Published online Oct 27, 2022. doi: 10.4240/wjgs.v14.i10.1141
Peer-review started: August 23, 2022
First decision: September 26, 2022
Revised: September 28, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: October 27, 2022
Processing time: 63 Days and 3.1 Hours
Split liver transplantation (SLT) is a complex procedure. The left-lateral and right tri-segment splits are the most common surgical approaches and are based on the Couinaud liver segmentation theory. Notably, the liver surface following right tri-segment splits may exhibit different degrees of ischemic changes related to the destruction of the local portal vein blood flow topology. There is currently no consensus on preoperative evaluation and predictive strategy for hepatic segmental necrosis after SLT.
To investigate the application of the topological approach in liver segmentation based on 3D visualization technology in the surgical planning of SLT.
Clinical data of 10 recipients and 5 donors who underwent SLT at Shenzhen Third People’s Hospital from January 2020 to January 2021 were retrospectively analyzed. Before surgery, all the donors were subjected to 3D modeling and evaluation. Based on the 3D-reconstructed models, the liver splitting procedure was simulated using the liver segmentation system described by Couinaud and a blood flow topology liver segmentation (BFTLS) method. In addition, the volume of the liver was also quantified. Statistical indexes mainly included the hepatic vasculature and expected volume of split grafts evaluated by 3D models, the actual liver volume, and the ischemia state of the hepatic segments during the actual surgery.
Among the 5 cases of split liver surgery, the liver was split into a left-lateral segment and right tri-segment in 4 cases, while 1 case was split using the left and right half liver splitting. All operations were successfully implemented according to the preoperative plan. According to Couinaud liver segmentation system and BFTLS methods, the volume of the left lateral segment was 359.00 ± 101.57 mL and 367.75 ± 99.73 mL, respectively, while that measured during the actual surgery was 397.50 ± 37.97 mL. The volume of segment IV (the portion of ischemic liver lobes) allocated to the right tri-segment was 136.31 ± 86.10 mL, as determined using the topological approach to liver segmentation. However, during the actual surgical intervention, ischemia of the right tri-segment section was observed in 4 cases, including 1 case of necrosis and bile leakage, with an ischemic liver volume of 238.7 mL.
3D visualization technology can guide the preoperative planning of SLT and improve accuracy during the intervention. The simulated operation based on 3D visualization of blood flow topology may be useful to predict the degree of ischemia in the liver segment and provide a reference for determining whether the ischemic liver tissue should be removed during the surgery.
Core Tip: This is the first study to explore the application of the topological approach of liver segmentation based on 3D visualization technology in surgical planning of split liver transplantation. Clinical data of 10 recipients and 5 donors were analyzed. Couinaud liver segmentation and blood flow topology liver segmentation (BFTLS) methods were used to simulate operation, respectively. The volume of segment IV (the portion of ischemic liver lobes) allocated to the right tri-segment was 136.31 ± 86.10 mL as determined using BFTLS. Results showed that the approach of BFTLS may be useful to predict the range of ischemia in the liver section.
- Citation: Zhao D, Zhang KJ, Fang TS, Yan X, Jin X, Liang ZM, Tang JX, Xie LJ. Topological approach of liver segmentation based on 3D visualization technology in surgical planning for split liver transplantation. World J Gastrointest Surg 2022; 14(10): 1141-1149
- URL: https://www.wjgnet.com/1948-9366/full/v14/i10/1141.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i10.1141
The Couinaud liver segmentation is based on the distribution of the Glisson system in the liver and the division of the hepatic vein system. Three hepatic veins are used as vertical planes to form the main longitudinal fissure, and the liver is divided into different liver segments by the left and right branches of the portal vein. This segmentation method provides an anatomical basis for the clinical imaging diagnosis of liver diseases and has been widely used in clinical practice[1-3]. However, only 30%-50% of the segmented results are consistent with the actual anatomy of the liver as the segmented results are derived from the cast liver specimen ex vivo, and the variation in hepatic blood vessels is not taken into account[4,5]. The blood flow topology segmentation method is based on the blood flow topology of the hepatic portal vein[6,7]. Therefore, this method can truly reflect the anatomical structure of the liver and is the theoretical basis of anatomical hepatectomy via indocyanine green fluorescence imaging[8-10].
Split liver transplantation (SLT) is complex, and the commonly used surgical technique is the left-lateral segment and right tri-segment splits, which are implemented based on the theory of Couinaud liver segmentation. Previous studies uncovered that the right tri-segment liver surface might show different degrees of ischemic changes related to the destruction of the local portal vein blood flow topology following the intervention[11-13].
However, opinions diverge on the management of ischemia found on the surface tissues of the liver segment following SLT, as well as liver tissue necrosis, infection, and bile leakage[11,14]. Some experts postulate that the direct resection of segment IV of the ischemic liver tissue is necessary, while others believe no treatment is needed. This difference in opinion is due to a lack of preoperative evaluation and predictive strategy for hepatic segmental necrosis after SLT and a dearth of relevant publications worldwide. Therefore, this study aimed to investigate the application value of 3D visualization technology in the surgical planning of SLTs.
A retrospective analysis was performed on 10 patients who underwent SLT in the Third People’s Hospital of Shenzhen from January 2020 to January 2021 and the corresponding data of 5 donors. All cases in this study were performed after the approval of the Hospital Ethics Committee. The livers were donated after the death of the donors.
For each organ donor, preoperative blood routine, liver function, kidney function, coagulation function, tumor markers, and infection-related tests, as well as abdominal Doppler ultrasound and liver computed tomography (CT) angiography examination, were performed. A preoperative 3D visualization model of the liver was constructed for each case. The model was acquired by importing high-quality THIN-layer enhanced CT DICOM data into the medical 3D reconstruction software: (1) For organ reconstruction: The region-growing method was used to perform a 3D reconstruction of the liver, tumor, pancreas and spleen; and (2) For vascular reconstruction: The segmentation based on threshold method was used to perform a 3D reconstruction of the portal vein, hepatic artery and hepatic vein[15]. The model was utilized to evaluate the vascular pattern and hepatectomy simulation.
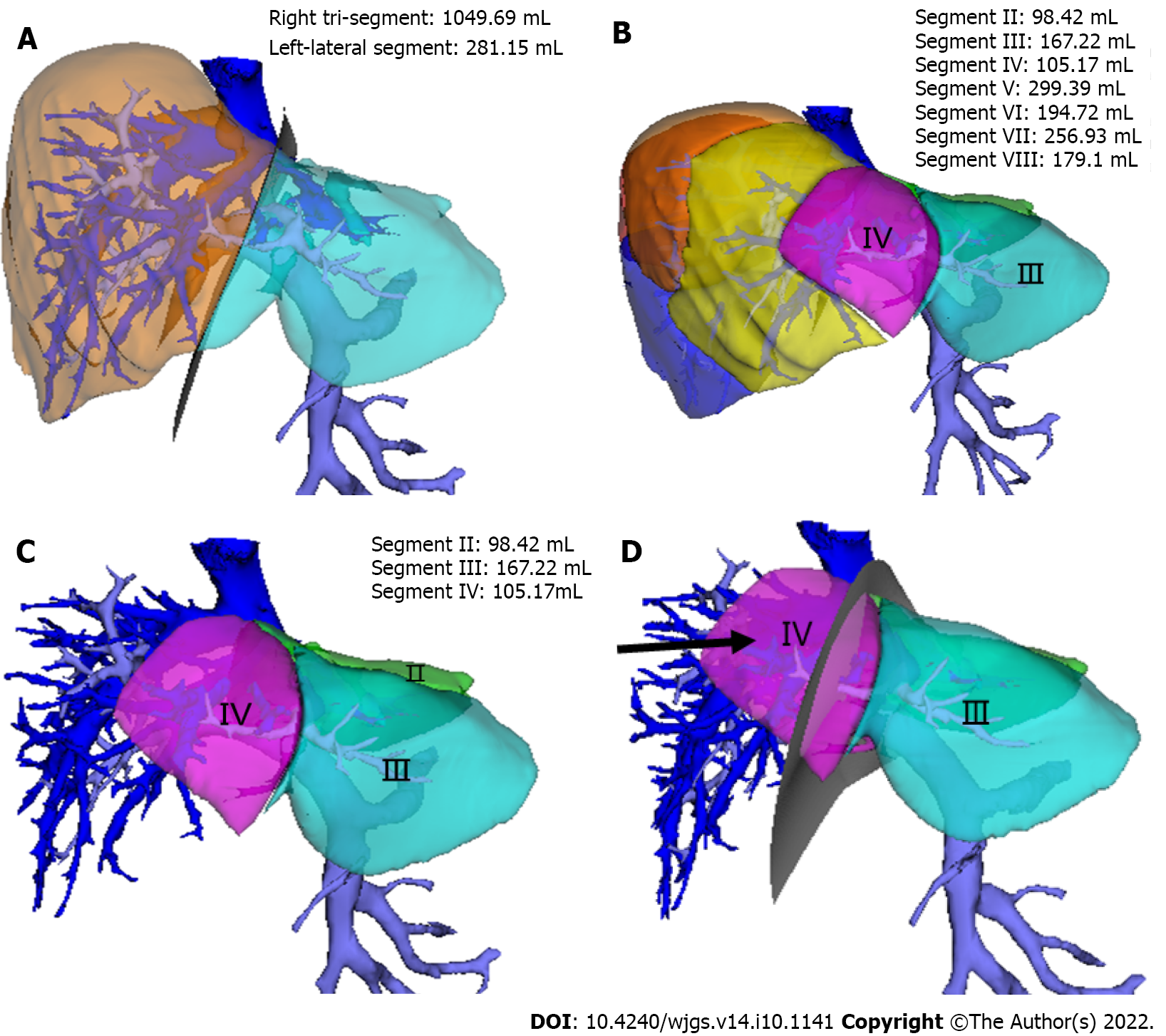
(1) The SLT procedure was simulated on a 3D visualization model and included a segment of the hepatic artery, portal vein, and hepatic vein and the disconnection of the liver parenchyma; and (2) The SLT procedure was simulated according to the Couinaud liver segmentation and blood flow topology liver segmentation (BFTLS) methods. The volume of the two liver segments and the ischemic volume were also quantified (Figure 1).
The combination of in-situ and ex vivo splitting was used for liver splitting. The surgical methods included left-lateral and right tri-segment splits, and left and right hepatic splits. During the operation, Doppler ultrasound was employed to identify and mark the shape of the middle hepatic vein, and a cavitron ultrasonic surgical aspirator and an ultrasonic knife were used to separate the liver parenchyma.
The classification of hepatic vasculatures was based on 3D visualization technology, the hepatic and ischemic volume was estimated using simulated surgery, and hepatic ischemia was measured during the actual surgery.
Preoperative hepatic vascular evaluation and simulated surgical results. The preoperative 3D visualization model revealed that all the donor hepatic portal veins were Cheng et al’s type I[16], the hepatic arteries were Michels[17] type I, the middle hepatic vein and left hepatic vein shared trunk in 5 cases, and a single hepatic vein of segment IV directly flowed into the inferior vena cava in 1 case. Among the 5 simulated operations, 4 cases were split into left-lateral segment and right tri-segment; 1 case was split into left and right half liver, and the middle hepatic vein was split by median segmentation.
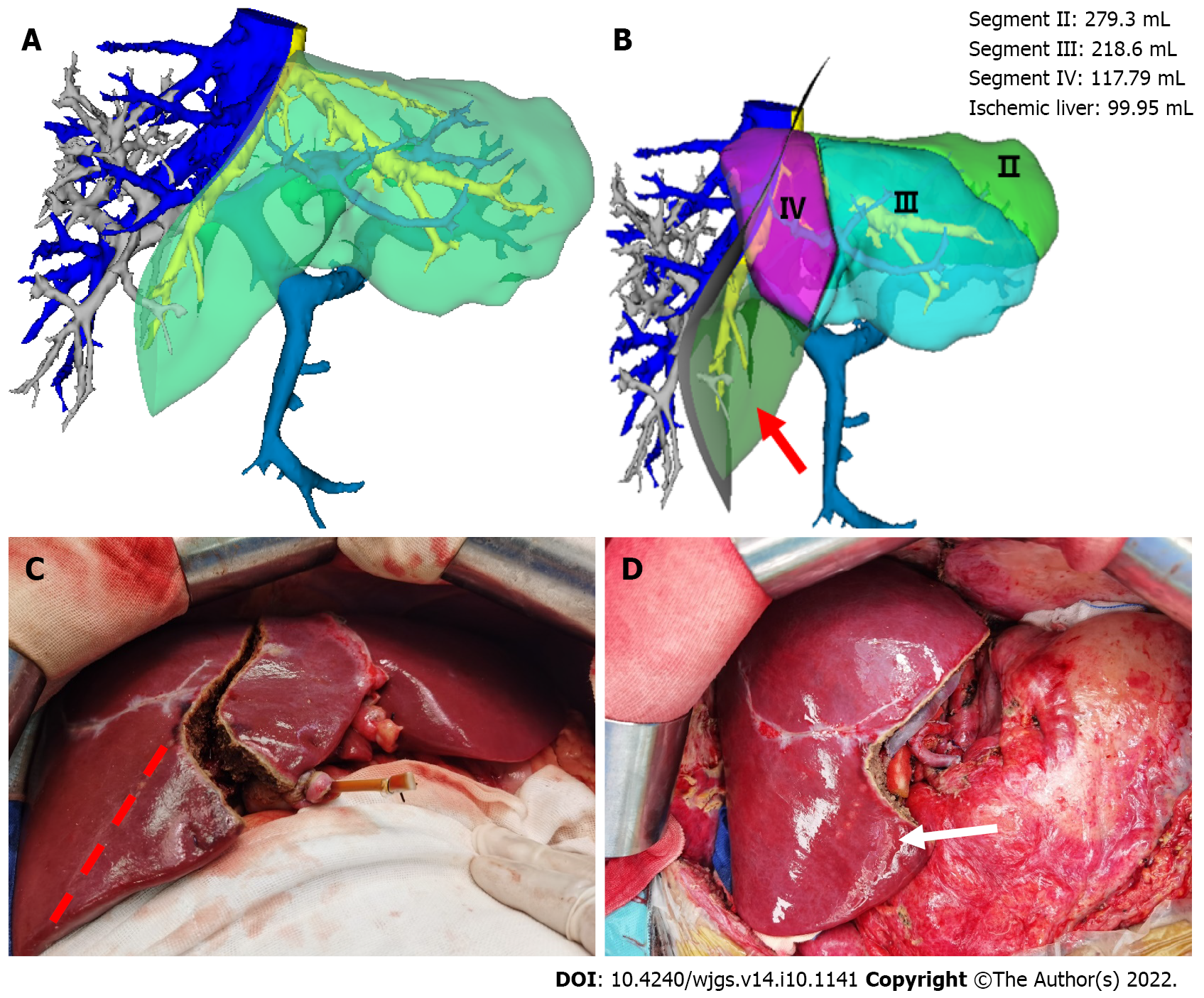
The results revealed that the resection plane simulated by the Couinaud liver segmentation method or BFTLS method was inconsistent, the former was flat, while the latter was irregularly shaped. 4 cases were simulated using left-lateral and right tri-segment splits. As measured by the above two liver segmentation methods, the volumes of the left-lateral segments were 359.00 ± 101.57 mL and 367.75 ± 99.73 mL, respectively. According to the BFTLS method, the volume of segment IV (i.e., the ischemic part of the liver) cleaving to the right tri-segment was 136.31 ± 86.10 mL. 1 case of left and right liver splitting was simulated, and median segmentation of the middle hepatic vein was performed after strictly evaluating two adult recipients with low body weight. The operation was simulated according to the above two segmentation methods. 99.95 mL of tissues in segment IV was assigned to the left half of the liver according to Couinaud classification; if splitting was carried out according to this method, 99.95 mL of liver tissues might experience postoperative ischemia or even necrosis.
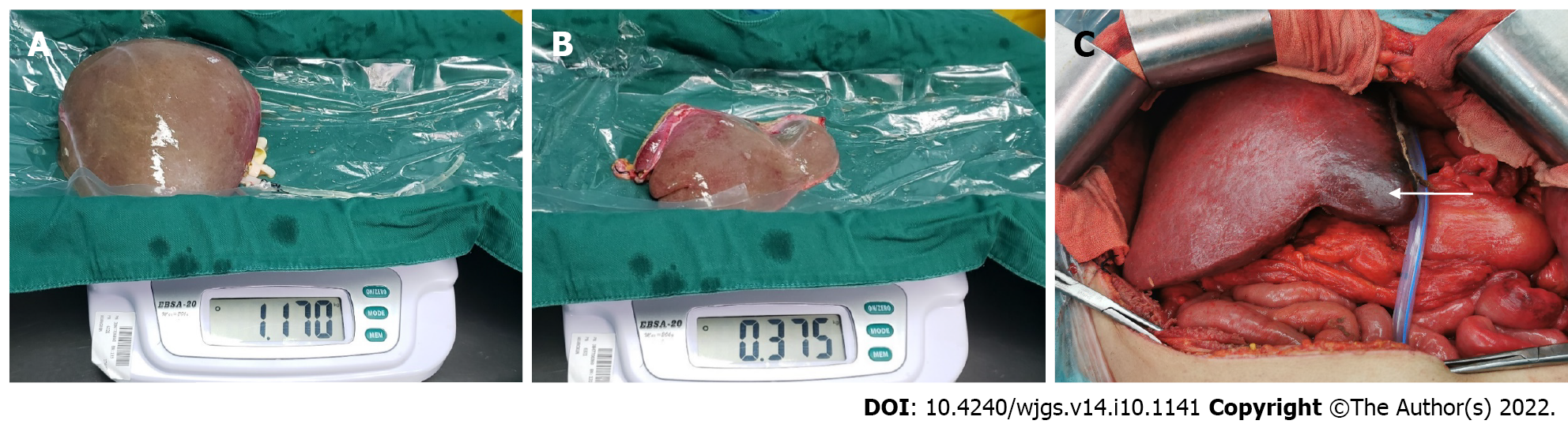
In practice, in-situ and ex vivo splits were performed successfully according to the preoperative plan. According to the Couinaud liver segmentation method, left-lateral and right tri-segment splitting was performed in 4 cases. The left branch of the portal vein, the main trunk of the left hepatic artery, and the left branch of the hepatic vein were distributed to the left-lateral segment. The hepatic artery and portal vein branches entering segment IV of the liver were severed. The actual volume of the left-lateral segment was 397.50 ± 37.97 mL. The liver sections of the 4 cases exhibited ischemic changes after the operation (Figure 2). 1 case experienced necrosis of the liver section and bile leakage and underwent reoperation to remove the necrotic tissues. The volume of the ischemic liver calculated before the operation was 238.7 mL.
In the other case, the operation was performed by left and right half liver splitting based on the portal vein BFTLS method. The middle hepatic vein was segmented in the middle, and the donor’s external iliac vein was used to reconstruct the middle hepatic vein of the left and right halves of the liver. No apparent changes in hepatic sectional ischemia were detected post-surgery (Figure 3).
The operation was successfully completed in all 10 patients corresponding to the split livers, and postoperative biliary leakage occurred in 1 case without small-for-size syndrome. During the perioperative period, 1 patient who underwent a right tri-segment split suffered from a sudden intracerebral hemorrhage on the 7th postoperative day and died on the 18th postoperative day.
Couinaud liver segmentation is an artificial segmentation method based on anatomical markers of the liver. Moreover, its segmentation plane limits the drainage area of the hepatic vein and does not consider the topology of portal vein branches in the liver[6,7,18]. For instance, when a vascular variation occurs in the liver, multiple portal or hepatic vein branches may co-exist within the same Couinaud liver segment. However, the BFTLS approach used herein was based on the topological structural relationship of the hepatic portal vein, which can truly display anatomical structural relationships in the liver[6,7]. Indeed, this concept is also widely used in clinical practice[19-21].
The initial aim of SLT is to save two lives with a single liver. However, inappropriate preoperative evaluation of the liver donor or splitting method may bring a well-functional liver into 2 marginal donors, which may delay the recovery of graft function and even lead to graft failure or recipient death[22,23]. Therefore, compared with other hepatobiliary surgeries, adequate preoperative evaluation of SLT is monumental. At the time of preoperative donor evaluation, enhanced CT of the liver must be performed, with initial vascular and biliary evaluation followed by re-evaluation based on the 3D visual model. If the donor has significant portal vein variation, SLT is not recommended to ensure the safety of both recipients. Herein, only donors with good liver function and no significant anatomical variation were included in the SLT cohort. All the patients undergoing SLT in our center underwent an initial simulation using the 3D visualization model, and the portal vein, hepatic vein, and hepatic artery were segmented accordingly. Furthermore, the liver volume was also calculated so that a detailed preoperative plan could be drawn up.
In this study, all patients underwent simulated surgeries using the Couinaud liver segmentation method and BFTLS method, and the measured volumes of the left-lateral segment were 359.00 ± 101.57 mL and 367.75 ± 99.73 mL, respectively. Moreover, according to the above two methods, the volume of the segment IV (the portion of the ischemic liver lobe) allocated to the right tri-segment was 136.31 ± 86.10 mL, obtained by adding the volume of segments Ⅱ/Ⅲ/Ⅳ minus the volume of the left-lateral segment. Based on these data, we can obtain a detailed evaluation of the surgery, predict the degree of ischemic tissue changes and necrosis in the liver segments, as well as assess the need to remove ischemic segments during liver transplantation.
Hepatic segmental ischemic necrosis is extremely common following SLT, mainly because the branches of the portal vein[13] and hepatic artery[14,24,25] entering this part of the liver are not connected, and the corresponding hepatic vein[26-28] may also be cut off in some cases. Therefore, this part of the liver may undergo pathological changes such as hepatocyte ischemia, necrosis, fibrosis, and atrophy, and in some cases, tissue necrosis and bile leakage. Indeed, one of the 5 cases in this study suffered from ischemic tissue necrosis and bile leakage on the surface of the right tri-segment. The necrotic tissue was eventually resected by reoperation in that particular case, with a preoperative ischemic liver volume of 238.7 mL. The ischemic areas of the right tri-segment in the other 3 cases, calculated preoperatively, were 76.9 mL, 54.4 mL, and 175.3 mL, respectively. Therefore, we postulate that if the scope of hepatic segmental ischemia can be accurately determined before the operation, hepatic segmental tissue necrosis can be predicted in advance, avoiding reoperation and alleviating the pain and economic burden of patients.
In conclusion, in the case of the left-lateral segment and right tri-segment splits, preoperative evaluation based on three-dimensional visualization technology could calculate the ischemic range of the right tri-segment. Judging by the results, the operator could predict the postoperative ischemic range and make clinical judgments accordingly. For instance, when the branches of the hepatic artery and portal vein of segment IV supplying the right tri-segment are disconnected, and the calculated ischemic range is large, the operator can directly remove this section during the operation to avoid further damage to the body due to tissue necrosis, infection or bile leakage. Nevertheless, due to the small number of cases, it was not possible to determine a specific cut-off value to predict the likelihood of postoperative hepatic ischemic necrosis. Therefore, it is imperative to include a large number of cases for future clinical or multi-center research.
Split liver transplantation (SLT) is complex, and the commonly used surgical technique is the left-lateral segment and right tri-segment splits, which is implemented based on Couinaud liver segmentation. The right tri-segment liver surface may have different degrees of ischemic changes after SLT, which was related to the destruction of the local portal vein blood flow topology.
To our best knowledge, opinions diverge on the management of ischemia in surface tissues of the liver segment following SLT and there was no a consensus of pre-operative evaluation and predictive strategy for hepatic segmental necrosis after SLT worldwide.
Herein, we sought to investigate the application of the topological approach of liver segmentation based on 3D visualization technology in the surgical planning of SLT.
A retrospective analysis was performed on 10 recipients and 5 donors who underwent SLT from January 2020 to January 2021. All the donor livers were subjected to 3D modeling and evaluation before surgery, based on which the liver splitting procedure was simulated by the Couinaud liver segmentation and blood flow topology liver segmentation (BFTLS) methods respectively, and the volume of the liver was calculated. Clinical data were analyzed, including the hepatic vasculature and expected volume of split grafts evaluated by 3D models, the actual liver volume, and the ischemia state of hepatic section in actual surgery.
The donor liver was split into a left-lateral segment and right tri-segment in 4 cases, while 1 case was split by left and right half liver splitting. According to Couinaud liver segmentation and BFTLS methods, the volume of the left lateral segment was 359.00 ± 101.57 mL and 367.75 ± 99.73 mL, respectively. The volume of segment IV (the portion of ischemic liver lobes) allocated to the right tri-segment was 136.31 ± 86.10 mL as determined using the topological approach to liver segmentation. Yet, during the actual operations, ischemia of the right tri-segment section was observed in 4 cases, including 1 case of necrosis of the surfaces cut and bile leakage.
The application of the topological approach of liver segmentation based on 3D visualization technology may be useful to predict the range of ischemia in the liver section and provide a basis for determining whether the ischemic liver tissue should be removed during the surgery.
However, the follow-up studies with large samples are still warranted due to the relatively small number of cases.
We thank all the liver donors and patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aseni P, Italy; Han B, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Couinaud C. [The anatomy of the liver]. Ann Ital Chir. 1992;63:693-697. [PubMed] |
| 2. | Juza RM, Pauli EM. Clinical and surgical anatomy of the liver: a review for clinicians. Clin Anat. 2014;27:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Lebre MA, Vacavant A, Grand-Brochier M, Rositi H, Abergel A, Chabrot P, Magnin B. Automatic segmentation methods for liver and hepatic vessels from CT and MRI volumes, applied to the Couinaud scheme. Comput Biol Med. 2019;110:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Ortale JR, Naves De Freitas Azevedo CH, Mello De Castro C. Anatomy of the intrahepatic ramification of the portal vein in the right hemiliver. Cells Tissues Organs. 2000;166:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Sakamoto Y, Kokudo N, Kawaguchi Y, Akita K. Clinical Anatomy of the Liver: Review of the 19th Meeting of the Japanese Research Society of Clinical Anatomy. Liver Cancer. 2017;6:146-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 6. | Zahlten C, Jürgens H, Evertsz CJ, Leppek R, Peitgen HO, Klose KJ. Portal vein reconstruction based on topology. Eur J Radiol. 1995;19:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Cho A, Okazumi S, Miyazawa Y, Makino H, Miura F, Ohira G, Yoshinaga Y, Tohma T, Kudo H, Matsubara K, Ryu M, Ochiai T. Proposal for a reclassification of liver based anatomy on portal ramifications. Am J Surg. 2005;189:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, Panganiban KM, Perini MV, Shah SR, Wang H, Xu Y, Suh KS, Kokudo N. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg. 2021;274:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 9. | Urade T, Sawa H, Iwatani Y, Abe T, Fujinaka R, Murata K, Mii Y, Man-I M, Oka S, Kuroda D. Laparoscopic anatomical liver resection using indocyanine green fluorescence imaging. Asian J Surg. 2020;43:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Kim YS, Choi SH. Pure Laparoscopic Living Donor Right Hepatectomy Using Real-Time Indocyanine Green Fluorescence Imaging. J Gastrointest Surg. 2019;23:1711-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Yersiz H, Renz JF, Farmer DG, Hisatake GM, McDiarmid SV, Busuttil RW. One hundred in situ split-liver transplantations: a single-center experience. Ann Surg. 2003;238:496-505; discussion 506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Maggi U, Caccamo L, Reggiani P, Lauro R, Bertoli P, Camagni S, Paterson IM, Rossi G. Hypoperfusion of segment 4 in right in situ split-liver transplantation. Transplant Proc. 2010;42:1240-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Maurer R, Rivoire M, Basso V, Meeus P, Peyrat P, Dupré A. Portal supply of segment IV of the liver based on CT-scan. Surg Radiol Anat. 2017;39:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Alghamdi T, Viebahn C, Justinger C, Lorf T. Arterial Blood Supply of Liver Segment IV and Its Possible Surgical Consequences. Am J Transplant. 2017;17:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Zhao D, Lau WY, Zhou W, Yang J, Xiang N, Zeng N, Liu J, Zhu W, Fang C. Impact of three-dimensional visualization technology on surgical strategies in complex hepatic cancer. Biosci Trends. 2018;12:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Cheng YF, Huang TL, Lee TY, Chen TY, Chen CL. Variation of the intrahepatic portal vein; angiographic demonstration and application in living-related hepatic transplantation. Transplant Proc. 1996;28:1667-1668. [PubMed] |
| 17. | Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 551] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Xiang N, Fang C, Fan Y, Yang J, Zeng N, Liu J, Zhu W. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience. Int J Clin Exp Med. 2015;8:18873-18878. [PubMed] |
| 19. | Ni ZK, Lin D, Wang ZQ, Jin HM, Li XW, Li Y, Huang H. Precision Liver Resection: Three-Dimensional Reconstruction Combined with Fluorescence Laparoscopic Imaging. Surg Innov. 2021;28:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Nakaseko Y, Ishizawa T, Saiura A. Fluorescence-guided surgery for liver tumors. J Surg Oncol. 2018;118:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Terasawa M, Ishizawa T, Mise Y, Inoue Y, Ito H, Takahashi Y, Saiura A. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg Endosc. 2017;31:5111-5118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Reyes J, Gerber D, Mazariegos GV, Casavilla A, Sindhi R, Bueno J, Madariaga J, Fung JJ. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg. 2000;35:283-9; discussion 289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Bowring MG, Massie AB, Schwarz KB, Cameron AM, King EA, Segev DL, Mogul DB. Survival Benefit of Split-Liver Transplantation for Pediatric and Adult Candidates. Liver Transpl. 2022;28:969-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Jin GY, Yu HC, Lim HS, Moon JI, Lee JH, Chung JW, Cho BH. Anatomical variations of the origin of the segment 4 hepatic artery and their clinical implications. Liver Transpl. 2008;14:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Sommacale D, Farges O, Ettorre GM, Lebigot P, Sauvanet A, Marty J, Durand F, Belghiti J. In situ split liver transplantation for two adult recipients. Transplantation. 2000;69:1005-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Cheng YF, Chen CL, Haung TL, Lee TY, Chen TY, Chen YS, Liu PP, Chiang YC, Eng HL, Wang CC, Cheung HK, Jawan B, Goto S. Post-transplant changes of segment 4 after living related liver transplantation. Clin Transplant. 1998;12:476-481. [PubMed] |
| 27. | Zhang J, Guo X, Qiao Q, Zhao J, Wang X. Anatomical Study of the Hepatic Veins in Segment 4 of the Liver Using Three-Dimensional Visualization. Front Surg. 2021;8:702280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Lubezky N, Oyfe I, Contreras AG, Rocca JP, Rudow DL, Keegan T, Taouli B, Kim-Schluger L, Florman S, Schiano T, Facciuto M. Segment 4 and the left lateral segment regeneration pattern after resection of the middle hepatic vein in a living donor right hepatectomy. HPB (Oxford). 2015;17:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |