Published online Oct 27, 2022. doi: 10.4240/wjgs.v14.i10.1120
Peer-review started: June 24, 2022
First decision: August 1, 2022
Revised: August 19, 2022
Accepted: September 22, 2022
Article in press: September 22, 2022
Published online: October 27, 2022
Processing time: 122 Days and 22.6 Hours
Hemorrhoids are a common anal condition and can afflict an individual at any age. Epidemiological survey results in China show that the prevalence of anorectal diseases is as high as 50.1% among which 98.08% of patients have hemorrhoid symptoms.
To assess long-term efficacy and safety of cap-assisted endoscopic sclerotherapy (CAES) with long injection needle for internal hemorrhoids.
This study was retrospective. Data from patients with symptomatic internal hemorrhoids treated with CAES using endoscopic long injection needle from April 2016 to December 2019 were collected. Patients were telephoned and followed at two time points, December 2020 and 2021, to evaluate the impro
Two hundreds and one patients with internal hemorrhoids underwent CAES with the long needle. The first median follow-up was performed 33 mo post-operatively. Symptoms improved in 87.5% of patients after the first CAES. Efficacy did not decrease with treatment time extension. Fifty-four patients underwent colonoscopy after the first CAES treatment of which 21 underwent CAES again, and 4 underwent hemorrhoidectomy. At the first follow-up, 62.7% of patients had both improved hemorrhoid grades and symptoms, and 27.4% had a significant improvement in both parameters. At the second follow-up, 61.7% of the patients showed satisfactory improvement in their hemorrhoid grade and symptoms when compared with pre-surgery values. 90% of patients reported CAES was painless, and 85% were satisfied/very satisfied with CAES treatment outcomes.
The present study based on the largest sample size reported the long-term follow-up of the treatment for internal hemorrhoid with the CAES using endoscopic long injection needle. Our findings demonstrate that CAES should be a micro-invasive endoscopic technology yields satisfactory long-term efficacy and safety.
Core Tip: Cap-assisted endoscopic sclerotherapy (CAES) is a novel procedure to process flexible endoscopic sclerotherapy. Data from patients with symptomatic internal hemorrhoids treated with CAES using endoscopic long injection needle from April 2016 to December 2019 were collected. Patients were telephoned and followed at two time points, December 2020 and 2021, to evaluate the improvements in symptoms, complications, recurrence, and satisfaction. The present study based on the largest sample size reported the long-term follow-up of the treatment for internal hemorrhoid with the CAES using endoscopic long injection needle. Our findings demonstrate that CAES should be a micro-invasive endoscopic technology yields satisfactory long-term efficacy and safety.
- Citation: Xie YT, Yuan Y, Zhou HM, Liu T, Wu LH, He XX. Long-term efficacy and safety of cap-assisted endoscopic sclerotherapy with long injection needle for internal hemorrhoids. World J Gastrointest Surg 2022; 14(10): 1120-1130
- URL: https://www.wjgnet.com/1948-9366/full/v14/i10/1120.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i10.1120
Hemorrhoids are a common anal condition that can afflict an individual at any age. Epidemiological survey results in China show that the prevalence of anorectal diseases is as high as 50.1% among which 98.08% of patients have hemorrhoid symptoms. Epidemiological survey results in the United States show that the prevalence rate of hemorrhoids was higher than 50%, and the risk of hemorrhoids was highest among people aged 45-65 with 44.7% of patients affected by bleeding, pain, prolapse, and other symptoms affecting their life quality[1-3].
Injection sclerotherapy is a safe and simple treatment for internal hemorrhoids. However, traditional hardening agent injection therapy is performed via an anoscopy, which may present iatrogenic risks due to incorrect injection location[4,5]. Three milestones in the history of flexible endoscopic sclerotherapy have been reported. Ponsky et al[6] in 1991 reported the flexible endoscopic injection of 23.4% saline, with 5-mm retractable needle, and retroflexed position for symptomatic hemorrhoids. Tomiki et al[7] in 2014 reported the flexible endoscopic injection of aluminum potassium sulfate and tannic acid, with 5-mm retractable needle, retroflexed and anterograde position, and endoscopic cap. Zhang et al[5] in 2015 reported cap-assisted endoscopic sclerotherapy (CAES) using a Lauromacrogol injection with a 10-20 mm retractable needle, normal position, endoscopic cap, and proper air delivery for improving endoscopic exposure.
CAES is a novel procedure to process flexible endoscopic sclerotherapy. The special design of the CAES endoscopic needle (generally using a long needle) helps accurately control the injection angle, direction, and depth under direct vision, and avoids iatrogenic injury caused by ectopic injection[5,8]. Although this technique has become a widely used flexible endoscopy procedure in China with expert recommendations[9], few studies have reported long-term follow-up studies of more than one year. This study retrospectively analyzed the clinical data of patients with internal hemorrhoids treated with long needle CAES from April 2016 to December 2019 in our hospital to explore the long-term clinical efficacy and safety of long needle CAES in the treatment of internal hemorrhoids.
This study was a single-center study. Patients with symptomatic internal hemorrhoids who received CAES treatment at the First Affiliated Hospital of Guangdong Pharmaceutical University from April 2016 to December 2019 were included in this study and followed by telephone.
No gender or age restrictions were placed on study participants. All patients underwent complete bowel preparation and signed an informed consent for colonoscopy diagnosis and treatment.
Patients with external hemorrhoids, asymptomatic internal hemorrhoids, perianal abscesses, anal stenosis, anal fistulas, malignancies involving the anal canal, pregnancy, coagulation dysfunction, decompensated cirrhosis, cerebrovascular accident, and other diseases, such as dementia, stroke, and mental retardation were excluded. Patients who did not comply with follow-ups were also excluded.
All patients completed the coagulation function examination. To prepare for concurrent endoscopic treatments, such as a bowel polypectomy, aspirin is generally discontinued for 5 d and other antiplatelet drugs for 7 d if possible. All patients signed informed consents for colonoscopy diagnosis and treatment. All patients underwent pre-operative intestinal preparation for CAES to meet the requirements of colonoscopy for diagnosis and treatment. If polyps were found during colonoscopy, treatment of polyps was completed before CAES treatment started. The One physician who was familiar with endoscopic operation and two assistants performed the procedures. All endoscopists had experience in with more than 200 endoscopes.
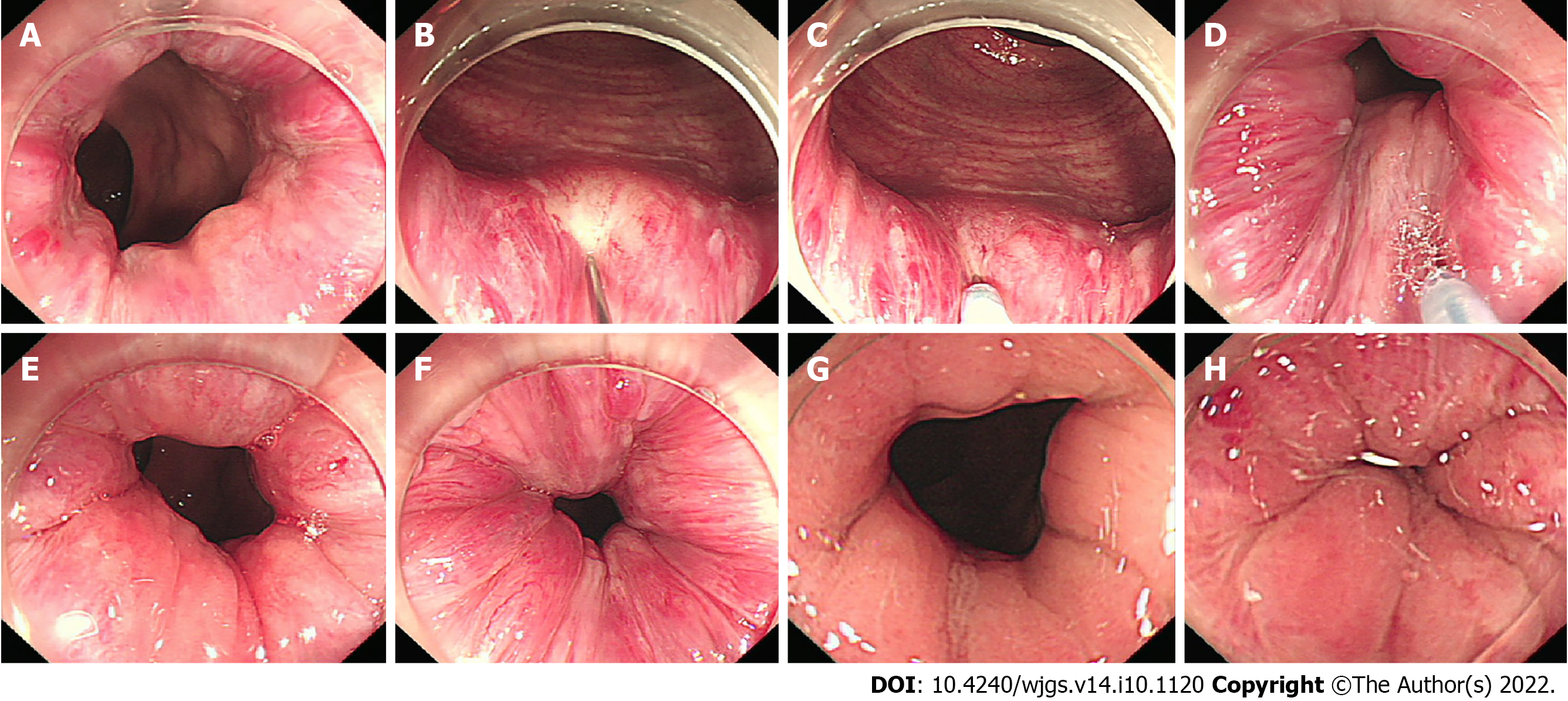
A short, straight transparent cap was installed at the front end of the endoscope,and an appropriate amount of gas was injected into the rectum. With the help of the transparent cap, the internal hemorrhoids with a blue-purple surface were visible. Under conditions of full exposure, a long needle, such as an injection needle with a diameter of 22 G and a length of 14 mm, was used (DT-EN-W122-14, Detian, Changzhou, China). Lauromacrogol (10 mL:100 mg, 1%, Tianyu, Xi’an, China) was injected into the base of the hemorrhoids. The injection point was located above the dentate line. According to the location of the left, posterior, right, and anterior anus (LPRA), the inclined plane injection was selected for an endoscopic direction of 6 o’clock, and 1-2 mL Lauromacrogol was injected into each injection site (Figure 1). The clockwise order should be followed when choosing the injection sites. The sclerosing agent is injected into submucosal layer within 5 s. After the injection, the needle was left in situ, or the needle sheath was pressed for 10 to 20 s to avoid bleeding at the injection site. Very rapid injections and more than a 2 mL injection in one site are not permitted. After retracting the endoscope, a finger massage around the anal ring was performed to help disperse the drug.
Patients were asked to maintain a horizontal position for at least 2 h after surgery, and routine use of antibiotics and hemostatic drugs was not required after surgery. Laxative agents, such as lactulose, were given after surgery to keep the stool soft and thin. The changes in the condition and vital signs were strictly monitored and handle defecation difficulties, bleeding, infection, ulcers, and other issues were addressed in a timely manner.
Patients self-reported bleeding and other symptoms were taken as the basis for with three classes of evaluation criteria: (1) Excellent, very satisfied no or mild symptoms; (2) good, significant improvement, occasional symptoms; or (3) poor, no improvement, even worse symptoms.
The symptoms and concomitant symptoms before and after treatment, including anal bleeding, anal pain, anal prolapse, defecation difficulties, anal distension, anal pruritus, anal dampness, and others, were evaluated according to the presence/absence of medical records.
Several parameters related to satisfaction with the procedure and outcome were rated: (1) Great satisfaction; (2) satisfaction; (3) general; (4) less satisfaction; and (5) very dissatisfied. Pain level of CAES was rated according to certain levels: (1) No pain; (2) mild and tolerable; and (3) serious and intolerable. Patients’ recommendations to undergo the procedure (Would you like to recommend it to other patients?) were based on yes or no answers.
The changes in internal hemorrhoids before, after, and before and during the first CAES and follow-up were compared in patients who returned to the hospital for the second CAES.
SPSS 25.0 was used for systematic analysis of the data, and a chi-squared test was used to analyze the data differences between the two groups before and after treatment. P < 0.05 was considered statistically significant.
A total of 201 patients with internal hemorrhoids who underwent CAES treatment were admitted to our hospital from April 2016 to December 2019. These patients were followed by telephone between December 2020 and December 2021. In December 2020, 201 patients were followed, and none were lost to follow-up. In December 2021, 149 patients were followed up of which 52 were lost to follow-up. The patient did not use Ayurveda, Chinese medicine, or herbal medicines during follow-up. Prior to the first treatment, patients with hemorrhoids based on Goligher classification were divided into four stages: (1) 88 patients with stage I hemorrhoids; (2) 53 patients with stage II hemorrhoids; (3) 50 patients with stage III hemorrhoids; and (4) 10 patients with stage IV hemorrhoids (Table 1).
| Basic situation | Follow-up of 2020 (n = 201) | Follow-up of 2021 (n = 149) |
| Median follow-up | 33 (24-45) | 45 (34-57) |
| Age | 54.71 ± 13.016 | 54.77 ± 13.495 |
| Gender, n (%) | ||
| Male | 116 (57.7) | 92 (61.7) |
| Female | 85 (42.3) | 57 (38.3) |
| Hemorrhoids installment, n (%) | ||
| Stage Ⅰ | 88 (43.8) | 67 (45.0) |
| Stage Ⅱ | 53 (26.4) | 38 (25.5) |
| Stage Ⅲ | 50 (24.8) | 37 (24.8) |
| Stage Ⅳ | 10 (5.0) | 7 (4.7) |
At the first follow-up, 62.7% (126/201) patients showed satisfactory improvement in hemorrhoid grade and symptoms when compared with pre-operative parameters, and 27.4% (55/201) patients had significant improvement in grade and symptoms of hemorrhoids compared with pre-operative parameters but occasionally had symptoms. Twenty out of 201 (9.9%) patients experienced the same grade and/or no improvement or even worsening of symptoms (Table 2).
| Efficacy | Excellent | Good | Poor | χ2 | P value |
| Stage Ⅰ | 54 (61.4) | 26 (29.5) | 8 (9.1) | ||
| Stage Ⅱ | 35 (66.1) | 13(24.5) | 5 (9.4) | 8.90 | 0.177 |
| Stage Ⅲ | 34 (68.0) | 13 (26.0) | 3 (6.0) | ||
| Stage Ⅳ | 3 (30.0) | 3 (30.0) | 4 (40.0) |
Fifty-four patients underwent colonoscopy after CAES treatment (Figure 2), and 21 of those patients underwent CAES treatment again. Four additional patients underwent hemorrhoidectomy. At the second follow-up, 61.7% (92/149) of the patients had satisfactory improvement in hemorrhoid grade and symptoms when compared with the pre-operative level, and 33.6% (50/149) of the patients had significant improvement in hemorrhoid grade and symptoms compared with the pre-operative level but occasionally had symptoms. Seven out of 149 patients (4.7%) showed no improvement or even deterioration (Table 3). In our long-term follow-up, we did not identify patients who developed ulcers after treatment.
| Efficacy | Excellent | Good | Poor | χ2 | P value |
| Stage Ⅰ | 46 (68.6) | 18 (26.9) | 3 (4.5) | ||
| Stage Ⅱ | 22 (57.9) | 13(34.2) | 3 (7.9) | 4.78 | 0.572 |
| Stage Ⅲ | 20 (54.1) | 16 (43.2) | 1 (2.7) | ||
| Stage Ⅳ | 4 (57.1) | 3 (42.9) | 0 (0) |
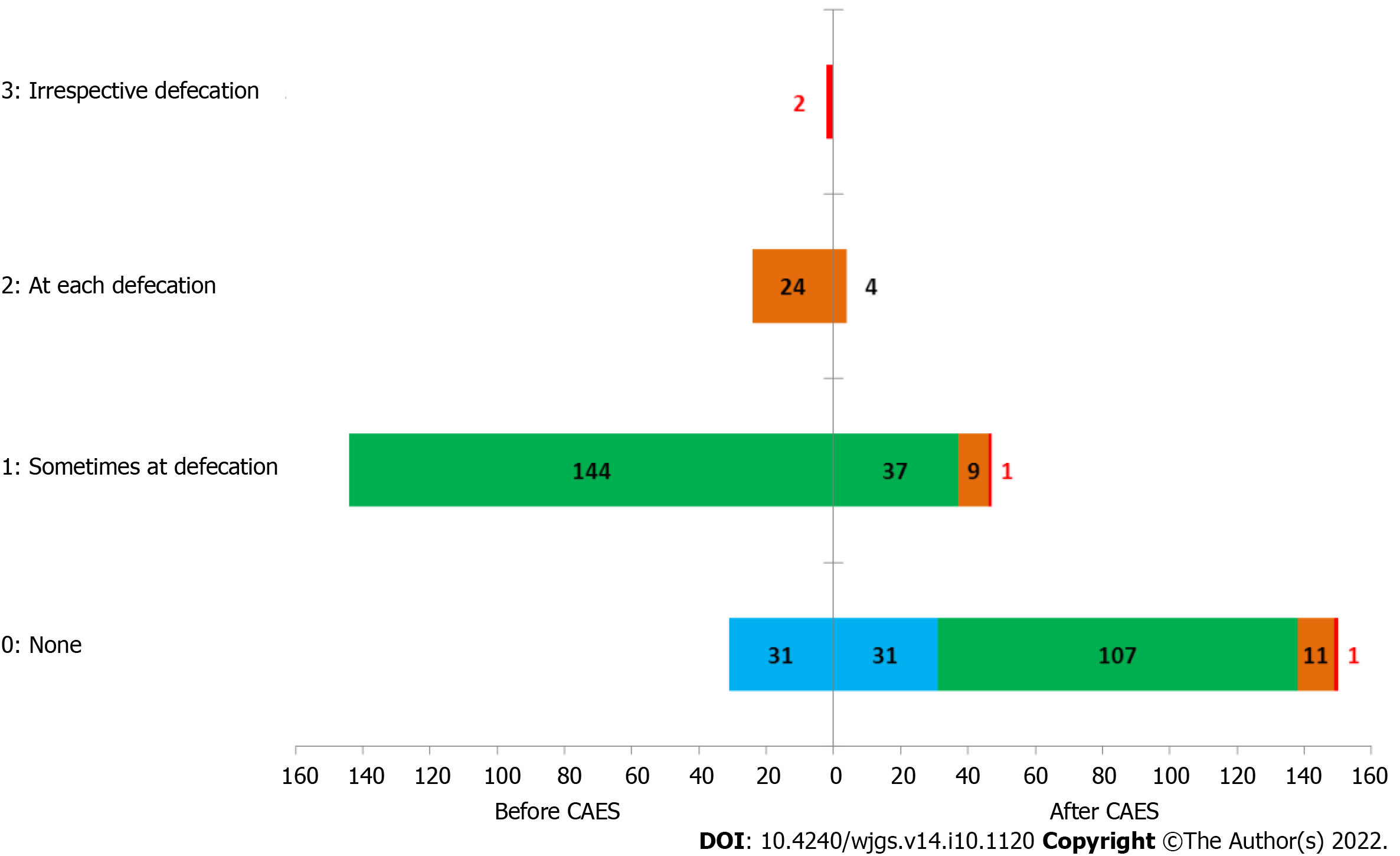
In terms of internal hemorrhoid improvements, anal bleeding was taken as an example. At the first follow-up, 31 patients had no bleeding either before or after treatment. Bleeding frequency ranged from occasional occurrence in the first defecation samples to asymptomatic after treatment in 107 patients, and 37 patients had no change. Bleeding frequency varied at each defecation before treatment to asymptomatic after treatment in 11 patients, occasionally in nine patients, and no change in four patients after treatment. Bleeding frequency ranged from pretreatment with or without defecation to occasionally in one patient after treatment to asymptomatic in one patient (Figure 3).
126 patients had no anal prolapse either before or after treatment, 57 patients had significant improvement in symptoms, 14 patients had no changes in symptoms either before or after treatment, and 4 patients reported symptom worsening after treatment. 10 patients had stage 4 internal hemorrhoids, 5 patients showed no improvement in prolapse symptoms, 3 patients with less prolapse than before, and 2 patients without prolapse.
131 patients had no pain either before or after treatment, 49 patients had significant improvement in symptoms after treatment, 20 patients had no change in symptoms after treatment, and 1 patient had aggravation of symptoms after treatment. One hundred and forty patients had no distension either before or after treatment, 47 patients’ symptoms improved significantly after treatment, 12 patients’ symptoms did not change after treatment, and 2 patients reported worsening symptoms after treatment. 59 patients had no pruritus either before or after treatment, 28 patients had significant improvement in symptoms after treatment, 11 patients had no change in symptoms after treatment, and 3 patients had aggravation of post-treatment symptoms. 176 patients had no dampness either before or after treatment, 18 patients had significant improvement in symptoms, 5 patients had no changes in symptoms either before or after treatment, and 2 patients had aggravation of symptoms after treatment.
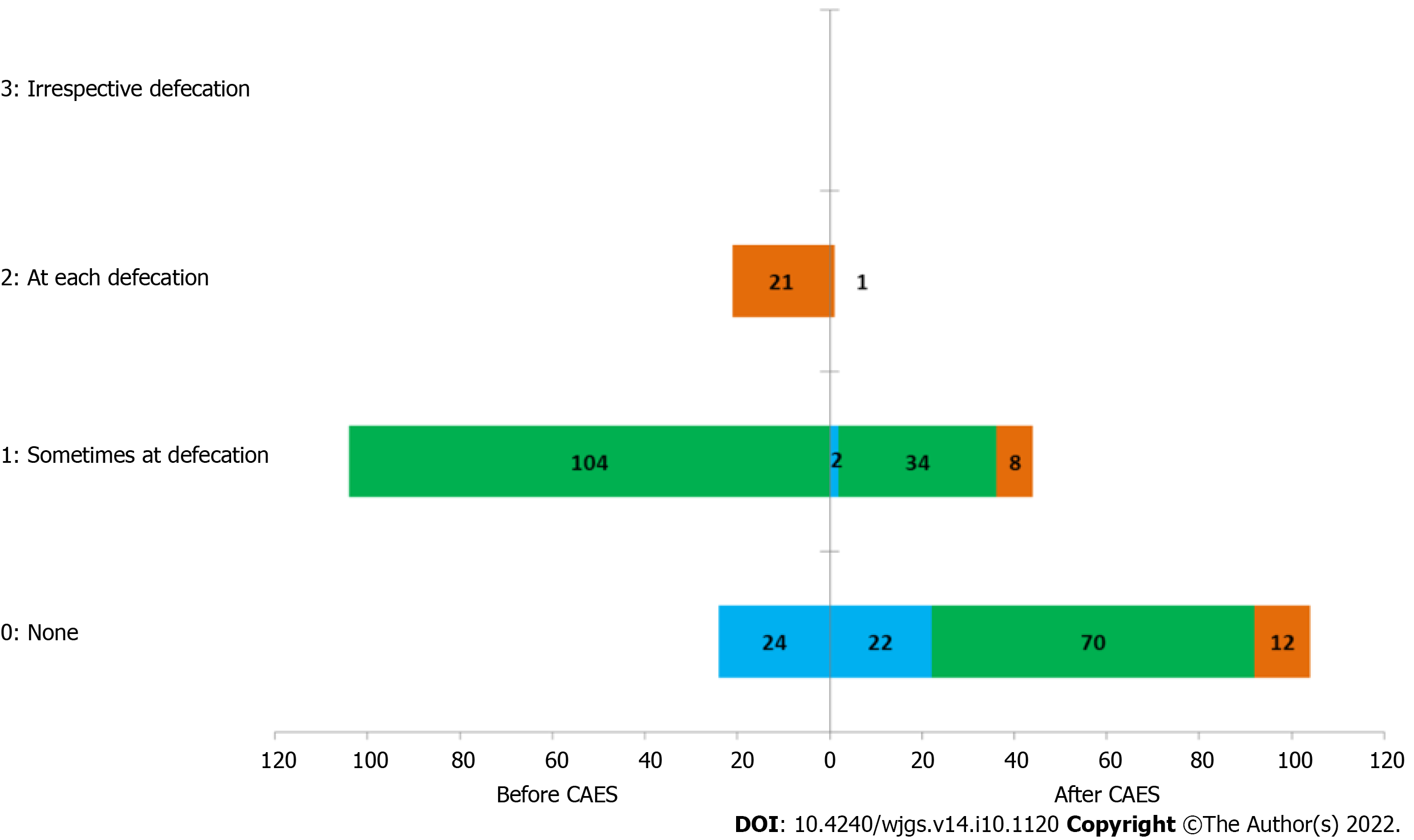
At the second follow-up, 22 patients had no bleeding before or after treatment, 90 patients had significant improvement in symptoms, 35 patients had no change in symptoms before or after treatment, and 2 patients had aggravation of symptoms (Figure 4). 94 patients had no prolapse before and after treatment, 41 patients had significant improvement in prolapse symptoms, 8 patients had no change in symptoms before or after treatment, and 6 patients had symptom worsening. 92 patients had no pain either before or after treatment, 38 patients had significant improvement in pain symptoms after treatment, 10 patients had no change in symptoms after treatment, and 9 patients had aggravation of symptoms after treatment. 94 patients had no distension either before or after treatment, 37 patients had significant improvement in distension symptoms, 8 patients had no change in symptoms either before or after treatment, and 10 patients had symptom aggravation. No pruritus was found in 110 patients either before or after treatment, 25 patients improved significantly after treatment, 5 patients’ pruritis symptoms did not change either before or after treatment, and 9 patients’ symptoms worsened after treatment. No dampness in 118 patients either before or after treatment was found, 15 patients improved significantly, 4 patients’ dampness symptoms did not change either before or after treatment, and 12 patients’ symptoms worsened.
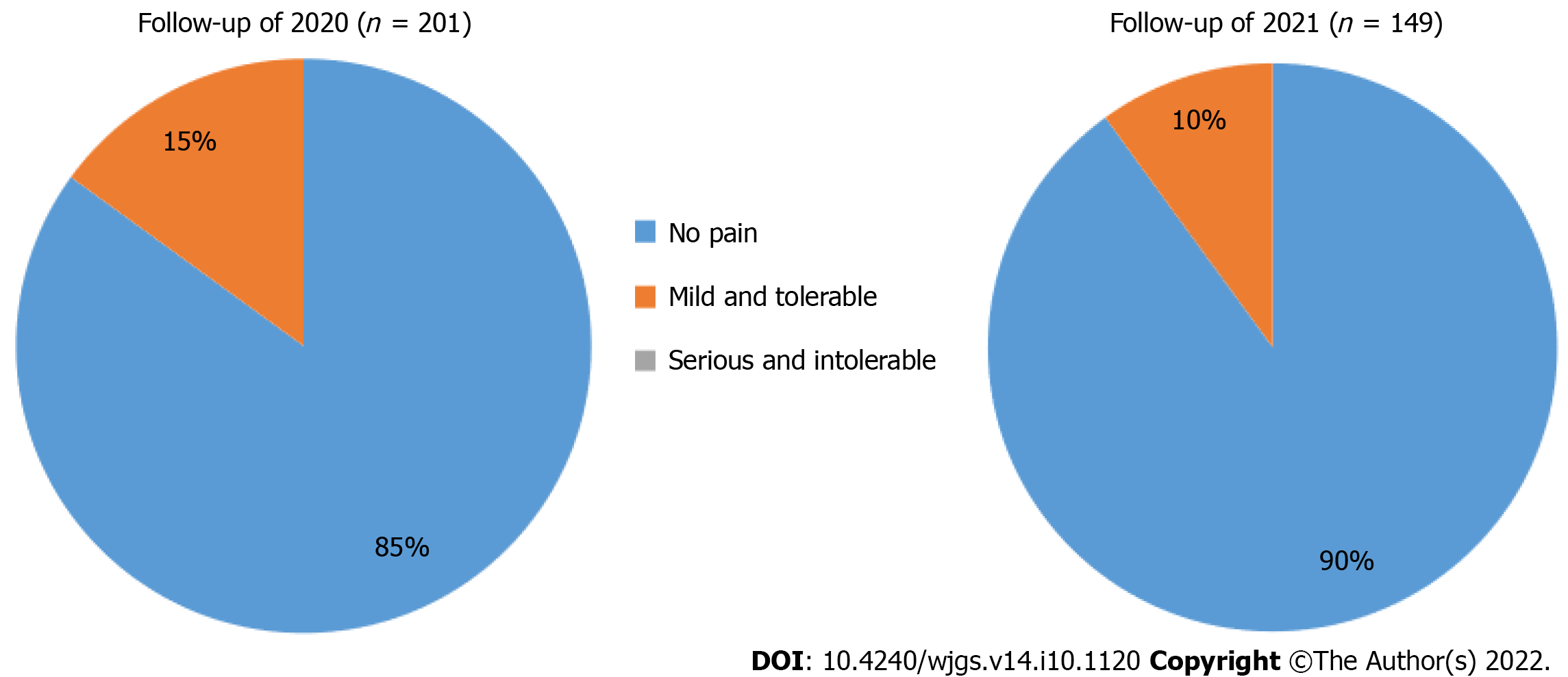
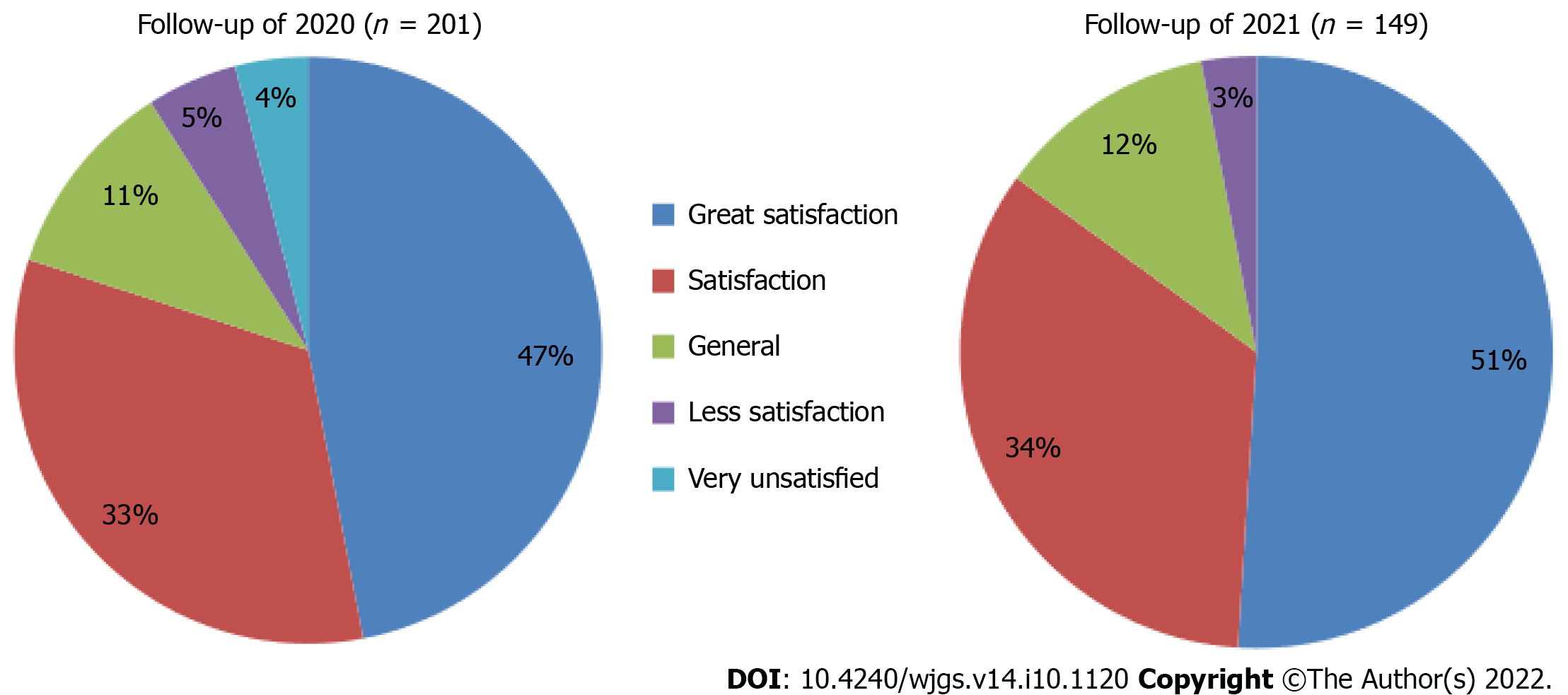
At the second follow-up, 90% of patients reported CAES was painless (Figure 5), and 85% were satisfied/very satisfied with CAES treatment outcomes (Figure 6).
As for the pathogenesis of internal hemorrhoids, Thomson[10] proposed the “theory of anal cushion moving down,” which has been widely recognized. Internal hemorrhoids are abnormal vascular pads covered by columnar epithelium located in the anal canal above the dentate line. The hemorrhoid pad shrinks during defecation to facilitate stool excretion. During periods of non-defecation, the arterial hemorrhoid pad becomes hyperemic and swollen and then seals the anus[11-13]. The American Society of Digestive Endoscopy recommends that endoscopic treatment of hemorrhoids include rubber band ligation (RBL), infrared coagulation, and/or injection sclerotherapy.
Polidocanol is a commonly used injection sclerotherapy that has been widely used for injection sclerotherapy of internal hemorrhoids[14,15]. Polidocanol is injected into the anus mirror or via colorectal colonoscopy under direct intravascular injection of pathological changes and causes minimal damage to the mucous membrane tube and anal cushion. However, local intravascular thrombosis resulting from vascular endothelial injury leading to aseptic inflammatory that eventually translates into fiber cords led to occlusion of artery branch blood vessels on rectal hemorrhoids that eventually shrank. In addition, this fibrous action can fix the loose mucous membrane to the anal muscle wall, thereby reducing the symptoms of prolapsed hemorrhoids.
A longer injection needle has advantages as the sclerosing agent injected with short injection needle cannot form hardening pile in the base of hemorrhoids, and the shallow injection depth of the sclerosing agent can easily cause ulcer formation. With the help of a transparent cap, Zhang et al[5] used a long needle for submucosal injection and achieved satisfactory efficacy. In 2021, Zhang et al[9] on behalf of the CAES-LPRA Study Group released the expert recommendation concerning flexible endoscopic positioning methods. Briefly, endoscopic residual effusion or injected water is the sign for determining the left anus under the left lateral decubitus position. Along the clockwise direction, LPRA are recommended to replace the typical lithotomy position for the precise direction description on the anal lesions and for endoscopic therapy.
The LPRA anal positioning method helps the endoscopist distinguish between injected and un-injected sites and avoids the use of tracers[9]. Our group routinely uses long needles for multi-point injection therapy according to the LPRA anal positioning method. This study evaluated the long-term efficacy and safety of long needle CAES for symptomatic internal hemorrhoids. In Zhang’s et al report[5,8,9,16,17], the follow-up for CAES treatment did not exceed one year. In our study, the time for following CAES treatment was up to 5 years. This study presents the largest sample size reported so far in the treatment of internal hemorrhoids with CAES long needles. In this study, patients treated with CAES were divided into three effect grades: (1) Excellent (no or mild symptoms); (2) good (obviously improved but occasionally symptomatic); and (3) invalid (no improvement, even worse symptoms). In the 2020 follow-up results, 90.1% of patients with internal hemorrhoids, including 94% of patients with stage III internal hemorrhoids, achieved good or excellent results. At the 2021 follow-up, 95.3% of patients with internal hemorrhoids, including 97.3% of patients with stage Ⅲ internal hemorrhoids achieved good or excellent outcomes. Zhang et al’s study found that CAES was effective for stages Ⅰ and Ⅱ and a portion of stage Ⅲ internal hemorrhoids[5]. However, our study found that symptoms improved significantly before and after CAES treatment, and no difference in the long-term efficacy of the treatment in stages Ⅰ-Ⅲ was found (Tables 2 and 3). No statistically significant difference in internal hemorrhoid staging between the four groups in 2020 was found. In 2021, no statistically significant differences in the efficacy of CAES in the treatment of stages Ⅰ-Ⅳ internal hemorrhoids were found, that is, it is not considered that CAES produces different long-term efficacies for different stages of internal hemorrhoids. According to the improvement in symptoms and overall evaluation of follow-up, it was found that as the follow-up years increased, no statistically significant difference between the two groups of patients followed up in 2020 and 2021 was noted, indicating that the curative effect of patients treated with CAES did not decline with the extension of treatment years, and the long-term curative effect was stable.
The limitation of this study is the lack of dose difference analysis although the same hardener was used for all patients. Although the operators in the center are experienced, individual technical differences were not considered in this study. Although CAES is a locally minimally invasive treatment, human conditions (such as constipation and advanced age) were not included in the analysis. Since the LPRA method for the location description of anal lesions was published in 2021[9], this study did not analyze the relationship between disease efficacy, safety, and disease site.
In general, post-operative bleeding is the most common complication of hemorrhoids, but in this study, no complications, such as anal bleeding, anal fistula, and anal stenosis occurred after CAES was performed with a long needle. When compared with RBL and hemorrhoidectomy, long-needle CAES appears to be less likely to cause pain. During the follow-up in 2020, 85% of patients believed that long-needle CAES was painless after treatment, and 80% of patients were satisfied or very satisfied with CAES treatment. During the follow-up in 2021, 90% of patients reported that CAES treatment with long needle was painless, and 85% of patients were satisfied or very satisfied with CAES treatment. Patients reported that the pain level during CAES treatment did not increase with the increase in treatment years. This study further confirms that CAES is a simple and effective treatment for internal hemorrhoids that requires no anesthesia and is less painful. Ninety-three percent of patients were willing to recommend CAES treatment to other patients.
The present study based on the largest sample size reported the long-term follow-up of the treatment for internal hemorrhoid with the CAES using endoscopic long injection needle. Our findings demonstrate that CAES should be a micro-invasive endoscopic technology yields satisfactory long-term efficacy and safety.
Hemorrhoids are a common anal condition and can afflict an individual at any age. Cap-assisted endoscopic sclerotherapy (CAES) is a novel procedure to process flexible endoscopic sclerotherapy.
There are few previous studies discussing CAES in the treatment of internal hemorrhoids with large sample size and long-term follow-up, so this study can make up for the shortcomings of previous theories.
Long-term efficacy and safety of CAES with long injection needle for internal hemorrhoids were assessed.
This retrospective analysis of data from patients with symptomatic internal hemorrhoids treated with CAES using endoscopic long injection needle from April 2016 to December 2019 were collected. Patients were telephoned and followed at two time points, December 2020 and 2021, to evaluate the impro
Two hundred and one patients with internal hemorrhoids underwent CAES with the long needle, At the first follow-up, 62.7% of patients had both improved hemorrhoid grades and symptoms, and 27.4% had a significant improvement in both parameters. At the second follow-up, 61.7% of the patients showed satisfactory improvement in their hemorrhoid grade and symptoms when compared with pre-surgery values. 90% of patients reported CAES was painless, and 85% were satisfied/very satisfied with CAES treatment outcomes.
The present study based on the largest sample size reported the long-term follow-up of the treatment for internal hemorrhoid with the CAES using endoscopic long injection needle. Our findings demonstrate that CAES should be a micro-invasive endoscopic technology yields satisfactory long-term efficacy and safety.
The improvement of symptoms, complications, recurrence and satisfaction of patients with symptomatic hemorrhoids were cure by CAES.
We sincerely thank Dr. Fa-Ming Zhang (Nanjing Medical University) for his guidance on this manuscript. We would like to thank all patients in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elfeki H, Egypt; Gupta R, India S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Ray-Offor E, Amadi S. Hemorrhoidal disease: Predilection sites, pattern of presentation, and treatment. Ann Afr Med. 2019;18:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 2. | Internal Hemorrhoids Cooperative Group of Chinese Society of Digestive Endoscopology. Chinese digestive endoscopic practice guidelines and operation consensus for internal hemorrhoids. Zhonghua Xiaohua Neijing Zazhi. 2021;38:676-687. [DOI] [Full Text] |
| 3. | Gallo G, Martellucci J, Sturiale A, Clerico G, Milito G, Marino F, Cocorullo G, Giordano P, Mistrangelo M, Trompetto M. Consensus statement of the Italian society of colorectal surgery (SICCR): management and treatment of hemorrhoidal disease. Tech Coloproctol. 2020;24:145-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 4. | Jacobs D. Clinical practice. Hemorrhoids. N Engl J Med. 2014;371:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 5. | Zhang T, Xu LJ, Xiang J, He Z, Peng ZY, Huang GM, Ji GZ, Zhang FM. Cap-assisted endoscopic sclerotherapy for hemorrhoids: Methods, feasibility and efficacy. World J Gastrointest Endosc. 2015;7:1334-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Ponsky JL, Mellinger JD, Simon IB. Endoscopic retrograde hemorrhoidal sclerotherapy using 23.4% saline: a preliminary report. Gastrointest Endosc. 1991;37:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Tomiki Y, Ono S, Aoki J, Takahashi R, Sakamoto K. Endoscopic sclerotherapy with aluminum potassium sulfate and tannic acid for internal hemorrhoids. Endoscopy. 2014;46 Suppl 1 UCTN:E114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Zhang T, Long CY, Cui BT, He Z, Peng ZY, Huang GM, Zhang FM. Cap-assisted endoscopic sclerotherapy for hemorrhoids: a prospective study (with video). Zhonghua Xiaohua Neijing Zazhi. 2017;34:709-712. [DOI] [Full Text] |
| 9. | Zhang FM, Wu KC, Li JN, Wang X, He XX, Wan R, Chen SY; CAES-LPRA Study Group. Rationale, new anus positioning methods, and updated protocols: Expert recommendations on cap-assisted endoscopic sclerotherapy for hemorrhoids from China Gut Conference. Chin Med J (Engl). 2021;134:2675-2677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Thomson WH. The nature of haemorrhoids. Br J Surg. 1975;62:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 328] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Pata F, Sgró A, Ferrara F, Vigorita V, Gallo G, Pellino G. Anatomy, Physiology and Pathophysiology of Haemorrhoids. Rev Recent Clin Trials. 2021;16:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | van Tol RR, Kleijnen J, Watson AJM, Jongen J, Altomare DF, Qvist N, Higuero T, Muris JWM, Breukink SO. European Society of ColoProctology: guideline for haemorrhoidal disease. Colorectal Dis. 2020;22:650-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 13. | Aoki T, Hirata Y, Yamada A, Koike K. Initial management for acute lower gastrointestinal bleeding. World J Gastroenterol. 2019;25:69-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (4)] |
| 14. | Watanabe T, Ohno M, Tahara K, Tomonaga K, Ogawa K, Takezoe T, Fuchimoto Y, Fujino A, Kanamori Y. Efficacy and safety of sclerotherapy with polidocanol in children with internal hemorrhoids. Pediatr Int. 2021;63:813-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Mishra S, Sahoo AK, Elamurugan TP, Jagdish S. Polidocanol versus phenol in oil injection sclerotherapy in treatment of internal hemorrhoids: A randomized controlled trial. Turk J Gastroenterol. 2020;31:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | ASGE Technology Committee; Siddiqui UD, Barth BA, Banerjee S, Bhat YM, Chauhan SS, Gottlieb KT, Konda V, Maple JT, Murad FM, Pfau P, Pleskow D, Tokar JL, Wang A, Rodriguez SA. Devices for the endoscopic treatment of hemorrhoids. Gastrointest Endosc. 2014;79:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Wu X, Wen Q, Cui B, Liu Y, Zhong M, Yuan Y, Wu L, Zhang X, Hu Y, Lv M, Wu Q, He S, Jin Y, Tian S, Wan R, Wang X, Xu L, Bai J, Huang G, Ji G, Zhang F. Cap-assisted endoscopic sclerotherapy for internal hemorrhoids: technique protocol and study design for a multi-center randomized controlled trial. Ther Adv Gastrointest Endosc. 2020;13:2631774520925636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |