efficacy of Cyclophosphamide treatment for immunoglobulin G4-related disease with addition of glucocorticoids. Sci Rep. 2017;7:6195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Yunyun F, Yu P, Panpan Z, Xia Z, Linyi P, Jiaxin Z, Li Z, Shangzhu Z, Jinjing L, Di W, Yamin L, Xiaowei L, Huadan X, Xuan Z, Xiaofeng Z, Fengchun Z, Yan Z, Wen Z. Efficacy and safety of low dose Mycophenolate mofetil treatment for immunoglobulin G4-related disease: a randomized clinical trial. Rheumatology (Oxford). 2019;58:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 28. | El Euch M, Hddad S, Mahfoudhi M, Maktouf H, Ben Hamida F, Jaziri F, Ben Abdelghani K, Turki S, Ben Abdallah T. A Case of Type 1 Autoimmune Pancreatitis (AIP), a Form of IgG4-Related Disease (IgG4-RD). Am J Case Rep. 2017;18:822-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |