Published online Oct 27, 2022. doi: 10.4240/wjgs.v14.i10.1089
Peer-review started: June 16, 2022
First decision: September 4, 2022
Revised: September 16, 2022
Accepted: October 14, 2022
Article in press: October 14, 2022
Published online: October 27, 2022
Processing time: 131 Days and 8.2 Hours
Clinically relevant postoperative pancreatic fistula (CR-POPF) has continued to compromise patient recovery post-pancreatectomy despite decades of research seeking to improve risk prediction and diagnosis. The current diagnostic criteria for CR-POPF requires elevated drain fluid amylase to present alongside POPF-related complications including infection, haemorrhage and organ failure. These worrying sequelae necessitate earlier and easily obtainable biomarkers capable of reflecting evolving CR-POPF. Drain fluid has recently emerged as a promising source of biomarkers as it is derived from the pancreas and hence, capable of reflecting its postoperative condition. The present review aims to summarise the current knowledge of CR-POPF drain fluid biomarkers and identify gaps in the field to invigorate future research in this critical area of clinical need. These findings may provide robust diagnostic alternatives for CR-POPF and hence, to clarify their clinical utility require further reports detailing their diagnostic and/or predictive accuracy.
Core Tip: This review demonstrates the potential for drain fluid biomarkers to overcome the limitations of the current diagnostic definition of clinically relevant postoperative pancreatic fistula. Numerous future directions for drain fluid research have been identified, where ideally, new biomarkers would report the accuracy of surgery-specific, risk-stratified cut-offs to clarify their clinical utility. Hence, decisions regarding drain removal and further monitoring can accordingly be made to either expediate or make recovery safer respectively. These improvements will invariably bolster pancreatic ductal adenocarcinoma survival outcomes by tapering the high morbidity of pancreatectomies and ensuring better quality of life for patients.
- Citation: Rykina-Tameeva N, Samra JS, Sahni S, Mittal A. Drain fluid biomarkers for prediction and diagnosis of clinically relevant postoperative pancreatic fistula: A narrative review. World J Gastrointest Surg 2022; 14(10): 1089-1106
- URL: https://www.wjgnet.com/1948-9366/full/v14/i10/1089.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i10.1089
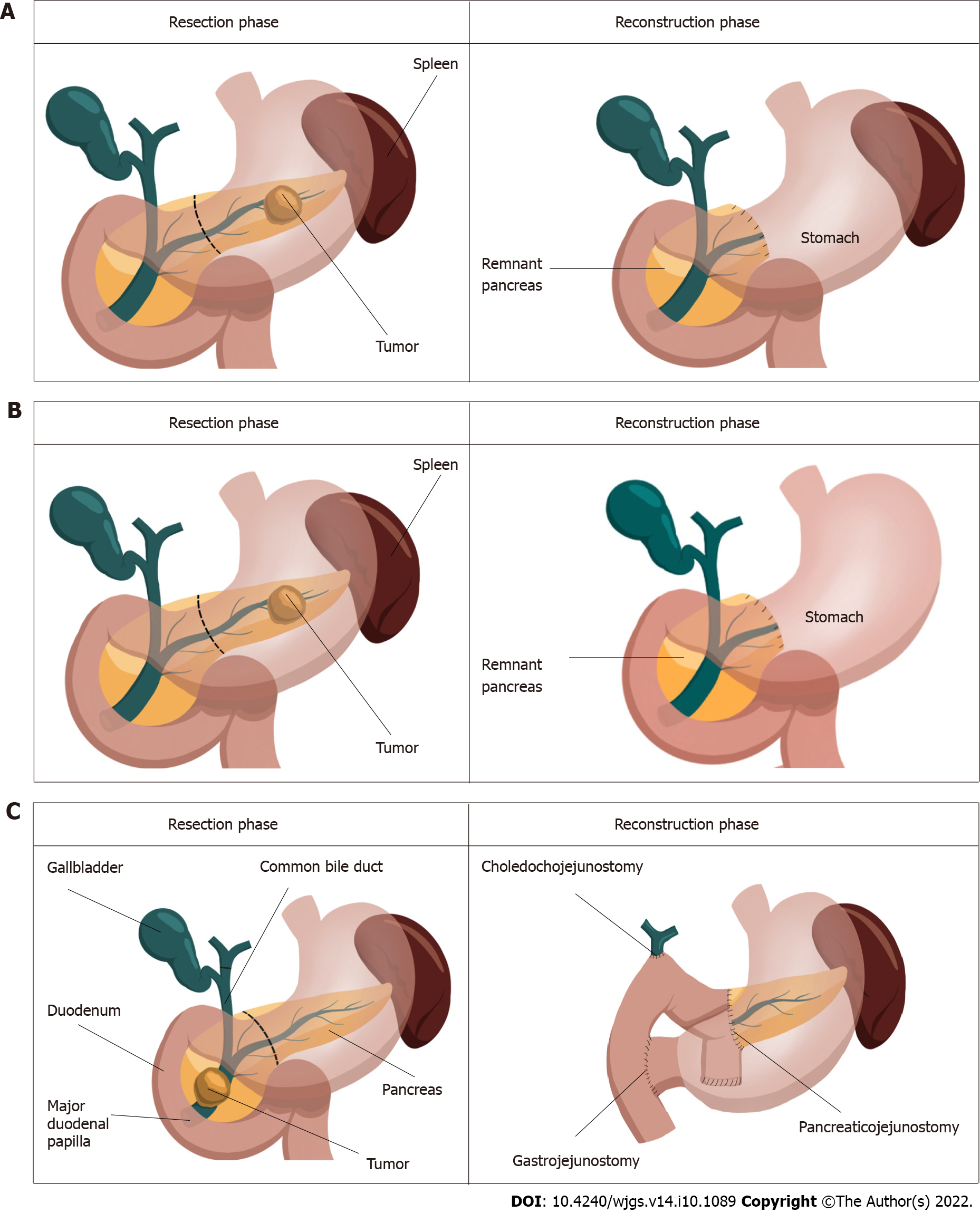
Pancreatic cancer represents a grave diagnosis in which incidence closely parallels mortality[1]. Manifesting as adenocarcinomas, neuroendocrine tumors, acinar carcinomas, colloid carcinomas, pancreatoblastomas and solid-pseudopapillary neoplasms, pancreatic cancer is predicted to be the second most diagnosed cancer by 2030[2]. Both the challenges of early diagnosis and treatment contribute to its dismal prognosis, whereby its failure to manifest symptoms early and resistance to conventional treatments leaves surgery as the only curative option[3,4]. The greatest contributor to the burden of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC) which occurs in 90% of cases and has the highest fatality rate of all solid tumors[3,5]. The majority of PDACs develop in the head of the pancreas (60%-70%) and require pancreaticoduodenectomy. The remainder arise in the body and tail (15% of cases each) which require a distal pancreatectomy to excise the tumor[6] (Figure 1). As only 20%-25% of PDAC patients are diagnosed with resectable disease, maximising their surgical outcomes is of utmost importance, particularly as 5-year survival can improve from < 7% without surgery[3] to 39% after surgery[7]. Necessarily, this involves minimising surgical complications, not only to improve recovery, but to avoid increasing the challenges of cancer which already include compromised nutrition, immunity, metabolism as well as mental and financial wellbeing[8-10]. Clinically relevant postoperative pancreatic fistula (CR-POPF) has persisted as the leading cause of postoperative morbidity and mortality despite decades of improving pancreatectomy techniques and perioperative care[11-15]. Affecting up to 50% of cases[16], CR-POPF has been shown to increase readmission rates, length of stay, health-related costs and particularly relevant for pancreatic cancer patients, potentiate recurrence and delay the delivery of adjuvant therapy, both of which can compromise the curative intent of surgery[17-22].
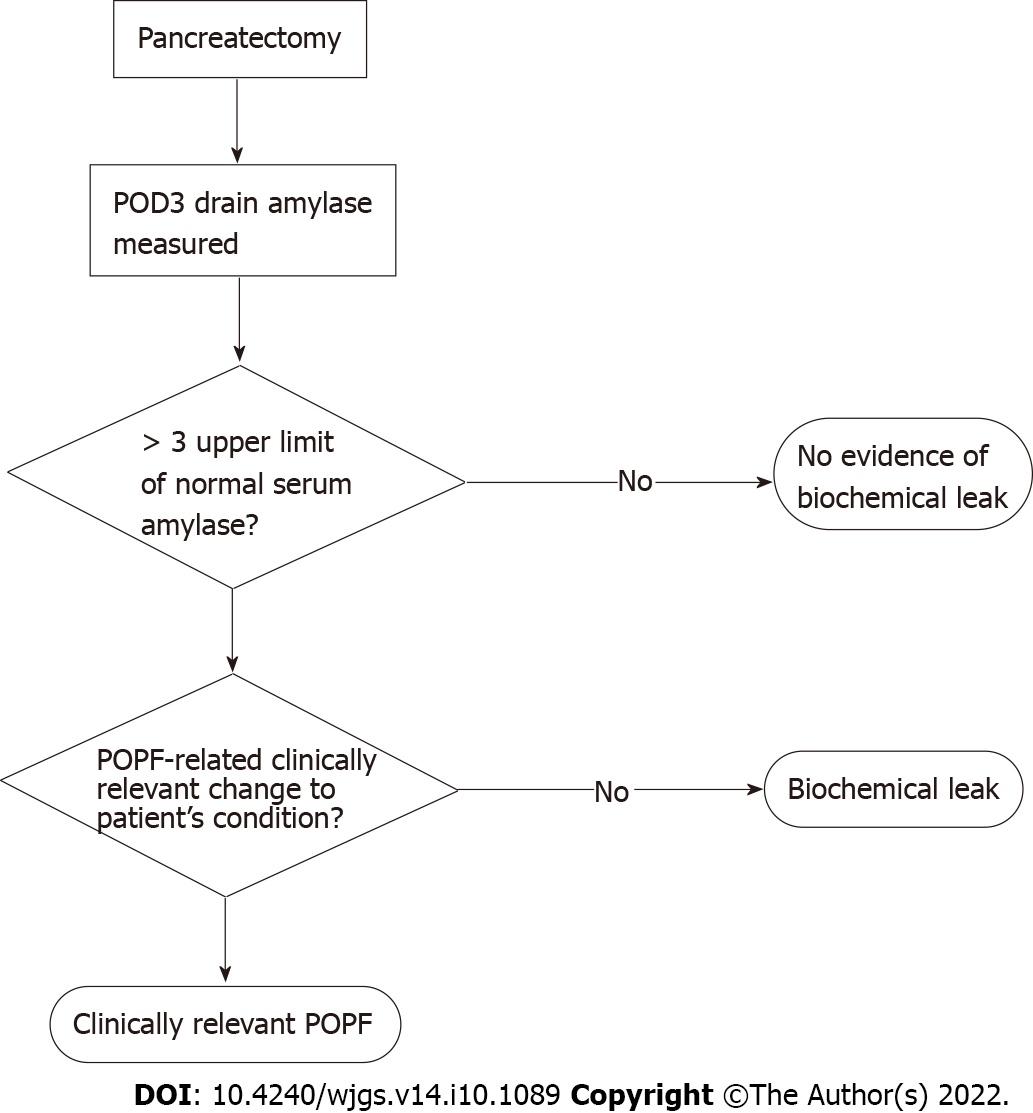
POPF was initially stratified into grades A-C[23], with grade A since being reclassified by the International Study Group on Pancreatic Surgery (ISGPS) as a biochemical leak in favour of recognising the clinically relevant grades B and C[24]. CR-POPF is diagnosed once drain amylase on postoperative day (POD) three exceeds three times the upper limit of normal for serum amylase and the patient develops a clinically relevant change in their condition, necessitating intervention (Figure 2). Grade B fistulae are characterised by prolonged drainage exceeding three weeks, pharmaceutical interventions, additional imaging and infections. Grade C sequelae are more severe, potentiating sepsis, organ failure and in up to 35% of cases, death[25]. This definition does not require imaging to confirm a diagnosis of CR-POPF, particularly as intra-abdominal fluid collections may be transiently increased after surgery. Imaging, however, may be necessary for planning interventions in confirmed cases[26]. Recently, non-contrast-enhanced computed tomography paired with machine learning has been shown capable of evaluating pancreatic texture to predict CR-POPF, doing so with a sensitivity of 0.96 and specificity of 0.98[27]. Similarly, transabdominal pancreatic ultrasound elastography has been associated with CR-POPF, occurring more in patients with softer parenchyma (P = 0.002)[28].
Pathophysiology of CR-POPF: Pancreatic fistulae often occur in pancreata with preserved exocrine function in which pancreatic enzymes are released and activated, damaging tissues, and potentiating systemic complications. Such glands are characterised by soft texture and at least normal acinar cell density at the surgical margin, both of which have been associated with CR-POPF after pancreatoduodenectomy and distal pancreatectomy[29-33]. In advanced PDAC, obstructive pancreatitis may develop[34], contributing to a firm parenchyma. Recently, neoadjuvant therapy has been explored as a potential protector against CR-POPF[35], being shown to favour a more fibrotic and acinar-deplete parenchyma[36]. However, pancreatic texture has been shown to not predict CR-POPF after distal pancreatectomy[37], emphasising the clinical importance of the distinct risk profiles for both resection types.
CR-POPF can develop following the reconstruction phase of surgery (Figure 1). In pancreatoduodenectomy, fistula is often attributed to failure of the pancreatoenteric anastomosis whereby pancreatic fluid destined for the duodenum leaks into the abdomen[38]. Leakage can also occur from the gland itself, in what is referred to as a parenchymal leak[39]. In distal pancreatectomy, increased pressure in the pancreatic duct due to obstruction at the sphincter of Oddi has been thought to result in pancreatic juice leakage[40,41]. As distal resections do not cause downstream obstruction of the pancreatic duct, they are not predisposed to leakage in the same way as pancreases after pancreatoduodenectomy. Splenic preservation has been seen effective in preventing CR-POPF, owing this to the avoidance of pancreatic ischemia secondary to splenic vessel ligation[42,43]. Indeed, the higher morbidity inherent to multi-visceral resection is avoided in spleen-preserving distal pancreatectomy. Moreover, the former facilitates shorter operative times, which may be advantageous given that operations exceeding 480 min were at greater risk of developing pancreatic fistula (P = 0.02)[44]. This finding however did not persist in pancreatoduodenectomy patients[31,45]. Whilst the location of the tumour matters in determining the surgical approach, the size of the tumour has not been shown to influence the development of CR-POPF in pancreatoduodenectomy patients[46] but has so in distal pancreatectomy patients undergoing staple closure (P = 0.009, univariate analysis)[47].
Drain biomarkers for CR-POPF: Predictive biomarkers have commonly been investigated in both drain fluid and blood. The operative placement of drains and close relationship of drain fluid to the pancreas highlights its potential as a convenient biofluid capable of reflecting CR-POPF risk. Hence, the present review synthesises all drain fluid biomarkers identified for the prediction and diagnosis of CR-POPF.
Drain amylase has been extensively explored given its evaluation being embedded in the current diagnostic pathway (Figure 2). Where diagnostic cut-offs have been defined for drain amylase, many measures of accuracy beyond sensitivity and specificity have been reported[48-57] (Table 1). While postoperative evaluation of drain amylase are common, earlier assessments may represent a simple way to improve the utility of drain amylase observations. Particularly, intraoperative measures provide an immediate assessment of pancreatic exocrine function and hence, its propensity to secrete erosive enzymes and predispose the pancreas to leak and subsequent CR-POPF. Indeed, Nahm et al[32] reported a significant association between intraoperative amylase concentration and CR-POPF with an area under the receiver operating characteristic curve (AUC) of 0.76 (P = 0.004) in their cohort of 61 pancreatectomy patients. The accuracy of intraoperative amylase has also been evaluated in surgery-exclusive cohorts with de Reuver et al[58] reporting an AUC of 0.83 in their cohort of 62 pancreatoduodenectomy patients. Wang et al[59] investigated this time point in 40 distal pancreatectomy patients, obtaining a sensitivity and specificity of 0.846 and 0.889 respectively for a cut-off of > 3089 U/L. These studies indicate that the enzymatic leak which can catalyse the development of a CR-POPF begins at the time of surgery, presenting an opportunity to expediate the diagnostic pathway which currently begins on POD3.
| Ref. | Cut-off (U/L) | Predictive | Evaluated (POD) | Patients | Surgery | CR-POPF (%) | Study design | Publication |
| Performance | n | |||||||
| Giovinazzo et al[48], 2018 | 350 | AUC = 0.92 | 1 | 568 | PD | NS | R | Abstract |
| Partelli et al[49], 2017 | 500 | AUC = 0.881, OR = 21.72 | 1 | 463 | PD | 13.82 | R | Abstract |
| Kerem et al[50], 2018 | 1363 | AUC = 0.91 | 1 | 135 | PD | 13.33 | R | Abstract |
| Kawai et al[51], 2009 | 5000 | P = 0.1002 (univariate) | 1 | 244 | PD | 28 | R | Full paper |
| Teixeira et al[52], 2018 | < 270 | Higher median values statistically predicted CR-POPF | 1 | 102 | PD | 25.5 | P | Full paper |
| 271-5000 | ||||||||
| > 5000 | ||||||||
| Mimura et al[53], 2012 | 2000 | AUC = 0.81 | 1 | 240 | PD | 23.4 | R | Abstract |
| Mimura et al[53], 2012 | 100 | AUC = 0.86 | 5 | 240 | PD | 23.4 | R | Abstract |
| Kawaida et al[54], 2018 | 860 | P = 0.002 (univariate) | 3 | 75 | DP | 9.3 | P | Full paper |
| Recreo Baquedano et al[55], 2019 | < 400 | NPV = 0.968 | 3 | 278 | PD | 14 | P | Abstract |
| Newhook et al[56], 2020 | 49 | Sensitivity = 1 | 1 | 45 | DP | 24 | P | Full paper |
| Newhook et al[56], 2020 | 26 | Sensitivity = 1 | 3 | 45 | DP | 24 | P | Full paper |
| van Dongen et al[57], 2021 | 100 | Sensitivity = 1 | 2 | 285 | PD | 18.24 | R | Abstract |
To better facilitate earlier diagnosis of CR-POPF, the sensitivity and specificity of POD1 drain amylase has been widely reported with cut-offs ranging from 282 U/L - 5000U/L[34,49,60-79]. Beyond discrete cut-offs, Hiraki et al[80] found median drain amylase concentration in a prospective study of 30 pancreatoduodenectomy patients to have a sensitivity and specificity of 0.933 and 0.867 respectively. Moreover, Kühlbrey et al[81] found POD1 drain amylase to effectively predict CR-POPF after pancreatectomy returning AUCs of 0.829 (P < 0.001) and 0.637 (P < 0.01) for pancreatoduodenectomy and distal pancreatectomy respectively. This was corroborated by Wüster et al[82] who reported similar AUCs of 0.830 and 0.854 respectively. POD1 drain amylase concentrations have been noted as significantly higher in CR-POPF patients[32,83], correlated with CR-POPF following univariate analysis[84] and identified as an independent risk factor for CR-POPF after pancreatoduodenectomy[49,85-87]. In contrast, in a cohort of 74 pancreatectomy patients, no significant differences in POD1 drain amylase were found in patients who did and did not develop CR-POPF[88]. However, this study may have been underpowered as only nine (12.2%) patients developed CR-POPF.
The accuracy of POD2 drain amylase has been less explored with all reports evaluating pancreatoduodenectomy patients alongside POD1 drain[65,69] or serum amylase[57]. Sensitivity has been reported by two independent studies as 0.88, with the specificity of 0.83 in Ansorge et al’s work[69] surpassing Caputo’s group’s specificity of 0.74 when cut-offs of 314 U/L and 368 U/L were used respectively[65]. Odds ratios of 35 and 29 have further been reported in prospective studies[89,90]. While measuring on POD2 does allow greater time for the biochemical leak to develop thereby enhancing diagnostic accuracy, it does require a change to monitoring protocols which predominantly sample drain fluid on odd PODs (e.g., POD1, POD3 or POD5). Moreover, this relatively unexamined timepoint reflects current preferences to either assess drain amylase early on POD1 or to abide by the recommended testing day of POD3.
The close relationship of POD3 amylase to the ISGPS definition has resulted in few explorations of its true diagnostic performance[24]. Following pancreatoduodenectomy, POD3 drain amylase has been noted to be significantly higher in CR-POPF patients[91]. Diagnostic cut-offs for POD3 drain amylase have ranged between 26 U/L and 1026 U/L for distal pancreatectomy cohorts[54,56,92,93], 93-2820 U/L for pancreatoduodenectomy cohorts[55,56,83,84,94-99] and 200-3000 U/L in studies analysing the biomarker in pancreatectomy patients[16,56,75,100-103]. When cut-off accuracy was reported, sensitivity ranged from 0.316-1.00 and specificity, from 0.631-0.968[16,55,75,84,92,94-98,101-103], showing drain amylase alone does not completely include or exclude CR-POPF. This reinforces the importance of clinically relevant sequelae developing for accurate diagnosis as stipulated by the consensus definition[24]. POD4 drain amylase was found by Kosaka et al[104] to be significantly elevated in CR-POPF patients after pancreatoduodenectomy later defining a cut-off of 646 U/L as having an AUC of 0.87[105]. After distal pancreatectomy, Suzumura et al[106] identified ≥ 1200 U/L as the predictive cut-off and Hiyoshi’s group reported a sensitivity and specificity of 0.938 and 0.7 for a cut-off of ≥ 800 U/L[107]. POD5 drain amylase has been significantly correlated with CR-POPF post-pancreatoduodenectomy[108] where after distal pancreatectomy, a cut-off of > 1000 U/L was significantly associated with CR-POPF[109], with the cut-off of > 538 U/L by Coayla et al[98] predicting CR-POPF with a sensitivity and specificity of 0.86 and 0.91 respectively.
Median drain amylase levels post-pancreatoduodenectomy have been observed as significantly higher in CR-POPF patients[110] on POD1[52], POD2[57] and POD3[69]. Similarly, Moskovic et al[111] found median drain amylase concentration post-pancreatectomy to be significantly elevated on PODs 1-6 in CR-POPF patients. However, this offered no diagnostic advantage and given the wide day range, would prevent diagnosis on a designated day and limit early intervention. Both median and statistically derived cut-offs are limited in their ability to determine specific patient risk as they are summarised from entire patient cohorts which exhibit a spectrum of risk profiles.
Studies stratifying CR-POPF risk using drain amylase have been few and pancreatoduodenectomy exclusive. On POD1, Sutcliffe’s group reported drain amylase < 2000 U/L excluded grade C POPF with a negative predictive value (NPV) of 0.99[112], whereas Caputo et al[113] found POD1 drain amylase ≥ 807 U/L to significantly predict grade C POPF with a sensitivity and specificity of 0.727 and 0.644 respectively. However, Chiba et al[114] did not find drain amylase to be a significant grade C POPF risk factor during the first postoperative week, owing this potentially to the difficulty of ensuring adequate pancreatic juice drainage post-operatively. Drain amylase was similarly examined by Li et al[115] to better identify low and high-risk patients. Here, a POD1 cut-off of 921.7 U/L (AUC = 0.85) had a sensitivity and specificity of 0.789 and 0.828 whereas their POD3 cut-off of 4021.5 U/L had overall higher accuracy favouring specificity at 0.954 (sensitivity = 0.778). In low-risk patients undergoing PD, Newhook et al[56] reported a POD1 cut-off of 661 U/L and POD3 cut-off of 141 U/L could completely exclude CR-POPF when drain amylase was below these levels (sensitivity = 1). Amongst high-risk PD patients, the POD1 and POD3 cut-offs to exclude CR-POPF were < 136 U/L and < 93 U/L respectively. Whilst these cut-offs ensure no false negative results, they may be rarely encountered and hence, rarely utilised. As such, clinicians may prefer higher cut-offs, compromising on sensitivity, to clarify the danger of higher amylase levels more likely to be encountered in clinical practice. These risk-stratified approaches are a welcome advance on previous reports which have predominantly derived predictive cut-offs from entire patient populations, limiting targeted risk prediction. Similar investigations should be conducted in distal pancreatectomy cohorts to define risk-specific cut-offs in these patients. As such, accounting for the operation, patient risk and corresponding predictive drain amylase levels will help refine CR-POPF diagnosis, ultimately decreasing complication rates.
The majority of drain amylase investigations have reported the biomarker at singular timepoints, with others considering its accuracy across multiple PODs. Tzedakis’ group found in their cohort of pancreatectomy patients that drain amylase elevated beyond three times the upper limit of normal on POD1 and POD3 had a sensitivity of 0.974 and NPV of 0.971[101]. Similarly, Linnemann et al[116] reported a NPV of 0.95 in pancreatoduodenectomy patients with a peak drain amylase of 1000 U/L on PODs 1-3. These studies evidence a superior ability to exclude CR-POPF which may justify the additional monitoring of drain amylase which differs from the popular, singular day approach. Hence, early, and continued monitoring can strengthen the identification and selection of low-risk patients for accelerated recovery pathways. In pancreatoduodenectomy cohorts, numerous reports have investigated changes in drain amylase across the postoperative period. This measure possesses the potential to reflect existing and imminent CR-POPF risk. Dugalic et al[110] reported a moderate decline of < 50% between POD 1 and POD3 to be significantly associated with CR-POPF. Seemingly supporting these findings, Koizumi et al[117] found a notable decrease in drain amylase between POD1 and POD5 in patients without CR-POPF. This suggests the relative persistence of elevated drain amylase may be predictive of CR-POPF, a finding which corroborates reports of drain amylase being significantly elevated in CR-POPF patients during this time period[118,119]. However, Furukawa et al[120] identified a decline of pancreatic amylase of greater than 80% between POD1 and POD3 to be predictive of CR-POPF after pancreatoduodenectomy. Further into the postoperative period, Kuhara et al[121] appear to support this in identifying a decrease in drain POD5 amylase to a third of the POD3 level to be a significant risk factor for CR-POPF. To bolster day-specific tracing of CR-POPF risk, future studies should clarify these discrepancies and quantify the drain amylase changes that would indicate impending CR-POPF. Similar investigations in distal pancreatectomy cohorts are also warranted. Nobuoka et al[122] evaluated CR-POPF risk by considering the product of drain amylase and volume. This combined variable was found to be significantly higher in CR-POPF patients on POD1 and POD7. Extending this, Okano et al[119] evaluated the product of drain amylase and volume on POD3 and POD1 in ratio. Here, patients who did not develop CR-POPF had significantly lower values. Together, these indicate that involving drain volume in the assessment of CR-POPF risk may provide opposite findings to when drain amylase is exclusively evaluated, persisting at elevated levels and potentially decreasing, respectively.
Drain lipase has gained momentum as a potential accompaniment or replacement for drain amylase in diagnosing CR-POPF given its similar ability to capture the exocrine function of the remnant pancreas. Moreover, serum lipase assists in acute pancreatitis diagnosis[123,124], a postoperative complication which itself has been shown to independently predict CR-POPF[125,126]. Lipase drives intraperitoneal lipolysis which can exacerbate systemic inflammation and trigger multi-organ dysfunction specifically as the subsequent high systemic unsaturated fatty acid levels can cause mitochondrial toxicity[127], lipotoxicity[128] and kidney[129,130] or liver damage[131]. Diagnostic cut-offs have ranged from 4.88 U/L to 1000 U/L with the majority exploring both pancreatoduodenectomy and distal pancreatectomy patients[100-102,132]. However, pancreatoduodenectomy[97] and distal pancreatectomy[107] exclusive studies have also been conducted. Amongst these reports, the sensitivity and specificity has ranged from 0.8-0.938 and 0.649-0.95, respectively[97,100-102,107,132]. Suzuki et al[133] reported POD1 drain lipase levels to be an independent risk factor for CR-POPF (P = 0.037). Tzedakis et al[101] further considered the evolution of drain lipase and its relation to CR-POPF risk with sustained elevation of drain lipase across POD1 and POD3 having a sensitivity of 0.948 which was then confirmed in their validation cohort.
In the way of risk stratification, Frymerman et al[134] identified the combination of elevated POD3 and POD5 drain lipase (> 5000 U/L) and soft pancreatic texture to be predictive of grade C fistula. As this combination includes the most widely reported risk factor for CR-POPF, soft parenchyma, the contribution of elevated drain lipase to overall grade C risk remains unclear. Hence, drain lipase-exclusive risk stratification requires further investigation particularly during the early postoperative period as the majority of the aforementioned studies evaluated drain lipase on or after POD3.
The extent and character of drain fluid infection has been explored in surgery-specific and all-inclusive analyses of CR-POPF patients (Table 2). Pancreatoduodenectomy has been more extensively explored, with infection of the ascitic fluid and surgical site potentially explained by preoperative bile duct infection[135,136]. Moreover, the construction of the gastrointestinal anastomosis exposes the pancreas to the densely colonised duodenum and jejunum, causing intra-abdominal translocation of species that is further facilitated by bile and pancreatic outflow[137].
| Clinical condition | Pancreatoduodenectomy patients | Distal pancreatectomy patients |
| Present in CR-POPF patients | Fungi, gram-positive bacteria, Acinetobacter, Stenotrophomonas, Citrobacter spp, Staphylococcus, Enterococcus, Enterococcus faecalis Candida spp., Klebsiella, Klebsiella pneumoniae, Pseudomonas, Pseudomonas aeruginosa, Escherichia coli, Enterobacter cloacae | Fungi, Staphylococcus, Enterococcus, Pseudomonas, Acinetobacter, Stenotrophomonas, Escherichia coli and Klebsiella spp |
| Predictor of CR-POPF | Polymicrobial infections, Candida | |
| Predictor of grade C | Gram-negative rods, Candida |
Investigations in pancreatoduodenectomy cohorts: A significantly higher prevalence of CR-POPF in pancreatoduodenectomy patients with positive drain culture has been widely reported[138-144], where internal and preoperative biliary drainage, elevated drain amylase, combined colectomy and a longer duration of surgery have been identified as significant risk factors for contaminated drain fluid[140,145].
Kimura et al[146] identified contaminated drain fluid on POD1 and POD3 to be an independent risk factor for CR-POPF which has since been corroborated in the early postoperative period PODs 1-3[139], POD1[147,148] and POD3[142,149]. The accuracy of POD1 drain culture was reported by Hata et al[145] as having a sensitivity of 0.45 and specificity of 0.813 resulting in a positive predictive value (PPV) of 0.479, with specificity (0.99) similarly prevailing over sensitivity (0.32) in Morimoto et al’s analysis of POD3 drain fluid smear tests which reported a superior PPV of 0.89[142].
The great diversity of microorganisms in post-pancreatoduodenectomy drain fluid is evident in the wide identification of Enterococcus[137-139,141,148,150-153], Enterobacter[138,141,148,151,152], Pseu
During the first postoperative week, Chiba et al[114] found gram-positive bacteria to predominate in grade B POPF patients while gram-negative rods were identified an independent predictor for grade C fistula. As McMillan’s group isolated gram-negative organisms more commonly than gram positive (78.3% vs 68.1% respectively)[157], this could indicate that infections, being more commonly comprised of high-risk bacteria, predispose patients to more severe POPF. Indeed, Yamashita et al[154] isolated Pseudomonas aeruginosa exclusively in CR-POPF patients and identified the bacteria as the source of proteases which activated trypsin from trypsinogen. Belmouhand’s group corroborated this latter finding and further identified drain Enterobacter cloacae as a source of trypsin-activating proteases thereby contextualising the role of gram-negative rods in CR-POPF development[150].
Nagakawa et al[141] found the bacteria detected on POD1 and POD3 to be similar in CR-POPF patients. This taken with the consistent number of non-intestinal bacterium observed on POD3 and POD7 highlights an opportunity for early risk assessment on POD3 as clinicians could anticipate a CR-POPF diagnosis when diagnostic bacteria are first detected[149]. Hence, the concurrent assessment of drain culture alongside POD3 drain amylase may assist earlier CR-POPF diagnosis, potentially reducing the reliance on complication development as is stipulated by the current consensus definition. Hence, patient safety will be increased as despite developing CR-POPF, patients will not have to endure challenging sequelae prior to diagnosis.
Beyond individual microorganisms, Demir et al[158] reported patients presenting with both CR-POPF and positive drain culture had significantly more polymicrobial infections with De Pastena’s group noting the number of CR-POPF patients with polymicrobial infections to be significantly higher than those with biochemical leak (P = 0.003)[159]. The prevalence of polymicrobial infections in CR-POPF patients has ranged from 0.478-0.681, however their association with the complication has not been noted[157,159,160]. Belmouhand’s group did not find polymicrobial drain fluid infections to be associated with anastomosis leakage[150], neither did Maatman et al[161] find this for any postoperative complication.
Rather than investigating polymicrobial infections as a risk factor for CR-POPF, risk stratification would be best assisted by the specific identification of problematic bacteria within polymicrobial drain fluid samples. Hence, an exploration of microorganisms associated with CR-POPF naturally assists in this. Abe et al[162] reported Candida to be significantly associated with CR-POPF and an independent risk factor for grade C fistulae (P = 0.043) which supported McMillan et al’s findings where Candida was found in 87.3% of grade C cases for which microbiological data was available[157]. Here, Enterococcus and Staphylococcus were also detected, conflicting later findings by Belmouhand’s group who reported no significant difference in the severity of POPF when drain fluid was similarly contaminated[150]. The commonly identified Enterococcus and Enterobacter species have been detected on POD1[146] and proposed to originate from bile[140]. Abe et al[162] detected Enterococcus and Enterobacter species in drain fluid with Yamashita et al[138] specifically identifying Enterococcus faecalis and Enterobacter cloacae as precipitating CR-POPF. Interestingly, McMillan et al[157] found mortality to be significantly lower in patients with Enterobacter positive cultures despite it being widely identified in the drain fluid of CR-POPF patients. The inconclusive relevance of Enterobacter, Enterococcus and Staphylococcus to CR-POPF risk and concurrent identification of Candida in drain fluid confirms the findings of Candida as characteristic of polymicrobial infections[150] and more likely to appear in grade C POPF[162].
Investigations in distal pancreatectomy cohorts: Similar to pancreatoduodenectomy studies, distal pancreatectomy patients with positive drain culture have been associated with significantly higher rates of CR-POPF[163,164] with positive drain culture being an independent risk factor for the complication before POD3[165] and on POD4[166]. However, abdominal infection was not found to be a risk factor for CR-POPF by Sato et al[167] in their cohort of 49 patients which may have been underpowered. Yang et al[165] identified Staphylococcus, Enterococcus, Pseudomonas, Acinetobacter, Stenotrophomonas, E. coli and Klebsiella spp significantly more often in their CR-POPF patients. Here, 74.2% of patients contaminated with Staphylococcus and 92.9% of patients with Klebsiella subsequently developed CR-POPF. Loos et al[137] similarly identified Staphylococcus spp. and Enterococcus spp. most frequently in the drain fluid of CR-POPF patients. Harino et al[163] found Staphylococcus numbers to increase in patients when drains were removed after POD5, with Yang’s group reporting rapid increases in positive drain culture when drains remained between POD3 and POD7 with a prevalence of 21.6% and 73.3% respectively[165]. Hence, earlier drain removal may assist in curbing the growth of bacteria and its subsequent role in CR-POPF development.
Yang et al[165] also found fungi to be isolated significantly more often in distal pancreatectomy patients who developed CR-POPF, while Abe et al[162] noted the absence of Candida which contrasted findings after pancreatoduodenectomy. Hence, the distinct drain culture portfolios following each resection type facilitate the identification of specific high-risk bacteria, the predictive potential of which would be enhanced by identifying the day of earliest detection and strongest association with CR-POPF. Moreover, it should be investigated whether mere presence of certain bacteria is predictive of CR-POPF or if there is a level at which risk is higher. Here, additional understanding of the time course for bacterial growth would assist close monitoring of colony numbers to facilitate better complication anticipation and prevention.
Other biomolecules: Drain lipase activity has been indirectly explored through alternate biomarkers for CR-POPF. Indeed, POD1 drain glycerol (> 800 μmol/L) has been associated with CR-POPF after pancreatoduodenectomy[168]. Similarly, drain free fatty acid has been significantly associated with CR-POPF. In an ensuing rat model, intraperitoneal lipolysis resulted in greater pancreatic juice leakage which risks CR-POPF by eroding the parenchyma and irritating acute pancreatitis[131]. Being products of lipolysis, drain glycerol and free fatty acids could serve as surrogate biomarkers for drain lipase, and hence CR-POPF. To effectively compare the predictive performance of these newer biomarkers however, a better understanding of their accuracy is required. To determine their clinical utility, their accuracy should also be compared against drain amylase and lipase.
Further, trypsin activation peptide (TAP) as a surrogate measure for protease activation has been explored. Xiu et al[169] found the TAP to drain amylase ratio in pancreatoduodenectomy patients to be significantly higher in CR-POPF patients, with this predictive measure being significantly higher when compared to distal pancreatectomy and biochemical leak patients. Wüster’s group identified TAP and chymotrypsin elevation to be uniquely associated with distal pancreatectomy and pancreatoduodenectomy CR-POPF patients, respectively[82]. Irrespective of resection type, myeloperoxidase and trypsin activity were significantly elevated on PODs 1-2 and PODs 1-7, respectively. However, amongst the CR-POPF patients, elastase was not found to be significantly associated with the complication[82]. Ansorge et al[168] identified a significantly higher intraperitoneal lactate to pyruvate ratio in CR-POPF patients which increased significantly between POD1 and POD2 due to increased lactate and decreased pyruvate, thereby implicating metabolic disruption in the pathophysiology of CR-POPF. Hence, these emerging biomarkers may offer new opportunities for bolstering CR-POPF prediction particularly if combined with established risk factors in future predictive models.
Drain fluid appearance: Observations of “sinister” drain effluent are often relied upon to inform an assessment of CR-POPF risk[76] and were a criteria of the initial consensus definition[23]. Abnormal drain fluid can be brown, green, milky or unusually clear[65]. Non-serous fluid following pancreatoduodenectomy has been independently associated with CR-POPF on POD1, POD3 and POD4[170]. However, Kosaka et al[105] did not find drain fluid colour to significantly differ between CR-POPF and non-CR-POPF pancreatoduodenectomy patients on POD4 on multivariate analysis agreeing with Suzumura et al’s findings following distal pancreatectomy[106]. Drain turbidity has been significantly correlated with drain fluid amylase on POD5 and beyond[108], suggesting its early observation could anticipate later development of CR-POPF.
This review revealed the potential for drain fluid biomarkers to overcome the limitations of the current diagnostic definition which necessitates a reactive management approach[24]. Numerous future directions for drain fluid research include investigating and confirming the accuracy of drain biomarkers in novel and established contexts respectively (Table 3). Through this, reports of biomarkers can specifically detail the accuracy of surgery-specific, risk-stratified cut-offs to clarify their clinical utility. Hence, decisions regarding drain removal and further monitoring can accordingly be made to either expediate or protect patient recovery respectively. Clarifying the clinical utility of drain bio
| Drain biomarker | To investigate | To confirm |
| Amylase | Accuracy of intraoperative predictive cut-offs in pancreatoduodenectomy patients | Diagnostic accuracy of proposed cut-offs |
| Accuracy of POD2 cut-offs in distal pancreatectomy patients | The change in postoperative drain amylase required to be predictive of CR-POPF in pancreatoduodenectomy patients | |
| Accuracy of POD4 cut-offs in surgery specific cohorts | If a persistently high value for drain amylase x drain volume postoperatively is predictive of CR-POPF | |
| Accuracy of risk-stratified cut-offs in distal pancreatectomy patients | ||
| The change in postoperative drain amylase required to be predictive of CR-POPF in distal pancreatectomy patients | ||
| When drain amylase has the highest predictive accuracy | ||
| Lipase | The change in postoperative drain lipase required to be predictive of CR-POPF in surgery specific cohorts and its accuracy | Diagnostic accuracy of proposed cut-offs |
| The accuracy of predictive cut-offs before POD3 in surgery specific cohorts | Diagnostic value of drain lipase in multi-factorial predictive models | |
| Accuracy of risk-stratified cut-offs in surgery specific cohorts | When drain amylase has the highest predictive accuracy | |
| Drain culture | Bacteria within polymicrobial drain fluid samples which predict grade B and C POPF in surgery specific cohorts | Clinical relevance of Enterobacter, Enterococcus and Staphylococcus to CR-POPF risk in pancreatoduodenectomy patients |
| When particular microorganisms are most predictive of CR-POPF | ||
| The concentrations of high-risk bacteria that accurately predict CR-POPF in surgery specific cohorts | ||
| Other biomolecules | Accuracy of predictive cut-offs for each biomarker in surgery specific cohorts | |
| Accuracy of novel enzymes compared to drain amylase and lipase in matched surgical cohorts and PODs | ||
| Fluid appearance | Accuracy on specific days before POD3 |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W, China; Kitamura K, Japan; Sharma V, India S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5134] [Article Influence: 466.7] [Reference Citation Analysis (0)] |
| 3. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1343] [Article Influence: 149.2] [Reference Citation Analysis (2)] |
| 4. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1730] [Article Influence: 192.2] [Reference Citation Analysis (1)] |
| 5. | Christenson ES, Jaffee E, Azad NS. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol. 2020;21:e135-e145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 6. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1261] [Article Influence: 180.1] [Reference Citation Analysis (39)] |
| 7. | Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 439] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 8. | Tumas J, Tumiene B, Jurkeviciene J, Jasiunas E, Sileikis A. Nutritional and immune impairments and their effects on outcomes in early pancreatic cancer patients undergoing pancreatoduodenectomy. Clin Nutr. 2020;39:3385-3394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Carrato A, Falcone A, Ducreux M, Valle JW, Parnaby A, Djazouli K, Alnwick-Allu K, Hutchings A, Palaska C, Parthenaki I. A Systematic Review of the Burden of Pancreatic Cancer in Europe: Real-World Impact on Survival, Quality of Life and Costs. J Gastrointest Cancer. 2015;46:201-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 10. | Gilliland TM, Villafane-Ferriol N, Shah KP, Shah RM, Tran Cao HS, Massarweh NN, Silberfein EJ, Choi EA, Hsu C, McElhany AL, Barakat O, Fisher W, Van Buren G. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 11. | Peters JH, Carey LC. Historical review of pancreaticoduodenectomy. Am J Surg. 1991;161:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, Anderson FA, Tseng JF. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Gleeson EM, Shaikh MF, Shewokis PA, Clarke JR, Meyers WC, Pitt HA, Bowne WB. WHipple-ABACUS, a simple, validated risk score for 30-day mortality after pancreaticoduodenectomy developed using the ACS-NSQIP database. Surgery. 2016;160:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Ecker BL, McMillan MT, Allegrini V, Bassi C, Beane JD, Beckman RM, Behrman SW, Dickson EJ, Callery MP, Christein JD, Drebin JA, Hollis RH, House MG, Jamieson NB, Javed AA, Kent TS, Kluger MD, Kowalsky SJ, Maggino L, Malleo G, Valero V 3rd, Velu LKP, Watkins AA, Wolfgang CL, Zureikat AH, Vollmer CM Jr. Risk Factors and Mitigation Strategies for Pancreatic Fistula After Distal Pancreatectomy: Analysis of 2026 Resections From the International, Multi-institutional Distal Pancreatectomy Study Group. Ann Surg. 2019;269:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 15. | Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): A systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 2017;96:e6858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 16. | Kanda M, Fujii T, Takami H, Suenaga M, Inokawa Y, Yamada S, Kobayashi D, Tanaka C, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Kodera Y. Novel diagnostics for aggravating pancreatic fistulas at the acute phase after pancreatectomy. World J Gastroenterol. 2014;20:8535-8544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Nagai S, Fujii T, Kodera Y, Kanda M, Sahin TT, Kanzaki A, Hayashi M, Sugimoto H, Nomoto S, Takeda S, Morita S, Nakao A. Recurrence pattern and prognosis of pancreatic cancer after pancreatic fistula. Ann Surg Oncol. 2011;18:2329-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Mosquera C, Vohra NA, Fitzgerald TL, Zervos EE. Discharge with Pancreatic Fistula after Pancreaticoduodenectomy Independently Predicts Hospital Readmission. Am Surg. 2016;82:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Williamsson C, Ansari D, Andersson R, Tingstedt B. Postoperative pancreatic fistula-impact on outcome, hospital cost and effects of centralization. HPB (Oxford). 2017;19:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Watanabe Y, Nishihara K, Matsumoto S, Okayama T, Abe Y, Nakano T. Effect of postoperative major complications on prognosis after pancreatectomy for pancreatic cancer: a retrospective review. Surg Today. 2017;47:555-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Mackay TM, Smits FJ, Roos D, Bonsing BA, Bosscha K, Busch OR, Creemers GJ, van Dam RM, van Eijck CHJ, Gerhards MF, de Groot JWB, Groot Koerkamp B, Haj Mohammad N, van der Harst E, de Hingh IHJT, Homs MYV, Kazemier G, Liem MSL, de Meijer VE, Molenaar IQ, Nieuwenhuijs VB, van Santvoort HC, van der Schelling GP, Stommel MWJ, Ten Tije AJ, de Vos-Geelen J, Wit F, Wilmink JW, van Laarhoven HWM, Besselink MG; Dutch Pancreatic Cancer Group. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: a nationwide analysis. HPB (Oxford). 2020;22:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Dhayat SA, Tamim ANJ, Jacob M, Ebeling G, Kerschke L, Kabar I, Senninger N. Postoperative pancreatic fistula affects recurrence-free survival of pancreatic cancer patients. PLoS One. 2021;16:e0252727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 24. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2956] [Article Influence: 369.5] [Reference Citation Analysis (35)] |
| 25. | McMillan MT, Allegrini V, Asbun HJ, Ball CG, Bassi C, Beane JD, Behrman SW, Berger AC, Bloomston M, Callery MP, Christein JD, Dickson E, Dixon E, Drebin JA, Fernandez-Del Castillo C, Fisher WE, Fong ZV, Haverick E, Hollis RH, House MG, Hughes SJ, Jamieson NB, Kent TS, Kowalsky SJ, Kunstman JW, Malleo G, McElhany AL, Salem RR, Soares KC, Sprys MH, Valero V 3rd, Watkins AA, Wolfgang CL, Zureikat AH, Vollmer CM Jr. Incorporation of Procedure-specific Risk Into the ACS-NSQIP Surgical Risk Calculator Improves the Prediction of Morbidity and Mortality After Pancreatoduodenectomy. Ann Surg. 2017;265:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Malleo G, Pulvirenti A, Marchegiani G, Butturini G, Salvia R, Bassi C. Diagnosis and management of postoperative pancreatic fistula. Langenbecks Arch Surg. 2014;399:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Kambakamba P, Mannil M, Herrera PE, Müller PC, Kuemmerli C, Linecker M, von Spiczak J, Hüllner MW, Raptis DA, Petrowsky H, Clavien PA, Alkadhi H. The potential of machine learning to predict postoperative pancreatic fistula based on preoperative, non-contrast-enhanced CT: A proof-of-principle study. Surgery. 2020;167:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Marasco G, Ricci C, Buttitta F, Dajti E, Ravaioli F, Ingaldi C, Alberici L, Serra C, Festi D, Colecchia A, Casadei R. Is Ultrasound Elastography Useful in Predicting Clinically Relevant Pancreatic Fistula After Pancreatic Resection? Pancreas. 2020;49:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Peng YP, Zhu XL, Yin LD, Zhu Y, Wei JS, Wu JL, Miao Y. Risk factors of postoperative pancreatic fistula in patients after distal pancreatectomy: a systematic review and meta-analysis. Sci Rep. 2017;7:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Wang GQ, Yadav DK, Jiang W, Hua YF, Lu C. Risk Factors for Clinically Relevant Postoperative Pancreatic Fistula (CR-POPF) after Distal Pancreatectomy: A Single Center Retrospective Study. Can J Gastroenterol Hepatol. 2021;2021:8874504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 919] [Article Influence: 70.7] [Reference Citation Analysis (2)] |
| 32. | Nahm CB, Brown KM, Townend PJ, Colvin E, Howell VM, Gill AJ, Connor S, Samra JS, Mittal A. Acinar cell density at the pancreatic resection margin is associated with post-pancreatectomy pancreatitis and the development of postoperative pancreatic fistula. HPB (Oxford). 2018;20:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Laaninen M, Bläuer M, Vasama K, Jin H, Räty S, Sand J, Nordback I, Laukkarinen J. The risk for immediate postoperative complications after pancreaticoduodenectomy is increased by high frequency of acinar cells and decreased by prevalent fibrosis of the cut edge of pancreas. Pancreas. 2012;41:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Kawai M, Kondo S, Yamaue H, Wada K, Sano K, Motoi F, Unno M, Satoi S, Kwon AH, Hatori T, Yamamoto M, Matsumoto J, Murakami Y, Doi R, Ito M, Miyakawa S, Shinchi H, Natsugoe S, Nakagawara H, Ohta T, Takada T. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Dahdaleh FS, Naffouje SA, Hanna MH, Salti GI. Impact of Neoadjuvant Systemic Therapy on Pancreatic Fistula Rates Following Pancreatectomy: a Population-Based Propensity-Matched Analysis. J Gastrointest Surg. 2021;25:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Rykina-Tameeva N, Nahm CB, Mehta S, Gill AJ, Samra JS, Mittal A. Neoadjuvant therapy for pancreatic cancer changes the composition of the pancreatic parenchyma. HPB (Oxford). 2020;22:1631-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Eshmuminov D, Karpovich I, Kapp J, Töpfer A, Endhardt K, Oberkofler C, Petrowsky H, Lenggenhager D, Tschuor C, Clavien PA. Pancreatic fistulas following distal pancreatectomy are unrelated to the texture quality of the pancreas. Langenbecks Arch Surg. 2021;406:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 38. | Nahm CB, Connor SJ, Samra JS, Mittal A. Postoperative pancreatic fistula: a review of traditional and emerging concepts. Clin Exp Gastroenterol. 2018;11:105-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 39. | Nguyen JH. Distinguishing between parenchymal and anastomotic leakage at duct-to-mucosa pancreatic reconstruction in pancreaticoduodenectomy. World J Gastroenterol. 2008;14:6648-6654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Hashimoto Y, Traverso LW. After distal pancreatectomy pancreatic leakage from the stump of the pancreas may be due to drain failure or pancreatic ductal back pressure. J Gastrointest Surg. 2012;16:993-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Hackert T, Klaiber U, Hinz U, Kehayova T, Probst P, Knebel P, Diener MK, Schneider L, Strobel O, Michalski CW, Ulrich A, Sauer P, Büchler MW. Sphincter of Oddi botulinum toxin injection to prevent pancreatic fistula after distal pancreatectomy. Surgery. 2017;161:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D, Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 43. | Balzano G, Zerbi A, Cristallo M, Di Carlo V. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005;9:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Kleeff J, Diener MK, Z'graggen K, Hinz U, Wagner M, Bachmann J, Zehetner J, Müller MW, Friess H, Büchler MW. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007;245:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 45. | Chen JY, Feng J, Wang XQ, Cai SW, Dong JH, Chen YL. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol. 2015;21:5926-5933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Tang T, Tan Y, Xiao B, Zu G, An Y, Zhang Y, Chen W, Chen X. Influence of Body Mass Index on Perioperative Outcomes Following Pancreaticoduodenectomy. J Laparoendosc Adv Surg Tech A. 2021;31:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Yoo HJ, Paik KY, Oh JS. Is there any different risk factor for clinical relevant pancreatic fistula according to the stump closure method following left-sided pancreatectomy? Ann Hepatobiliary Pancreat Surg. 2019;23:385-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Giovinazzo F, Dalla Riva GV, Greener D, Morano C, Linneman R, Besselink M, Abu Hilal M. A learning machine method to predict post-operative pancreatic fistula after pancreaticoduodenectomy based on amylases value in the drains: A multicentre database analysis of 1638 patients. HPB. 2018;20:S828. [DOI] [Full Text] |
| 49. | Partelli S, Pecorelli N, Muffatti F, Belfiori G, Crippa S, Piazzai F, Castoldi R, Marmorale C, Balzano G, Falconi M. Early Postoperative Prediction of Clinically Relevant Pancreatic Fistula after Pancreaticoduodenectomy: usefulness of C-reactive Protein. HPB (Oxford). 2017;19:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Kerem M, Dikmen K, Bostanci H, Ermis I, Buyukkasap AC. Predictive effect of postoperative 1st day drain amylase value on the development of pancreatic fistula that occurs after pancreaticoduodenectomy: A prospective clinicalal study. HPB. 2018;20:S645. [DOI] [Full Text] |
| 51. | Kawai M, Tani M, Hirono S, Ina S, Miyazawa M, Yamaue H. How do we predict the clinically relevant pancreatic fistula after pancreaticoduodenectomy? World J Surg. 2009;33:2670-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Teixeira UF, Rodrigues PD, Goldoni MB, Sampaio JA, Fontes PRO, Waechter FL. Early drain fluid amylase is useful to predict pancreatic fistula after pancreatoduodenectomy: Lessons learned from a southern brazilian center. Arq Gastroenterol. 2018;55:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Mimura T, Niguma T, Kojima T. Free orals: Biliary. HPB. 2012;14 (Suppl 2):107-287. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Kawaida H, Kono H, Watanabe M, Hosomura N, Amemiya H, Fujii H. Risk factors of postoperative pancreatic fistula after distal pancreatectomy using a triple-row stapler. Surg Today. 2018;48:95-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Recreo Baquedano A, Sánchez Acedo P, Zazpe Ripa C, Herrera J, Tarifa Castilla A, Fernández San José B, Pelegrín Esteban I. Predictive value of amylase determination in drainages for pancreatic fistula after pancreaticoduodenectomy. Pancreatology. 2019;19 (Suppl 2):S183. [DOI] [Full Text] |
| 56. | Newhook TE, Vega EA, Vreeland TJ, Prakash L, Dewhurst WL, Bruno ML, Kim MP, Ikoma N, Vauthey JN, Katz MH, Lee JE, Tzeng CD. Early postoperative drain fluid amylase in risk-stratified patients promotes tailored post-pancreatectomy drain management and potential for accelerated discharge. Surgery. 2020;167:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | van Dongen JC, Merkens S, Aziz MH, Groot Koerkamp B, van Eijck CHJ. The value of serum amylase and drain fluid amylase to predict postoperative pancreatic fistula after pancreatoduodenectomy: a retrospective cohort study. Langenbecks Arch Surg. 2021;406:2333-2341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 58. | de Reuver PR, Gundara J, Hugh TJ, Samra JS, Mittal A. Intra-operative amylase in peri-pancreatic fluid independently predicts for pancreatic fistula post pancreaticoduodectomy. HPB (Oxford). 2016;18:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Wang W, Qian H, Lin J, Weng Y, Zhang J, Wang J. Has the pancreatic fistula already occurred in the operation? Surg Open Sci. 2019;1:38-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Yu YD, Kim DS, Jung SW, Yoon YI. "Detecting pancreatic fistula beforehand": Utilization of drain fluid amylase measurement on the first postoperative day following pancreaticoduodenectomy. Korean Liver Society Spring/Autumn Conference (KASL). 2017;1:53-54. |
| 61. | Pinter Carvalheiro da Silva Boteon A, Longatto Boteon Y, Dasari B, Isaac J, Marudanayagam R, Mirza DF, Muiesan P, John Roberts K, Sutcliffe RP. Early predictors of clinically relevant post-operative pancreatic fistula after pancreaticoduodenectomy. HPB. 2018;20 (Suppl 2):S625. [DOI] [Full Text] |
| 62. | Mintziras I, Maurer E, Kanngiesser V, Bartsch DK. C-reactive protein and drain amylase accurately predict clinically relevant pancreatic fistula after partial pancreaticoduodenectomy. Int J Surg. 2020;76:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Bertens KA, Crown A, Clanton J, Alemi F, Alseidi AA, Biehl T, Helton WS, Rocha FG. What is a better predictor of clinically relevant postoperative pancreatic fistula (CR-POPF) following pancreaticoduodenectomy (PD): postoperative day one drain amylase (POD1DA) or the fistula risk score (FRS)? HPB (Oxford). 2017;19:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Ven Fong Z, Correa-Gallego C, Ferrone CR, Veillette GR, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Early Drain Removal--The Middle Ground Between the Drain Versus No Drain Debate in Patients Undergoing Pancreaticoduodenectomy: A Prospective Validation Study. Ann Surg. 2015;262:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 65. | Caputo D, Angeletti S, Ciccozzi M, Cartillone M, Cascone C, La Vaccara V, Coppola A, Coppola R. Role of drain amylase levels assay and routinary postoperative day 3 abdominal CT scan in prevention of complications and management of surgical drains after pancreaticoduodenectomy. Updates Surg. 2020;72:727-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Aleassa EM, Sharma G, Malik S, Morris-Stiff G. Lower is high enough: New suggested threshold for postoperative day 1 drain-fluid-amylase post pancreatoduodenectomy. HPB. 2018;20 (Suppl 2):S633. [DOI] [Full Text] |
| 67. | Peng JS, Ko JS, Chalikonda S, Wey JS, Walsh RM, Morris-Stiff G. Use of postoperative day 1 drain amylase levels to predict postoperative pancreatic fistulas. J Am Coll Surg. 2016;223:e147-e148. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Takeishi K, Maeda T, Yamashita Y, Tsujita E, Itoh S, Harimoto N, Ikegami T, Yoshizumi T, Shirabe K, Maehara Y. A Cohort Study for Derivation and Validation of Early Detection of Pancreatic Fistula After Pancreaticoduodenectomy. J Gastrointest Surg. 2016;20:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Ansorge C, Nordin J, Strommer L, Lundell Lars, Rangelova E, Blomberg J, Del Chiaro M, Segersvard R. The diagnostic value of pancreatic amylase analyses from prophylactic abdominal drainage in identifying pancreatic fistula following pancreaticoduodenectomy. Pancreatology. 2013;13:S82. [DOI] [Full Text] |
| 70. | Daniel F, Tamim H, Hosni M. Ueg week 2018 poster presentations. UEG. 2018;6:A423. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 71. | Maggino L, Malleo G, Bassi C, Allegrini V, Beane JD, Beckman RM, Chen B, Dickson EJ, Drebin JA, Ecker BL, Fraker DL, House MG, Jamieson NB, Javed AA, Kowalsky SJ, Lee MK, McMillan MT, Roses RE, Salvia R, Valero V 3rd, Velu LKP, Wolfgang CL, Zureikat AH, Vollmer CM Jr. Identification of an Optimal Cut-off for Drain Fluid Amylase on Postoperative Day 1 for Predicting Clinically Relevant Fistula After Distal Pancreatectomy: A Multi-institutional Analysis and External Validation. Ann Surg. 2019;269:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 72. | Lee CW, Pitt HA, Riall TS, Ronnekleiv-Kelly SS, Israel JS, Leverson GE, Parmar AD, Kilbane EM, Hall BL, Weber SM. Low drain fluid amylase predicts absence of pancreatic fistula following pancreatectomy. J Gastrointest Surg. 2014;18:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | El Nakeeb A, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, Hamdy E, Atef E, El Hanafy E, El-Geidie A, Abdel Wahab M, Abdallah T. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg. 2013;37:1405-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 74. | Casadei R, Ricci C, Taffurelli G, Pacilio CA, Di Marco M, Pagano N, Serra C, Calculli L, Santini D, Minni F. Prospective validation of a preoperative risk score model based on pancreatic texture to predict postoperative pancreatic fistula after pancreaticoduodenectomy. Int J Surg. 2017;48:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Kosaka H, Satoi S, Yamamoto T, Hirooka S, Yamaki S, Kotsuka M, Sakaguchi T, Inoue K, Matsui Y, Sekimoto M. Clinical impact of the sequentially-checked drain removal criteria on postoperative outcomes after pancreatectomy: a retrospective study. J Hepatobiliary Pancreat Sci. 2019;26:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | McMillan MT, Malleo G, Bassi C, Allegrini V, Casetti L, Drebin JA, Esposito A, Landoni L, Lee MK, Pulvirenti A, Roses RE, Salvia R, Vollmer CM Jr. Multicenter, Prospective Trial of Selective Drain Management for Pancreatoduodenectomy Using Risk Stratification. Ann Surg. 2017;265:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 77. | McMillan MT, Malleo G, Bassi C, Butturini G, Salvia R, Roses RE, Lee MK, Fraker DL, Drebin JA, Vollmer CM Jr. Drain Management after Pancreatoduodenectomy: Reappraisal of a Prospective Randomized Trial Using Risk Stratification. J Am Coll Surg. 2015;221:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 78. | Trudeau MT, Casciani F, Ecker BL, Maggino L, Seykora TF, Puri P, McMillan MT, Miller B, Pratt WB, Asbun HJ, Ball CG, Bassi C, Behrman SW, Berger AC, Bloomston MP, Callery MP, Castillo CF, Christein JD, Dillhoff ME, Dickson EJ, Dixon E, Fisher WE, House MG, Hughes SJ, Kent TS, Malleo G, Salem RR, Wolfgang CL, Zureikat AH, Vollmer CM; on the behalf of the Pancreas Fistula Study Group. The Fistula Risk Score Catalog: Toward Precision Medicine for Pancreatic Fistula After Pancreatoduodenectomy. Ann Surg. 2022;275:e463-e472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 79. | Yamane H, Abe T, Amano H, Hanada K, Minami T, Kobayashi T, Fukuda T, Yonehara S, Nakahara M, Ohdan H, Noriyuki T. Visceral Adipose Tissue and Skeletal Muscle Index Distribution Predicts Severe Pancreatic Fistula Development After Pancreaticoduodenectomy. Anticancer Res. 2018;38:1061-1066. [PubMed] [DOI] [Full Text] |
| 80. | Hiraki M, Miyoshi A, Sadashima E, Shinkai Y, Yasunami M, Manabe T, Kitahara K, Noshiro H. The novel early predictive marker presepsin for postoperative pancreatic fistula: A pilot study. Exp Ther Med. 2020;20:2298-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 81. | Kühlbrey CM, Samiei N, Sick O, Makowiec F, Hopt UT, Wittel UA. Pancreatitis After Pancreatoduodenectomy Predicts Clinically Relevant Postoperative Pancreatic Fistula. J Gastrointest Surg. 2017;21:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 82. | Wüster C, Shi H, Kühlbrey CM, Biesel EA, Hopt UT, Fichtner-Feigl S, Wittel UA. Pancreatic Inflammation and Proenzyme Activation Are Associated With Clinically Relevant Postoperative Pancreatic Fistulas After Pancreas Resection. Ann Surg. 2020;272:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Sugimoto M, Takahashi S, Gotohda N, Kato Y, Kinoshita T, Shibasaki H, Konishi M. Schematic pancreatic configuration: a risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2013;17:1744-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Gruppo M, Angriman I, Martella B, Spolverato YC, Zingales F, Bardini R. Perioperative albumin ratio is associated with post-operative pancreatic fistula. ANZ J Surg. 2018;88:E602-E605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Hanaki T, Uejima C, Amisaki M, Yosuke A, Tokuyasu N, Honjo S, Sakamoto T, Saito H, Ikeguchi M, Fujiwara Y. The attenuation value of preoperative computed tomography as a novel predictor for pancreatic fistula after pancreaticoduodenectomy. Surg Today. 2018;48:598-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Iida H, Tani M, Maehira H, Mori H, Kitamura N, Miyake T, Kaida S, Shimizu T. Postoperative Pancreatic Swelling Predicts Pancreatic Fistula after Pancreaticoduodenectomy. Am Surg. 2019;85:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Sakamoto K, Tokuhisa Y, Tokumitsu Y. Risk factors of pancreatic fistula after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2017;24:A387. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Singh H, Singh MK, Gupta V, Kochhar R, Medhi B, Yadav TD. The value of post-operative measurement of day one drain fluid amylase, serum amylase and serum crp as predictor of pancreatic fistula in pancreatic surgery. HPB. 2018;20 (Suppl 2):S617. [DOI] [Full Text] |
| 89. | Segersvard R, Blomberg J, Del Chiaro M, Rengelova E, Ansorge C. The diagnostic value of abdominal drainage in the individual risk assessment for pancreatic fistula following pancreaticoduodenectomy. Pancreatology. 2013;13:S8-S9. [DOI] [Full Text] |
| 90. | Ansorge C, Nordin JZ, Lundell L, Strömmer L, Rangelova E, Blomberg J, Del Chiaro M, Segersvärd R. Diagnostic value of abdominal drainage in individual risk assessment of pancreatic fistula following pancreaticoduodenectomy. Br J Surg. 2014;101:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Kawaida H, Watanabe M, Hosomura N. The predictive factors for postoperative pancreatic fistula after pancreaticoduodenectomy for soft pancreas. J Hepatobiliary Pancreat Sci. 2017;24 (Suppl 1):A278. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 92. | Yoshino J, Ban D, Ogura T, Ogawa K, Ono H, Mitsunori Y, Kudo A, Tanaka S, Tanabe M. The Clinical Implications of Peripancreatic Fluid Collection After Distal Pancreatectomy. World J Surg. 2019;43:2069-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Ohira G, Amano R, Kimura K. Analysis of pancreatic fistula after distal pancreatectomy. J Hepatobiliary Pancreat Sci. 2017;24 (Suppl 1):A279. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Iwasaki T, Nara S, Kishi Y, Esaki M, Takamoto T, Shimada K. Proposal of a Clinically Useful Criterion for Early Drain Removal After Pancreaticoduodenectomy. J Gastrointest Surg. 2021;25:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Araki M, Yasuda T, Yoshioka Y. Abstracts of papers submitted to the joint 43rd meeting of the american pancreatic association and the 17th meeting of the international association of pancreatology, october 31-november 3, 2012, miami, florida. Pancreas. 2012;41:1344-1416. [DOI] [Full Text] |
| 96. | Srivastava M, Kumaran V, Nundy S. Does drain amylase <666 iu/L on the third post-operative day effectively predicts the absence of a high-impact postoperative pancreatic fistula following pancreaticoduodenectomy? HPB. 2016;18 (Suppl 1):e111. [DOI] [Full Text] |
| 97. | Facy O, Chalumeau C, Poussier M, Binquet C, Rat P, Ortega-Deballon P. Diagnosis of postoperative pancreatic fistula. Br J Surg. 2012;99:1072-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Coayla G, Rodriguez C, Targarona J, Marcos JC, Hernandez R, Quijano J, Rivero L, Barreda L. Amylase value in drains after pancreatoduodenectomy as predictive factor of postoperative pancreatic fistula. Pancreatology. 2017;17:S39. [DOI] [Full Text] |
| 99. | Ceroni M, Galindo J, Guerra JF, Salinas J, Martínez J, Jarufe N. Amylase level in drains after pancreatoduodenectomy as a predictor of clinically significant pancreatic fistula. Pancreas. 2014;43:462-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Griffith D, Hanna T, Wong K, Reece-Smith A, Aroori S, Bowles M, Stell D, Briggs C. Comparison of lipase and amylase for diagnosing post-operative pancreatic fistulae. ANZ J Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 101. | Tzedakis S, Sauvanet A, Schiavone R, Razafinimanana M, Cauchy F, Rouet J, Dousset B, Gaujoux S. What should we trust to define, predict and assess pancreatic fistula after pancreatectomy? Pancreatology. 2020;20:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 102. | Roy M, Ban E, Mohandas S, Mownah O, Banerjee A, Valente R, Abraham AT, Kocher H, Bhattacharya S, Hutchins RR. Comparison of drain fluid lipase with drain fluid amylase in the context of post-operative pancreatic fistula. HPB. 2018;20:S674. [DOI] [Full Text] |
| 103. | Noji T, Nakamura T, Ambo Y, Suzuki O, Nakamura F, Kishida A, Hirano S, Kondo S, Kashimura N. Clinically relevant pancreas-related infectious complication after pancreaticoenteral anastomosis could be predicted by the parameters obtained on postoperative day 3. Pancreas. 2012;41:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Kosaka H, Kuroda N, Suzumura K. Abstracts of papers submitted to the international symposium on pancreas cancer 2012, cosponsored by the japan pancreas society, october 4-6, 2012, kyoto, japan. Pancreas. 2012;41:1140-1163. [DOI] [Full Text] |
| 105. | Kosaka H, Kuroda N, Suzumura K, Asano Y, Okada T, Fujimoto J. Multivariate logistic regression analysis for prediction of clinically relevant pancreatic fistula in the early phase after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2014;21:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Suzumura K, Iida K, Iwama H, Kawabata Y. Prediction of clinically relevant pancreatic fistula in the early phase after distal pancreatectomy. J Pancreas. 2019;20:121-125. |
| 107. | Hiyoshi M, Wada T, Tsuchimochi Y, Hamada T, Yano K, Imamura N, Fujii Y, Nanashima A. Usefulness of drain lipase to predict postoperative pancreatic fistula after distal pancreatectomy. Indian J Surg. 2020;82:1-2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 108. | Rajkamal R, Mathew J, Subramaniayer M, Ramesh H. Drain fluid amylase levels after pancreaticoduodenectomy for cancer: Correlation with outcomes and complications and evolution of a uniform grading system. HPB. 2019;21:S416-S417. [DOI] [Full Text] |
| 109. | Ridaura Capellino N, Protti Ruiz GP, Dopazo Taboada C, Blanco Cuso L, Pando E, Caralt M, Balsells J, Charco R. Drain fluid amylase on post-operative day 5 as pronostic factor of grade b-c pancreatic fistula after distal pancreatectomy. HPB. 2018;20:S601-S602. [DOI] [Full Text] |
| 110. | Dugalic VD, Knezevic DM, Obradovic VN, Gojnic-Dugalic MG, Matic SV, Pavlovic-Markovic AR, Dugalic PD, Knezevic SM. Drain amylase value as an early predictor of pancreatic fistula after cephalic duodenopancreatectomy. World J Gastroenterol. 2014;20:8691-8699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Moskovic DJ, Hodges SE, Wu MF, Brunicardi FC, Hilsenbeck SG, Fisher WE. Drain data to predict clinically relevant pancreatic fistula. HPB (Oxford). 2010;12:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Sutcliffe RP, Hamoui M, Pitchaimuthu M, Isaac J, Marudanayagam R, Mirza DF, Muiesan P, John Roberts K. First postoperative day drain fluid amylase greater than 2000 iu/L predicts grade c pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2014;101:7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Caputo D, Coppola A, Cascone C, Angeletti S, Ciccozzi M, La Vaccara V, Coppola R. Preoperative systemic inflammatory biomarkers and postoperative day 1 drain amylase value predict grade C pancreatic fistula after pancreaticoduodenectomy. Ann Med Surg (Lond). 2020;57:56-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | Chiba N, Ochiai S, Yokozuka K, Gunji T, Sano T, Tomita K, Tsutsui R, Kawachi S. Risk Factors for Life-threatening Grade C Postoperative Pancreatic Fistula After Pancreatoduodenectomy Compared to Grade B. Anticancer Res. 2019;39:2199-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 115. | Li Y, Zhou F, Zhu DM, Zhang ZX, Yang J, Yao J, Wei YJ, Xu YL, Li DC, Zhou J. Novel risk scoring system for prediction of pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol. 2019;25:2650-2664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 116. | Linnemann RJA, Patijn GA, van Rijssen LB, Besselink MG, Mungroop TH, de Hingh IH, Kazemier G, Festen S, de Jong KP, van Eijck CHJ, Scheepers JJG, van der Kolk M, Dulk MD, Bosscha K, Busch OR, Boerma D, van der Harst E, Nieuwenhuijs VB; Dutch Pancreatic Cancer Group. The role of abdominal drainage in pancreatic resection - A multicenter validation study for early drain removal. Pancreatology. 2019;19:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 117. | Koizumi M, Sata N, Taguchi M. Management of pancreatic anastomotic leakage by measuring drainage amylase and volume after pylorus preserving pancreatoduodenectomy. Pancreas. 2015;44:1387-1388. [DOI] [Full Text] |
| 118. | Hiyoshi M, Chijiiwa K, Fujii Y, Imamura N, Nagano M, Ohuchida J. Usefulness of drain amylase, serum C-reactive protein levels and body temperature to predict postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2013;37:2436-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 119. | Okano K, Kakinoki K, Suto H, Oshima M, Kashiwagi H, Yamamoto N, Akamoto S, Fujiwara M, Takama T, Usuki H, Hagiike M, Suzuki Y. Persisting ratio of total amylase output in drain fluid can predict postoperative clinical pancreatic fistula. J Hepatobiliary Pancreat Sci. 2011;18:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 120. | Furukawa K, Gocho T, Shirai Y, Iwase R, Haruki K, Fujiwara Y, Shiba H, Misawa T, Yanaga K. The Decline of Amylase Level of Pancreatic Juice After Pancreaticoduodenectomy Predicts Postoperative Pancreatic Fistula. Pancreas. 2016;45:1474-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Kuhara K, Shiozawa S, Usui T, Tsuchiya A, Miyauchi T, Kono T, Shimojima Y, Yamaguchi K, Yokomizo H, Shimakawa T, Yoshimatsu K, Katsube T, Naritaka Y. [Analysis of the Relationship between the Change of Drain Amylase Value and Postoperative Pancreatic Fistula after Pancreaticoduodenectomy]. Gan To Kagaku Ryoho. 2017;44:1729-1731. [PubMed] |
| 122. | Nobuoka D, Gotohda N, Konishi M, Nakagohri T, Takahashi S, Kinoshita T. The correlation between postoperative pancreatic fistula and volume of amylase discharge in drainage fluid after pancreaticoduodenectomy. Japanese J Gastroenter Surg. 2010;43:351-358. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 123. | Connor S. Defining post-operative pancreatitis as a new pancreatic specific complication following pancreatic resection. HPB (Oxford). 2016;18:642-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 124. | Birgin E, Reeg A, Téoule P, Rahbari NN, Post S, Reissfelder C, Rückert F. Early postoperative pancreatitis following pancreaticoduodenectomy: what is clinically relevant postoperative pancreatitis? HPB (Oxford). 2019;21:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 125. | Andrianello S, Bannone E, Marchegiani G, Malleo G, Paiella S, Esposito A, Salvia R, Bassi C. Characterization of postoperative acute pancreatitis (POAP) after distal pancreatectomy. Surgery. 2021;169:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 126. | Ikenaga N, Ohtsuka T, Nakata K, Watanabe Y, Mori Y, Nakamura M. Clinical significance of postoperative acute pancreatitis after pancreatoduodenectomy and distal pancreatectomy. Surgery. 2021;169:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 127. | Noel P, Patel K, Durgampudi C, Trivedi RN, de Oliveira C, Crowell MD, Pannala R, Lee K, Brand R, Chennat J, Slivka A, Papachristou GI, Khalid A, Whitcomb DC, DeLany JP, Cline RA, Acharya C, Jaligama D, Murad FM, Yadav D, Navina S, Singh VP. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut. 2016;65:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 128. | Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT, Furlan A, Behari J, Liu S, McHale T, Nichols L, Papachristou GI, Yadav D, Singh VP. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 318] [Article Influence: 24.5] [Reference Citation Analysis (2)] |
| 129. | Ishola DA Jr, Post JA, van Timmeren MM, Bakker SJ, Goldschmeding R, Koomans HA, Braam B, Joles JA. Albumin-bound fatty acids induce mitochondrial oxidant stress and impair antioxidant responses in proximal tubular cells. Kidney Int. 2006;70:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Arany I, Clark JS, Reed DK, Juncos LA, Dixit M. Role of p66shc in renal toxicity of oleic acid. Am J Nephrol. 2013;38:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Uchida Y, Masui T, Nakano K, Yogo A, Sato A, Nagai K, Anazawa T, Takaori K, Tabata Y, Uemoto S. Clinical and experimental studies of intraperitoneal lipolysis and the development of clinically relevant pancreatic fistula after pancreatic surgery. Br J Surg. 2019;106:616-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Müssle B, Oehme F, Schade S, Sommer M, Bogner A, Hempel S, Pochhammer J, Kahlert C, Distler M, Weitz J, Welsch T. Drain Amylase or Lipase for the Detection of POPF-Adding Evidence to an Ongoing Discussion. J Clin Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 133. | Suzuki S, Shimoda M, Shimazaki J, Oshiro Y, Nishida K, Shiihara M, Izumo W, Yamamoto M. Drain Lipase Levels and Decreased Rate of Drain Amylase Levels as Independent Predictors of Pancreatic Fistula with Nomogram After Pancreaticoduodenectomy. World J Surg. 2021;45:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 134. | Frymerman AS, Schuld J, Ziehen P, Kollmar O, Justinger C, Merai M, Richter S, Schilling MK, Moussavian MR. Impact of postoperative pancreatic fistula on surgical outcome--the need for a classification-driven risk management. J Gastrointest Surg. 2010;14:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 135. | Kitahata Y, Kawai M, Tani M, Hirono S, Okada K, Miyazawa M, Shimizu A, Yamaue H. Preoperative cholangitis during biliary drainage increases the incidence of postoperative severe complications after pancreaticoduodenectomy. Am J Surg. 2014;208:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 136. | Akashi M, Nagakawa Y, Hosokawa Y, Takishita C, Osakabe H, Nishino H, Katsumata K, Akagi Y, Itoi T, Tsuchida A. Preoperative cholangitis is associated with increased surgical site infection following pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2020;27:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 137. | Loos M, Strobel O, Legominski M, Dietrich M, Hinz U, Brenner T, Heininger A, Weigand MA, Büchler MW, Hackert T. Postoperative pancreatic fistula: Microbial growth determines outcome. Surgery. 2018;164:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 138. | Yamashita K, Kato D, Sasaki T, Shiwaku H, Ishii F, Naito S, Yamashita Y, Hasegawa S. Contaminated drainage fluid and pancreatic fistula after pancreatoduodenectomy: A retrospective study. Int J Surg. 2018;52:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 139. | Yang F, Jin C, Li J, Di Y, Zhang J, Fu D. Clinical significance of drain fluid culture after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2018;25:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 140. | Sugiura T, Mizuno T, Okamura Y, Ito T, Yamamoto Y, Kawamura I, Kurai H, Uesaka K. Impact of bacterial contamination of the abdominal cavity during pancreaticoduodenectomy on surgical-site infection. Br J Surg. 2015;102:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 141. | Nagakawa Y, Matsudo T, Hijikata Y, Kikuchi S, Bunso K, Suzuki Y, Kasuya K, Tsuchida A. Bacterial contamination in ascitic fluid is associated with the development of clinically relevant pancreatic fistula after pancreatoduodenectomy. Pancreas. 2013;42:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Morimoto M, Honjo S, Sakamoto T, Yagyu T, Uchinaka E, Amisaki M, Watanabe J, Yamamoto M, Fukumoto Y, Tokuyasu N, Ashida K, Saito H, Fujiwara Y. Bacterial smear test of drainage fluid after pancreaticoduodenectomy can predict postoperative pancreatic fistula. Pancreatology. 2019;19:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 143. | Sato A, Masui T, Nakano K, Sankoda N, Anazawa T, Takaori K, Kawaguchi Y, Uemoto S. Abdominal contamination with Candida albicans after pancreaticoduodenectomy is related to hemorrhage associated with pancreatic fistulas. Pancreatology. 2017;17:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Uemura K, Murakami Y, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, Sasaki H, Okada K, Ohge H, Sueda T. Activation of pancreatic enzyme plus bacterial infection plays an important role in the pathogenic mechanism of clinically relevant popf after pancreaticoduodenectomy. Gastroenterology. 2013;144:S1082. [DOI] [Full Text] |
| 145. | Hata T, Mizuma M, Motoi F, Nakagawa K, Masuda K, Ishida M, Morikawa T, Hayashi H, Kamei T, Naitoh T, Unno M. Early postoperative drainage fluid culture positivity from contaminated bile juice is predictive of pancreatic fistula after pancreaticoduodenectomy. Surg Today. 2020;50:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 146. | Kimura N, Ishido K, Kudo D, Wakiya T, Odagiri T, Wakasa T, Toyoki Y, Hakamada K. Causative effect of bacterial infection on pancreatic fistula and appropriate timing of drain removal after pancreaticoduodenectomy. Pancreatology. 2016;16:S109-S110. [DOI] [Full Text] |
| 147. | Taniguchi K, Matsuyama R, Yabushita Y, Homma Y, Ota Y, Mori R, Morioka D, Endo I. Prophylactic drain management after pancreaticoduodenectomy without focusing on the drain fluid amylase level: A prospective validation study regarding criteria for early drain removal that do not include the drain fluid amylase level. J Hepatobiliary Pancreat Sci. 2020;27:950-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 148. | Osakabe H, Nagakawa Y, Takishita C, Hijikata Y, Kiya Y, Akashi M, Nishino H, Nakajima T, Shirota T, Sahara Y, Hosokawa Y. Relationship Between Drain Fluid Culture and Clinically Relevant Post-Operative Pancreatic Fistula After Pancreatectomy. Proceedings of the joint 50th anniversary meeting of the American pancreatic association and Japan pancreas society; 2019 Nov 6-9; Maui, Hawaii. Miami: American Pancreatic Association, 2019: 1401-1564. |
| 149. | Abstracts of the Twelfth Annual Americas Hepato-Pancreato-Biliary Congress. March 7-11, 2012. Miami Beach, Florida, USA. HPB (Oxford). 2012;14 Suppl 1:1-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 150. | Belmouhand M, Krohn PS, Svendsen LB, Henriksen A, Hansen CP, Achiam MP. The occurrence of Enterococcus faecium and faecalis is significantly associated with anastomotic leakage after pancreaticoduodenectomy. Scand J Surg. 2018;107:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 151. | Kimura N, Ishido K, Nagase H, Kudo K, Hakamada K. Peripancreatic bacterial contamination can trigger the development of pancreatic fistula after pancreaticoduodenectomy. HPB. 2019;21:S453-S454. [DOI] [Full Text] |
| 152. | Imamura M, Kimura Y, Meguro M, Nishidate T, Ito T, Kyuno D, Ishii M, Kawamoto M, Mizuguchi T, Hirata K. The correlation between postoperative pancreatic fistula and bacterial infection after the pancreatoduodenectomy: Importance of the ivr of the early postoperative period. HPB. 2014;16:626. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 154. | Yamashita K, Sasaki T, Itoh R, Kato D, Hatano N, Soejima T, Ishii K, Takenawa T, Hiromatsu K, Yamashita Y. Pancreatic fistulae secondary to trypsinogen activation by Pseudomonas aeruginosa infection after pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2015;22:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 155. | Yamashita K, Kato D, Sasaki T, Ishii F, Okada H, Hirano Y, Hayashi T, Yamada T, Hasegawa S. Clinical impact of bacterial contamination of intra-abdominal discharge on the incidence of pancreatic fistula after pancreati coduodenectomy. United European Gastroenterol J. 2019;7:303. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 156. | Abstracts of Papers Submitted to the 49th Annual Meeting of the American Pancreatic Association, October 31-November 3, 2018, Miami Beach, Florida. Pancreas. 2018;47:1370-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 157. | McMillan MT, Vollmer CM Jr, Asbun HJ, Ball CG, Bassi C, Beane JD, Berger AC, Bloomston M, Callery MP, Christein JD, Dixon E, Drebin JA, Castillo CF, Fisher WE, Fong ZV, Haverick E, House MG, Hughes SJ, Kent TS, Kunstman JW, Malleo G, McElhany AL, Salem RR, Soares K, Sprys MH, Valero V 3rd, Watkins AA, Wolfgang CL, Behrman SW. The Characterization and Prediction of ISGPF Grade C Fistulas Following Pancreatoduodenectomy. J Gastrointest Surg. 2016;20:262-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 158. | Demir E, Abdelhai K, Demir IE, Jäger C, Scheufele F, Schorn S, Rothe K, Friess H, Ceyhan GO. Association of bacteria in pancreatic fistula fluid with complications after pancreatic surgery. BJS Open. 2020;4:432-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 159. | De Pastena M, Paiella S, Marchegiani G, Malleo G, Ciprani D, Gasparini C, Secchettin E, Salvia R, Bassi C. Postoperative infections represent a major determinant of outcome after pancreaticoduodenectomy: Results from a high-volume center. Surgery. 2017;162:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 160. | Takishita C. Preoperative cholangitis is associated with the development of clinically relevant surgical site infection (ssi) that cause pancreatic fistula after pancreatoduodenectomy. HPB. 2016;18:e455. [DOI] [Full Text] |
| 161. | Maatman TK, Weber DJ, Qureshi B, Ceppa EP, Nakeeb A, Schmidt CM, Zyromski NJ, House MG. Does the Microbiology of Bactibilia Drive Postoperative Complications After Pancreatoduodenectomy? J Gastrointest Surg. 2020;24:2544-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 162. | Abe K, Kitago M, Shinoda M, Yagi H, Abe Y, Oshima G, Hori S, Yokose T, Endo Y, Kitagawa Y. High risk pathogens and risk factors for postoperative pancreatic fistula after pancreatectomy; a retrospective case-controlled study. Int J Surg. 2020;82:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 163. | Harino T, Noguchi K, Yanagimoto Y. Clinical Significance of Drainage Culture Contamination in Pancreatic Fistula After Distal Pancreatectomy. Proceedings of the joint 50th anniversary meeting of the American pancreatic association and Japan pancreas society, November 6-9, 2019, Maui, Hawaii. Miami: American Pancreatic Association, 2019: 1401-1564. |
| 164. | Mori R, Matsuyama R, Ota Y, Hiratani S, Goto K, Miyake K, Sawada Y, Kumamoto T, Takeda K, Endo I. The risk factors of postoperative pancreatic fistula after distal pancreatectomy. Pancreatology. 2016;16:S127. [DOI] [Full Text] |
| 165. | Yang F, Jin C, Hao S, Fu D. Drain Contamination after Distal Pancreatectomy: Incidence, Risk Factors, and Association with Postoperative Pancreatic Fistula. J Gastrointest Surg. 2019;23:2449-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 166. | Osakabe H, Nagakawa Y, Kozono S, Takishita C, Nakagawa N, Nishino H, Suzuki K, Shirota T, Hosokawa Y, Akashi M, Ishizaki T, Katsumata K, Tsuchida A. Causative bacteria associated with a clinically relevant postoperative pancreatic fistula infection after distal pancreatectomy. Surg Today. 2021;51:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 167. | Sato A, Masui T, Nakano K, Ito T, Anazawa T, Kawaguchi M, Kawaguchi Y, Takaori K, Uemoto S. Is drainage culture useful in predicting postoperative pancreatic fistula after distal pancreatectomy? Pancreatology. 2016;16:S79. [DOI] [Full Text] |
| 168. | Ansorge C, Regner S, Segersvärd R, Strömmer L. Early intraperitoneal metabolic changes and protease activation as indicators of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2012;99:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 169. | Xiu D, Wang H, Li M. The research of drainage trypsin activation peptide (TAP) in post-operation pancreatic fistula (POPF). HPB. 2019;21:S446. [DOI] [Full Text] |
| 170. | Uemura K, Murakami Y, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, Sasaki H, Ohge H, Sueda T. Indicators for proper management of surgical drains following pancreaticoduodenectomy. J Surg Oncol. 2014;109:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 171. | Rollin N, Cassese G, Pineton DE Chambrun G, Serrand C, Navarro F, Blanc P, Panaro F, Valats JC. An easy-to-use score to predict clinically relevant postoperative pancreatic fistula after distal pancreatectomy. Minerva Surg. 2022;77:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 172. | Li B, Pu N, Chen Q, Mei Y, Wang D, Jin D, Wu W, Zhang L, Lou W. Comprehensive Diagnostic Nomogram for Predicting Clinically Relevant Postoperative Pancreatic Fistula After Pancreatoduodenectomy. Front Oncol. 2021;11:717087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 173. | Shen J, Guo F, Sun Y, Zhao J, Hu J, Ke Z, Zhang Y, Jin X, Wu H. Predictive nomogram for postoperative pancreatic fistula following pancreaticoduodenectomy: a retrospective study. BMC Cancer. 2021;21:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |