Published online Oct 27, 2022. doi: 10.4240/wjgs.v14.i10.1161
Peer-review started: June 21, 2022
First decision: July 12, 2022
Revised: August 8, 2022
Accepted: October 5, 2022
Article in press: October 5, 2022
Published online: October 27, 2022
Processing time: 125 Days and 22.2 Hours
Enterocutaneous fistula (ECF) is an abnormal communication between the skin and the gastrointestinal tract and is associated with considerable morbidity and mortality. To diagnose ECF, X-ray fistulography and abdominal computed tomography (CT) with intravenous or oral contrast are generally used. If the anatomic details obtained from CT are insufficient, CT fistulography may help diagnose and determine the extent of the abnormal channel. However, CT fistulography is seldom performed in patients with insufficient evidence of a fistula.
A 35-year-old man with a prior appendectomy presented with purulence over the abdominal wall without gastrointestinal tract symptoms or a visible opening on the abdominal surface. His history and physical examination were negative for nausea, diarrhea, muscle guarding, and bloating. Local abdominal tenderness and redness over a purulent area were noted, which led to the initial diagnosis of cellulitis. He was admitted to our hospital with a diagnosis of cellulitis. We performed a minimal incision on the carbuncle to collect the pus. The bacterial culture of the exudate resulted positive for Enterococcus sp. ECF was thus suspected, and we arranged a CT scan for further investigation. CT images before intravenous contrast administration showed that the colon was in close contact with the abdominal wall. Therefore, we conducted CT fistulography by injecting contrast dye into the carbuncle during the CT scan. The images showed an accumulation of the contrast agent within the subcutaneous tissues, suggesting the formation of an abscess. The contrast dye tracked down through the muscles and peritoneum into the colon, delineating a channel connecting the subcutaneous abscess with the colon. This evidence confirmed cecocutaneous fistula and avoided misdiagnosing ECF without gastrointestinal tract symptoms as cellulitis. The patient underwent laparoscopic right hemicolectomy with re-anastomosis of the ileum and transverse colon.
CT fistulography can rule out ECF in cases presenting as cellulitis if examinations are suggestive.
Core Tip: Computed tomography (CT) fistulography is seldom performed on patients with insufficient evidence of fistula; however, it provides more accurate anatomical details than X-ray fistulography and abdominal CT. A 35-year-old man with swelling and purulence over the abdominal wall was admitted to our hospital under the diagnosis of cellulitis. Serial examinations suggested a possible enterocutaneous fistula (ECF); thus, we performed CT fistulography. Images showed the subcutaneous contrast agent tracked down through the muscle and peritoneum into the cecum, confirming a cecocutaneous fistula. CT fistulography may rule out ECF in patients presenting with cellulitis if examinations are suggestive.
- Citation: Wu TY, Lo KH, Chen CY, Hu JM, Kang JC, Pu TW. Cecocutaneous fistula diagnosed by computed tomography fistulography: A case report. World J Gastrointest Surg 2022; 14(10): 1161-1168
- URL: https://www.wjgnet.com/1948-9366/full/v14/i10/1161.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i10.1161
Enterocutaneous fistula (ECF) is an abnormal channel connecting the skin and the gastrointestinal tract. It occurs most often after abdominal surgery (75%-85%), and only 15%-25% of cases occur spontaneously[1,2]. X-ray fistulography with oral contrast and abdominal computed tomography (CT) with intravenous or oral contrast are generally used to diagnose ECF. If radiographic findings from X-ray fistulography and CT scan are insufficient, CT fistulography is a good alternative[3,4]. However, CT fistulography is seldom used without strong evidence of ECF.
Herein, we present the case of a 35-year-old man admitted to the hospital with presumed abdominal wall cellulitis. However, a series of events provided evidence supporting the diagnosis of ECF. Therefore, we elected to conduct CT fistulography, which provides more accurate anatomical details compared to a simple CT scan.
Abdominal wall swelling and pain for a week.
A 35-year-old man presented to the outpatient department with painful swelling and mass formation over the right lower quadrant abdominal wall for one week. No fever or gastrointestinal symptoms were reported. Oral intake and defecation were normal. The patient was hospitalized with a suspected diagnosis of cellulitis.
The patient was diagnosed with acute appendicitis and underwent an appendectomy 15 years ago in 2006. No history of underlying abdominal malignancy, inflammatory bowel disease, abdominal trauma, or other gastrointestinal diseases was reported.
There was no family history of abdominal neoplasms, inflammatory bowel disease, or ECF. The patient exhibited normal social functioning and self-care.
The vital signs, including body temperature, were within the normal ranges. Abdominal palpation revealed a mass over the right lower abdominal wall without visible opening and suspected abscess formation. Local abdominal tenderness and redness over purulence were noted. No nausea, diarrhea, muscle guarding, or bloating was observed, which led to the initial diagnosis of cellulitis.
In outpatient department, we performed a minimal incision of the lesion to collect discharge for bacterial culture and blood analysis was also performed. Lab reports revealed a white blood cell count of 20.17 × 103/μL, 79.9% neutrophils and 12.4% lymphocytes, and a serum C-reactive protein concentration of 19.60 mg/dL. The bacterial culture of the lesion pus revealed the presence of Enterococcus sp. Based on these results, ECF was suspected.
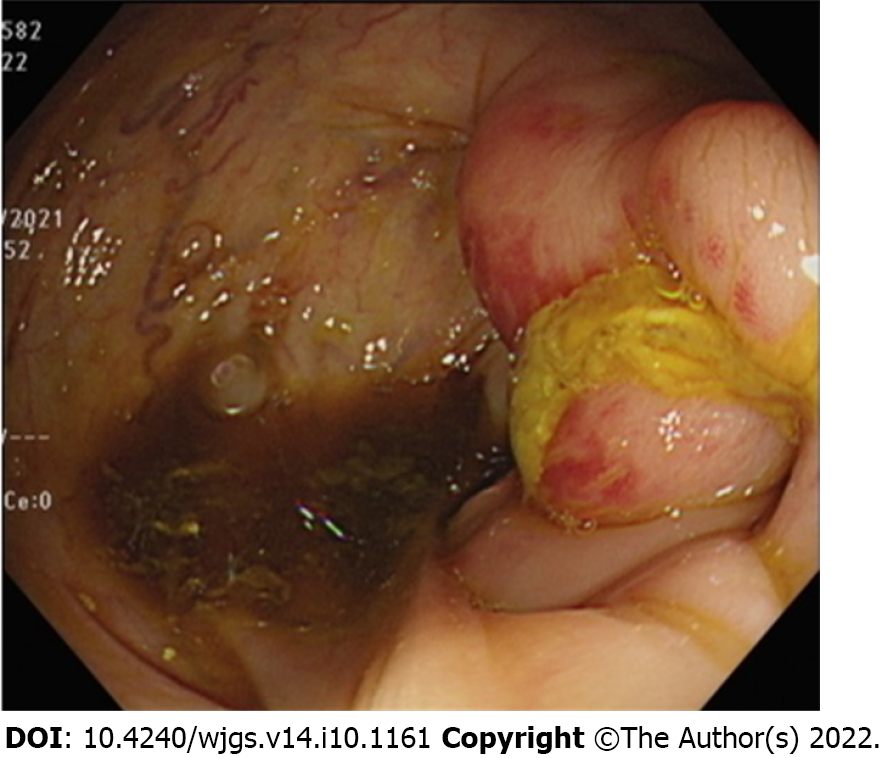
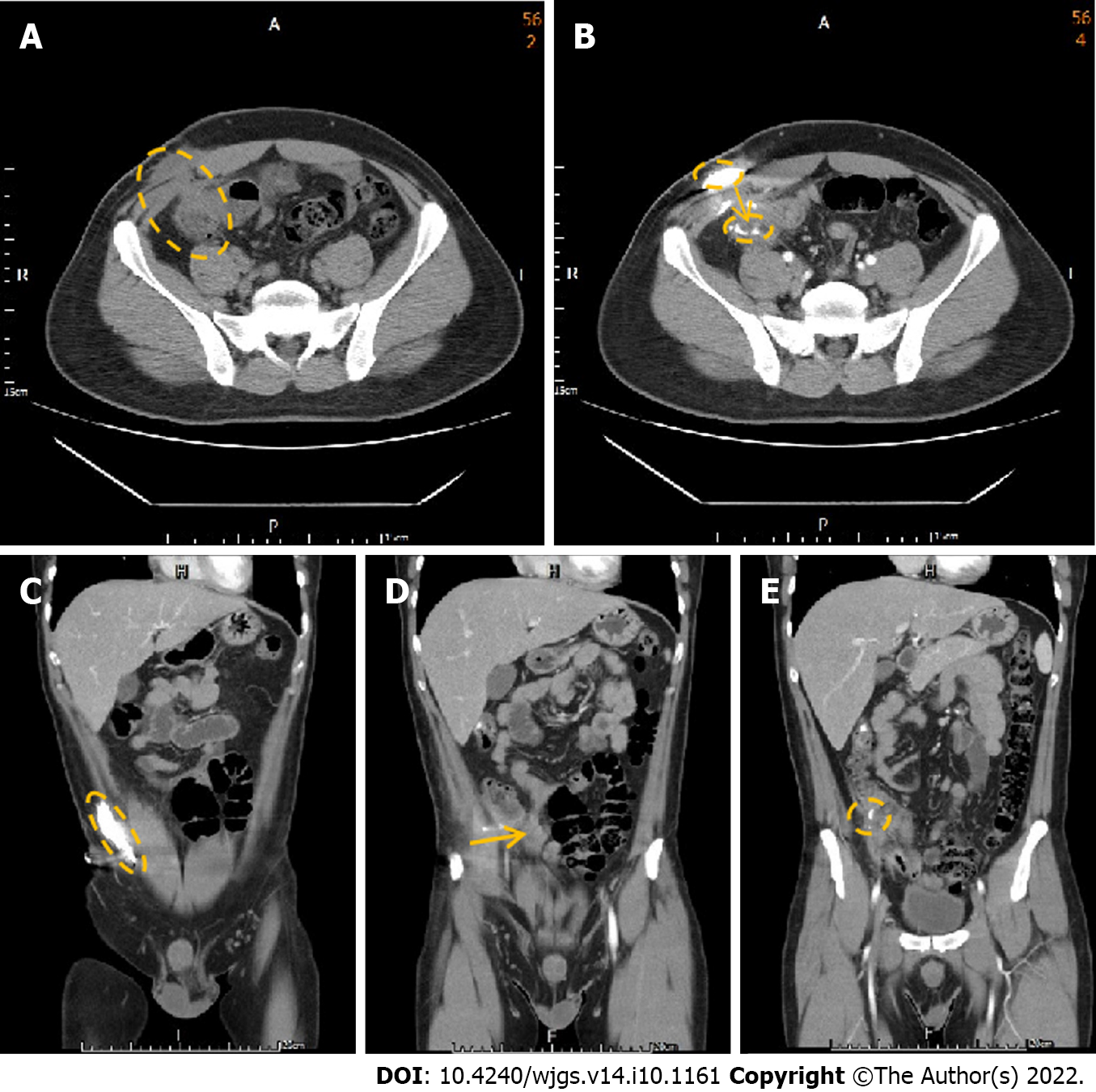
We performed a colonoscopy and observed inflammation in the ileocecal valve region (Figure 1). CT of the abdomen in the axial view suggested that the colon was in close contact with the abdominal wall (Figure 2A), highly suggestive of ECF. We conducted CT fistulography by injecting contrast dye into the carbuncle for a more detailed radiographic view and a definitive diagnosis. Images after contrast administration showed the presence of contrast dye in both the abdominal wall and the colon (Figure 2B). Coronal images showed contrast dye retention in the subcutaneous area (Figure 2C) with penetration through the abdominal wall (Figure 2D ) into the colon (Figure 2E).
The laboratory results and the imaging examinations indicated the existence of a cecocutaneous fistula. Therefore, we performed a diagnostic laparoscopy, during which we could observe severe adhesion of the colon to the abdominal wall. This evidence allowed us to formulate the final diagnosis of the cecocutaneous fistula.
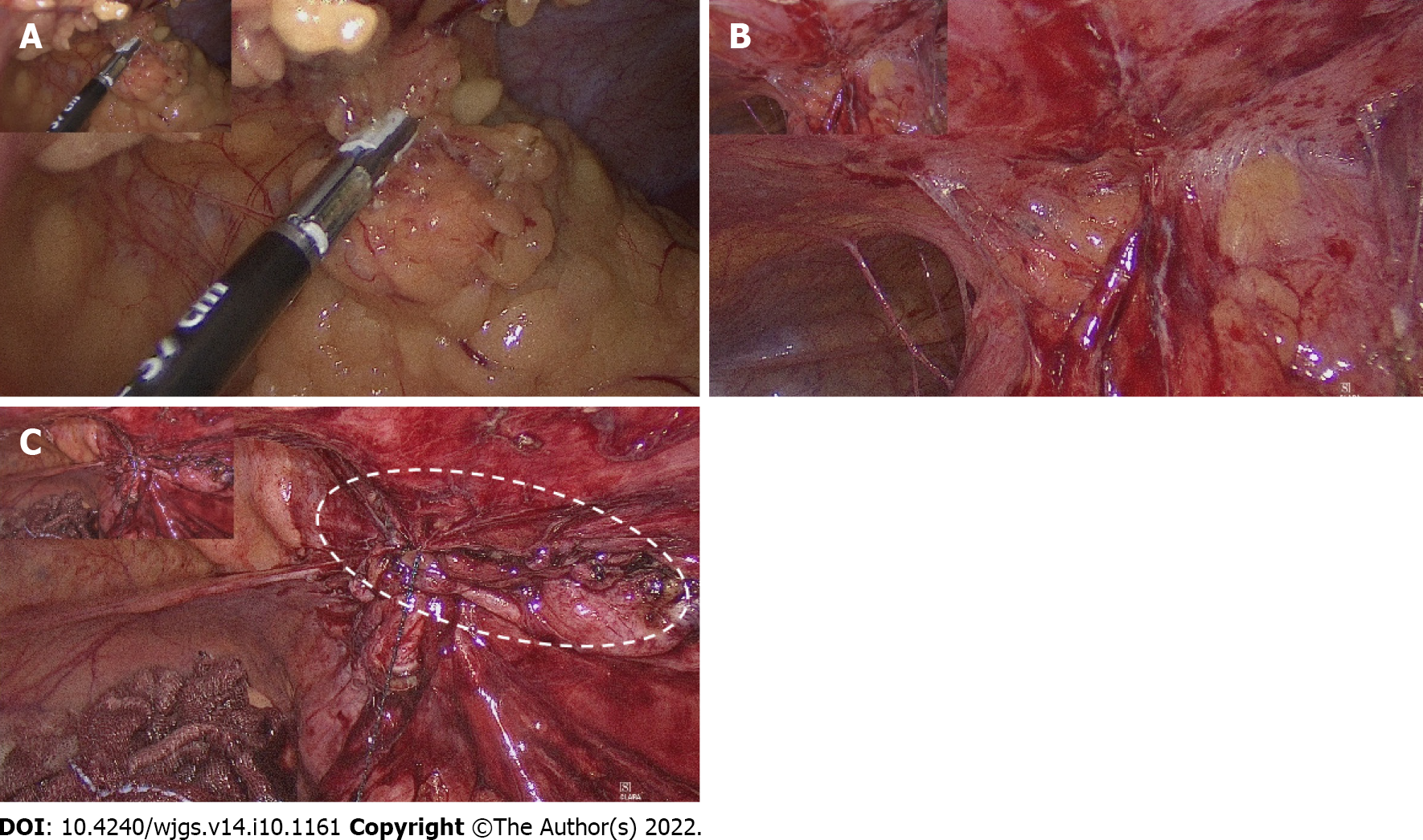
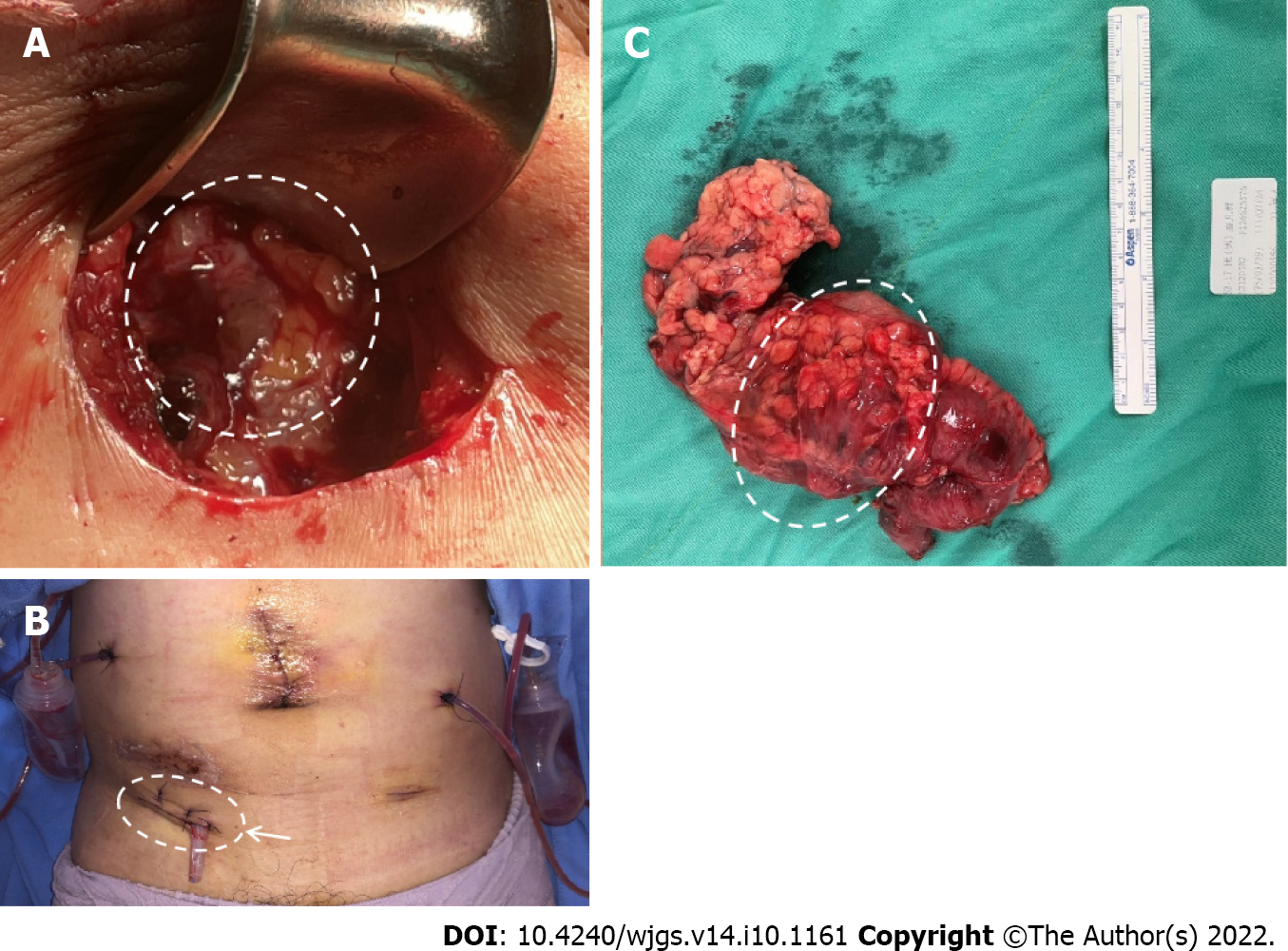
The patient underwent laparoscopic right hemicolectomy, reanastomosis of ileum and transverse colon, and peritoneal repair. During surgery, severe adhesion of the colon to the abdominal wall was noted (Figure 3A). After tissue adhesiolysis (Figure 3B), colon resection, and reanastomosis, a peritoneal defect was reported. We thus performed peritoneal repair with a V-LOC suture line (COVIDIENTM 1-0 V-LOC, Medtronic, Ireland) to prevent hernia and adhesion (Figure 3C). Upon debridement of the infected abdominal wall, a fascial defect due to the outer opening of the fistula was noted (Figure 4A). Finally, a Penrose drainage tube was placed (Figure 4B). The surgical specimen consisted of the resected colon with attached peritoneum (Figure 4C). The histopathological results suggested a fistula with multifocal chronic inflammation. Microscopically, abscess formation with focal regeneration atypia of the soft tissue were found, with no granulomatous inflammation.
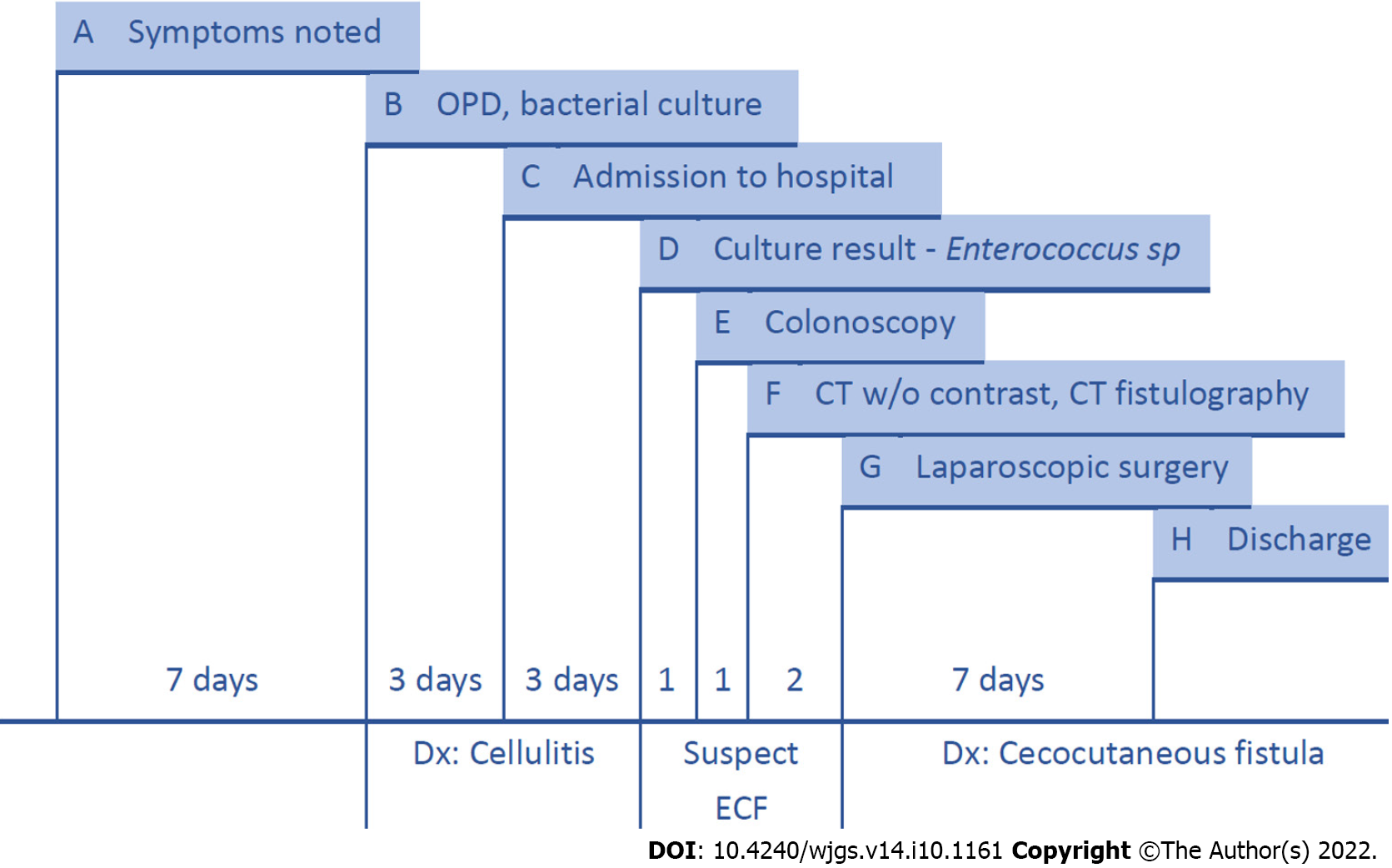
After surgery, we kept administering antibiotics with Ampicillin 1g + Sulbactam 0.5g IV Q6H and used chlorhexidine gluconate 2% solution for daily skin surface disinfection. The wound remained stable without contracting infections. Swelling and redness over the abdominal wall gradually improved. The highest temperature during the hospital stay was 37.3C on the fourth day after the operation. The final data before discharging were a white blood cell count of 11.44 × 103/μL, 68.9% neutrophils and 22.1% lymphocytes, and a serum C-reactive protein concentration of 1.99 mg/dL. The patient was discharged from the hospital one week after surgery. No wound infection or gastrointestinal symptoms were noted during outpatient follow-up 6 mo after surgery. The patient’s oral intake recovered due to the short course of treatment and the absence of any further surgical operation. The patient was satisfied with the treatment outcome, and further hospitalization was not required. The treatment timeline from admission to discharge is shown in Figure 5.
Although cecocutaneous fistulae are rare, they still contribute to considerable morbidity and mortality. Major etiological factors of cecocutaneous fistula include abdominal tuberculosis, neoplasm of the appendix or cecum, leakage from the appendiceal stump, and inflammatory bowel disease[1,5]. In addition, some cases of cecocutaneous fistula were reported as related to a previous appendectomy[6], while some others were related to an underlying stump appendix[7]. Investigation of the patient’s history was negative for abdominal malignancy, inflammatory bowel disease, abdominal trauma, or any other gastrointestinal disease. The histopathological report of the resected colon showed a fistula with multifocal chronic inflammation. Microscopically, it evidenced abscess formation with focal regeneration atypia of the soft tissues, with no granulomatous inflammation. The single abnormal finding in the history was the appendectomy in 2006, which was preliminarily compatible with the result of the diagnostic laparoscopy (cecum attached to the peritoneum). Appendectomy might have been a possible reason for the fistula.
Different protocols and modalities for the treatment of ECF have been reported in the literature[3,8,9]. Most of them include four phases: Treatment of sepsis, nutrition support, definition of fistula anatomy, and definitive intervention. Surgical intervention is not necessary in all cases; some fistulae close spontaneously. Patients with ECF associated with independent adverse factors conditioning non-spontaneous closure (including sepsis, high output, and multiple fistulae) may need surgical treatment[10]. Besides surgery, there are several other methods for the management of ECF, including negative pressure wound therapy (NPWT), stent placement, fibrin glues, and endoscopic management[5]. Stent placement plays an important role in the drainage of sepsis[11]. Recently, a 3D-printed patient-personalized fistula stent was successfully implanted in patients, reducing the fistula output[12]. NPWT has the advantage of lowering the effluent volume of enteric fistulae, in some cases leading to spontaneous closure; however, it often entails a longer treatment time. Fibrin glues are an option when the fistula has low-to-medium effluent volume, surgery is not possible, the fistula has complex branching, or is only accessible from a small external orifice[13,14]. Endoscopic minimally invasive management is emerging as a choice for gastrointestinal fistulae, and it may be safer and more effective than surgery[15,16]. Surgery is usually time-consuming and requires extensive adhesiolysis[17].
In this case, we suspected adhesions of the colon and peritoneum. The patient opted for a treatment that would ensure low recurrence and a prompt resolution. Considering his young age and his relatively stable condition, we finally opted for surgery rather than endoscopic management or conservative treatment. We performed laparoscopic resection with reanastomosis, which has a lower recurrence rate than oversewing surgery[18].
Despite the wealth of treatments for ECF, diagnosis has always been challenging. The diagnostic process generally includes X-ray fistulography and abdominal CT with intravenous contrast. X-ray fistulography has recently been replaced by abdominal CT, which better reveals the anatomy of the gastrointestinal tract and provides more information about the associated pathology. If anatomical details obtained using X-ray fistulography and CT are insufficient, CT fistulography helps to identify and determine the extent of the abnormal channel[3,4].
In this case, physical examination revealed a mass over the right lower abdominal wall without visible opening and suspected abscess formation. Absence of nausea, diarrhea, abdominal tenderness, muscle guarding, or bloating led to the initial diagnosis of cellulitis. Bacterial culture revealed Enterococcus sp., and CT images before administering the contrast agent showed that the colon was in close contact with the abdominal wall. ECF was thus highly suspected. Because CT with intravenous contrast agent may provide insufficient anatomical details concerning fistulae, we conducted CT fistulography by injecting contrast dye into the carbuncle. The resulting images showed definitive evidence of a cecocutaneous fistula.
Though CT fistulography is an option that provides more detailed anatomical information, it is still seldom utilized in patients with insufficient evidence of fistula[3]. ECF without gastrointestinal symptoms may mimic cellulitis. Once a fistula is suspected according to our diagnostic evaluation (e.g., bacterial culture, X-ray fistulography, CT without contrast), CT fistulography can be used to diagnose or rule out ECF.
A significant problem with CT fistulography is that the contrast agent sometimes cannot be administered through the fistula because of adhesions and continuous purulent discharge; in such cases, magnetic resonance imaging (MRI) may be considered. MRI has superior soft tissue discrimination. Magnetic resonance enterography (which is a variant of MRI, has been used to rule out small bowel pathology and delineate fistula anatomy. Magnetic resonance enterography has also been used to detect colon disease[19], but it was initially used for small bowel investigation. Therefore, its application as diagnostic imaging of the colon still warrants further evidence.
The current challenge is that a simple CT scan may provide insufficient anatomical details of the fistula[3]. Furthermore, most advanced examinations, including CT and MRI, are expensive. Thus, a fistula may get neglected following a plain CT scan, and CT fistulography/MRI may not be arranged. In this case, we suspected ECF due to the positive bacterial culture and performed CT fistulography. However, most physicians may not perform advanced examinations without evidence suggesting fistula. Therefore, several ECF cases may be neglected or misdiagnosed. Despite the availability of various diagnostic methods, the indications for performing further examinations are pivotal in the process and require adequate discussion.
ECF without gastrointestinal symptoms or visible openings may be misdiagnosed as cellulitis. X-ray fistulography and abdominal CT sometimes provide insufficient anatomical details, thus leading to misdiagnosis. CT fistulography may rule out ECF in patients presenting with cellulitis if examinations are suggestive.
The authors wish to acknowledge the assistance of the people at the Department of Surgery, Tri-Service General Hospital Songsang Branch. This report would not have been possible without their efforts in data collection and interprofessional collaboration in treating this patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E, E
P-Reviewer: Dai DL, China; Mishra TS, India; Salimi M, Iran; Sica G, Italy; Singh R, India; Vyshka G, Albania S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Kumar P, Maroju NK, Kate V. Enterocutaneous fistulae: etiology, treatment, and outcome - a study from South India. Saudi J Gastroenterol. 2011;17:391-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Falconi M, Pederzoli P. The relevance of gastrointestinal fistulae in clinical practice: a review. Gut. 2001;49 Suppl 4:iv2-i10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Schecter WP, Hirshberg A, Chang DS, Harris HW, Napolitano LM, Wexner SD, Dudrick SJ. Enteric fistulas: principles of management. J Am Coll Surg. 2009;209:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Agrawal V, Prasad S. Appendico-cutaneous fistula: a diagnostic dilemma. Trop Gastroenterol. 2003;24:87-89. [PubMed] |
| 5. | Ghimire P. Management of Enterocutaneous Fistula: A Review. JNMA J Nepal Med Assoc. 2022;60:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Genier F, Plattner V, Letessier E, Armstrong O, Heloury Y, Le Neel JC. [Post-appendectomy fistulas of the cecum. Apropos of 22 cases]. J Chir (Paris). 1995;132:393-398. [PubMed] |
| 7. | Agostinho N, Bains HK, Sardelic F. Enterocutaneous Fistula Secondary to Stump Appendicitis. Case Rep Surg. 2017;2017:6135251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Datta V, Engledow A, Chan S, Forbes A, Cohen CR, Windsor A. The management of enterocutaneous fistula in a regional unit in the United kingdom: a prospective study. Dis Colon Rectum. 2010;53:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Gribovskaja-Rupp I, Melton GB. Enterocutaneous Fistula: Proven Strategies and Updates. Clin Colon Rectal Surg. 2016;29:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Martinez JL, Luque-de-Leon E, Mier J, Blanco-Benavides R, Robledo F. Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes. World J Surg. 2008;32:436-43; discussion 444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Alexander RJ, Nash GF. Enterocutaneous fistula stent. Ann R Coll Surg Engl. 2009;91:619-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Huang JJ, Ren JA, Wang GF, Li ZA, Wu XW, Ren HJ, Liu S. 3D-printed "fistula stent" designed for management of enterocutaneous fistula: An advanced strategy. World J Gastroenterol. 2017;23:7489-7494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Assenza M, Rossi D, De Gruttola I, Ballanti C. Enterocutaneous fistula treatment: case report and review of the literature. G Chir. 2018;39:143-151. [PubMed] |
| 14. | Avalos-González J, Portilla-deBuen E, Leal-Cortés CA, Orozco-Mosqueda A, Estrada-Aguilar Mdel C, Velázquez-Ramírez GA, Ambriz-González G, Fuentes-Orozco C, Guzmán-Gurrola AE, González-Ojeda A. Reduction of the closure time of postoperative enterocutaneous fistulas with fibrin sealant. World J Gastroenterol. 2010;16:2793-2800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Rogalski P, Daniluk J, Baniukiewicz A, Wroblewski E, Dabrowski A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015;21:10542-10552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Cereatti F, Grassia R, Drago A, Conti CB, Donatelli G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J Gastroenterol. 2020;26:4198-4217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 17. | Bhama AR. Evaluation and Management of Enterocutaneous Fistula. Dis Colon Rectum. 2019;62:906-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Lynch AC, Delaney CP, Senagore AJ, Connor JT, Remzi FH, Fazio VW. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Somwaru AS, Khanijow V, Katabathina VS. Magnetic resonance enterography, colonoscopy, and fecal calprotectin correlate in colonic Crohn's disease. BMC Gastroenterol. 2019;19:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |